A Starch- and Sucrose-Reduced Diet Has Similar Efficiency as Low FODMAP in IBS—A Randomized Non-Inferiority Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients
2.3. Dietary Advice
2.4. Questionnaires
2.4.1. Study Questionnaire
2.4.2. Rome IV Questionnaire
2.4.3. Irritable Bowel Syndrome-Severity Scoring System
2.4.4. Visual Analog Scale for Irritable Bowel Syndrome
2.5. Statistical Analyses
3. Results
3.1. Basal Characteristics
3.2. Gastrointestinal and Extraintestinal Symptoms
3.3. Anthropometric Data
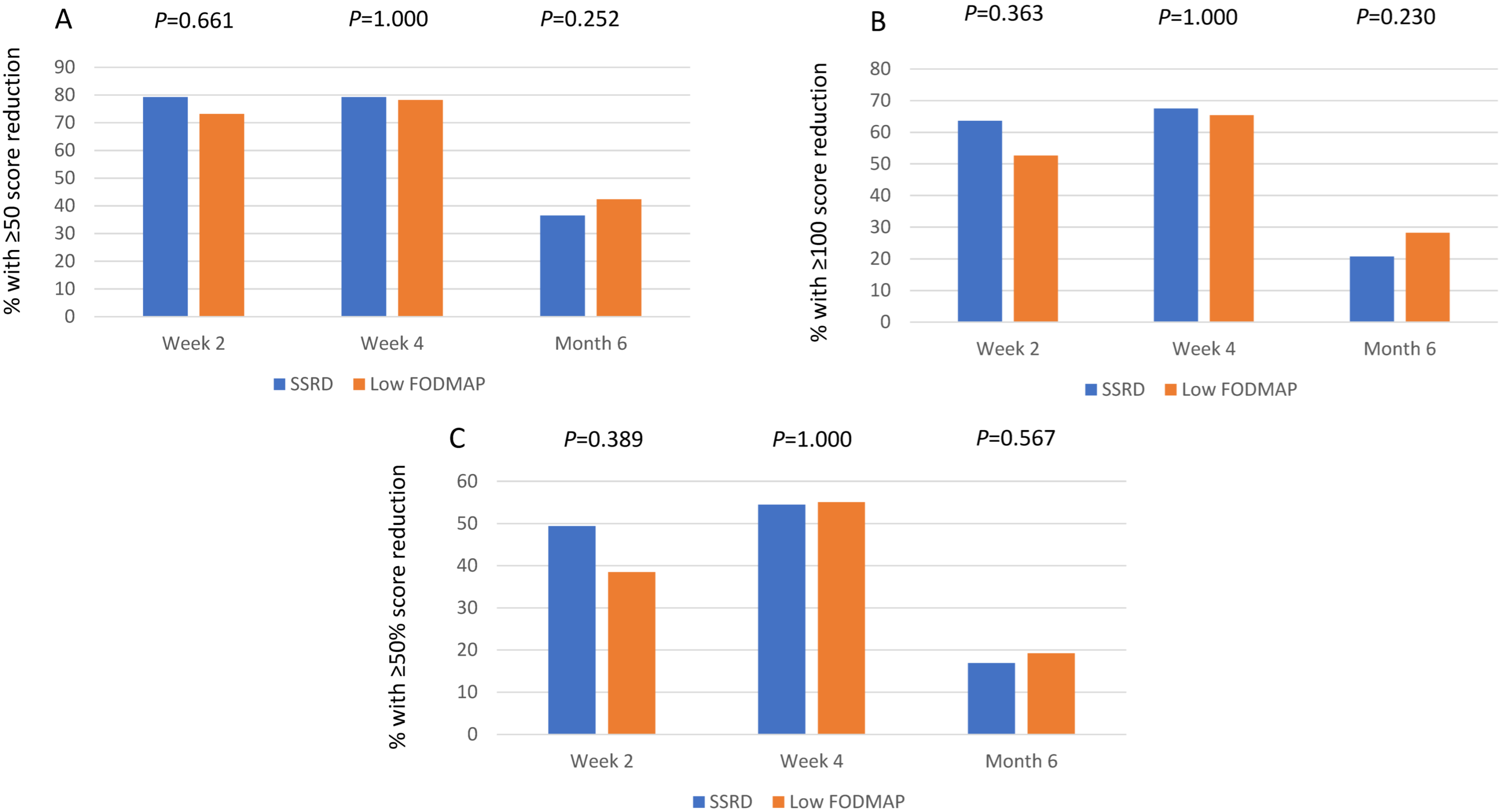
3.4. Follow-Up
3.5. Safety Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lacy, B.E.; Mearin, F.; Chang, L.; Chey, W.D.; Lembo, A.J.; Simren, M.; Spiller, R. Bowel disorders. Gastroenterology 2016, 150, 1393–1407. [Google Scholar] [CrossRef]
- Sperber, A.D.; Bangdiwala, S.I.; Drossman, D.A.; Ghoshal, U.C.; Simren, M.; Tack, J.; Whitehead, W.E.; Dumitrascu, D.L.; Fang, X.; Fukudo, S.; et al. Worldwide prevalence and burden of functional gastrointestinal disorders, results of Rome foundation global study. Gastroenterology 2021, 160, 99–114. [Google Scholar] [CrossRef]
- Hayes, P.A.; Fraher, M.H.; Quigley, E.M. Irritable bowel syndrome: The role of food in pathogenesis and management. Gastroenterol. Hepatol. 2014, 10, 164–174. [Google Scholar]
- Algera, J.; Colomier, E.; Simrén, M. The Dietary Management of Patients with Irritable Bowel Syndrome: A Narrative Review of the Existing and Emerging Evidence. Nutrients 2019, 11, 2162. [Google Scholar] [CrossRef]
- Mitchell, H.; Porter, J.; Gibson, P.R.; Barrett, J.; Garg, M. Review article: Implementation of a diet low in FODMAPs for patients with irritable bowel syndrome-directions for future research. Aliment. Pharmacol. Ther. 2019, 49, 124–139. [Google Scholar] [CrossRef] [PubMed]
- Whelan, K.; Martin, L.D.; Staudacher, H.M.; Lomer, M.C.E. The low FODMAP diet in the management of irritable bowel syndrome: An evidence-based review of FODMAP restriction, reintroduction and personalisation in clinical practice. J. Hum. Nutr. Diet. 2018, 31, 239–255. [Google Scholar] [CrossRef] [PubMed]
- Staudacher, H.M.; Whelan, K. The low FODMAP diet: Recentadvances in understanding its mechanisms and efficacy in IBS. Gut 2017, 66, 1517–1527. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Zhang, H.; Chen, S.; Wu, P.; Chen, M. Global research progress of visceral hypersensitivity and irritable bowel syndrome: Bibliometrics and visualized analysis. Front. Pharmacol. 2023, 14, 1175057. [Google Scholar] [CrossRef]
- Maagaard, L.; Ankersen, D.V.; Végh, Z.; Burisch, J.; Jensen, L.; Pedersen, N.; Munkholm, P. Follow-up of patients with functional bowel symptoms treated with a low FODMAP diet. World J. Gastroenterol. 2016, 22, 4009–4019. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Ralph, F.S.E.; Irving, P.M.; Whelan, K.; Lomer, M.C.E. Nutrient intake, diet quality, and diet diversity in irritable bowel syndrome and the impact of the low FODMAP diet. J. Acad. Nutr. Diet. 2020, 120, 535–547. [Google Scholar] [CrossRef]
- O’Keeffe, M.; Jansen, C.; Martin, L.; Williams, M.; Seamark, L.; Staudacher, H.M.; Irving, P.M.; Whelan, K.; Lomer, M.C. Long-term impact of the low-FODMAP diet on gastrointestinal symptoms, dietary intake, patient acceptability, and healthcare utilization in irritable bowel syndrome. Neurogastroenterol. Motil. 2018, 30, e13154. [Google Scholar] [CrossRef]
- Danialifar, T.F.; Chumpitazi, B.P.; Mehta, D.I.; Di Lorenzo, C. Genetic and acquired sucrase-isomaltase deficiency: A clinical review. J. Pediatr. Gastroenterol. Nutr. 2024, 78, 774–782. [Google Scholar] [CrossRef]
- Henström, M.; Diekmann, L.; Bonfiglio, F.; Hadizadeh, F.; Kuech, E.M.; von Köckritz-Blickwede, M.; Thingholm, L.B.; Zheng, T.; Assadi, G.; Dierks, C.; et al. Functional variants in the sucrase-isomaltase gene associate with increased risk of irritable bowel syndrome. Gut 2018, 67, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Etxebarria, K.; Zheng, T.; Bonfiglio, F.; Bujanda, L.; Dlugosz, A.; Lindberg, G.; Schmidt, P.T.; Karling, P.; Ohlsson, B.; Simren, M.; et al. Increased Prevalence of Rare Sucrase-isomaltase Pathogenic Variants in Irritable Bowel Syndrome Patients. Clin. Gastroenterol. Hepatol. 2018, 16, 1673–1676. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.A.; Oloyede, H.; Gold, B.D.; Mohammed, A.; Elser, H.E. Clinical Characteristics of Disaccharidase Deficiencies Among Children Undergoing Upper Endoscopy. J. Pediatr. Gastroenterol. Nutr. 2018, 66 (Suppl. 3), S56–S60. [Google Scholar] [CrossRef] [PubMed]
- McMeans, A.R. Congenital sucrase-isomaltase deficiency: Diet assessment and education guidelines. J. Pediatr. Gastroenterol. Nutr. 2012, 55 (Suppl. 2), S37–S39. [Google Scholar] [CrossRef] [PubMed]
- Treem, W.R.; McAdams, L.; Stanford, L.; Kastoff, G.; Justinich, C.; Hyams, J. Sacrosidase therapy for congenital sucrase-isomaltase deficiency. J. Pediatr. Gastroenterol. Nutr. 1999, 28, 137–142. [Google Scholar]
- Cordain, L.; Eaton, S.B.; Sebastian, A.; Mann, N.; Lindeberg, S.; Watkins, B.A.; O’Keefe, J.H.; Brand-Miller, J. Origins and evolution of the Western diet: Health implications for the 21st century. Am. J. Clin. Nutr. 2005, 81, 341–354. [Google Scholar] [CrossRef]
- Senftleber, N.K.; Skøtt Pedersen, K.; Schnoor Jørgensen, C.; Pedersen, H.; Bjerg Christensen, M.M.; Kabel Madsen, E.; Andersen, K.; Jørsboe, E.; Gillum, M.P.; Frøst, M.B.; et al. The effect of sucrase-isomaltase deficiency on metabolism, food intake and preferences: Protocol for a dietary intervention study. Int. J. Circumpolar Health 2023, 82, 2178067. [Google Scholar] [CrossRef]
- Nilholm, C.; Roth, B.; Ohlsson, B. A Dietary Intervention with Reduction of Starch and Sucrose Leads to Reduced Gastrointestinal and Extra-Intestinal Symptoms in IBS Patients. Nutrients 2019, 11, 1662. [Google Scholar] [CrossRef]
- Gayoso, L.; Garcia-Etxebarria, K.; Arzallus, T.; Montalvo, I.; Lizasoain, J.; D’Amato, M.; Etxeberria, U.; Bujanda, L. The effect of starch- and sucrose-reduced diet accompanied by nutritional and culinary recommendations on the symptoms of irritable bowel syndrome patients with diarrhoea. Ther. Adv. Gastroenterol. 2023, 16, 175628482311566. [Google Scholar] [CrossRef] [PubMed]
- (Choosing Your Foods—CSID Cares). Available online: https://www.csidcares.org (accessed on 12 February 2024).
- Nilholm, C.; Larsson, E.; Sonestedt, E.; Roth, B.; Ohlsson, B. Assessment of a 4-Week Starch- and Sucrose-Reduced Diet and Its Effects on Gastrointestinal Symptoms and Inflammatory Parameters among Patients with Irritable Bowel Syndrome. Nutrients 2021, 13, 416. [Google Scholar] [CrossRef] [PubMed]
- Roth, B.; Ohlsson, B. Challenges of recruitment processes to a randomized dietary trial in irritable bowel syndrome. F1000Research 2024, 13, 323. [Google Scholar] [CrossRef]
- Palsson, O.S.; Whitehead, W.E.; Van Tilburg, M.A.L.; Chang, L.; Chey, W.; Crowell, M.D.; Keefer, L.; Lembo, A.J.; Parkman, H.P.; Rao, S.S.; et al. Development and validation of the Rome IV diagnostic questionnaire for adults. Gastroenterology 2016, 150, 1481–1491. [Google Scholar] [CrossRef]
- Francis, C.Y.; Morris, J.; Whorwell, P.J. The irritable bowel severity scoring system: A simple method of monitoring irritable bowel syndrome and its progress. Aliment. Pharmacol. Ther. 1997, 11, 395–402. [Google Scholar] [CrossRef]
- Bengtsson, M.; Ohlsson, B.; Ulander, K. Development and psychometric testing of the Visual Analogue Scale for Irritable Bowel Syndrome (VAS-IBS). BMC Gastroenterol. 2007, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Laboratoriemedicin. Available online: http://www.analysportalen-labmedicin.skane.se (accessed on 12 April 2021).
- Staudacher, H.M.; Rossi, M.; Kaminski, T.; Dimidi, E.; Ralph, F.S.E.; Wilson, B.; Martin, L.D.; Louis, P.; Lomer, M.C.E.; Irving, P.M.; et al. Long-term personalized low FODMAP diet improves symptoms and maintains luminal Bifidobacteria abundance in irritable bowel syndrome. Neurogastroenterol. Motil. 2022, 34, e14241. [Google Scholar] [CrossRef]
- Available online: www.livsmedelsverket.se (accessed on 3 April 2024).
- Perry, L.; Morgan, J.; Reid, F.; Brunton, J.; O’Brien, A.; Luck, A.; Lacey, H. Screening for symptoms of eating disorders: Reliability of the SCOFF screening tool with written compared to oral delivery. Int. J. Eat. Disord. 2002, 32, 466–472. [Google Scholar] [CrossRef]
- Bengtsson, M.; Persson, J.; Sjölund, K.; Ohlsson, B. Further validation of the visual analogue scale for irritable bowel syndrome after use in clinical practice. Gastroenterol. Nurs. 2013, 36, 188–198. [Google Scholar] [CrossRef]
- McDonald, J.H. Handbook of Biological Statistics, 3rd ed.; Sparky House Publishing: Baltimore, MD, USA, 2014. [Google Scholar]
- Algera, J.P.; Demir, D.; Törnblom, H.; Nybacka, S.; Simrén, M.; Störsrud, S. Low FODMAP diet reduces gastrointestinal symptoms in irritable bowel syndrome and clinical response could be predicted by symptom severity: A randomized crossover trial. Clin. Nutr. 2022, 41, 2792–2800. [Google Scholar] [CrossRef]
- Pereyra, F.; Bustos Fernández, L.M.; Schlottmann, F.; Zamora, R.; Marconi, A.; Steinberg, L.; Pereyra, L. Prevalence of extra-intestinal symptoms according to irritable bowel syndrome subtype. Neurogastroenterol. Motil. 2024, 36, e14796. [Google Scholar] [CrossRef] [PubMed]
- Nybacka, S.; Törnblom, H.; Josefsson, A.; Hreinsson, J.P.; Böhn, L.; Frändemark, Å.; Weznaver, C.; Störsrud, S.; Simrén, M. A low FODMAP diet plus traditional dietary advice versus a low-carbohydrate diet versus pharmacological treatment in irritable bowel syndrome (CARIBS): A single-centre, single-blind, randomised controlled trial. Lancet Gastroenterol. Hepatol. 2024, 9, 507–520. [Google Scholar] [CrossRef] [PubMed]
- Nilholm, C.; Larsson, E.; Roth, B.; Gustafsson, R.; Ohlsson, B. Irregular Dietary Habits with a High Intake of Cereals and Sweets Are Associated with More Severe Gastrointestinal Symptoms in IBS Patients. Nutrients 2019, 11, 1279. [Google Scholar] [CrossRef]
- Torices, L.; Zamfir-Taranu, A.; Esteban-Blanco, C.; Bozzarelli, I.; Bonfiglio, F.; D’Amato, M. Human CAZyme genes polymorphism and risk of IBS: A population-based study. Gut 2024, online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Ng, Q.X.; Yau, C.E.; Yaow, C.Y.L.; Chong, R.I.H.; Chong, N.Z.; Teoh, S.E.; Lim, Y.L.; Soh, A.Y.S.; Ng, W.K.; Thumboo, J. What Has Longitudinal ‘Omics’ Studies Taught Us about Irritable Bowel Syndrome? A Systematic Review. Metabolites 2023, 13, 484. [Google Scholar] [CrossRef]
- Saidi, K.; Nilholm, C.; Roth, B.; Ohlsson, B. A carbohydrate-restricted diet for patients with irritable bowel syndrome lowers serum C-peptide, insulin, and leptin without any correlation with symptom reduction. Nutr. Res. 2021, 86, 23–36. [Google Scholar] [CrossRef]
- El-Salhy, M.; Gundersen, D. Diet in irritable bowel syndrome. Nutr. J. 2015, 14, 36. [Google Scholar] [CrossRef]
- Bek, S.; Teo, Y.N.; Tan, X.H.; Fan, K.H.R.; Siah, K.T.H. Association between irritable bowel syndrome and micronutrients: A systematic review. J. Gastroenterol. Hepatol. 2022, 37, 1485–1497. [Google Scholar] [CrossRef]
- Luger, M.; Lafontan, M.; Bes-Rastrollo, M.; Winzer, E.; Yumuk, V.; Farpour-Lambert, N. Sugar-Sweetened Beverages and Weight Gain in Children and Adults: A Systematic Review from 2013 to 2015 and a Comparison with Previous Studies. Obes. Facts 2017, 10, 674–693. [Google Scholar] [CrossRef]
- Garcia, M.M.; Corrales, P.; Huerta, M.Á.; Czachorowski, M.J.; López-Miranda, V.; Medina-Gómez, G.; Cobos, E.J.; Goicoechea, C.; Molina-Álvarez, M. Adults with excess weight or obesity, but not with overweight, report greater pain intensities than individuals with normal weight: A systematic review and meta-analysis. Front. Endocrinol. 2024, 15, 1340465. [Google Scholar] [CrossRef]
- Shi, X.; Deng, G.; Wen, H.; Lin, A.; Wang, H.; Zhu, L.; Mou, W.; Liu, Z.; Li, X.; Zhang, J.; et al. Role of body mass index and weight change in the risk of cancer: A systematic review and meta-analysis of 66 cohort studies. J. Glob. Health 2024, 14, 04067. [Google Scholar] [CrossRef]
- Zia, J.K.; Lenhart, A.; Yang, P.L.; Heitkemper, M.M.; Baker, J.; Keefer, L.; Saps, M.; Cuff, C.; Hungria, G.; Videlock, E.J.; et al. Risk factors for abdominal pain-related disorders of gut-brain interaction in adults and children: A systematic review. Gastroenterology 2022, 163, 995–1023. [Google Scholar] [CrossRef]
- Guo, Y.; Niu, K.; Momma, H.; Kobayashi, Y.; Chujo, M.; Otomo, A.; Fukudo, S.; Nagatomi, R. Irritable bowel syndrome is positively related to metabolic syndrome: A population-based cross-sectional study. PLoS ONE 2014, 9, e112289. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Liang, Z.; Ma, J.; Hu, D.; Yao, F.; Qin, P. Total sugar, added sugar, fructose, and sucrose intake and all-cause, cardiovascular, and cancer mortality: A systematic review and dose-response meta-analysis of prospective cohort studies. Nutrition 2023, 111, 112032. [Google Scholar] [CrossRef]
- Sultan, N.; Foyster, M.; Tonkovic, M.; Noon, D.; Burton-Murray, H.; Biesiekierski, J.R.; Tuck, C.J. Presence and characteristics of disordered eating and orthorexia in irritable bowel syndrome. Neurogastroenterol. Motil. 2024, 36, e14797. [Google Scholar] [CrossRef]
- Whelan, K.; Staudacher, H. Low FODMAP diet in irritable bowel syndrome: A review of recent clinical trials and meta-analyses. Curr. Opin. Clin. Nutr. Metab. Care 2022, 25, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Staudacher, H.M.; Mahoney, S.; Canale, K.; Opie, R.S.; Loughman, A.; So, D.; Beswick, L.; Hair, C.; Jacka, F.N. Clinical trial: A Mediterranean diet is feasible and improves gastrointestinal and psychological symptoms in irritable bowel syndrome. Aliment. Pharmacol. Ther. 2024, 59, 492–503. [Google Scholar] [CrossRef] [PubMed]

| Parameters | SSRD N = 77 | Low FODMAP N = 78 | p-Value |
|---|---|---|---|
| Age (year) | 41.0 (29.5–53.0) | 43.0 (33.8–56.0) | 0.227 |
| Gender (male/female) (n,%) | 15 (19.5)/62 (80.5) | 10 (12.8)/68 (87.2) | 0.283 |
| Weight (kg) | 71.5 (63.6–82.8) | 68.6 (63.0–83.4) | 0.389 |
| BMI (kg/m2) | 25.1 (22.6–28.4) | 24.7 (22.1–27.6) | 0.479 |
| Disease duration (year) | 16 (7–27) | 20 (10–30) | 0.261 |
| Education (n,%) | 0.585 | ||
| Primary school | 5 (6.5) | 2 (2.6) | |
| Secondary school | 10 (13.0) | 13 (16.7) | |
| Education after secondary school | 20 (26.0) | 17 (21.8) | |
| Examination at university | 42 (54.5) | 46 (59.0) | |
| Occupation (n,%) | 0.963 | ||
| Working full time | 48 (62.3) | 46 (59.0) | |
| Working 99–51% | 6 (7.8) | 9 (11.5) | |
| Working 50% | 1 (1.3) | 1 (1.3) | |
| Studying | 10 (13.0) | 10 (12.8) | |
| Sick leave | 3 (3.9) | 2 (2.6) | |
| Unemployment | 3 (3.9) | 2 (2.6) | |
| Retirement | 6 (7.8) | 8 (10.3) | |
| Marital status (n,%) | 0.837 | ||
| Living alone | 16 (20.8) | 14 (17.9) | |
| Living together | 55 (71.4) | 56 (71.8) | |
| Other | 6 (7.8) | 8 (10.3) | |
| Smoking (n,%) | 0.086 | ||
| Never | 42 (54.5) | 43 (55.1) | |
| Former | 26 (33.8) | 28 (35.9) | |
| Present un regular | 2 (2.6) | 6 (7.7) | |
| Present regular | 7 (9.1) | 1 (1.3) | |
| Alcohol intake for 1 week (standard glass) (n,%) | 1.000 | ||
| Missing | 1 | ||
| <1 | 34 (44.2) | 33 (42.3) | |
| 1–4 | 28 (36.4) | 29 (37.2) | |
| 5–9 | 13 (16.9) | 13 (16.7) | |
| 10–14 | 1 (1.3) | 2 (2.6) | |
| ≥15 | 1 (1.3) | 0 | |
| Physical activity for 1 week (n,%) | 0.475 | ||
| Missing | 1 | ||
| No time | 10 (13.0) | 8 (10.4) | |
| <30 min | 11 (14.3) | 14 (18.2) | |
| 30–60 min | 16 (20.8) | 14 (18.2 | |
| 60–90 min | 8 (10.4) | 16 (20.8) | |
| 90–120 min | 8 (10.4) | 8 (10.4) | |
| >120 min | 24 (31.2) | 17 (22.1) |
| SSRD N = 77 | Low FODMAP N = 78 | p-Value * | |||||
|---|---|---|---|---|---|---|---|
| VAS-IBS (mm) | Value | p-Value | Difference | Value | p-Value | Difference | |
| Abdominal pain 5 (1–13) | |||||||
| Baseline | 47 (28–64) | - | - | 50 (32–65) | - | - | 0.368 |
| 2 weeks | 17 (8–30) | <0.001 | −27 (−47–(−9)) | 22 (13–40) | <0.001 | −19 (−36–(−3)) | 0.252 |
| 4 weeks | 16 (0–31) | <0.001 | −24 (−44–(−9)) | 13 (0–27) | <0.001 | −30 (−53–(−8)) | 0.425 |
| 6 months | 32 (19–63) | 0.003 | −6 (−28–3) | 30 (16–54) | <0.001 | −16 (−38–4) | 0.270 |
| Diarrhea 3 (0–10) | |||||||
| Baseline | 53 (19–73) | - | - | 37 (4–74) | - | - | 0.245 |
| 2 weeks | 15 (4–48) | <0.001 | −14 (−51–0) | 10 (0–37) | <0.001 | −14 (−38–0) | 0.793 |
| 4 weeks | 17 (3–39) | <0.001 | −8 (−48–2) | 8 (0–24) | <0.001 | −16 (−53–0) | 0.633 |
| 6 months | 31 (8–68) | <0.001 | −12 (−39–0) | 11 (3–44) | 0.008 | −8 (−30–7) | 0.457 |
| Constipation 6 (2–16) | |||||||
| Baseline | 53 (6–72) | - | - | 54 (10–76) | - | - | 0.439 |
| 2 weeks | 16 (2–50) | <0.001 | −12 (−36–2) | 20 (0–68) | 0.02 | −6 (−28–4) | 0.510 |
| 4 weeks | 16 (2–43) | <0.001 | −8 (−46–0) | 21 (0–55) | <0.001 | −13 (−33–0) | 0.815 |
| 6 months | 22 (0–61) | 0.121 | −3 (−24–12) | 42 (2–70) | 0.022 | −4 (−32–3) | 0.528 |
| Bloating and flatulence 10 (2–23) | |||||||
| Baseline | 73 (58–88) | - | - | 73 (54–86) | - | - | 0.677 |
| 2 weeks | 34 (18–53) | <0.001 | −37 (−53–(−9)) | 23 (13–50) | <0.001 | −39 (−56–(−16)) | 0.469 |
| 4 weeks | 24 (10–54) | <0.001 | −43 (−63–(−11) | 19 (8–50) | <0.001 | −44 (−61–(−25)) | 0.359 |
| 6 months | 62 (30–75) | 0.002 | −15 (−38–14) | 56 (33–70) | <0.001 | −18 (−33–(−3)) | 0.416 |
| Vomiting and nausea 2 (0–4) | |||||||
| Baseline | 13 (2–34) | - | - | 13 (1–36) | - | - | 0.957 |
| 2 weeks | 6 (0–12) | <0.001 | −7 (−20–0) | 4 (0–12) | <0.001 | −7 (−22–0) | 0.773 |
| 4 weeks | 3 (0–12) | <0.001 | −6 (−15–0) | 0 (0–11) | <0.001 | −7 (−21–0) | 0.743 |
| 6 months | 8 (1–21) | 0.002 | −4 (−14–1) | 5 (0–21) | 0.009 | −2 (−17–1) | 0.892 |
| Intestinal symptom’s influence on daily life 2 (0–14) | |||||||
| Baseline | 74 (57–84) | - | - | 70 (54–84) | - | - | 0.694 |
| 2 weeks | 29 (15–60) | <0.001 | −36 (−52–(−11)) | 30 (17–60) | <0.001 | −28 (−50–(−15)) | 0.688 |
| 4 weeks | 24 (12–62) | <0.001 | −30 (−60–(−10)) | 22 (10–50) | <0.001 | −33 (−53–(−18)) | 0.593 |
| 6 months | 40 (23–76) | <0.001 | −12 (−45–0) | 48 (24–68) | <0.001 | −26 (−43–(−2)) | 0.492 |
| Psychological well-being 5 (2–15) | |||||||
| Baseline | 39 (15–65) | - | - | 45 (16–59) | - | - | 0.708 |
| 2 weeks | 24 (11–42) | <0.001 | −7 (−26–0) | 27 (8–46) | 0.021 | −4 (−25–5) | 0.339 |
| 4 weeks | 20 (5–32) | <0.001 | −12 (−29–(−2) | 18 (2–34) | <0.001 | −13 (−32–1) | 0.788 |
| 6 months | 22 (11–50) | 0.009 | −6 (−20–4) | 26 (8–39) | 0.043 | −2 (−30–7) | 0.911 |
| IBS-SSS | |||||||
| Total IBS-SSS | |||||||
| Baseline | 301 (233–348) | - | - | 300 (238–360) | - | - | 0.845 |
| 2 weeks | 136 (87–223) | <0.001 | −138 (−212–(−82)) | 151 (100–232) | <0.001 | −110 (−188–(−68)) | 0.310 |
| 4 weeks | 119 (66–230) | <0.001 | −146 (−240–(−88)) | 116 (63–176) | <0.001 | −153 (−231–(−90)) | 0.585 |
| 6 months | 204 (146–234) | <0.001 | −55 (−130–4) | 220 (144–301) | <0.001 | −93 (−181–(−20)) | 0.069 |
| SSRD | p-Value * | Low FODMAP | p-Value * | p-Value ** | |
|---|---|---|---|---|---|
| Baseline | N = 77 | N = 78 | 0.078 | ||
| IBS-C | 14 (18.2) | 12 (15.4) | |||
| IBS-D | 29 (37.7) | 15 (19.2) | |||
| IBS-M | 22 (28.6) | 32 (41.0) | |||
| IBS-U | 2 (2.6) | 5 (6.4) | |||
| FBD | 10 (13.0) | 14 (17.9) | |||
| Week 4 | 0.621 | ||||
| IBS-C | 8 (10.4) | 10 (12.8) | |||
| IBS-D | 8 (10.4) | 7 (9.0) | |||
| IBS-M | 14 (18.2) | 9 (11.5) | |||
| IBS-U | 0 | 2 (2.6) | |||
| FBD | 23 (29.9) | 25 (32.1) | |||
| Healthy | 19 (24.7) | 19 (24.4) | |||
| Missing | 5 (6.5) | <0.001 | 6 (7.7) | <0.001 | |
| Month 6 | 0.198 | ||||
| IBS-C | 6 (7.8) | 11 (14.1) | |||
| IBS-D | 8 (10.4) | 6 (7.7) | |||
| IBS-M | 12 (15.6) | 9 (11.5) | |||
| IBS-U | 1 (1.3) | 3 (3.8) | |||
| FBD | 23 (29.9) | 13 (16.7) | |||
| Healthy | 3 (3.9) | 7 (9.0) | |||
| Missing | 24 (31.2) | <0.001 | 29 (37.2) | 0.046 |
| SSRD N = 77 | Low FODMAP N = 78 | p-Value * | |||||
|---|---|---|---|---|---|---|---|
| Extraintestinal IBS-SSS | Value | p-Value | Difference | Value | p-Value | Difference | |
| Difficulties to eat a whole meal | |||||||
| Baseline | 10 (2–26) | - | - | 6 (0–22) | - | - | 0.267 |
| 2 weeks | 4 (0–12) | 0.002 | −3 (−13–3) | 3 (0–13) | 0.005 | −2 (−11–0) | 0.951 |
| 4 weeks | 2 (0–13) | <0.001 | −4 (−16–1) | 0 (0–9) | <0.001 | −3 (−12–0) | 0.629 |
| 6 months | 4 (0–14) | 0.074 | −2 (−13–4) | 2 (0–18) | 0.053 | −1 (−10–0) | 0.940 |
| Headache | |||||||
| Baseline | 33 (10–66) | - | - | 27 (9–58) | - | - | 0.737 |
| 2 weeks | 14 (5–36) | <0.001 | −5 (−30–2) | 15 (2–47) | <0.001 | −6 (−22–0) | 0.993 |
| 4 weeks | 14 (2–32) | <0.001 | −9 (−27–2) | 12 (0–35) | <0.001 | −9 (−31–0) | 0.855 |
| 6 months | 24 (10–55) | 0.185 | −1 (−21–8) | 20 (4–50) | 0.001 | −4 (−21–2) | 0.324 |
| Back pain | |||||||
| Baseline | 20 (4–50) | - | - | 28 (4–65) | - | - | 0.395 |
| 2 weeks | 6 (0–29) | <0.001 | −6 (−21–0) | 14 (0–39) | <0.001 | −2 (−26–2) | 0.409 |
| 4 weeks | 6 (0–30) | <0.001 | −7 (−22–0) | 4 (0–35) | <0.001 | −7 (−32–0) | 0.998 |
| 6 months | 23 (4–58) | 0.157 | −4 (−18–8) | 24 (4–70) | 0.675 | 0 (−14–12) | 0.501 |
| Fatigue | |||||||
| Baseline | 57 (30–81) | - | - | 74 (48–84) | - | - | 0.055 |
| 2 weeks | 33 (16–68) | <0.001 | −14 (−27–0) | 47 (19–70) | <0.001 | −12 (−32–0) | 0.660 |
| 4 weeks | 27 (9–56) | <0.001 | −18 (−32–(−2)) | 37 (14–60) | <0.001 | −19 (−38–(−3)) | 0.712 |
| 6 months | 49 (18–68) | 0.004 | −7 (−20–4) | 48 (19–69) | <0.001 | −13 (−27–0) | 0.128 |
| Belching/excess wind | |||||||
| Baseline | 72 (48–85) | - | - | 75 (52–87) | - | - | 0.621 |
| 2 weeks | 24 (10–66) | <0.001 | −21 (−51––(−6)) | 37 (14–66) | <0.001 | −23 (−44–(−6)) | 0.804 |
| 4 weeks | 14 (6–40) | <0.001 | −41 (−67–(−6)) | 21 (8–45) | <0.001 | −41 (−59–(−19)) | 0.878 |
| 6 months | 47 (20–68) | <0.001 | −13 (−33–(−2)) | 48 (22–70) | <0.001 | −15 (−37–(−2)) | 0.599 |
| Reflux | |||||||
| Baseline | 20 (7–50) | - | - | 20 (2–60) | - | - | 0.678 |
| 2 weeks | 5 (0–18) | <0.001 | −12 (−27–0) | 7 (0–35) | <0.001 | −6 (−30–0) | 0.274 |
| 4 weeks | 4 (0–20) | <0.001 | −12 (−28–0) | 3 (0–26) | <0.001 | −10 (−30–0) | 0.945 |
| 6 months | 11 (4–26) | 0.002 | −6 (−20–2) | 21 (2–54) | 0.013 | −3 (−18–2) | 0.727 |
| Urinary urgency | |||||||
| Baseline | 14 (2–64) | - | - | 22 (4–64) | - | - | 0.491 |
| 2 weeks | 7 (0–24) | <0.001 | −7 (−24–0) | 7 (0–33) | <0.001 | −8 (−29–0) | 0.598 |
| 4 weeks | 4 (0–23) | <0.001 | −6 (−34–0) | 3 (0–22) | <0.001 | −13 (−44–0) | 0.290 |
| 6 months | 18 (0–44) | 0.003 | −7 (−22–1) | 16 (0–53) | 0.003 | −7 (−19–0) | 0.975 |
| Leg pain | |||||||
| Baseline | 2 (0–9) | - | - | 0 (0–18) | - | - | 0.995 |
| 2 weeks | 1 (0–15) | 0.281 | 0 (−4–2) | 0 (0–10) | 0.036 | 0 (−3–0) | 0.776 |
| 4 weeks | 0 (0–7) | 0.024 | 0 (−4–0) | 0 (0–5) | 0.005 | 0 (−4–0) | 0.564 |
| 6 months | 2 (0–12) | 0.774 | 0 (−2–2) | 0 (0–14) | 0.573 | 0 (−2–2) | 0.682 |
| Muscle/joint pain | |||||||
| Baseline | 25 (5–56) | - | - | 30 (4–72) | - | - | 0.506 |
| 2 weeks | 11 (0–46) | 0.002 | −5 (−20–1) | 18 (0–57) | 0.014 | −1 (−20–4) | 0.616 |
| 4 weeks | 13 (0–30) | <0.001 | −10 (−27–0) | 12 (0–39) | <0.001 | −3 (−35–1) | 0.699 |
| 6 months | 23 (5–53) | 0.032 | −3 (−17–4) | 19 (4–70) | 0.084 | −2 (−18–7) | 0.755 |
| Total extraintestinal IBS-SSS | |||||||
| Baseline | 160 (110–208) | - | - | 172 (120–242) | - | - | 0.268 |
| 2 weeks | 96 (50–154) | <0.001 | −60 (−89–(−20)) | 115 (55–156) | <0.001 | −54 (−82–(−30)) | 0.852 |
| 4 weeks | 91 (28–140) | <0.001 | −72 (−112–(−41)) | 77 (44–136) | <0.001 | −83 (−118–(−44)) | 0.408 |
| 6 months | 127 (71–191) | <0.001 | −44 (−75–2) | 133 (78–214) | <0.001 | −36 (−59–(−10)) | 0.977 |
| SSRD N = 77 | Low FODMAP N = 78 | p-Value * | |||||
|---|---|---|---|---|---|---|---|
| Variables | Value | p-Value | Difference | Value | p-Value | Difference | |
| Weight (kg) | |||||||
| Baseline | 71.5 (63.6–82.8) | - | - | 68.6 (63–83.4) | - | - | 0.513 |
| 4 weeks | 70 (63.2–81) | <0.001 | −1.6 (−2.4–(−0.4)) | 67.8 (62.5–82.7) | <0.001 | −0.8 (−1.6–(−0.1)) | 0.006 |
| 6 months | 74.1 (66.6–85.7) | 0.516 | −0.2 (−1.4–1.2) | 68.6 (62.8–80.8) | 0.079 | −0.3 (−1.6–0.6) | 0.438 |
| BMI (kg/m2) | |||||||
| Baseline | 25.14 (22.64–28.45) | - | - | 24.68 (22.13–27.64) | - | - | 0.538 |
| 4 weeks | 24.8 (21.97–27.6) | <0.001 | −0.55 (−0.86–(−0.15)) | 24.63 (22–27.32) | <0.001 | −0.26 (−0.56–(−0.03)) | 0.005 |
| 6 months | 25.95 (22.66–28.57) | 0.504 | −0.07 (−0.53–0.44) | 25.08 (22.05–26.76) | 0.089 | −0.11 (−0.54–0.23) | 0.526 |
| Waist circumference (cm) | |||||||
| Baseline | 88 (76–97) | - | - | 86 (79–94.8) | - | - | 0.831 |
| 4 weeks | 86 (75–94) | <0.001 | −2 (−4–0) | 85 (79–93) | <0.001 | −2 (−3–1) | 0.981 |
| 6 months | 89 (77.5–97) | 0.022 | −1 (−4–1) | 85.5 (80–93.5) | 0.038 | −1 (−3–1) | 0.758 |
| Systolic Blood Pressure (mmHg) | |||||||
| Baseline | 125 (114–139) | 126 (116–139) | 0.657 | ||||
| 4 weeks | 123 (114–135) | 0.097 | −2 (−10–6) | 124 (113–135) | 0.024 | −3 (−8–3) | 0.762 |
| 6 months | 127 (116–138) | 0.588 | −1 (−7–6) | 126 (117–136) | 0.138 | −3 (−12–8) | 0.399 |
| Diastolic Blood Pressure (mmHg) | |||||||
| Baseline | 81 (72–88) | 81 (74–90) | 0.403 | ||||
| 4 weeks | 78 (70–84) | 0.006 | −3 (−6–3) | 80 (73–85) | <0.001 | −4 (−8–2) | 0.225 |
| 6 months | 80 (76–86) | 0.575 | −2 (−6–5) | 80 (72–86) | 0.044 | −1 (−10–3) | 0.190 |
| Sugar craving (mm) | |||||||
| Baseline | 66 (40–85) | - | - | 60 (29–80) | - | - | 0.384 |
| 4 weeks | 34 (17–67) | <0.001 | −15 (−41–0) | 41 (22–69) | 0.001 | −8 (−23–5) | 0.050 |
| 6 months | 53 (31–72) | 0.058 | −7 (−23–10) | 48 (28–72) | 0.246 | −2 (−10–8) | 0.448 |
| Saturation (mm) | |||||||
| Baseline | 74 (52–93) | - | - | 77 (68–86) | - | - | 0.833 |
| 4 weeks | 83 (69–93) | 0.107 | 4 (−12–24) | 80 (62–90) | 0.688 | 1 (−10–12) | 0.261 |
| 6 months | 77 (69–90) | 0.473 | 0 (−12–16) | 72 (62–87) | 0.464 | −2 (−13–16) | 0.275 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roth, B.; Nseir, M.; Jeppsson, H.; D’Amato, M.; Sundquist, K.; Ohlsson, B. A Starch- and Sucrose-Reduced Diet Has Similar Efficiency as Low FODMAP in IBS—A Randomized Non-Inferiority Study. Nutrients 2024, 16, 3039. https://doi.org/10.3390/nu16173039
Roth B, Nseir M, Jeppsson H, D’Amato M, Sundquist K, Ohlsson B. A Starch- and Sucrose-Reduced Diet Has Similar Efficiency as Low FODMAP in IBS—A Randomized Non-Inferiority Study. Nutrients. 2024; 16(17):3039. https://doi.org/10.3390/nu16173039
Chicago/Turabian StyleRoth, Bodil, Mohamed Nseir, Håkan Jeppsson, Mauro D’Amato, Kristina Sundquist, and Bodil Ohlsson. 2024. "A Starch- and Sucrose-Reduced Diet Has Similar Efficiency as Low FODMAP in IBS—A Randomized Non-Inferiority Study" Nutrients 16, no. 17: 3039. https://doi.org/10.3390/nu16173039
APA StyleRoth, B., Nseir, M., Jeppsson, H., D’Amato, M., Sundquist, K., & Ohlsson, B. (2024). A Starch- and Sucrose-Reduced Diet Has Similar Efficiency as Low FODMAP in IBS—A Randomized Non-Inferiority Study. Nutrients, 16(17), 3039. https://doi.org/10.3390/nu16173039







