Effects of Different Exercises Combined with Different Dietary Interventions on Body Composition: A Systematic Review and Network Meta-Analysis
Abstract
:1. Introduction
2. Methods
2.1. Protocol and Registration
2.2. Search Strategy
2.3. Inclusion Criteria and Exclusion Criteria
- (1)
- Must be a randomized controlled trial and include a group receiving a combination of exercise and dietary intervention.
- (2)
- Must include a control group, which could be no intervention, exercise only, or dietary intervention only.
- (3)
- Participants must be adults aged 18–65 years.
- (4)
- Participants must be healthy individuals.
- (5)
- Outcome measures must include at least one of the following: body weight, BMI, fat mass, or fat percentage.
- (1)
- Studies that are not randomized controlled trials.
- (2)
- Non-human studies.
- (3)
- Non-original studies, including reviews, letters, case reports, or papers that do not provide accurate and clear data.
- (4)
- Studies where participants are children, elderly, or individuals with any diseases.
2.4. Data Extraction
2.5. Risk of Bias Assessment
2.6. Statistical Analysis
3. Results
3.1. Search Results and Study Selection
3.2. Study Characteristics
3.3. Risk of Bias in Included Studies
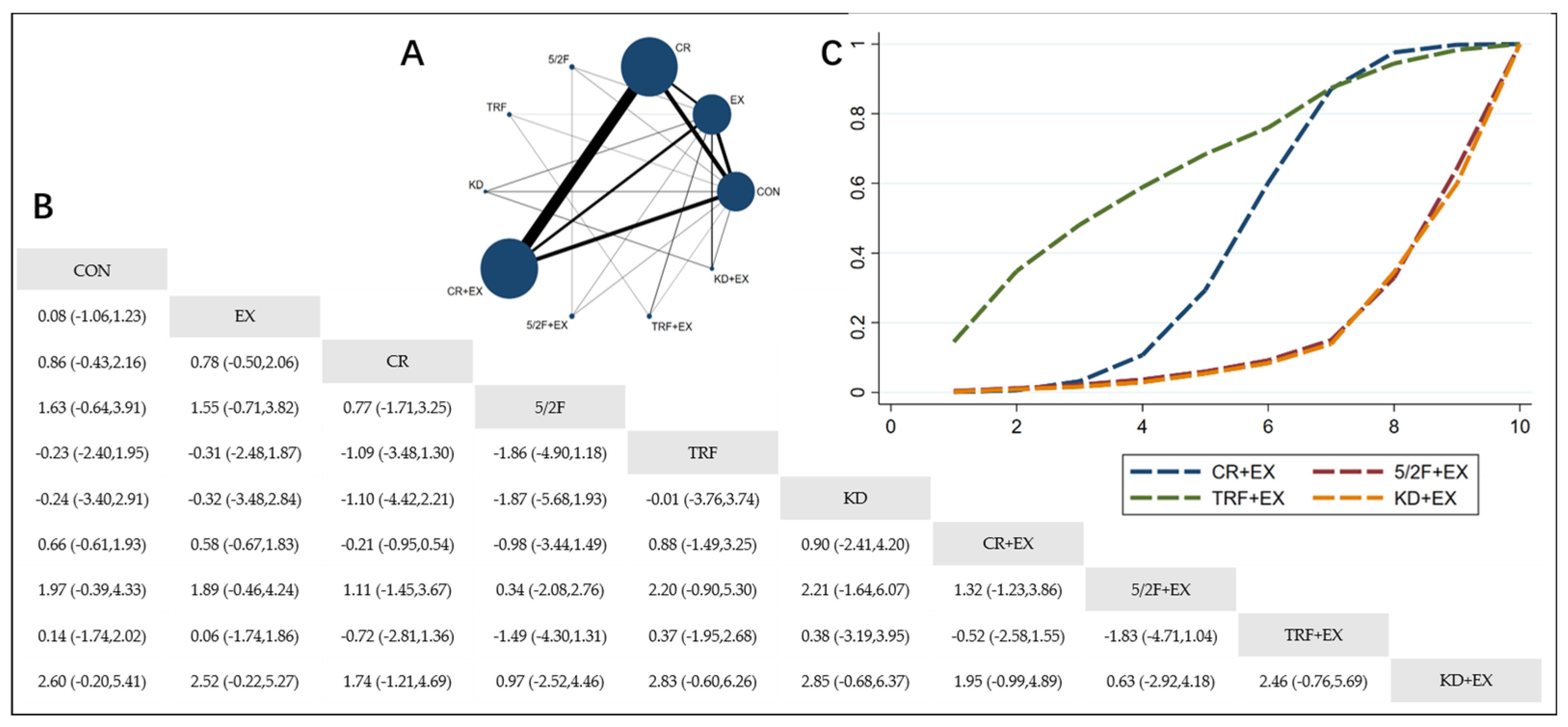
3.4. Effects of the Interventions
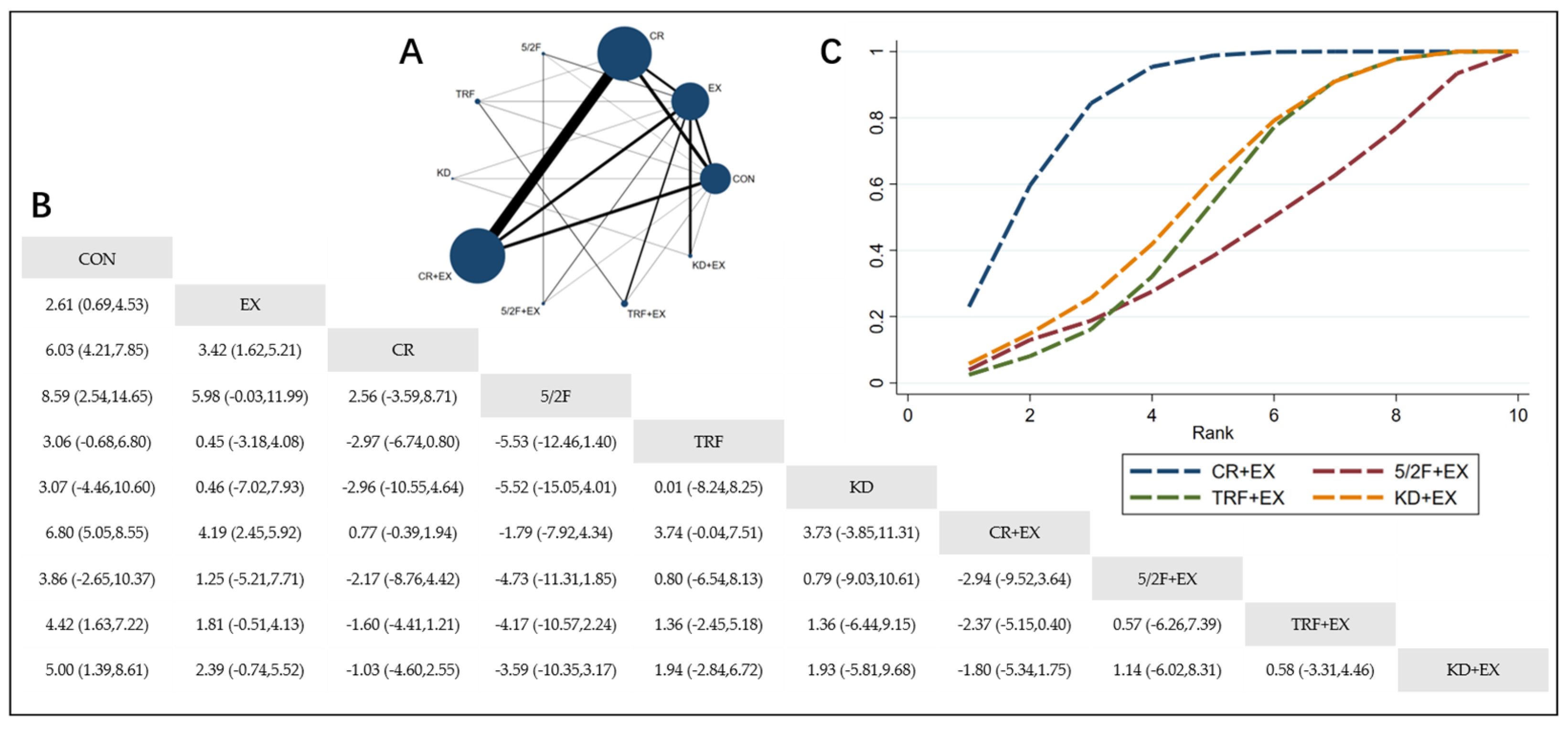
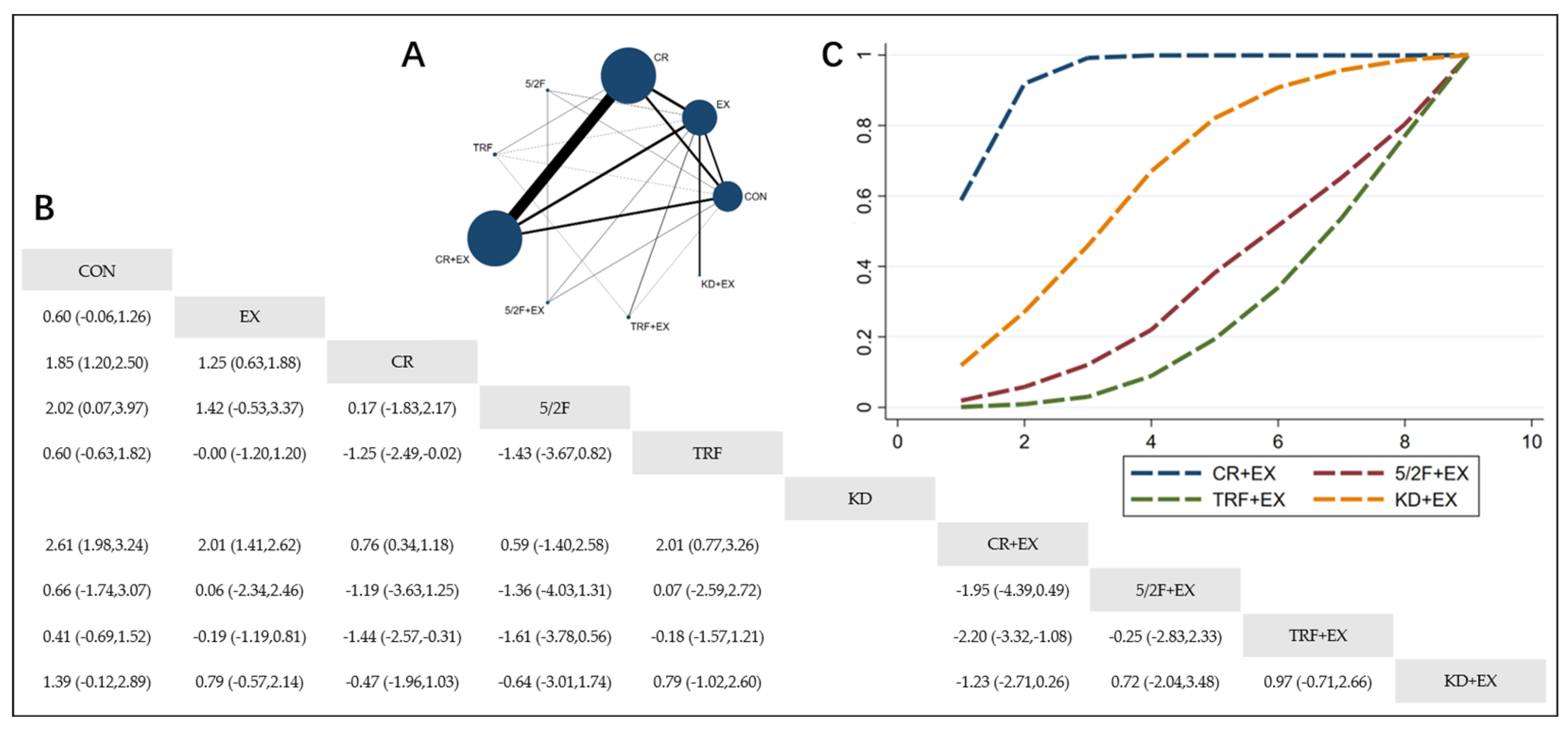
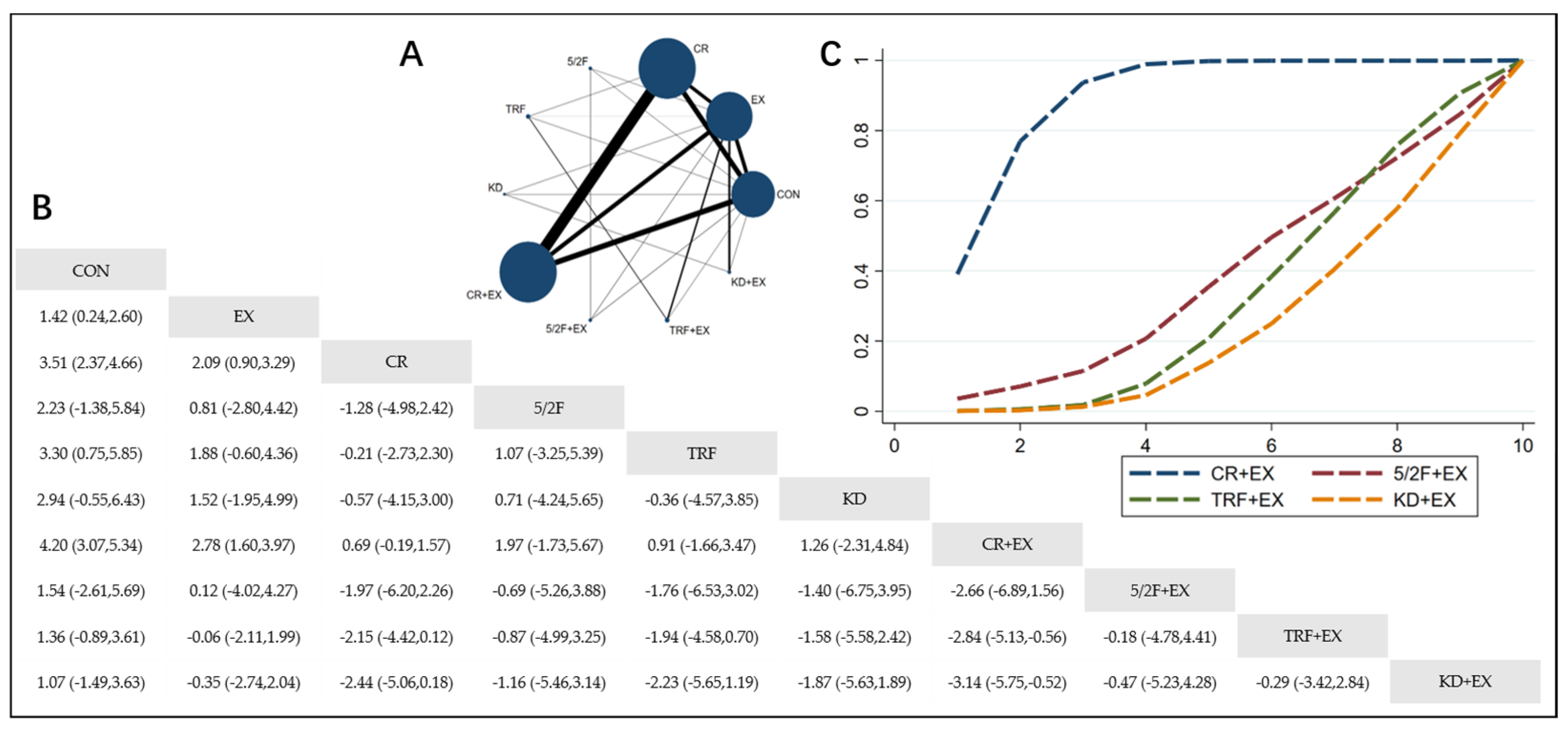
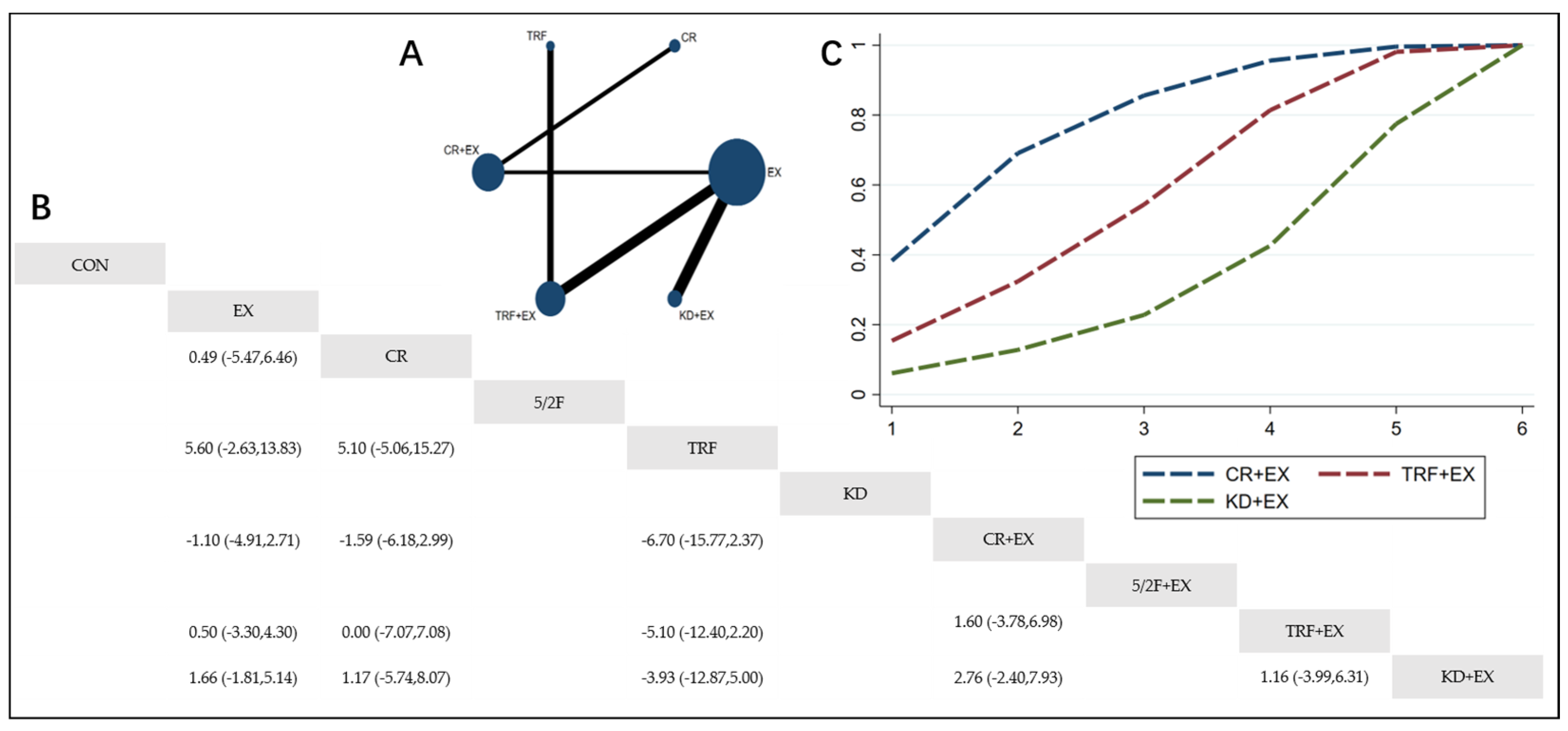
3.4.1. The Effect of Different Dietary Interventions Combined with Exercise on Body Weight
3.4.2. The Effect of Different Dietary Interventions Combined with Exercise on BMI
3.4.3. The Effect of Different Dietary Interventions Combined with Exercise on Body Fat Percentage
3.4.4. The Effect of Different Dietary Interventions Combined with Exercise on Lean Body Mass
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kong, Z.; Sun, S.; Shi, Q.; Zhang, H.; Tong, T.K.; Nie, J. Short-Term Ketogenic Diet Improves Abdominal Obesity in Overweight/Obese Chinese Young Females. Front. Physiol. 2020, 11, 856. [Google Scholar] [CrossRef]
- Johnstone, A.M.; Horgan, G.W.; Murison, S.D.; Bremner, D.M.; Lobley, G.E. Effects of a High-Protein Ketogenic Diet on Hunger, Appetite, and Weight Loss in Obese Men Feeding Ad Libitum. Am. J. Clin. Nutr. 2008, 87, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Varady, K.A.; Bhutani, S.; Church, E.C.; Klempel, M.C. Short-Term Modified Alternate-Day Fasting: A Novel Dietary Strategy for Weight Loss and Cardioprotection in Obese Adults12. Am. J. Clin. Nutr. 2009, 90, 1138–1143. [Google Scholar] [CrossRef]
- Sutton, E.F.; Beyl, R.; Early, K.S.; Cefalu, W.T.; Ravussin, E.; Peterson, C.M. Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes. Cell Metab. 2018, 27, 1212–1221. [Google Scholar] [CrossRef] [PubMed]
- Harvie, M.N.; Pegington, M.; Mattson, M.P.; Frystyk, J.; Dillon, B.; Evans, G.; Cuzick, J.; Jebb, S.A.; Martin, B.; Cutler, R.G.; et al. The Effects of Intermittent or Continuous Energy Restriction on Weight Loss and Metabolic Disease Risk Markers: A Randomized Trial in Young Overweight Women. Int. J. Obes. 2011, 35, 714–727. [Google Scholar] [CrossRef]
- Parvaresh, A.; Razavi, R.; Abbasi, B.; Yaghoobloo, K.; Hassanzadeh, A.; Mohammadifard, N.; Safavi, S.M.; Hadi, A.; Clark, C.C.T. Modified Alternate-Day Fasting vs. Calorie Restriction in the Treatment of Patients with Metabolic Syndrome: A Randomized Clinical Trial. Complement. Ther. Med. 2019, 47, 102187. [Google Scholar] [CrossRef]
- Sundfør, T.M.; Svendsen, M.; Tonstad, S. Effect of Intermittent versus Continuous Energy Restriction on Weight Loss, Maintenance and Cardiometabolic Risk: A Randomized 1-Year Trial. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Gabel, K.; Hoddy, K.K.; Haggerty, N.; Song, J.; Kroeger, C.M.; Trepanowski, J.F.; Panda, S.; Varady, K.A. Effects of 8-Hour Time Restricted Feeding on Body Weight and Metabolic Disease Risk Factors in Obese Adults: A Pilot Study. Nutr. Healthy Aging 2018, 4, 345–353. [Google Scholar] [CrossRef]
- Lettieri-Barbato, D.; Cannata, S.M.; Casagrande, V.; Ciriolo, M.R.; Aquilano, K. Time-controlled fasting prevents aging-like mitochondrial changes induced by persistent dietary fat overload in skeletal muscle. PLoS ONE 2018, 13, e0195912. [Google Scholar] [CrossRef]
- Lanza, I.R.; Zabielski, P.; Klaus, K.A.; Morse, D.M.; Heppelmann, C.J.; Bergen, H.R.; Dasari, S.; Walrand, S.; Short, K.R.; Johnson, M.L.; et al. Chronic Caloric Restriction Preserves Mitochondrial Function in Senescence without Increasing Mitochondrial Biogenesis. Cell Metab. 2012, 16, 777–788. [Google Scholar] [CrossRef]
- Miller, V.J.; Villamena, F.A.; Volek, J.S. Nutritional Ketosis and Mitohormesis: Potential Implications for Mitochondrial Function and Human Health. J. Nutr. Metab. 2018, 2018, 5157645. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.B.; Summer, W.; Cutler, R.G.; Martin, B.; Hyun, D.-H.; Dixit, V.D.; Pearson, M.; Nassar, M.; Telljohann, R.; Maudsley, S.; et al. Alternate Day Calorie Restriction Improves Clinical Findings and Reduces Markers of Oxidative Stress and Inflammation in Overweight Adults with Moderate Asthma. Free Radic. Biol. Med. 2007, 42, 665–674. [Google Scholar] [CrossRef]
- Cienfuegos, S.; Gabel, K.; Kalam, F.; Ezpeleta, M.; Wiseman, E.; Pavlou, V.; Lin, S.; Oliveira, M.L.; Varady, K.A. Effects of 4- and 6-h Time-Restricted Feeding on Weight and Cardiometabolic Health: A Randomized Controlled Trial in Adults with Obesity. Cell Metab. 2020, 32, 366–378. [Google Scholar] [CrossRef] [PubMed]
- Manning, P.J.; Sutherland, W.H.F.; Walker, R.J.; Williams, S.M.; De Jong, S.A.; Ryalls, A.R.; Berry, E.A. Effect of High-Dose Vitamin E on Insulin Resistance and Associated Parameters in Overweight Subjects. Diabetes Care 2004, 27, 2166–2171. [Google Scholar] [CrossRef] [PubMed]
- Rains, J.L.; Jain, S.K. Oxidative Stress, Insulin Signaling, and Diabetes. Free Radic. Biol. Med. 2011, 50, 567–575. [Google Scholar] [CrossRef]
- Houstis, N.; Rosen, E.D.; Lander, E.S. Reactive Oxygen Species Have a Causal Role in Multiple Forms of Insulin Resistance. Nature 2006, 440, 944–948. [Google Scholar] [CrossRef]
- Zaulkffali, A.S.; Md Razip, N.N.; Syed Alwi, S.S.; Abd Jalil, A.; Abd Mutalib, M.S.; Gopalsamy, B.; Chang, S.K.; Zainal, Z.; Ibrahim, N.N.; Zakaria, Z.A.; et al. Vitamins D and E Stimulate the PI3K-AKT Signalling Pathway in Insulin-Resistant SK-N-SH Neuronal Cells. Nutrients 2019, 11, 2525. [Google Scholar] [CrossRef]
- Wang, S.; Huang, M.; You, X.; Zhao, J.; Chen, L.; Wang, L.; Luo, Y.; Chen, Y. Gut Microbiota Mediates the Anti-Obesity Effect of Calorie Restriction in Mice. Sci. Rep. 2018, 8, 13037. [Google Scholar] [CrossRef]
- Fabbiano, S.; Suárez-Zamorano, N.; Chevalier, C.; Lazarević, V.; Kieser, S.; Rigo, D.; Leo, S.; Veyrat-Durebex, C.; Gaïa, N.; Maresca, M.; et al. Functional Gut Microbiota Remodeling Contributes to the Caloric Restriction-Induced Metabolic Improvements. Cell Metab. 2018, 28, 907–921. [Google Scholar] [CrossRef]
- Liu, Z.; Dai, X.; Zhang, H.; Shi, R.; Hui, Y.; Jin, X.; Zhang, W.; Wang, L.; Wang, Q.; Wang, D.; et al. Gut Microbiota Mediates Intermittent-Fasting Alleviation of Diabetes-Induced Cognitive Impairment. Nat. Commun. 2020, 11, 855. [Google Scholar] [CrossRef]
- Paoli, A.; Mancin, L.; Bianco, A.; Thomas, E.; Mota, J.F.; Piccini, F. Ketogenic Diet and Microbiota: Friends or Enemies? Genes 2019, 10, 534. [Google Scholar] [CrossRef] [PubMed]
- Kalaany, N.Y.; Sabatini, D.M. Tumours with PI3K Activation Are Resistant to Dietary Restriction. Nature 2009, 458, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Z.; Ren, Y.; Wang, Y.; Fang, J.; Yue, H.; Ma, S.; Guan, F. Aging and Age-related Diseases: From Mechanisms to Therapeutic Strategies. Biogerontology 2021, 22, 165–187. [Google Scholar] [CrossRef] [PubMed]
- O’Flanagan, C.H.; Smith, L.A.; McDonell, S.B.; Hursting, S.D. When Less May Be More: Calorie Restriction and Response to Cancer Therapy. BMC Med. 2017, 15, 106. [Google Scholar] [CrossRef]
- Meydani, S.N.; Das, S.K.; Pieper, C.F.; Lewis, M.R.; Klein, S.; Dixit, V.D.; Gupta, A.K.; Villareal, D.T.; Bhapkar, M.; Huang, M.; et al. Long-Term Moderate Calorie Restriction Inhibits Inflammation without Impairing Cell-Mediated Immunity: A Randomized Controlled Trial in Non-Obese Humans. Aging 2016, 8, 1416–1431. [Google Scholar] [CrossRef]
- Das, J.K.; Banskota, N.; Candia, J.; Griswold, M.E.; Orenduff, M.; de Cabo, R.; Corcoran, D.L.; Das, S.K.; De, S.; Huffman, K.M.; et al. Calorie Restriction Modulates the Transcription of Genes Related to Stress Response and Longevity in Human Muscle: The CALERIE Study. Aging Cell 2023, 22, e13963. [Google Scholar] [CrossRef]
- Dorling, J.L.; Ravussin, E.; Redman, L.M.; Bhapkar, M.; Huffman, K.M.; Racette, S.B.; Das, S.K.; Apolzan, J.W.; Kraus, W.E.; Höchsmann, C.; et al. Effect of 2 Years of Calorie Restriction on Liver Biomarkers: Results from the CALERIE Phase 2 Randomized Controlled Trial. Eur. J. Nutr. 2021, 60, 1633–1643. [Google Scholar] [CrossRef]
- Kraus, W.E.; Bhapkar, M.; Huffman, K.M.; Pieper, C.F.; Krupa Das, S.; Redman, L.M.; Villareal, D.T.; Rochon, J.; Roberts, S.B.; Ravussin, E.; et al. 2 Years of Calorie Restriction and Cardiometabolic Risk (CALERIE): Exploratory Outcomes of a Multicentre, Phase 2, Randomised Controlled Trial. Lancet Diabetes Endocrinol. 2019, 7, 673–683. [Google Scholar] [CrossRef]
- Martin, C.K.; Bhapkar, M.; Pittas, A.G.; Pieper, C.F.; Das, S.K.; Williamson, D.A.; Scott, T.; Redman, L.M.; Stein, R.; Gilhooly, C.H.; et al. Effect of Calorie Restriction on Mood, Quality of Life, Sleep, and Sexual Function in Healthy Nonobese Adults: The CALERIE 2 Randomized Clinical Trial. JAMA Intern. Med. 2016, 176, 743–752. [Google Scholar] [CrossRef]
- Brogi, S.; Tabanelli, R.; Puca, S.; Calderone, V. Intermittent Fasting: Myths, Fakes and Truth on This Dietary Regimen Approach. Foods 2024, 13, 1960. [Google Scholar] [CrossRef]
- Varady, K.A.; Cienfuegos, S.; Ezpeleta, M.; Gabel, K. Clinical Application of Intermittent Fasting for Weight Loss: Progress and Future Directions. Nat. Rev. Endocrinol. 2022, 18, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Trepanowski, J.F.; Kroeger, C.M.; Barnosky, A.; Klempel, M.C.; Bhutani, S.; Hoddy, K.K.; Gabel, K.; Freels, S.; Rigdon, J.; Rood, J.; et al. Effect of Alternate-Day Fasting onWeight Loss, Weight Maintenance, and Cardioprotection Among Metabolically Healthy Obese Adults. JAMA Intern. Med. 2017, 177, 930–938. [Google Scholar] [CrossRef]
- Varady, K.A.; Bhutani, S.; Klempel, M.C.; Kroeger, C.M.; Trepanowski, J.F.; Haus, J.M.; Hoddy, K.K.; Calvo, Y. Alternate Day Fasting for Weight Loss in Normal Weight and Overweight Subjects: A Randomized Controlled Trial. Nutr. J. 2013, 12, 146. [Google Scholar] [CrossRef] [PubMed]
- Ravussin, E.; Redman, L.M.; Rochon, J.; Das, S.K.; Fontana, L.; Kraus, W.E.; Romashkan, S.; Williamson, D.A.; Meydani, S.N.; Villareal, D.T.; et al. A 2-Year Randomized Controlled Trial of Human Caloric Restriction: Feasibility and Effects on Predictors of Health Span and Longevity. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 1097–1104. [Google Scholar] [CrossRef]
- Willoughby, D.; Hewlings, S.; Kalman, D. Body Composition Changes in Weight Loss: Strategies and Supplementation for Maintaining Lean Body Mass, a Brief Review. Nutrients 2018, 10, 1876. [Google Scholar] [CrossRef]
- Heymsfield, S.B.; Cristina Gonzalez, M.C.; Shen, W.; Redman, L.; Thomas, D. Weight Loss Composition Is One-Fourth Fat-Free Mass: A Critical Review and Critique of This Widely Cited Rule. Obes. Rev. 2014, 15, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Pownall, H.J.; Bray, G.A.; Wagenknecht, L.E.; Walkup, M.P.; Heshka, S.; Hubbard, V.S.; Hill, J.; Kahn, S.E.; Nathan, D.M.; Schwartz, A.V.; et al. Changes in Body Composition over Eight Years in a Randomized Trial of a Lifestyle Intervention: The Look AHEAD Study. Obesity (Silver Spring) 2015, 23, 565–572. [Google Scholar] [CrossRef]
- Simeone, T.A.; Simeone, K.A.; Stafstrom, C.E.; Rho, J.M. Do Ketone Bodies Mediate the Anti-Seizure Effects of the Ketogenic Diet? Neuropharmacology 2018, 133, 233–241. [Google Scholar] [CrossRef]
- Key, M.N.; Szabo-Reed, A.N. Impact of Diet and Exercise Interventions on Cognition and Brain Health in Older Adults: A Narrative Review. Nutrients 2023, 15, 2495. [Google Scholar] [CrossRef]
- Lussier, D.M.; Woolf, E.C.; Johnson, J.L.; Brooks, K.S.; Blattman, J.N.; Scheck, A.C. Enhanced Immunity in a Mouse Model of Malignant Glioma Is Mediated by a Therapeutic Ketogenic Diet. BMC Cancer 2016, 16, 310. [Google Scholar] [CrossRef]
- Hirschberger, S.; Strauß, G.; Effinger, D.; Marstaller, X.; Ferstl, A.; Müller, M.B.; Wu, T.; Hübner, M.; Rahmel, T.; Mascolo, H.; et al. Very-low-carbohydrate Diet Enhances Human T-cell Immunity through Immunometabolic Reprogramming. EMBO Mol. Med. 2021, 13, e14323. [Google Scholar] [CrossRef]
- Talib, W.H.; Mahmod, A.I.; Kamal, A.; Rashid, H.M.; Alashqar, A.M.D.; Khater, S.; Jamal, D.; Waly, M. Ketogenic Diet in Cancer Prevention and Therapy: Molecular Targets and Therapeutic Opportunities. Curr. Issues Mol. Biol. 2021, 43, 558–589. [Google Scholar] [CrossRef] [PubMed]
- Heilbronn, L.K.; Smith, S.R.; Martin, C.K.; Anton, S.D.; Ravussin, E. Alternate-Day Fasting in Nonobese Subjects: Effects on Body Weight, Body Composition, and Energy Metabolism. Am. J. Clin. Nutr. 2005, 81, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Tinsley, G.M.; Moore, M.L.; Graybeal, A.J.; Paoli, A.; Kim, Y.; Gonzales, J.U.; Harry, J.R.; VanDusseldorp, T.A.; Kennedy, D.N.; Cruz, M.R. Time-Restricted Feeding plus Resistance Training in Active Females: A Randomized Trial. Am. J. Clin. Nutr. 2019, 110, 628–640. [Google Scholar] [CrossRef] [PubMed]
- Moro, T.; Tinsley, G.; Bianco, A.; Marcolin, G.; Pacelli, Q.F.; Battaglia, G.; Palma, A.; Gentil, P.; Neri, M.; Paoli, A. Effects of Eight Weeks of Time-Restricted Feeding (16/8) on Basal Metabolism, Maximal Strength, Body Composition, Inflammation, and Cardiovascular Risk Factors in Resistance-Trained Males. J. Transl. Med. 2016, 14, 290. [Google Scholar] [CrossRef]
- Selvaraj, S.; Kim, J.; Ansari, B.A.; Zhao, L.; Cvijic, M.E.; Fronheiser, M.; Vanjarapu, J.M.-R.; Kumar, A.A.; Suri, A.; Yenigalla, S.; et al. Body Composition, Natriuretic Peptides, and Adverse Outcomes in Heart Failure with Preserved and Reduced Ejection Fraction. JACC Cardiovasc. Imaging 2021, 14, 203–215. [Google Scholar] [CrossRef]
- Nicklas, B.J.; Brinkley, T.E.; Houston, D.K.; Lyles, M.F.; Hugenschmidt, C.E.; Beavers, K.M.; Leng, X. Effects of Caloric Restriction on Cardiorespiratory Fitness, Fatigue, and Disability Responses to Aerobic Exercise in Older Adults with Obesity: A Randomized Controlled Trial. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 1084–1090. [Google Scholar] [CrossRef]
- Carbone, S.; Billingsley, H.E.; Rodriguez-Miguelez, P.; Kirkman, D.L.; Garten, R.; Lee Franco, R.; Lee, D.; Lavie, C.J. Lean Mass Abnormalities in Heart Failure: The Role of Sarcopenia, Sarcopenic Obesity, and Cachexia. Curr. Probl. Cardiol. 2020, 45, 100417. [Google Scholar] [CrossRef]
- Brubaker, P.H.; Nicklas, B.J.; Houston, D.K.; Hundley, G.; Chen, H.; Molina, M.A.J.A.; Lyles, W.M.; Nelson, B.; Upadhya, B.; Newland, R.; et al. A Randomized, Controlled Trial of Resistance Training Added to Caloric Restriction Plus Aerobic Exercise Training in Obese Heart Failure with Preserved Ejection Fraction. Circ. Heart Fail. 2023, 16, e010161. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Sun, Y.; Zhang, X. Intermittent Fasting and Physical Exercise for Preventing Metabolic Disorders through Interaction with Gut Microbiota: A Review. Nutrients 2023, 15, 2277. [Google Scholar] [CrossRef]
- Tinsley, G.M.; Forsse, J.S.; Butler, N.K.; Paoli, A.; Bane, A.A.; La Bounty, P.M.; Morgan, G.B.; Grandjean, P.W. Time-Restricted Feeding in Young Men Performing Resistance Training: A Randomized Controlled Trial. Eur. J. Sport Sci. 2017, 17, 200–207. [Google Scholar] [CrossRef]
- Bhutani, S.; Klempel, M.C.; Kroeger, C.M.; Trepanowski, J.F.; Varady, K.A. Alternate Day Fasting and Endurance Exercise Combine to Reduce Body Weight and Favorably Alter Plasma Lipids in Obese Humans. Obesity (Silver Spring) 2013, 21, 1370–1379. [Google Scholar] [CrossRef] [PubMed]
- Ellison, R.C.; Myers, R.H.; Zhang, Y.; Djoussé, L.; Knox, S.; Williams, R.R.; Province, M.A. Effects of Similarities in Lifestyle Habits on Familial Aggregation of High Density Lipoprotein and Low Density Lipoprotein Cholesterol: The NHLBI Family Heart Study. Am. J. Epidemiol. 1999, 150, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Peven, J.C.; Jakicic, J.M.; Rogers, R.J.; Lesnovskaya, A.; Erickson, K.I.; Kang, C.; Zhou, X.; Porter, A.; Donofry, S.D.; Watt, J.C.; et al. The Effects of a 12-Month Weight Loss Intervention on Cognitive Outcomes in Adults with Overweight and Obesity. Nutrients 2020, 12, 2988. [Google Scholar] [CrossRef]
- Pratchayasakul, W.; Arunsak, B.; Suparan, K.; Sriwichaiin, S.; Chunchai, T.; Chattipakorn, N.; Chattipakorn, S.C. Combined Caloric Restriction and Exercise Provides Greater Metabolic and Neurocognitive Benefits than Either as a Monotherapy in Obesity with or without Estrogen Deprivation. J. Nutr. Biochem. 2022, 110, 109125. [Google Scholar] [CrossRef] [PubMed]
- Thonusin, C.; Pantiya, P.; Kongkaew, A.; Nawara, W.; Arunsak, B.; Sriwichaiin, S.; Chattipakorn, N.; Chattipakorn, S.C. Exercise and Caloric Restriction Exert Different Benefits on Skeletal Muscle Metabolism in Aging Condition. Nutrients 2023, 15, 5004. [Google Scholar] [CrossRef]
- Khalafi, M.; Azali Alamdari, K.; Symonds, M.E.; Rohani, H.; Sakhaei, M.H. A Comparison of the Impact of Exercise Training with Dietary Intervention versus Dietary Intervention Alone on Insulin Resistance and Glucose Regulation in Individual with Overweight or Obesity: A Systemic Review and Meta-Analysis. Crit. Rev. Food Sci. Nutr. 2023, 63, 9349–9363. [Google Scholar] [CrossRef]
- Khalafi, M.; Sakhaei, M.H.; Kazeminasab, F.; Rosenkranz, S.K.; Symonds, M.E. Exercise Training, Dietary Intervention, or Combined Interventions and Their Effects on Lipid Profiles in Adults with Overweight and Obesity: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 1662–1683. [Google Scholar] [CrossRef]
- Khalafi, M.; Hossein Sakhaei, M.; Kheradmand, S.; Symonds, M.E.; Rosenkranz, S.K. The Impact of Exercise and Dietary Interventions on Circulating Leptin and Adiponectin in Individuals Who Are Overweight and Those with Obesity: A Systematic Review and Meta-Analysis. Adv. Nutr. 2022, 14, 128–146. [Google Scholar] [CrossRef]
- Cheng, C.-C.; Hsu, C.-Y.; Liu, J.-F. Effects of Dietary and Exercise Intervention on Weight Loss and Body Composition in Obese Postmenopausal Women: A Systematic Review and Meta-Analysis. Menopause 2018, 25, 772–782. [Google Scholar] [CrossRef]
- Wu, T.; Gao, X.; Chen, M.; van Dam, R.M. Long-Term Effectiveness of Diet-plus-Exercise Interventions vs. Diet-Only Interventions for Weight Loss: A Meta-Analysis. Obes. Rev. 2009, 10, 313–323. [Google Scholar] [CrossRef]
- Khalafi, M.; Symonds, M.E.; Akbari, A. The Impact of Exercise Training versus Caloric Restriction on Inflammation Markers: A Systemic Review and Meta-Analysis. Crit. Rev. Food Sci. Nutr. 2022, 62, 4226–4241. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Farì, G.; Megna, M.; Scacco, S.; Ranieri, M.; Raele, M.V.; Chiaia Noya, E.; Macchiarola, D.; Bianchi, F.P.; Carati, D.; Panico, S.; et al. Hemp Seed Oil in Association with β-Caryophyllene, Myrcene and Ginger Extract as a Nutraceutical Integration in Knee Osteoarthritis: A Double-Blind Prospective Case-Control Study. Medicina (Kaunas) 2023, 59, 191. [Google Scholar] [CrossRef] [PubMed]
- Farì, G.; Santagati, D.; Pignatelli, G.; Scacco, V.; Renna, D.; Cascarano, G.; Vendola, F.; Bianchi, F.P.; Fiore, P.; Ranieri, M.; et al. Collagen Peptides, in Association with Vitamin C, Sodium Hyaluronate, Manganese and Copper, as Part of the Rehabilitation Project in the Treatment of Chronic Low Back Pain. Endocr. Metab. Immune Disord. Drug Targets 2022, 22, 108–115. [Google Scholar] [CrossRef]
- Phillips, N.E.; Mareschal, J.; Schwab, N.; Manoogian, E.N.C.; Borloz, S.; Ostinelli, G.; Gauthier-Jaques, A.; Umwali, S.; Gonzalez Rodriguez, E.; Aeberli, D.; et al. The Effects of Time-Restricted Eating versus Standard Dietary Advice on Weight, Metabolic Health and the Consumption of Processed Food: A Pragmatic Randomised Controlled Trial in Community-Based Adults. Nutrients 2021, 13, 1042. [Google Scholar] [CrossRef]
- Conceição, E.; Orcutt, M.; Mitchell, J.; Engel, S.; LaHaise, K.; Jorgensen, M.; Woodbury, K.; Hass, N.; Garcia, L.; Wonderlich, S. Characterization Of Eating Disorders After Bariatric Surgery: A Case Series Study. Int. J. Eat. Disord. 2013, 46, 274–279. [Google Scholar] [CrossRef]
- Fawcett, E.; Van Velthoven, M.H.; Meinert, E. Long-Term Weight Management Using Wearable Technology in Overweight and Obese Adults: Systematic Review. JMIR Mhealth Uhealth 2020, 8, e13461. [Google Scholar] [CrossRef]
- Przulj, D.; Ladmore, D.; Smith, K.M.; Phillips-Waller, A.; Hajek, P. Time Restricted Eating as a Weight Loss Intervention in Adults with Obesity. PLoS ONE 2021, 16, e0246186. [Google Scholar] [CrossRef]
- Vidmar, A.P.; Naguib, M.; Raymond, J.K.; Salvy, S.J.; Hegedus, E.; Wee, C.P.; Goran, M.I. Time-Limited Eating and Continuous Glucose Monitoring in Adolescents with Obesity: A Pilot Study. Nutrients 2021, 13, 3697. [Google Scholar] [CrossRef]
- Ross, R.; Janiszewski, P.M. Is Weight Loss the Optimal Target for Obesity-Related Cardiovascular Disease Risk Reduction? Can. J. Cardiol. 2008, 24, 25D–31D. [Google Scholar] [CrossRef] [PubMed]
- Davidson, L.E.; Hunt, S.C.; Adams, T.D. Fitness versus Adiposity in Cardiovascular Disease Risk. Eur. J. Clin. Nutr. 2019, 73, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Eglseer, D.; Traxler, M.; Embacher, S.; Reiter, L.; Schoufour, J.D.; Weijs, P.J.M.; Voortman, T.; Boirie, Y.; Cruz-Jentoft, A.; Bauer, S. Nutrition and Exercise Interventions to Improve Body Composition for Persons with Overweight or Obesity Near Retirement Age: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2023, 14, 516–538. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Y.; Seo, D.S.; Jang, Y. Metabolic Effects of Ketogenic Diets: Exploring Whole-Body Metabolism in Connection with Adipose Tissue and Other Metabolic Organs. Int. J. Mol. Sci. 2024, 25, 7076. [Google Scholar] [CrossRef]
- Kuchkuntla, A.R.; Shah, M.; Velapati, S.; Gershuni, V.M.; Rajjo, T.; Nanda, S.; Hurt, R.T.; Mundi, M.S. Ketogenic Diet: An Endocrinologist Perspective. Curr. Nutr. Rep. 2019, 8, 402–410. [Google Scholar] [CrossRef]
- Weber, D.D.; Aminzadeh-Gohari, S.; Tulipan, J.; Catalano, L.; Feichtinger, R.G.; Kofler, B. Ketogenic Diet in the Treatment of Cancer—Where Do We Stand? Mol. Metab. 2019, 33, 102–121. [Google Scholar] [CrossRef]
- Qiu, H.; Kan, C.; Han, F.; Luo, Y.; Qu, N.; Zhang, K.; Ma, Y.; Hou, N.; Wu, D.; Sun, X.; et al. Metagenomic and Metabolomic Analysis Showing the Adverse Risk-Benefit Trade-off of the Ketogenic Diet. Lipids Health Dis. 2024, 23, 207. [Google Scholar] [CrossRef]
- Virto, N.; Río, X.; Méndez-Zorrilla, A.; García-Zapirain, B. Non Invasive Techniques for Direct Muscle Quality Assessment after Exercise Intervention in Older Adults: A Systematic Review. BMC Geriatr. 2024, 24, 642. [Google Scholar] [CrossRef]
- Sandireddy, R.; Sakthivel, S.; Gupta, P.; Behari, J.; Tripathi, M.; Singh, B.K. Systemic Impacts of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) and Metabolic Dysfunction-Associated Steatohepatitis (MASH) on Heart, Muscle, and Kidney Related Diseases. Front. Cell Dev. Biol. 2024, 12, 1433857. [Google Scholar] [CrossRef]
- Furrer, R.; Handschin, C. Molecular Aspects of the Exercise Response and Training Adaptation in Skeletal Muscle. Free Radic. Biol. Med. 2024, 223, 53–68. [Google Scholar] [CrossRef]
- Genton, L.; Karsegard, V.L.; Chevalley, T.; Kossovsky, M.P.; Darmon, P.; Pichard, C. Body Composition Changes over 9 Years in Healthy Elderly Subjects and Impact of Physical Activity. Clin. Nutr. 2011, 30, 436–442. [Google Scholar] [CrossRef]
- Moradell, A.; Gomez-Cabello, A.; Mañas, A.; Gesteiro, E.; Pérez-Gómez, J.; González-Gross, M.; Casajús, J.A.; Ara, I.; Vicente-Rodríguez, G. Longitudinal Changes in the Body Composition of Non-Institutionalized Spanish Older Adults after 8 Years of Follow-Up: The Effects of Sex, Age, and Organized Physical Activity. Nutrients 2024, 16, 298. [Google Scholar] [CrossRef] [PubMed]
- Paoli, A.; Bianco, A.; Grimaldi, K.A. The Ketogenic Diet and Sport: A Possible Marriage? Exerc. Sport Sci. Rev. 2015, 43, 153–162. [Google Scholar] [CrossRef]
- Phinney, S.D. Ketogenic Diets and Physical Performance. Nutr. Metab. 2004, 1, 2. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Elías, V.E.; Ortega, J.F.; Nelson, R.K.; Mora-Rodriguez, R. Relationship between Muscle Water and Glycogen Recovery after Prolonged Exercise in the Heat in Humans. Eur. J. Appl. Physiol. 2015, 115, 1919–1926. [Google Scholar] [CrossRef] [PubMed]
- Manolis, A.S.; Manolis, T.A.; Manolis, A.A.; Melita, H. Diet and Sudden Death: How to Reduce the Risk. Curr. Vasc. Pharmacol. 2022, 20, 383–408. [Google Scholar] [CrossRef]
- Kenig, S.; Petelin, A.; Poklar Vatovec, T.; Mohorko, N.; Jenko-Pražnikar, Z. Assessment of Micronutrients in a 12-Wk Ketogenic Diet in Obese Adults. Nutrition 2019, 67–68, 110522. [Google Scholar] [CrossRef]
- Burén, J.; Svensson, M.; Liv, P.; Sjödin, A. Effects of a Ketogenic Diet on Body Composition in Healthy, Young, Normal-Weight Women: A Randomized Controlled Feeding Trial. Nutrients 2024, 16, 2030. [Google Scholar] [CrossRef]
- Noakes, M.; Foster, P.R.; Keogh, J.B.; James, A.P.; Mamo, J.C.; Clifton, P.M. Comparison of Isocaloric Very Low Carbohydrate/High Saturated Fat and High Carbohydrate/Low Saturated Fat Diets on Body Composition and Cardiovascular Risk. Nutr. Metab. 2006, 3, 7. [Google Scholar] [CrossRef]
- Brehm, B.J.; Spang, S.E.; Lattin, B.L.; Seeley, R.J.; Daniels, S.R.; D’Alessio, D.A. The Role of Energy Expenditure in the Differential Weight Loss in Obese Women on Low-Fat and Low-Carbohydrate Diets. J. Clin. Endocrinol. Metab. 2005, 90, 1475–1482. [Google Scholar] [CrossRef]
- Tinsley, G.M.; Willoughby, D.S. Fat-Free Mass Changes During Ketogenic Diets and the Potential Role of Resistance Training. Int. J. Sport Nutr. Exerc. Metab. 2016, 26, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Ashtary-Larky, D.; Bagheri, R.; Asbaghi, O.; Tinsley, G.M.; Kooti, W.; Abbasnezhad, A.; Afrisham, R.; Wong, A. Effects of Resistance Training Combined with a Ketogenic Diet on Body Composition: A Systematic Review and Meta-Analysis. Crit. Rev. Food Sci. Nutr. 2022, 62, 5717–5732. [Google Scholar] [CrossRef]
- Thomson, D.M. The Role of AMPK in the Regulation of Skeletal Muscle Size, Hypertrophy, and Regeneration. Int. J. Mol. Sci. 2018, 19, 3125. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.-J.; Schell, J.R.; Chocron, E.S.; Varmazyad, M.; Xu, G.; Chen, W.H.; Martinez, G.M.; Dong, F.F.; Sreenivas, P.; Trevino, R.; et al. Ketogenic Diet Induces P53-Dependent Cellular Senescence in Multiple Organs. Sci. Adv. 2024, 10, eado1463. [Google Scholar] [CrossRef] [PubMed]
- Andersen, R.E.; Wadden, T.A.; Herzog, R.J. Changes in Bone Mineral Content in Obese Dieting Women. Metabolism 1997, 46, 857–861. [Google Scholar] [CrossRef]
- Andreou, E.; Philippou, C.; Papandreou, D. Effects of an Intervention and Maintenance Weight Loss Diet with and without Exercise on Anthropometric Indices in Overweight and Obese Healthy Women. Ann. Nutr. Metab. 2011, 59, 187–192. [Google Scholar] [CrossRef]
- Apekey, T.A.; Morris, A.E.J.; Fagbemi, S.; Griffiths, G.J. Benefits of Moderate-Intensity Exercise during a Calorie-Restricted Low-Fat Diet. Health Educ. J. 2012, 71, 154–164. [Google Scholar] [CrossRef]
- Brinkley, T.E.; Wang, X.; Kume, N.; Mitsuoka, H.; Nicklas, B.J. Caloric Restriction, Aerobic Exercise Training and Soluble Lectin-like Oxidized LDL Receptor-1 Levels in Overweight and Obese Post-Menopausal Women. Int. J. Obes. 2011, 35, 793–799. [Google Scholar] [CrossRef]
- Brochu, M.; Malita, M.F.; Messier, V.; Doucet, E.; Strychar, I.; Lavoie, J.M.; Prud’homme, D.; Rabasa-Lhoret, R. Resistance Training Does Not Contribute to Improving the Metabolic Profile after a 6-Month Weight Loss Program in Overweight and Obese Postmenopausal Women. J. Clin. Endocrinol. Metab. 2009, 94, 3226–3233. [Google Scholar] [CrossRef]
- Campbell, K.L.; Foster-Schubert, K.E.; Alfano, C.M.; Wang, C.-C.; Wang, C.-Y.; Duggan, C.R.; Mason, C.; Imayama, I.; Kong, A.; Xiao, L.; et al. Reduced-Calorie Dietary Weight Loss, Exercise, and Sex Hormones in Postmenopausal Women: Randomized Controlled Trial. J. Clin. Oncol. 2012, 30, 2314–2326. [Google Scholar] [CrossRef]
- Cho, A.R.; Moon, J.Y.; Kim, S.; An, K.Y.; Oh, M.; Jeon, J.Y.; Jung, D.H.; Choi, M.H.; Lee, J.W. Effects of Alternate Day Fasting and Exercise on Cholesterol Metabolism in Overweight or Obese Adults: A Pilot Randomized Controlled Trial. Metab. Clin. Exp. 2019, 93, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, T.; Paulsen, S.K.; Bruun, J.M.; Ploug, T.; Pedersen, S.B.; Richelsen, B. Diet-Induced Weight Loss and Exercise Alone and in Combination Enhance the Expression of Adiponectin Receptors in Adipose Tissue and Skeletal Muscle, but Only Diet-Induced Weight Loss Enhanced Circulating Adiponectin. J. Clin. Endocrinol. Metab. 2010, 95, 911–919. [Google Scholar] [CrossRef]
- Civitarese, A.E.; Carling, S.; Heilbronn, L.K.; Hulver, M.H.; Ukropcova, B.; Deutsch, W.A.; Smith, S.R.; Ravussin, E. Calorie Restriction Increases Muscle Mitochondrial Biogenesis in Healthy Humans. PLoS Med. 2007, 4, e76. [Google Scholar] [CrossRef] [PubMed]
- Cooke, M.B.; Deasy, W.; Ritenis, E.J.; Wilson, R.A.; Stathis, C.G. Effects of Intermittent Energy Restriction Alone and in Combination with Sprint Interval Training on Body Composition and Cardiometabolic Biomarkers in Individuals with Overweight and Obesity. Int. J. Environ. Res. Public Health 2022, 19, 7969. [Google Scholar] [CrossRef]
- Cooper, J.N.; Columbus, M.L.; Shields, K.J.; Asubonteng, J.; Meyer, M.L.; Sutton-Tyrrell, K.; Goodpaster, B.H.; DeLany, J.P.; Jakicic, J.M.; Barinas-Mitchell, E. Effects of an Intensive Behavioral Weight Loss Intervention Consisting of Caloric Restriction with or without Physical Activity on Common Carotid Artery Remodeling in Severely Obese Adults. Metab. Clin. Exp. 2012, 61, 1589–1597. [Google Scholar] [CrossRef]
- Correia, J.M.; Santos, P.D.G.; Pezarat-Correia, P.; Minderico, C.S.; Infante, J.; Mendonca, G.V. Effect of Time-Restricted Eating and Resistance Training on High-Speed Strength and Body Composition. Nutrients 2023, 15, 285. [Google Scholar] [CrossRef] [PubMed]
- Cox, K.L.; Burke, V.; Morton, A.R.; Beilin, L.J.; Puddey, I.B. The Independent and Combined Effects of 16 Weeks of Vigorous Exercise and Energy Restriction on Body Mass and Composition in Free-Living Overweight Men—A Randomized Controlled Trial. Metab.-Clin. Exp. 2003, 52, 107–115. [Google Scholar] [CrossRef]
- Del Corral, P.; Chandler-Laney, P.C.; Casazza, K.; Gower, B.A.; Hunter, G.R. Effect of Dietary Adherence with or without Exercise on Weight Loss: A Mechanistic Approach to a Global Problem. J. Clin. Endocrinol. Metab. 2009, 94, 1602–1607. [Google Scholar] [CrossRef]
- Duggan, C.; De Dieu Tapsoba, J.; Shivappa, N.; Harris, H.R.; Hébert, J.R.; Wang, C.Y.; McTiernan, A. Changes in Dietary Inflammatory Index Patterns with Weight Loss in Women: A Randomized Controlled Trial. Cancer Prev. Res. 2021, 14, 85–94. [Google Scholar] [CrossRef]
- Fontana, L.; Villareal, D.T.; Weiss, E.P.; Racette, S.B.; Steger-May, K.; Klein, S.; Holloszy, J.O. Calorie Restriction or Exercise: Effects on Coronary Heart Disease Risk Factors. A Randomized, Controlled Trial. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E197–E202. [Google Scholar] [CrossRef]
- Foster-Schubert, K.E.; Alfano, C.M.; Duggan, C.R.; Xiao, L.; Campbell, K.L.; Kong, A.; Bain, C.E.; Wang, C.Y.; Blackburn, G.L.; McTiernan, A. Effect of Diet and Exercise, Alone or Combined, on Weight and Body Composition in Overweight-to-Obese Postmenopausal Women. Obesity 2012, 20, 1628–1638. [Google Scholar] [CrossRef]
- Galedari, M.; Azarbayjani, M.A.; Peeri, M. Effects of Type of Exercise along with Caloric Restriction on Plasma Apelin 36 and HOMA-IR in Overweight Men. Sci. Sports 2017, 32, e137–e145. [Google Scholar] [CrossRef]
- García-Unciti, M.; Izquierdo, M.; Idoate, F.; Gorostiaga, E.; Grijalba, A.; Ortega-Delgado, F.; Martínez-Labari, C.; Moreno-Navarrete, J.M.; Forga, L.; Fernández-Real, J.M.; et al. Weight-Loss Diet Alone or Combined with Progressive Resistance Training Induces Changes in Association between the Cardiometabolic Risk Profile and Abdominal Fat Depots. Ann. Nutr. Metab. 2012, 61, 296–304. [Google Scholar] [CrossRef]
- Geliebter, A.; Maher, M.M.; Gerace, L.; Gutin, B.; Heymsfield, S.B.; Hashim, S.A. Effects of Strength or Aerobic Training on Body Composition, Resting Metabolic Rate, and Peak Oxygen Consumption in Obese Dieting Subjects. Am. J. Clin. Nutr. 1997, 66, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Geliebter, A.; Ochner, C.N.; Dambkowski, C.L.; Hashim, S.A. Obesity-Related Hormones and Metabolic Risk Factors: A Randomized Trial of Diet plus Either Strength or Aerobic Training versus Diet Alone in Overweight Participants. J. Diabetes Obes. 2014, 1, 1–7. [Google Scholar] [PubMed]
- Glud, M.; Christiansen, T.; Larsen, L.H.; Richelsen, B.; Bruun, J.M. Changes in Circulating BDNF in Relation to Sex, Diet, and Exercise: A 12-Week Randomized Controlled Study in Overweight and Obese Participants. J. Obes. 2019, 2019, 4537274. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; DeLany, J.P.; Otto, A.D.; Kuller, L.; Vockley, J.; South-Paul, J.E.; Thomas, S.B.; Brown, J.; McTigue, K.; Hames, K.C.; et al. Effects of Diet and Physical Activity Interventions on Weight Loss and Cardiometabolic Risk Factors in Severely Obese Adults A Randomized Trial. JAMA-J. Am. Med. Assoc. 2010, 304, 1795–1802. [Google Scholar] [CrossRef] [PubMed]
- Gregory, R.M. A Low-Carbohydrate Ketogenic Diet Combined with 6-Weeks of Crossfit Training Improves Body Composition and Performance. Int. J. Sports Exerc. Med. 2017, 3, 054. [Google Scholar] [CrossRef]
- Gutierrez-Lopez, L.; Garcia-Sanchez, J.R.; Rincon-Viquez Mde, J.; Lara-Padilla, E.; Sierra-Vargas, M.P.; Olivares-Corichi, I.M. Hypocaloric Diet and Regular Moderate Aerobic Exercise Is an Effective Strategy to Reduce Anthropometric Parameters and Oxidative Stress in Obese Patients. Obes. Facts 2012, 5, 12–22. [Google Scholar] [CrossRef]
- Hadizadeh, M.; Gan, W.Y.; Mohafez, H.; Sugajima, Y. Impact of Ketogenic Diet on Body Composition during Resistance Training among Untrained Individuals. Open Sports Sci. J. 2020, 13, 114–119. [Google Scholar] [CrossRef]
- Haganes, K.L.; Silva, C.P.; Eyjolfsdottir, S.K.; Steen, S.; Grindberg, M.; Lydersen, S.; Hawley, J.A.; Moholdt, T. Time-Restricted Eating and Exercise Training Improve HbA1c and Body Composition in Women with Overweight/Obesity: A Randomized Controlled Trial. Cell Metab. 2022, 34, 1457–1471. [Google Scholar] [CrossRef] [PubMed]
- Hosny, I.A.; Elghawabi, H.S.; Younan, W.B.; Sabbour, A.A.; Gobrial, M.A. Beneficial Impact of Aerobic Exercises on Bone Mineral Density in Obese Premenopausal Women under Caloric Restriction. Skelet. Radiol. 2012, 41, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, J.; Izquierdo, M.; Martínez-Labari, C.; Ortega, F.; Grijalba, A.; Forga, L.; Idoate, F.; García-Unciti, M.; Fernández-Real, J.M.; Gorostiaga, E.M. Resistance Training Improves Cardiovascular Risk Factors in Obese Women Despite a Significative Decrease in Serum Adiponectin Levels. Obesity 2010, 18, 535–541. [Google Scholar] [CrossRef]
- Isenmann, E.; Dissemond, J.; Geisler, S. The Effects of a Macronutrient-Based Diet and Time-Restricted Feeding (16:8) on Body Composition in Physically Active Individuals-A 14-Week Randomised Controlled Trial. Nutrients 2021, 13, 3122. [Google Scholar] [CrossRef] [PubMed]
- Jo, E.; Worts, P.R.; Elam, M.L.; Brown, A.F.; Khamoui, A.V.; Kim, D.H.; Yeh, M.C.; Ormsbee, M.J.; Prado, C.M.; Cain, A.; et al. Resistance Training during a 12-Week Protein Supplemented VLCD Treatment Enhances Weight-Loss Outcomes in Obese Patients. Clin. Nutr. 2019, 38, 372–382. [Google Scholar] [CrossRef]
- Keawtep, P.; Sungkarat, S.; Boripuntakul, S.; Sa-nguanmoo, P.; Wichayanrat, W.; Chattipakorn, S.C.; Worakul, P. Effects of Combined Dietary Intervention and Physical-Cognitive Exercise on Cognitive Function and Cardiometabolic Health of Postmenopausal Women with Obesity: A Randomized Controlled Trial. Int. J. Behav. Nutr. Phys. Act. 2024, 21, 28. [Google Scholar] [CrossRef] [PubMed]
- Keenan, S.; Cooke, M.B.; Chen, W.S.; Wu, S.; Belski, R. The Effects of Intermittent Fasting and Continuous Energy Restriction with Exercise on Cardiometabolic Biomarkers, Dietary Compliance, and Perceived Hunger and Mood: Secondary Outcomes of a Randomised, Controlled Trial. Nutrients 2022, 14, 3071. [Google Scholar] [CrossRef] [PubMed]
- Kerksick, C.M.; Roberts, M.D.; Campbell, B.I.; Galbreath, M.M.; Taylor, L.W.; Wilborn, C.D.; Lee, A.; Dove, J.; Bunn, J.W.; Rasmussen, C.J.; et al. Differential Impact of Calcium and Vitamin D on Body Composition Changes in Post-Menopausal Women Following a Restricted Energy Diet and Exercise Program. Nutrients 2020, 12, 713. [Google Scholar] [CrossRef]
- Kirkwood, L.; Aldujaili, E.; Drummond, S. Effects of Advice on Dietary Intake and/or Physical Activity on Body Composition, Blood Lipids and Insulin Resistance Following a Low-Fat, Sucrose-Containing, High-Carbohydrate, Energy-Restricted Diet. Int. J. Food Sci. Nutr. 2007, 58, 383–397. [Google Scholar] [CrossRef]
- Kotarsky, C.J.; Johnson, N.R.; Mahoney, S.J.; Mitchell, S.L.; Schimek, R.L.; Stastny, S.N.; Hackney, K.J. Time-Restricted Eating and Concurrent Exercise Training Reduces Fat Mass and Increases Lean Mass in Overweight and Obese Adults. Physiol. Rep. 2021, 9, e14868. [Google Scholar] [CrossRef]
- Larson-Meyer, D.E.; Newcomer, B.R.; Heilbronn, L.K.; Volaufova, J.; Smith, S.R.; Alfonso, A.J.; Lefevre, M.; Rood, J.C.; Williamson, D.A.; Ravussin, E. Effect of 6-Month Calorie Restriction and Exercise on Serum and Liver Lipids and Markers of Liver Function. Obesity 2008, 16, 1355–1362. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.T.; Chen, S.Y.; Ji, H.Y.; Dai, Z.Q. Effects of Time-Restricted Feeding and Walking Exercise on the Physical Health of Female College Students with Hidden Obesity: A Randomized Trial. Front. Public Health 2023, 11, 1020887. [Google Scholar] [CrossRef]
- Maaloul, R.; Marzougui, H.; Ben Dhia, I.; Ghroubi, S.; Tagougui, S.; Kallel, C.; Driss, T.; Elleuch, M.H.; Ayadi, F.; Turki, M.; et al. Effectiveness of Ramadan Diurnal Intermittent Fasting and Concurrent Training in the Management of Obesity: Is the Combination Worth the Weight? Nutr. Metab. Cardiovasc. Dis. 2023, 33, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Marks, B.L.; Ward, A.; Morris, D.H.; Castellani, J.; Rippe, J.M. Fat-free mass is maintained in women following a moderate diet and exercise program. Med. Sci. Sports Exerc. 1995, 27, 1243–1251. [Google Scholar] [CrossRef]
- Martin, C.K.; Heilbronn, L.K.; de Jonge, L.; DeLany, J.P.; Volaufova, J.; Anton, S.D.; Redman, L.M.; Smith, S.R.; Ravussin, E. Effect of Calorie Restriction on Resting Metabolic Rate and Spontaneous Physical Activity. Obesity 2007, 15, 2964–2973. [Google Scholar] [CrossRef]
- Martínez-Rodríguez, A.; Rubio-Arias, J.A.; García-De Frutos, J.M.; Vicente-Martínez, M.; Gunnarsson, T.P. Effect of High-Intensity Interval Training and Intermittent Fasting on Body Composition and Physical Performance in Active Women. Int. J. Environ. Res. Public Health 2021, 18, 6431. [Google Scholar] [CrossRef]
- McNeil, J.; Schwartz, A.; Rabasa-Lhoret, R.; Lavoie, J.M.; Brochu, M.; Doucet, É. Changes in Leptin and Peptide YY Do Not Explain the Greater-Than-Predicted Decreases in Resting Energy Expenditure After Weight Loss. J. Clin. Endocrinol. Metab. 2015, 100, E443–E452. [Google Scholar] [CrossRef] [PubMed]
- Meckling, K.A.; Sherfey, R. A Randomized Trial of a Hypocaloric High-Protein Diet, with and without Exercise, on Weight Loss, Fitness, and Markers of the Metabolic Syndrome in Overweight and Obese Women. Appl. Physiol. Nutr. Metab. 2007, 32, 743–752. [Google Scholar] [CrossRef]
- Mediano, M.F.; Barbosa, J.S.; Moura, A.S.; Willett, W.C.; Sichieri, R. A Randomized Clinical Trial of Home-Based Exercise Combined with a Slight Caloric Restriction on Obesity Prevention among Women. Prev. Med. 2010, 51, 247–252. [Google Scholar] [CrossRef]
- Messier, V.; Rabasa-Lhoret, R.; Doucet, E.; Brochu, M.; Lavoie, J.M.; Karelis, A.; Prud’homme, D.; Strychar, I. Effects of the Addition of a Resistance Training Programme to a Caloric Restriction Weight Loss Intervention on Psychosocial Factors in Overweight and Obese Post-Menopausal Women: A Montreal Ottawa New Emerging Team Study. J. Sports Sci. 2010, 28, 83–92. [Google Scholar] [CrossRef]
- Miller, T.; Mull, S.; Aragon, A.A.; Krieger, J.; Schoenfeld, B.J. Resistance Training Combined with Diet Decreases Body Fat While Preserving Lean Mass Independent of Resting Metabolic Rate: A Randomized Trial. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 46–54. [Google Scholar] [CrossRef]
- Moreno, B.; Bellido, D.; Sajoux, I.; Goday, A.; Saavedra, D.; Crujeiras, A.B.; Casanueva, F.F. Comparison of a Very Low-Calorie-Ketogenic Diet with a Standard Low-Calorie Diet in the Treatment of Obesity. Endocrine 2014, 47, 793–805. [Google Scholar] [CrossRef] [PubMed]
- Moro, T.; Tinsley, G.; Pacelli, F.Q.; Marcolin, G.; Bianco, A.; Paoli, A. Twelve Months of Time-Restricted Eating and Resistance Training Improves Inflammatory Markers and Cardiometabolic Risk Factors. Med. Sci. Sports Exerc. 2021, 53, 2577–2585. [Google Scholar] [CrossRef]
- Murakami, T.; Horigome, H.; Tanaka, K.; Nakata, Y.; Katayama, Y.; Matsui, A. Effects of Diet with or without Exercise on Leptin and Anticoagulation Proteins Levels in Obesity. Blood Coagul. Fibrinolysis 2007, 18, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Nakata, Y.; Ohkawara, K.; Lee, D.J.; Okura, T.; Tanaka, K. Effects of Additional Resistance Training during Diet-Induced Weight Loss on Bone Mineral Density in Overweight Premenopausal Women. J. Bone Miner. Metab. 2008, 26, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Nicklas, B.J.; Wang, X.W.; You, T.J.; Lyles, M.F.; Demons, J.; Easter, L.; Berry, M.J.; Lenchik, L.; Carr, J.J. Effect of Exercise Intensity on Abdominal Fat Loss during Calorie Restriction in Overweight and Obese Postmenopausal Women: A Randomized, Controlled Trial. Am. J. Clin. Nutr. 2009, 89, 1043–1052. [Google Scholar] [CrossRef]
- Nieman, D.C.; Nehlsen-Cannarella, S.L.; Henson, D.A.; Koch, A.J.; Butterworth, D.E.; Fagoaga, O.R.; Utter, A. Immune Response to Exercise Training and/or Energy Restriction in Obese Women. Med. Sci. Sports Exerc. 1998, 30, 679–686. [Google Scholar] [CrossRef]
- Paoli, A.; Cenci, L.; Pompei, P.; Sahin, N.; Bianco, A.; Neri, M.; Caprio, M.; Moro, T. Effects of Two Months of Very Low Carbohydrate Ketogenic Diet on Body Composition, Muscle Strength, Muscle Area, and Blood Parameters in Competitive Natural Body Builders. Nutrients 2021, 13, 374. [Google Scholar] [CrossRef]
- Piacenza, F.; Malavolta, M.; Basso, A.; Costarelli, L.; Giacconi, R.; Ravussin, E.; Redman, L.M.; Mocchegiani, E. Effect of 6-Month Caloric Restriction on Cu Bound to Ceruloplasmin in Adult Overweight Subjects. J. Nutr. Biochem. 2015, 26, 876–882. [Google Scholar] [CrossRef]
- Pureza, I.; Melo, I.S.V.; Macena, M.L.; Praxedes, D.R.S.; Vasconcelos, L.G.L.; Silva, A.E.; Florêncio, T.; Bueno, N.B. Acute Effects of Time-Restricted Feeding in Low-Income Women with Obesity Placed on Hypoenergetic Diets: Randomized Trial. Nutrition 2020, 77, 110796. [Google Scholar] [CrossRef]
- Rhyu, H.-S.; Cho, S.-Y. The Effect of Weight Loss by Ketogenic Diet on the Body Composition, Performance-Related Physical Fitness Factors and Cytokines of Taekwondo Athletes. J. Exerc. Rehabil. 2014, 10, 326–331. [Google Scholar] [CrossRef]
- Richardson, C.E.; Tovar, A.P.; Davis, B.A.; Van Loan, M.D.; Keim, N.L.; Casazza, G.A. An Intervention of Four Weeks of Time-Restricted Eating (16/8) in Male Long-Distance Runners Does Not Affect Cardiometabolic Risk Factors. Nutrients 2023, 15, 985. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.S.; Nicklas, B.J.; Dennis, K.E. Aerobic Exercise Maintains Regional Bone Mineral Density during Weight Loss in Postmenopausal Women. J. Appl. Physiol. 1998, 84, 1305–1310. [Google Scholar] [CrossRef]
- Sénéchal, M.; Bouchard, D.R.; Dionne, I.J.; Brochu, M. The Effects of Lifestyle Interventions in Dynapenic-Obese Postmenopausal Women. Menopause 2012, 19, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Serra, M.C.; Ryan, A.S. Bone Mineral Density Changes during Weight Regain Following Weight Loss with and without Exercise. Nutrients 2021, 13, 2848. [Google Scholar] [CrossRef] [PubMed]
- Silverman, N.E.; Nicklas, B.J.; Ryan, A.S. Addition of Aerobic Exercise to a Weight Loss Program Increases BMD, with an Associated Reduction in Inflammation in Overweight Postmenopausal Women. Calcif. Tissue Int. 2009, 84, 257–265. [Google Scholar] [CrossRef]
- Solomon, T.P.; Haus, J.M.; Marchetti, C.M.; Stanley, W.C.; Kirwan, J.P. Effects of Exercise Training and Diet on Lipid Kinetics during Free Fatty Acid-Induced Insulin Resistance in Older Obese Humans with Impaired Glucose Tolerance. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E552–E559. [Google Scholar] [CrossRef] [PubMed]
- St-Onge, M.; Rabasa-Lhoret, R.; Strychar, I.; Faraj, M.; Doucet, É.; Lavoie, J.M. Impact of Energy Restriction with or without Resistance Training on Energy Metabolism in Overweight and Obese Postmenopausal Women: A Montreal Ottawa New Emerging Team Group Study. Menopause 2013, 20, 194–201. [Google Scholar] [CrossRef]
- Tang, Z.; Ming, Y.; Wu, M.; Jing, J.; Xu, S.; Li, H.; Zhu, Y. Effects of Caloric Restriction and Rope-Skipping Exercise on Cardiometabolic Health: A Pilot Randomized Controlled Trial in Young Adults. Nutrients 2021, 13, 3222. [Google Scholar] [CrossRef]
- Tovar, A.P.; Richardson, C.E.; Keim, N.L.; Van Loan, M.D.; Davis, B.A.; Casazza, G.A. Four Weeks of 16/8 Time Restrictive Feeding in Endurance Trained Male Runners Decreases Fat Mass, without Affecting Exercise Performance. Nutrients 2021, 13, 2941. [Google Scholar] [CrossRef]
- Utter, A.C.; Nieman, D.C.; Shannonhouse, E.M.; Butterworth, D.E.; Nieman, C.N. Influence of Diet and/or Exercise on Body Composition and Cardiorespiratory Fitness in Obese Women. Int. J. Sport Nutr. 1998, 8, 213–222. [Google Scholar] [CrossRef]
- Valsdottir, T.D.; Ovrebo, B.; Falck, T.M.; Litleskare, S.; Johansen, E.I.; Henriksen, C.; Jensen, J. Low-Carbohydrate High-Fat Diet and Exercise: Effect of a 10-Week Intervention on Body Composition and CVD Risk Factors in Overweight and Obese Women-A Randomized Controlled Trial. Nutrients 2021, 13, 110. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Molina, S.; Petro, J.L.; Romance, R.; Kreider, R.B.; Schoenfeld, B.J.; Bonilla, D.A.; Benítez-Porres, J. Effects of a Ketogenic Diet on Body Composition and Strength in Trained Women. J. Int. Soc. Sports Nutr. 2020, 17, 19. [Google Scholar] [CrossRef]
- Vidić, V.; Ilić, V.; Toskić, L.; Janković, N.; Ugarković, D. Effects of Calorie Restricted Low Carbohydrate High Fat Ketogenic vs. Non-Ketogenic Diet on Strength, Body-Composition, Hormonal and Lipid Profile in Trained Middle-Aged Men. Clin. Nutr. 2021, 40, 1495–1502. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lyles, M.F.; You, T.; Berry, M.J.; Rejeski, W.J.; Nicklas, B.J. Weight Regain Is Related to Decreases in Physical Activity during Weight Loss. Med. Sci. Sports Exerc. 2008, 40, 1781–1788. [Google Scholar] [CrossRef]
- Wang, X.W.; You, T.J.; Murphy, K.; Lyles, M.F.; Nicklas, B.J. Addition of Exercise Increases Plasma Adiponectin and Release from Adipose Tissue. Med. Sci. Sports Exerc. 2015, 47, 2450–2455. [Google Scholar] [CrossRef] [PubMed]
- Weiss, E.P.; Jordan, R.C.; Frese, E.M.; Albert, S.G.; Villareal, D.T. Effects of Weight Loss on Lean Mass, Strength, Bone, and Aerobic Capacity. Med. Sci. Sports Exerc. 2017, 49, 206–217. [Google Scholar] [CrossRef]
- Wilson, J.M.; Lowery, R.P.; Roberts, M.D.; Sharp, M.H.; Joy, J.M.; Shields, K.A.; Partl, J.M.; Volek, J.S.; D’Agostino, D.P. Effects of Ketogenic Dieting on Body Composition, Strength, Power, and Hormonal Profiles in Resistance Training Men. J. Strength Cond. Res. 2020, 34, 3463–3474. [Google Scholar] [CrossRef]
- Yoshimura, E.; Kumahara, H.; Tobina, T.; Matsuda, T.; Ayabe, M.; Kiyonaga, A.; Anzai, K.; Higaki, Y.; Tanaka, H. Lifestyle Intervention Involving Calorie Restriction with or without Aerobic Exercise Training Improves Liver Fat in Adults with Visceral Adiposity. J. Obes. 2014, 2014, 197216. [Google Scholar] [CrossRef]
- You, T.; Disanzo, B.L.; Wang, X.; Yang, R.; Gong, D. Adipose Tissue Endocannabinoid System Gene Expression: Depot Differences and Effects of Diet and Exercise. Lipids Health Dis. 2011, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zajac, A.; Poprzecki, S.; Maszczyk, A.; Czuba, M.; Michalczyk, M.; Zydek, G. The Effects of a Ketogenic Diet on Exercise Metabolism and Physical Performance in Off-Road Cyclists. Nutrients 2014, 6, 2493–2508. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.G.; Huang, H.M.; Du, C.Y. Effect of a Combination of Aerobic Exercise and Dietary Modification on Liver Function in Overweight and Obese Men. J. Men’s Health 2021, 17, 176–182. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, M.J.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Y.; Gu, Y.; Li, Z.; He, B.; Zhang, L. Effects of Different Exercises Combined with Different Dietary Interventions on Body Composition: A Systematic Review and Network Meta-Analysis. Nutrients 2024, 16, 3007. https://doi.org/10.3390/nu16173007
Xie Y, Gu Y, Li Z, He B, Zhang L. Effects of Different Exercises Combined with Different Dietary Interventions on Body Composition: A Systematic Review and Network Meta-Analysis. Nutrients. 2024; 16(17):3007. https://doi.org/10.3390/nu16173007
Chicago/Turabian StyleXie, Yongchao, Yu Gu, Zhen Li, Bingchen He, and Lei Zhang. 2024. "Effects of Different Exercises Combined with Different Dietary Interventions on Body Composition: A Systematic Review and Network Meta-Analysis" Nutrients 16, no. 17: 3007. https://doi.org/10.3390/nu16173007
APA StyleXie, Y., Gu, Y., Li, Z., He, B., & Zhang, L. (2024). Effects of Different Exercises Combined with Different Dietary Interventions on Body Composition: A Systematic Review and Network Meta-Analysis. Nutrients, 16(17), 3007. https://doi.org/10.3390/nu16173007





