Preventive and Therapeutic Effects of Lactiplantibacillus plantarum HD02 and MD159 through Mast Cell Degranulation Inhibition in Mouse Models of Atopic Dermatitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Identification of New Probiotic Strains
2.2. Mice
2.3. Mast Cell Degranulation Assay
2.4. Passive Cutaneous Anaphylaxis (PCA) Model
2.5. MC903-Induced AD Model
2.6. House Dust Mite (HDM)-Induced AD Model
2.7. RNA Extraction and Quantitative RT-PCR (qRT-PCR) Analysis
2.8. Enzyme-Linked Immunosorbent Assay (ELISA)
2.9. Flow Cytometry Analysis
2.10. Histological Analysis
2.11. Whole-Genome Sequencing and Analysis
2.12. Statistical Analysis
3. Results
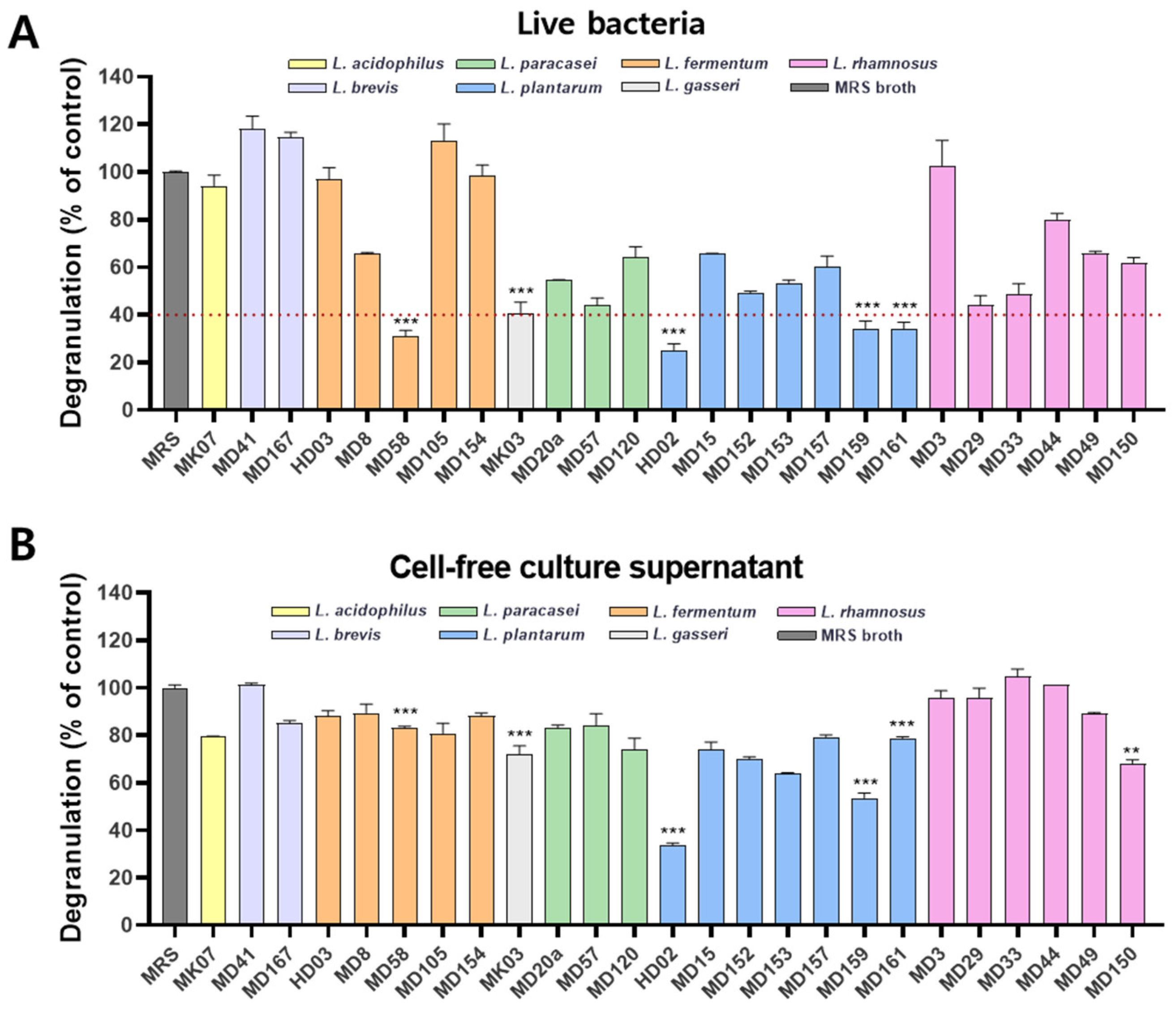
3.1. Identification of L. plantarum HD02 and MD159 as Potent Inhibitors of Mast Cell Degranulation
3.2. Whole-Genome Analysis of L. plantarum HD02 and MD159
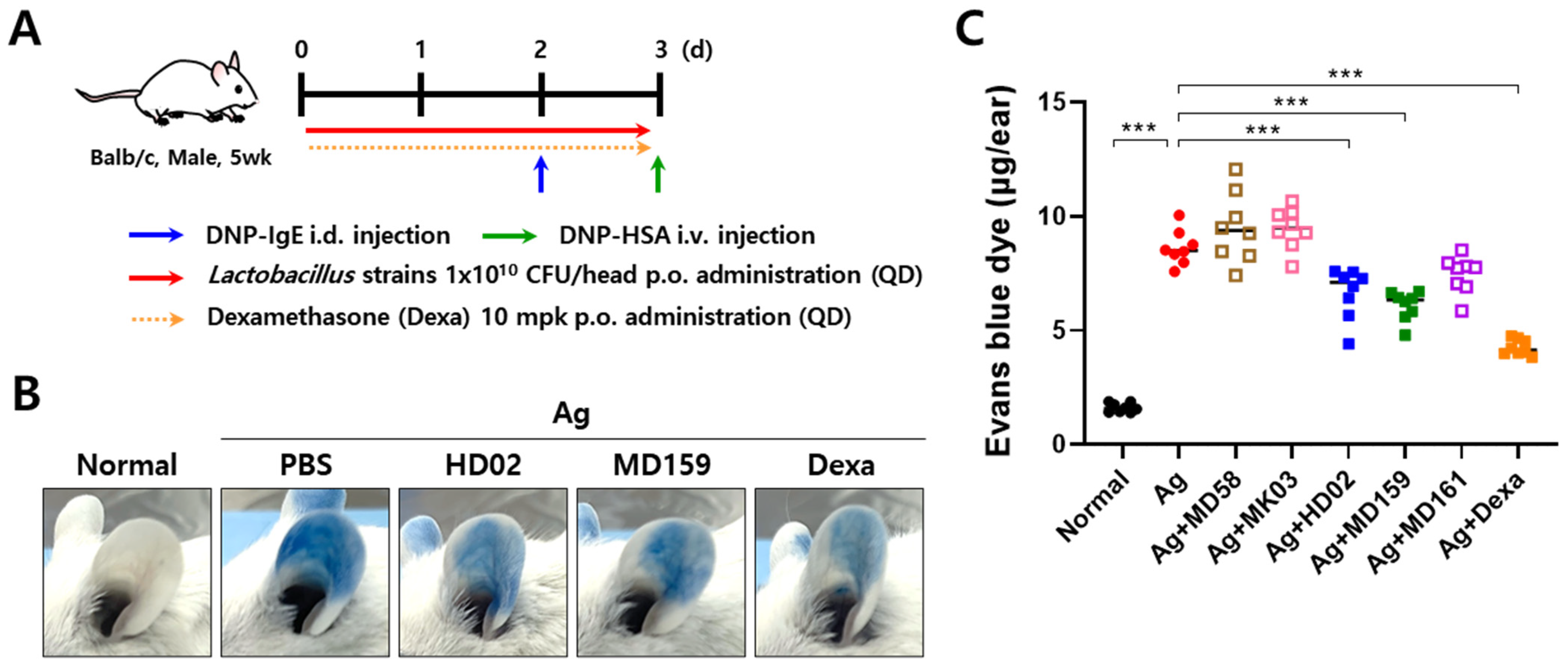
3.3. L. plantarum HD02 and MD159 Show Preventive Effects in an MC903-Induced AD Mouse Model
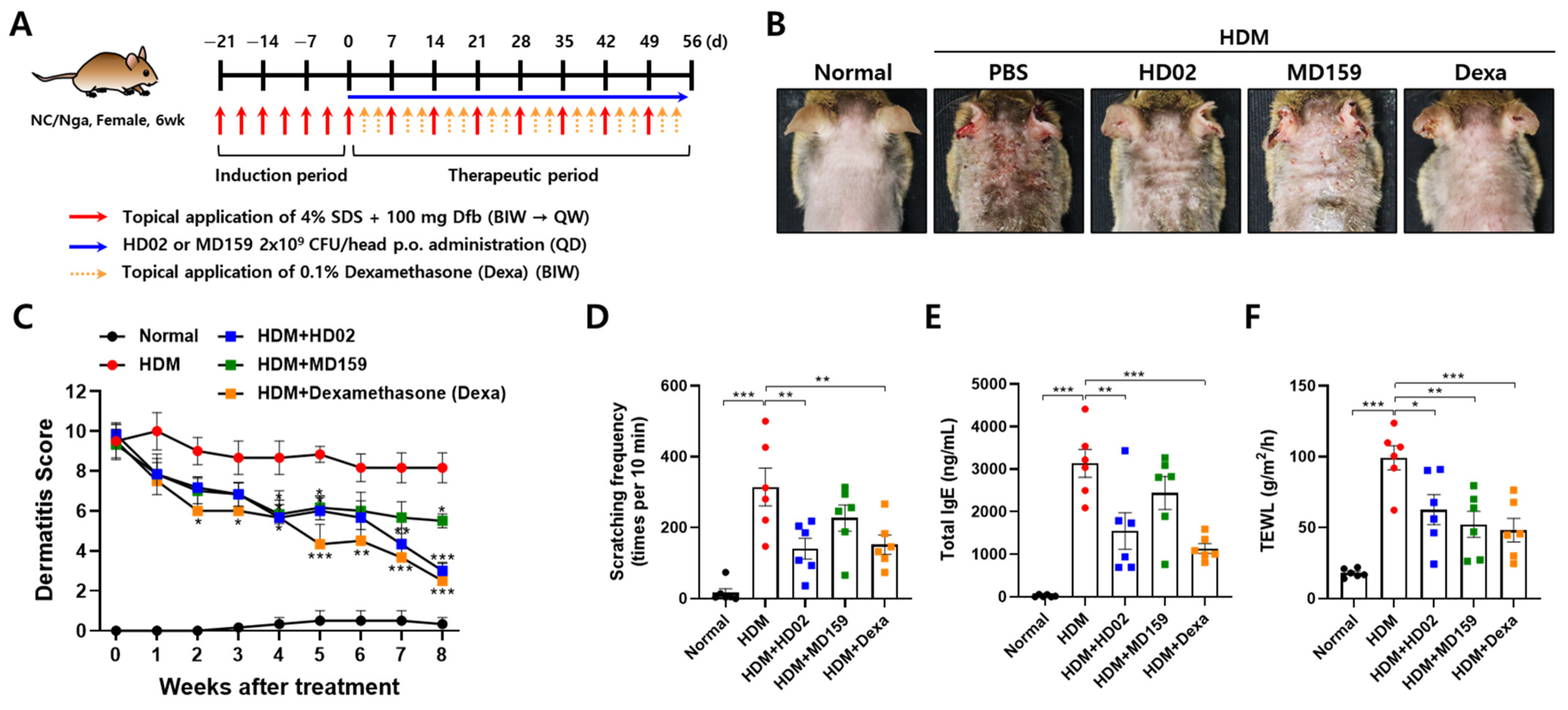
3.4. L. plantarum HD02 and MD159 Show Therapeutic Activity in an HDM-Induced AD Mouse Model
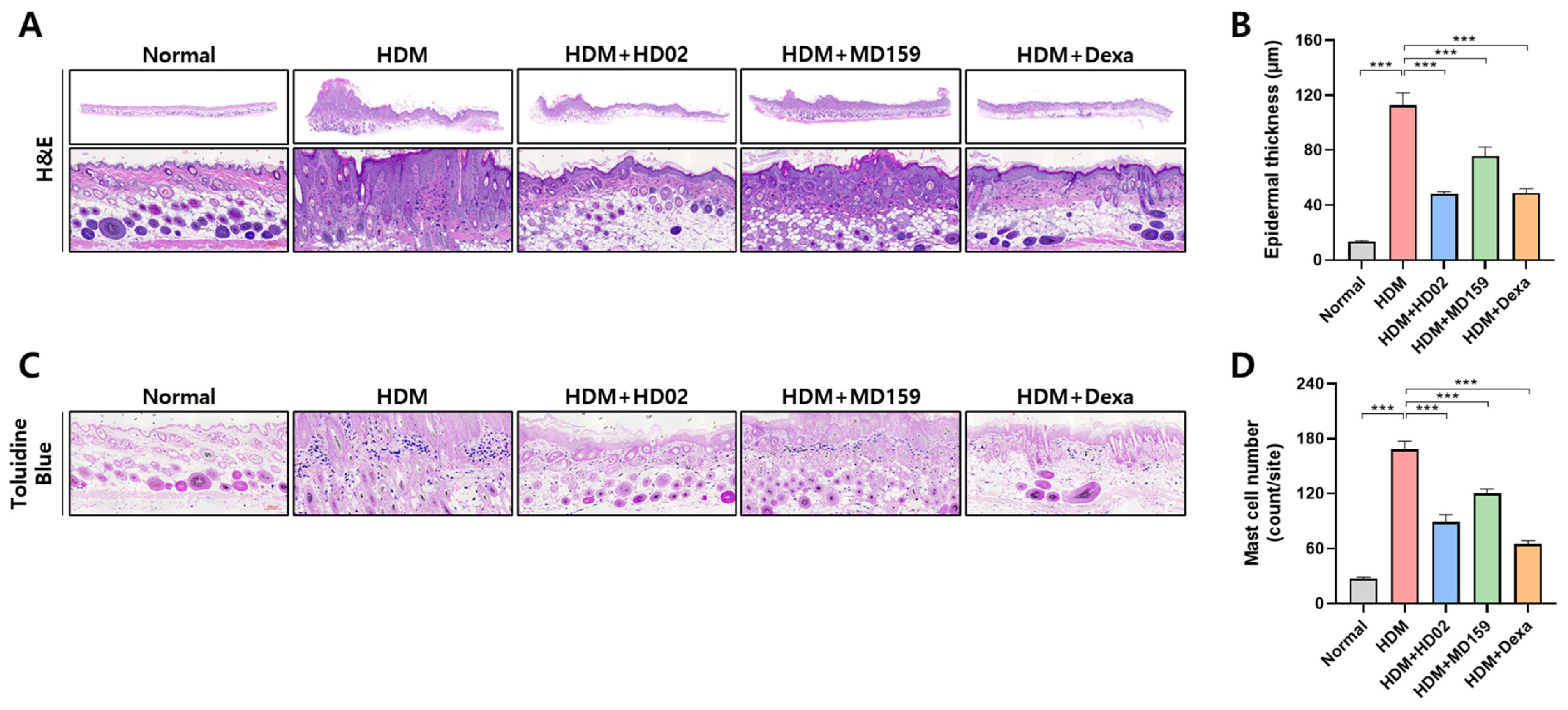
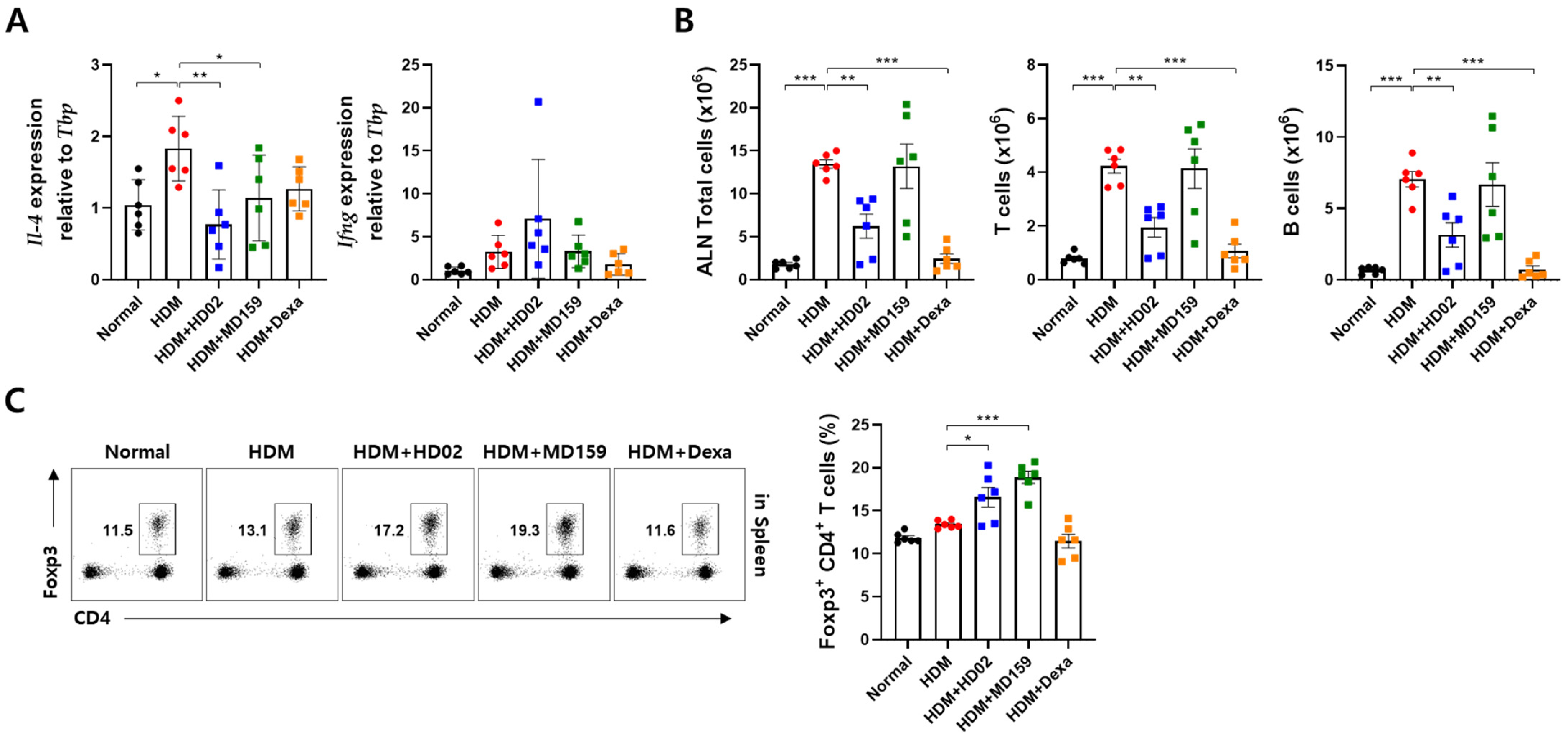
3.5. L. plantarum HD02 and MD159 Regulate Immune Responses in an HDM-Induced AD Mouse Model
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, B.G.; Kim, A.R.; Lee, D.; An, S.B.; Shim, Y.A.; Jang, M.H. Degranulation of Mast Cells as a Target for Drug Development. Cells 2023, 12, 1506. [Google Scholar] [CrossRef] [PubMed]
- Kolkhir, P.; Elieh-Ali-Komi, D.; Metz, M.; Siebenhaar, F.; Maurer, M. Understanding human mast cells: Lesson from therapies for allergic and non-allergic diseases. Nat. Rev. Immunol. 2022, 22, 294–308. [Google Scholar] [CrossRef] [PubMed]
- XOLAIR® (Omalizumab) Injection, for Subcutaneous Use. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/103976s5245lbl.pdf (accessed on 15 July 2024).
- Wang, H.H.; Li, Y.C.; Huang, Y.C. Efficacy of omalizumab in patients with atopic dermatitis: A systematic review and meta-analysis. J. Allergy Clin. Immunol. 2016, 138, 1719–1722.e1. [Google Scholar] [CrossRef] [PubMed]
- An, S.B.; Yang, B.G.; Jang, G.; Kim, D.Y.; Kim, J.; Oh, S.M.; Oh, N.; Lee, S.; Moon, J.Y.; Kim, J.A.; et al. Combined IgE neutralization and Bifidobacterium longum supplementation reduces the allergic response in models of food allergy. Nat. Commun. 2022, 13, 5669. [Google Scholar] [CrossRef] [PubMed]
- Ansaldo, E.; Farley, T.K.; Belkaid, Y. Control of immunity by the microbiota. Annu. Rev. Immunol. 2021, 39, 449–479. [Google Scholar] [CrossRef]
- Tanaka, M.; Nakayama, J. Development of the gut microbiota in infancy and its impact on health in later life. Allergol. Int. 2017, 66, 515–522. [Google Scholar] [CrossRef]
- Akagawa, S.; Kaneko, K. Gut microbiota and allergic diseases in children. Allergol. Int. 2022, 71, 301–309. [Google Scholar] [CrossRef]
- Cahenzli, J.; Koller, Y.; Wyss, M.; Geuking, M.B.; McCoy, K.D. Intestinal microbial diversity during early-life colonization shapes long-term IgE levels. Cell Host Microbe 2013, 14, 559–570. [Google Scholar] [CrossRef]
- Stefka, A.T.; Feehley, T.; Tripathi, P.; Qiu, J.; McCoy, K.; Mazmanian, S.K.; Tjota, M.Y.; Seo, G.Y.; Cao, S.; Theriault, B.R.; et al. Commensal bacteria protect against food allergen sensitization. Proc. Natl. Acad. Sci. USA 2014, 111, 13145–13150. [Google Scholar] [CrossRef]
- Kozakova, H.; Schwarzer, M.; Tuckova, L.; Srutkova, D.; Czarnowska, E.; Rosiak, I.; Hudcovic, T.; Schabussova, I.; Hermanova, P.; Zakostelska, Z.; et al. Colonization of germ-free mice with a mixture of three lactobacillus strains enhances the integrity of gut mucosa and ameliorates allergic sensitization. Cell Mol. Immunol. 2016, 13, 251–262. [Google Scholar] [CrossRef]
- Hajavi, J.; Esmaeili, S.A.; Varasteh, A.R.; Vazini, H.; Atabati, H.; Mardani, F.; Momtazi-Borojeni, A.A.; Hashemi, M.; Sankian, M.; Sahebkar, A. The immunomodulatory role of probiotics in allergy therapy. J. Cell Physiol. 2019, 234, 2386–2398. [Google Scholar] [CrossRef]
- Kim, J.H.; Jeun, E.J.; Hong, C.P.; Kim, S.H.; Jang, M.S.; Lee, E.J.; Moon, S.J.; Yun, C.H.; Im, S.H.; Jeong, S.G.; et al. Extracellular vesicle-derived protein from Bifidobacterium longum alleviates food allergy through mast cell suppression. J. Allergy Clin. Immunol. 2016, 137, 507–516.e8. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.E.; Bunyavanich, S. Role of the Microbiome in Food Allergy. Curr. Allergy Asthma Rep. 2018, 18, 27. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, J.; Wang, X.; Jin, Z.; Zhang, P.; Su, H.; Sun, X. Effect of Probiotics on Respiratory Tract Allergic Disease and Gut Microbiota. Front. Nutr. 2022, 9, 821900. [Google Scholar] [CrossRef]
- Hrestak, D.; Matijasic, M.; Cipcic Paljetak, H.; Ledic Drvar, D.; Ljubojevic Hadzavdic, S.; Peric, M. Skin Microbiota in Atopic Dermatitis. Int. J. Mol. Sci. 2022, 23, 3503. [Google Scholar] [CrossRef]
- Losol, P.; Wolska, M.; Wypych, T.P.; Yao, L.; O’Mahony, L.; Sokolowska, M. A cross talk between microbial metabolites and host immunity: Its relevance for allergic diseases. Clin. Transl. Allergy 2024, 14, e12339. [Google Scholar] [CrossRef]
- Hirata, S.I.; Kunisawa, J. Gut microbiome, metabolome, and allergic diseases. Allergol. Int. 2017, 66, 523–528. [Google Scholar] [CrossRef]
- Jeon, H.J.; Ban, O.H.; Bang, W.Y.; Yang, J.; Jung, Y.H. Trends, Functionalities, and Prospects of Probiotics. Microbiol. Biotechnol. Lett. 2022, 50, 465–476. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, A.R.; Kim, H.S.; Kim, H.W.; Park, Y.H.; You, J.S.; Park, Y.M.; Her, E.; Kim, H.S.; Kim, Y.M.; et al. Rhamnus davurica leaf extract inhibits Fyn activation by antigen in mast cells for anti-allergic activity. BMC Complement. Altern. Med. 2015, 15, 80. [Google Scholar] [CrossRef]
- Moosbrugger-Martinz, V.; Schmuth, M.; Dubrac, S. A Mouse Model for Atopic Dermatitis Using Topical Application of Vitamin D3 or of Its Analog MC903. Methods Mol. Biol. 2017, 1559, 91–106. [Google Scholar] [CrossRef]
- Yamamoto, M.; Haruna, T.; Yasui, K.; Takahashi, H.; Iduhara, M.; Takaki, S.; Deguchi, M.; Arimura, A. A novel atopic dermatitis model induced by topical application with dermatophagoides farinae extract in NC/Nga mice. Allergol. Int. 2007, 56, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Chin, C.S.; Alexander, D.H.; Marks, P.; Klammer, A.A.; Drake, J.; Heiner, C.; Clum, A.; Copeland, A.; Huddleston, J.; Eichler, E.E.; et al. Nonhybrid, finished microbial genome assemblies from long-read SMRT sequencing data. Nat. Methods 2013, 10, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Walker, B.J.; Abeel, T.; Shea, T.; Priest, M.; Abouelliel, A.; Sakthikumar, S.; Cuomo, C.A.; Zeng, Q.; Wortman, J.; Young, S.K.; et al. Pilon: An integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS ONE 2014, 9, e112963. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H.; Apweiler, R.; Bairoch, A.; Natale, D.A.; Barker, W.C.; Boeckmann, B.; Ferro, S.; Gasteiger, E.; Huang, H.; Lopez, R.; et al. The Universal Protein Resource (UniProt): An expanding universe of protein information. Nucleic Acids Res. 2006, 34, D187–D191. [Google Scholar] [CrossRef]
- Jain, C.; Rodriguez, R.L.; Phillippy, A.M.; Konstantinidis, K.T.; Aluru, S. High throughput ANI analysis of 90K prokaryotic genomes reveals clear species boundaries. Nat. Commun. 2018, 9, 5114. [Google Scholar] [CrossRef]
- Liu, B.; Zheng, D.; Jin, Q.; Chen, L.; Yang, J. VFDB 2019: A comparative pathogenomic platform with an interactive web interface. Nucleic Acids Res. 2019, 47, D687–D692. [Google Scholar] [CrossRef]
- Lebman, D.A.; Coffman, R.L. Interleukin 4 causes isotype switching to IgE in T cell-stimulated clonal B cell cultures. J. Exp. Med. 1988, 168, 853–862. [Google Scholar] [CrossRef]
- Burton, O.T.; Darling, A.R.; Zhou, J.S.; Noval-Rivas, M.; Jones, T.G.; Gurish, M.F.; Chatila, T.A.; Oettgen, H.C. Direct effects of IL-4 on mast cells drive their intestinal expansion and increase susceptibility to anaphylaxis in a murine model of food allergy. Mucosal Immunol. 2013, 6, 740–750. [Google Scholar] [CrossRef]
- Sehra, S.; Yao, Y.; Howell, M.D.; Nguyen, E.T.; Kansas, G.S.; Leung, D.Y.; Travers, J.B.; Kaplan, M.H. IL-4 regulates skin homeostasis and the predisposition toward allergic skin inflammation. J. Immunol. 2010, 184, 3186–3190. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, A.-R.; Jeon, S.-G.; Kim, H.-R.; Hong, H.; Yoon, Y.W.; Lee, B.-M.; Yoon, C.H.; Choi, S.J.; Jang, M.H.; Yang, B.-G. Preventive and Therapeutic Effects of Lactiplantibacillus plantarum HD02 and MD159 through Mast Cell Degranulation Inhibition in Mouse Models of Atopic Dermatitis. Nutrients 2024, 16, 3021. https://doi.org/10.3390/nu16173021
Kim A-R, Jeon S-G, Kim H-R, Hong H, Yoon YW, Lee B-M, Yoon CH, Choi SJ, Jang MH, Yang B-G. Preventive and Therapeutic Effects of Lactiplantibacillus plantarum HD02 and MD159 through Mast Cell Degranulation Inhibition in Mouse Models of Atopic Dermatitis. Nutrients. 2024; 16(17):3021. https://doi.org/10.3390/nu16173021
Chicago/Turabian StyleKim, A-Ram, Seong-Gak Jeon, Hyung-Ran Kim, Heeji Hong, Yong Won Yoon, Byung-Min Lee, Chung Hoo Yoon, Soo Jin Choi, Myoung Ho Jang, and Bo-Gie Yang. 2024. "Preventive and Therapeutic Effects of Lactiplantibacillus plantarum HD02 and MD159 through Mast Cell Degranulation Inhibition in Mouse Models of Atopic Dermatitis" Nutrients 16, no. 17: 3021. https://doi.org/10.3390/nu16173021
APA StyleKim, A.-R., Jeon, S.-G., Kim, H.-R., Hong, H., Yoon, Y. W., Lee, B.-M., Yoon, C. H., Choi, S. J., Jang, M. H., & Yang, B.-G. (2024). Preventive and Therapeutic Effects of Lactiplantibacillus plantarum HD02 and MD159 through Mast Cell Degranulation Inhibition in Mouse Models of Atopic Dermatitis. Nutrients, 16(17), 3021. https://doi.org/10.3390/nu16173021





