Efficacy, Safety, and Tolerability of a Very Low-Calorie Ketogenic Diet in Women with Obesity and Symptomatic Knee Osteoarthritis: A Pilot Interventional Study
Abstract
1. Introduction
2. Materials and Methods
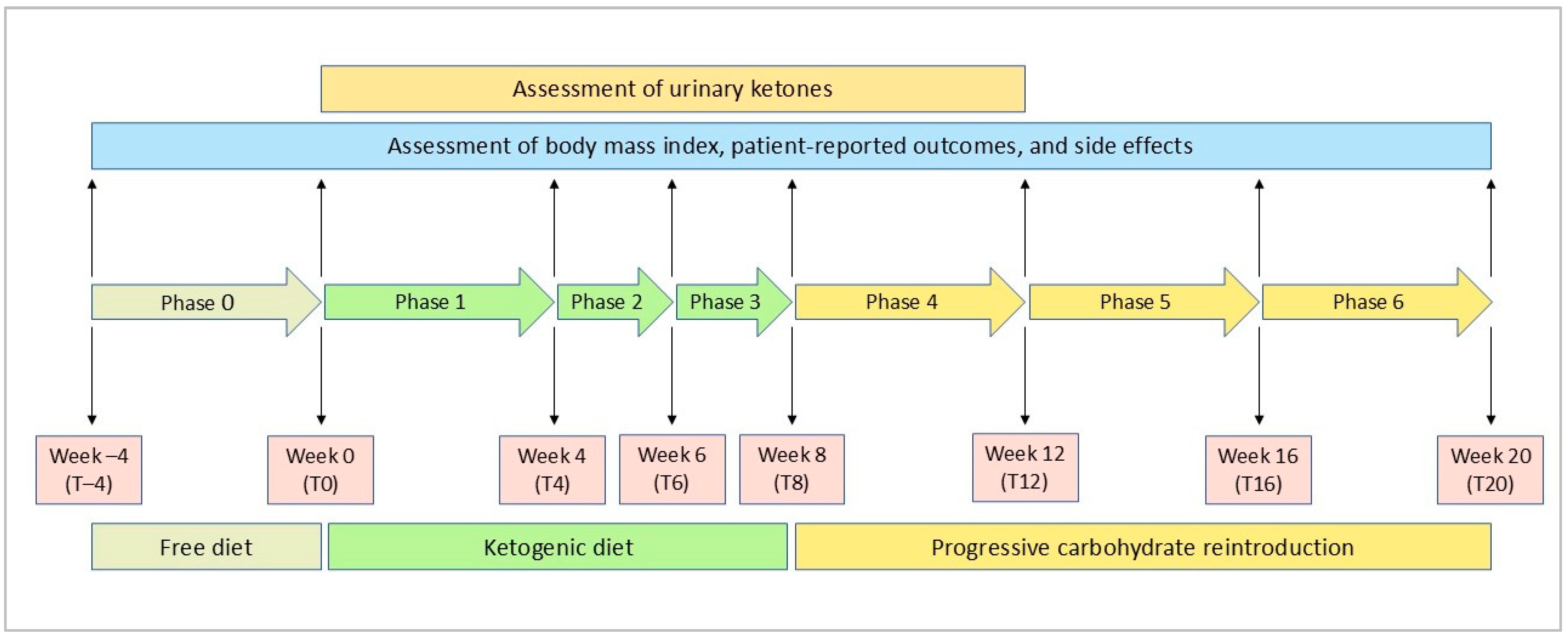
2.1. Study Design
2.2. Study Sample
2.3. Dietary Intervention
2.4. Adherence
2.5. Safety Monitoring
2.6. Study Outcomes
2.6.1. Body Mass Index (BMI)
2.6.2. Western Ontario and McMaster Universities (WOMAC) Osteoarthritis Index
2.6.3. EuroQoL-5 Dimensions (EQ-5D)
2.6.4. The 36-Item Short Form Health Survey (SF-36)
2.7. Statistical Analysis
2.8. Ethical Considerations
3. Results
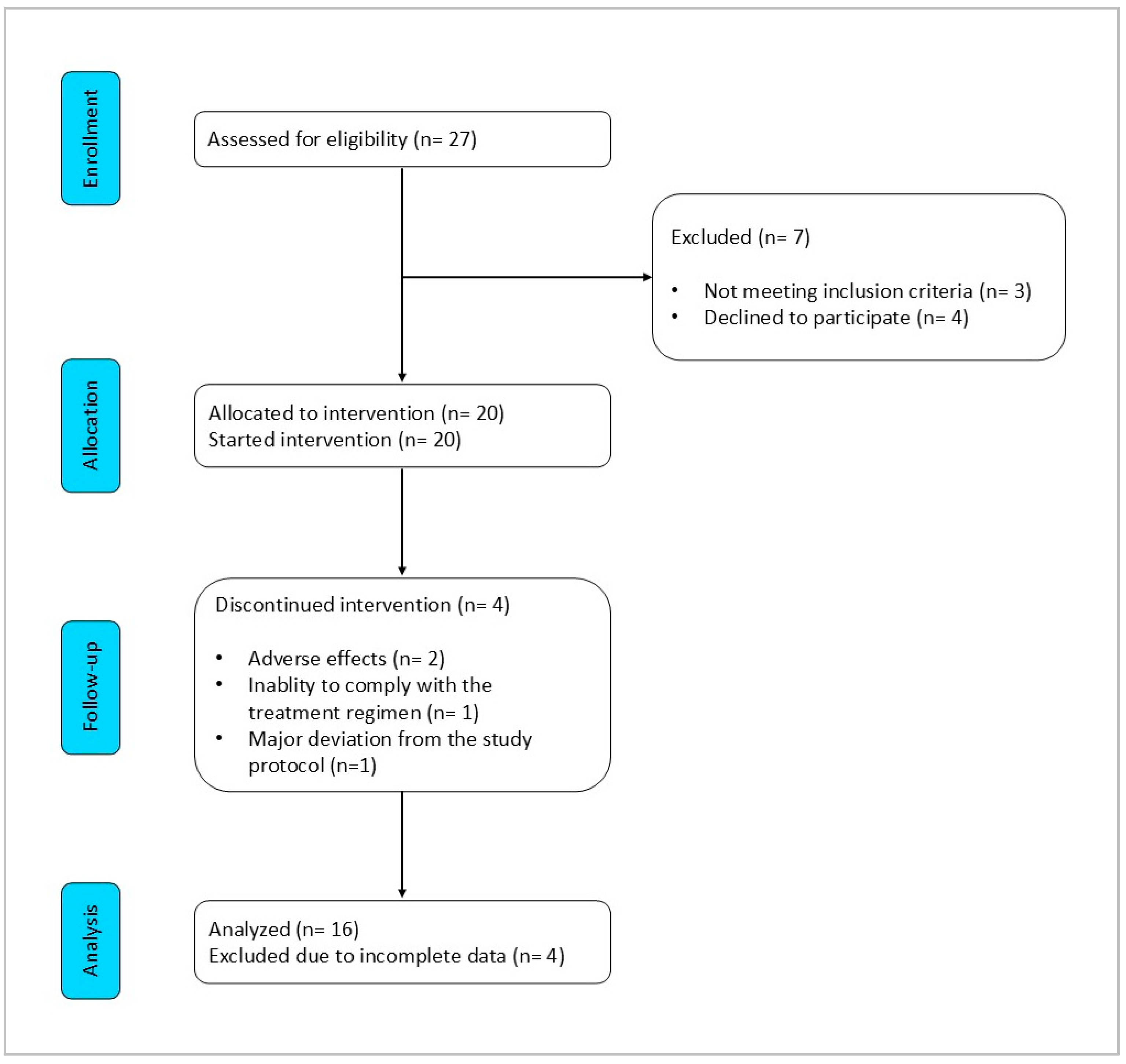
3.1. Characteristics of Patients
3.2. Adherence to VLCKD
3.3. Safety
3.4. Efficacy
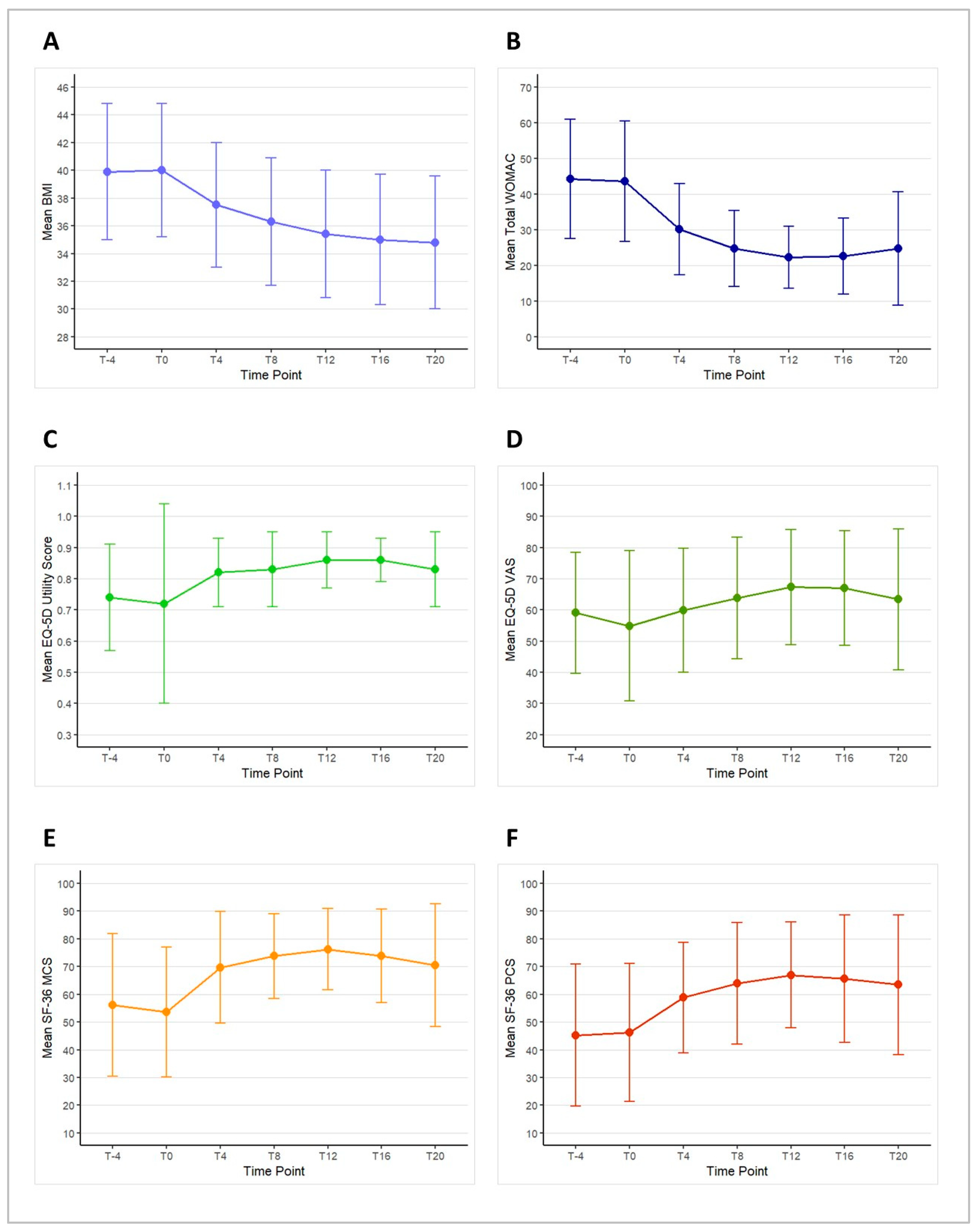
3.4.1. Change in BMI
3.4.2. Change in Total WOMAC
3.4.3. Change in Each WOMAC Domain
3.4.4. Change in EQ-5D
3.4.5. Change in SF-36
3.4.6. Association over Time between BMI and Patient-Reported Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moseng, T.; Vliet Vlieland, T.P.M.; Battista, S.; Beckwée, D.; Boyadzhieva, V.; Conaghan, P.G.; Costa, D.; Doherty, M.; Finney, A.G.; Georgiev, T.; et al. EULAR recommendations for the non-pharmacological core management of hip and knee osteoarthritis: 2023 update. Ann. Rheum. Dis. 2024, 83, 730–740. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Alhambra, D.; Judge, A.; Javaid, M.K.; Cooper, C.; Diez-Perez, A.; Arden, N.K. Incidence and risk factors for clinically diagnosed knee, hip and hand osteoarthritis: Influences of age, gender and osteoarthritis affecting other joints. Ann. Rheum. Dis. 2014, 73, 1659–1664. [Google Scholar] [CrossRef] [PubMed]
- Osteoarthritis Knee—Level 4 Cause|Institute for Health Metrics and Evaluation. Available online: https://www.healthdata.org/research-analysis/diseases-injuries-risks/factsheets/2021-osteoarthritis-knee-level-4-disease (accessed on 14 August 2024).
- Cui, A.; Li, H.; Wang, D.; Zhong, J.; Chen, Y.; Lu, H. Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies. EClinicalMedicine 2020, 29–30, 100587. [Google Scholar] [CrossRef]
- Billesberger, L.M.; Fisher, K.M.; Qadri, Y.J.; Boortz-Marx, R.L. Procedural Treatments for Knee Osteoarthritis: A Review of Current Injectable Therapies. Pain. Res. Manag. 2020, 2020, 3873098. [Google Scholar] [CrossRef]
- Gregori, D.; Giacovelli, G.; Minto, C.; Barbetta, B.; Gualtieri, F.; Azzolina, D.; Vaghi, P.; Rovati, L.C. Association of Pharmacological Treatments With Long-term Pain Control in Patients With Knee Osteoarthritis: A Systematic Review and Meta-analysis. JAMA 2018, 320, 2564. [Google Scholar] [CrossRef]
- Stewart, M.; Cibere, J.; Sayre, E.C.; Kopec, J.A. Efficacy of commonly prescribed analgesics in the management of osteoarthritis: A systematic review and meta-analysis. Rheumatol. Int. 2018, 38, 1985–1997. [Google Scholar] [CrossRef]
- Bennell, K.L.; Paterson, K.L.; Metcalf, B.R.; Duong, V.; Eyles, J.; Kasza, J.; Wang, Y.; Cicuttini, F.; Buchbinder, R.; Forbes, A.; et al. Effect of Intra-articular Platelet-Rich Plasma vs Placebo Injection on Pain and Medial Tibial Cartilage Volume in Patients With Knee Osteoarthritis: The RESTORE Randomized Clinical Trial. JAMA 2021, 326, 2021. [Google Scholar] [CrossRef]
- Hohmann, E.; Tetsworth, K.; Glatt, V. Is platelet-rich plasma effective for the treatment of knee osteoarthritis? A systematic review and meta-analysis of level 1 and 2 randomized controlled trials. Eur. J. Orthop. Surg. Traumatol. 2020, 30, 955–967. [Google Scholar] [CrossRef]
- Najm, A.; Alunno, A.; Gwinnutt, J.M.; Weill, C.; Berenbaum, F. Efficacy of intra-articular corticosteroid injections in knee osteoarthritis: A systematic review and meta-analysis of randomized controlled trials. Jt. Bone Spine 2021, 88, 105198. [Google Scholar] [CrossRef]
- Pereira, T.V.; Jüni, P.; Saadat, P.; Xing, D.; Yao, L.; Bobos, P.; Agarwal, A.; Hincapié, C.A.; Da Costa, B.R. Viscosupplementation for knee osteoarthritis: Systematic review and meta-analysis. BMJ 2022, 378, e069722. [Google Scholar] [CrossRef] [PubMed]
- Ciaffi, J.; Papalexis, N.; Vanni, E.; Miceli, M.; Faldini, C.; Scotti, L.; Zambon, A.; Salvarani, C.; Caporali, R.; Facchini, G.; et al. Minimally invasive interventional procedures for osteoarthritis and inflammatory arthritis: A systematic review and meta-analysis. Semin. Arthritis Rheum. 2024, 68, 152525. [Google Scholar] [CrossRef] [PubMed]
- Shang, Z.; Wanyan, P.; Zhang, B.; Wang, M.; Wang, X. A systematic review, umbrella review, and quality assessment on clinical translation of stem cell therapy for knee osteoarthritis: Are we there yet? Stem Cell Res. Ther. 2023, 14, 91. [Google Scholar] [CrossRef] [PubMed]
- Skou, S.T.; Roos, E.M. Physical therapy for patients with knee and hip osteoarthritis: Supervised, active treatment is current best practice. Clin. Exp. Rheumatol. 2019, 37 (Suppl. S120), 112–117. [Google Scholar] [PubMed]
- Cooper, C.; Snow, S.; McAlindon, T.E.; Kellingray, S.; Stuart, B.; Coggon, D.; Dieppe, P.A. Risk factors for the incidence and progression of radiographic knee osteoarthritis. Arthritis Rheum. 2000, 43, 995–1000. [Google Scholar] [CrossRef]
- Niu, J.; Zhang, Y.Q.; Torner, J.; Nevitt, M.; Lewis, C.E.; Aliabadi, P.; Sack, B.; Clancy, M.; Sharma, L.; Felson, D.T. Is obesity a risk factor for progressive radiographic knee osteoarthritis? Arthritis Rheum. 2009, 61, 329–335. [Google Scholar] [CrossRef]
- Losina, E.; Weinstein, A.M.; Reichmann, W.M.; Burbine, S.A.; Solomon, D.H.; Daigle, M.E.; Rome, B.N.; Chen, S.P.; Hunter, D.J.; Suter, L.G.; et al. Lifetime Risk and Age at Diagnosis of Symptomatic Knee Osteoarthritis in the US. Arthritis Care Res. 2013, 65, 703–711. [Google Scholar] [CrossRef]
- Kolasinski, S.L.; Neogi, T.; Hochberg, M.C.; Oatis, C.; Guyatt, G.; Block, J.; Callahan, L.; Copenhaver, C.; Dodge, C.; Felson, D.; et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Care Res. 2020, 72, 149–162. [Google Scholar] [CrossRef]
- Bannuru, R.R.; Osani, M.C.; Vaysbrot, E.E.; Arden, N.K.; Bennell, K.; Bierma-Zeinstra, S.M.A.; Kraus, V.B.; Lohmander, L.S.; Abbott, J.H.; Bhandari, M.; et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1578–1589. [Google Scholar] [CrossRef]
- Brophy, R.H.; Fillingham, Y.A. AAOS Clinical Practice Guideline Summary: Management of Osteoarthritis of the Knee (Nonarthroplasty), Third Edition. J. Am. Acad. Orthop. Surg. 2022, 30, e721–e729. Available online: https://journals.lww.com/10.5435/JAAOS-D-21-01233 (accessed on 13 August 2024). [CrossRef]
- Messier, S.P.; Mihalko, S.L.; Legault, C.; Miller, G.D.; Nicklas, B.J.; DeVita, P.; Beavers, D.P.; Hunter, D.J.; Lyles, M.F.; Eckstein, F.; et al. Effects of Intensive Diet and Exercise on Knee Joint Loads, Inflammation, and Clinical Outcomes Among Overweight and Obese Adults With Knee Osteoarthritis: The IDEA Randomized Clinical Trial. JAMA 2013, 310, 1263. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.D.; Kahan, S. Maintenance of Lost Weight and Long-Term Management of Obesity. Med. Clin. N. Am. 2018, 102, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.W.; Konz, E.C.; Frederich, R.C.; Wood, C.L. Long-term weight-loss maintenance: A meta-analysis of US studies. Am. J. Clin. Nutr. 2001, 74, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Law, L.; Heerey, J.L.; Devlin, B.L.; Brukner, P.; Kemp, J.L.; Attanayake, A.; Hulett, M.D.; De Livera, A.; Mosler, A.B.; Morris, H.G.; et al. Effectiveness of an anti-inflammatory diet versus low-fat diet for knee osteoarthritis: The FEAST randomised controlled trial protocol. BMJ Open 2024, 14, e079374. [Google Scholar] [CrossRef]
- Knights, A.J.; Redding, S.J.; Maerz, T. Inflammation in osteoarthritis: The latest progress and ongoing challenges. Curr. Opin. Rheumatol. 2023, 35, 128–134. [Google Scholar] [CrossRef]
- Dupuis, N.; Curatolo, N.; Benoist, J.; Auvin, S. Ketogenic diet exhibits anti-inflammatory properties. Epilepsia 2015, 56, e95–e98. Available online: https://onlinelibrary.wiley.com/doi/10.1111/epi.13038 (accessed on 14 August 2024). [CrossRef]
- Lorenzo, P.M.; Sajoux, I.; Izquierdo, A.G.; Gomez-Arbelaez, D.; Zulet, M.A.; Abete, I.; Castro, A.I.; Baltar, J.; Portillo, M.P.; Tinahones, F.J.; et al. Immunomodulatory effect of a very-low-calorie ketogenic diet compared with bariatric surgery and a low-calorie diet in patients with excessive body weight. Clin. Nutr. 2022, 41, 1566–1577. [Google Scholar] [CrossRef]
- Ji, J.; Fotros, D.; Sohouli, M.H.; Velu, P.; Fatahi, S.; Liu, Y. The effect of a ketogenic diet on inflammation-related markers: A systematic review and meta-analysis of randomized controlled trials. Nutr. Rev. 2024, nuad175. [Google Scholar] [CrossRef]
- Chow, Y.Y.; Chin, K.-Y. The Role of Inflammation in the Pathogenesis of Osteoarthritis. Mediat. Inflamm. 2020, 2020, 8293921. [Google Scholar] [CrossRef]
- Hu, N.; Gong, X.; Yin, S.; Li, Q.; Chen, H.; Li, Y.; Li, F.; Qing, L.; Yang, J.; Zhu, S.; et al. Saxagliptin suppresses degradation of type II collagen and aggrecan in primary human chondrocytes: A therapeutic implication in osteoarthritis. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3239–3245. [Google Scholar] [CrossRef]
- Kong, G.; Wang, J.; Li, R.; Huang, Z.; Wang, L. Ketogenic diet ameliorates inflammation by inhibiting the NLRP3 inflammasome in osteoarthritis. Arthritis Res. Ther. 2022, 24, 113. [Google Scholar] [CrossRef] [PubMed]
- Wheless, J.W. History of the ketogenic diet. Epilepsia 2008, 49, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Grochowska, K.; Przeliorz, A. The Effect of the Ketogenic Diet on the Therapy of Neurodegenerative Diseases and Its Impact on Improving Cognitive Functions. Dement. Geriatr. Cogn. Disord. Extra 2022, 12, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Ciaffi, J.; Mitselman, D.; Mancarella, L.; Brusi, V.; Lisi, L.; Ruscitti, P.; Cipriani, P.; Meliconi, R.; Giacomelli, R.; Borghi, C.; et al. The Effect of Ketogenic Diet on Inflammatory Arthritis and Cardiovascular Health in Rheumatic Conditions: A Mini Review. Front. Med. 2021, 8, 792846. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.D.; Aminzadeh-Gohari, S.; Tulipan, J.; Catalano, L.; Feichtinger, R.G.; Kofler, B. Ketogenic diet in the treatment of cancer—Where do we stand? Mol. Metab. 2020, 33, 102–121. [Google Scholar] [CrossRef]
- Ciaffi, J.; Lisi, L.; Mari, A.; Mancarella, L.; Brusi, V.; Pignatti, F.; Ricci, S.; Vitali, G.; Stefanelli, N.; Assirelli, E.; et al. Efficacy, safety and tolerability of very low-calorie ketogenic diet in obese women with fibromyalgia: A pilot interventional study. Front. Nutr. 2023, 10, 1219321. [Google Scholar] [CrossRef]
- Baylie, T.; Ayelgn, T.; Tiruneh, M.; Tesfa, K. Effect of Ketogenic Diet on Obesity and Other Metabolic Disorders: Narrative Review. Diabetes Metab. Syndr. Obes. 2024, 17, 1391–1401. [Google Scholar] [CrossRef]
- Castellana, M.; Conte, E.; Cignarelli, A.; Perrini, S.; Giustina, A.; Giovanella, L.; Giorgino, F.; Trimboli, P. Efficacy and safety of very low calorie ketogenic diet (VLCKD) in patients with overweight and obesity: A systematic review and meta-analysis. Rev. Endocr. Metab. Disord. 2020, 21, 5–16. [Google Scholar] [CrossRef]
- Caprio, M.; Infante, M.; Moriconi, E.; Armani, A.; Fabbri, A.; Mantovani, G.; Mariani, S.; Lubrano, C.; Poggiogalle, E.; Migliaccio, S.; et al. Very-low-calorie ketogenic diet (VLCKD) in the management of metabolic diseases: Systematic review and consensus statement from the Italian Society of Endocrinology (SIE). J. Endocrinol. Investig. 2019, 42, 1365–1386. [Google Scholar] [CrossRef]
- Strath, L.J.; Jones, C.D.; Philip George, A.; Lukens, S.L.; Morrison, S.A.; Soleymani, T.; Locher, J.L.; Gower, B.A.; Sorge, R.E. The Effect of Low-Carbohydrate and Low-Fat Diets on Pain in Individuals with Knee Osteoarthritis. Pain. Med. 2020, 21, 150–160. [Google Scholar] [CrossRef]
- Kellgren, J.H.; Lawrence, J.S. Radiological Assessment of Osteo-Arthrosis. Ann. Rheum. Dis. 1957, 16, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Tschon, M.; Contartese, D.; Pagani, S.; Borsari, V.; Fini, M. Gender and Sex Are Key Determinants in Osteoarthritis Not Only Confounding Variables. A Systematic Review of Clinical Data. J. Clin. Med. 2021, 10, 3178. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.N.; Arant, K.R.; Loeser, R.F. Diagnosis and Treatment of Hip and Knee Osteoarthritis: A Review. JAMA 2021, 325, 568. [Google Scholar] [CrossRef] [PubMed]
- Peat, G. Clinical classification criteria for knee osteoarthritis: Performance in the general population and primary care. Ann. Rheum. Dis. 2006, 65, 1363–1367. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, P.B. Dicarboxylic acids and the lipid metabolism. Dan. Med. Bull. 1984, 31, 121–145. [Google Scholar]
- Warren, S.E. False-positive urine ketone test with captopril. N. Engl. J. Med. 1980, 303, 1003–1004. [Google Scholar] [CrossRef]
- Nagel, D.; Seiler, D.; Hohenberger, E.F.; Ziegler, M. Investigations of ascorbic acid interference in urine test strips. Clin. Lab. 2006, 52, 149–153. [Google Scholar]
- Fitzgerald, G.K.; Hinman, R.S.; Zeni, J.; Risberg, M.A.; Snyder-Mackler, L.; Bennell, K.L. OARSI Clinical Trials Recommendations: Design and conduct of clinical trials of rehabilitation interventions for osteoarthritis. Osteoarthr. Cartil. 2015, 23, 803–814. [Google Scholar] [CrossRef]
- Ware, J.E.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef]
- Bellamy, N.; Buchanan, W.W.; Goldsmith, C.H.; Campbell, J.; Stitt, L.W. Validation study of WOMAC: A health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J. Rheumatol. 1988, 15, 1833–1840. [Google Scholar]
- Rabin, R.; Charro, F. de EQ-SD: A measure of health status from the EuroQol Group. Ann. Med. 2001, 33, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Clement, N.D.; Bardgett, M.; Weir, D.; Holland, J.; Gerrand, C.; Deehan, D.J. What is the Minimum Clinically Important Difference for the WOMAC Index After TKA? Clin. Orthop. Relat. Res. 2018, 476, 2005–2014. [Google Scholar] [CrossRef] [PubMed]
- Williams, V.J.; Piva, S.R.; Irrgang, J.J.; Crossley, C.; Fitzgerald, G.K. Comparison of Reliability and Responsiveness of Patient-Reported Clinical Outcome Measures in Knee Osteoarthritis Rehabilitation. J. Orthop. Sports Phys. Ther. 2012, 42, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Hmamouchi, I.; Allali, F.; Tahiri, L.; Khazzani, H.; Mansouri, L.E.; Ali Ou Alla, S.; Abouqal, R.; Hajjaj-Hassouni, N. Clinically important improvement in the WOMAC and predictor factors for response to non-specific non-steroidal anti-inflammatory drugs in osteoarthritic patients: A prospective study. BMC Res. Notes 2012, 5, 58. [Google Scholar] [CrossRef] [PubMed]
- Rabin, R.; Gudex, C.; Selai, C.; Herdman, M. From Translation to Version Management: A History and Review of Methods for the Cultural Adaptation of the EuroQol Five-Dimensional Questionnaire. Value Health 2014, 17, 70–76. [Google Scholar] [CrossRef]
- Finch, A.P.; Meregaglia, M.; Ciani, O.; Roudijk, B.; Jommi, C. An EQ-5D-5L value set for Italy using videoconferencing interviews and feasibility of a new mode of administration. Soc. Sci. Med. 2022, 292, 114519. [Google Scholar] [CrossRef]
- Apolone, G.; Mosconi, P. The Italian SF-36 Health Survey. J. Clin. Epidemiol. 1998, 51, 1025–1036. [Google Scholar] [CrossRef]
- Ito, K.; Murphy, D. Application of ggplot2 to Pharmacometric Graphics. CPT Pharmacom Syst. Pharma 2013, 2, 1–16. [Google Scholar] [CrossRef]
- General Assembly of the World Medical Association World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. J. Am. Coll. Dent. 2014, 81, 14–18.
- Liebensteiner, M.; Wurm, A.; Gamper, D.; Oberaigner, W.; Dammerer, D.; Krismer, M. Patient satisfaction after total knee arthroplasty is better in patients with pre-operative complete joint space collapse. Int. Orthop. (SICOT) 2019, 43, 1841–1847. [Google Scholar] [CrossRef]
- Leung, Y.-Y.; Thumboo, J.; Yeo, S.-J.; Wylde, V.; Tannant, A. Validation and interval scale transformation of the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) in patients undergoing knee arthroplasty, using the Rasch model. Osteoarthr. Cartil. Open 2022, 4, 100322. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wu, D.; Chang, Q.; Guo, Y.; Wang, C.; Fan, W. Intra-articular, single-shot co-injection of hyaluronic acid and corticosteroids in knee osteoarthritis: A randomized controlled trial. Exp. Ther. Med. 2018, 16, 1928–1934. Available online: http://www.spandidos-publications.com/10.3892/etm.2018.6371 (accessed on 19 August 2024). [CrossRef] [PubMed]
- Smith, J.L.; Innes, A.Q.; Burns, D.S.; Deniszczyc, D.; Selfe, J.; MacConville, S.; Deighton, K.; Kelly, B.M. A scalable 12-week exercise and education programme reduces symptoms and improves function and wellbeing in people with hip and knee osteoarthritis. Front. Rehabil. Sci. 2023, 4, 1147938. [Google Scholar] [CrossRef] [PubMed]
- Riecke, B.F.; Christensen, R.; Christensen, P.; Leeds, A.R.; Boesen, M.; Lohmander, L.S.; Astrup, A.; Bliddal, H. Comparing two low-energy diets for the treatment of knee osteoarthritis symptoms in obese patients: A pragmatic randomized clinical trial. Osteoarthr. Cartil. 2010, 18, 746–754. [Google Scholar] [CrossRef]
- Veronese, N.; Koyanagi, A.; Stubbs, B.; Cooper, C.; Guglielmi, G.; Rizzoli, R.; Punzi, L.; Rogoli, D.; Caruso, M.G.; Rotolo, O.; et al. Mediterranean diet and knee osteoarthritis outcomes: A longitudinal cohort study. Clin. Nutr. 2019, 38, 2735–2739. [Google Scholar] [CrossRef]
- Panunzi, S.; Maltese, S.; De Gaetano, A.; Capristo, E.; Bornstein, S.R.; Mingrone, G. Comparative efficacy of different weight loss treatments on knee osteoarthritis: A network meta-analysis. Obes. Rev. 2021, 22, e13230. [Google Scholar] [CrossRef]
- Bendridi, N.; Selmi, A.; Balcerczyk, A.; Pirola, L. Ketone Bodies as Metabolites and Signalling Molecules at the Crossroad between Inflammation and Epigenetic Control of Cardiometabolic Disorders. Int. J. Mol. Sci. 2022, 23, 14564. [Google Scholar] [CrossRef]
- Wu, Y.; Teng, Y.; Zhang, C.; Pan, Y.; Zhang, Q.; Zhu, X.; Liu, N.; Su, X.; Lin, J. The ketone body β-hydroxybutyrate alleviates CoCrMo alloy particles induced osteolysis by regulating NLRP3 inflammasome and osteoclast differentiation. J. Nanobiotechnol 2022, 20, 120. [Google Scholar] [CrossRef]
- Previtali, D.; Merli, G.; Di Laura Frattura, G.; Candrian, C.; Zaffagnini, S.; Filardo, G. The Long-Lasting Effects of “Placebo Injections” in Knee Osteoarthritis: A Meta-Analysis. Cartilage 2021, 13, 185S–196S. [Google Scholar] [CrossRef]
- Bihlet, A.R.; Byrjalsen, I.; Mundbjerg, K.; Rovsing, H.; Axelsen, T.M.; Andersen, J.R.; Metnik, A.; Bachtell, N.; Brett, A.; Alexandersen, P. A phase 2b double-blind placebo-controlled randomized clinical trial of SB-061, an aggrecan mimetic, in patients with symptomatic knee osteoarthritis. Osteoarthr. Cartil. 2024; in press. [Google Scholar] [CrossRef]
- Jones, I.A.; Togashi, R.; Wilson, M.L.; Heckmann, N.; Vangsness, C.T. Intra-articular treatment options for knee osteoarthritis. Nat. Rev. Rheumatol. 2019, 15, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W. The powerful placebo effect in osteoarthritis. Clin. Exp. Rheumatol. 2019, 37 (Suppl. S120), 118–123. [Google Scholar] [PubMed]
- Wen, X.; Luo, J.; Mai, Y.; Li, Y.; Cao, Y.; Li, Z.; Han, S.; Fu, Q.; Zheng, Q.; Ding, C.; et al. Placebo Response to Oral Administration in Osteoarthritis Clinical Trials and Its Associated Factors: A Model-Based Meta-analysis. JAMA Netw. Open 2022, 5, e2235060. [Google Scholar] [CrossRef] [PubMed]
- Bannuru, R.R.; McAlindon, T.E.; Sullivan, M.C.; Wong, J.B.; Kent, D.M.; Schmid, C.H. Effectiveness and Implications of Alternative Placebo Treatments: A Systematic Review and Network Meta-analysis of Osteoarthritis Trials. Ann. Intern. Med. 2015, 163, 365–372. [Google Scholar] [CrossRef]

| Inclusion Criteria |
|---|
| Age ≥ 18 years and ≤65 years |
| AND |
| Symptomatic knee osteoarthritis according to the American College of Rheumatology criteria [45] |
| AND |
| History of failure to achieve weight loss with standard hypocaloric diets |
| AND |
|
| OR |
|
| Exclusion Criteria |
|
| Characteristics | Patients (n = 16) |
|---|---|
| Age, years, mean ± SD | 57.3 ± 5.5 |
| BMI, kg/m2, mean ± SD | 40.0 ± 4.8 |
| BMI > 35, n (%) | 13 (81) |
| Comorbidities | |
| Hypertension, n (%) | 9 (56) |
| Hyperlipidemia, n (%) | 8 (50) |
| Non-alcoholic fatty liver disease, n (%) | 5 (31) |
| Polycystic ovary syndrome, n (%) | 1 (6) |
| Type 2 diabetes, n (%) | 1 (6) |
| Previous atherothrombotic events, n (%) | 1 (6) |
| Congestive heart failure, n (%) | 0 |
| Neurodegenerative disorders, n (%) | 0 |
| Medications | |
| Non-steroidal anti-inflammatory drugs, n (%) | 6 (38) |
| Paracetamol, n (%) | 9 (56) |
| Patient-reported outcomes | |
| Total WOMAC, mean ± SD | 43.6 ± 16.9 |
| WOMAC pain, mean ± SD | 8.0 ± 3.1 |
| WOMAC stiffness, mean ± SD | 4.2 ± 1.9 |
| WOMAC function, mean ± SD | 31.3 ± 13.0 |
| EQ-5D utility score, mean ± SD | 0.72 ± 0.32 |
| EQ-5D VAS, mean ± SD | 54.9 ± 24.0 |
| SF-36 MCS, mean ± SD | 53.6 ± 23.7 |
| SF-36 PCS, mean ± SD | 46.2 ± 24.8 |
| Patient | Time Point of the Study | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T12 | |
| OA1 | ● | ● | ●● | ●● | ● | ● | ||||
| OA3 | ● | ● | ●● | ● | ● | |||||
| OA4 | ●● | ●● | ●●● | ●●● | ●● | ● | ||||
| OA5 | ● | ●● | ● | ●● | ● | ● | ● | |||
| OA6 | ● | ● | ● | ● | ●● | ●● | ● | ● | ||
| OA7 | ● | ●● | ●●● | ●● | ●● | ● | ●● | ● | ● | |
| OA9 | ● | ● | ●● | ● | ● | ● | ||||
| OA10 | ● | ●● | ● | ●● | ●● | ● | ● | |||
| OA11 | ● | ● | ● | ●● | ●● | ●● | ● | ● | ● | |
| OA12 | ● | ● | ●● | ●●● | ●●● | ● | ● | ● | ||
| OA14 | ● | ● | ● | ●● | ● | ●● | ● | |||
| OA15 | ● | ● | ●● | ● | ● | ● | ● | |||
| OA16 | ●● | ●●● | ●●● | ●● | ●● | ● | ● | |||
| OA17 | ● | ● | ● | ● | ● | ● | ● | ● | ||
| OA19 | ● | ● | ●● | ● | ● | ● | ||||
| OA20 | ● | ●● | ●●● | ● | ● | ● | ||||
| Amount of ketones in the urine: | absent | ● small | ●● moderate | ●●● large | ||||||
| β Coefficient (95% CI) | p-Value | |
|---|---|---|
| Outcome: total WOMAC index over time | ||
| BMI | 1.43 (0.29 to 2.58) | 0.014 |
| Outcome: WOMAC pain over time | ||
| BMI | 0.26 (−0.17 to 0.54) | 0.066 |
| Outcome: WOMAC stiffness over time | ||
| BMI | 0.12 (0.00 to 0.23) | 0.044 |
| Outcome: WOMAC function over time | ||
| BMI | 1.07 (0.26 to 1.89) | 0.010 |
| Outcome: EQ-5D utility score over time | ||
| BMI | −0.01 (−0.17 to 0.00) | 0.055 |
| Outcome: EQ-5D VAS score over time | ||
| BMI | −1.74 (−3.09 to −0.39) | 0.012 |
| Outcome: SF-36 MCS over time | ||
| BMI | −1.35 (−3.15 to 0.45) | 0.140 |
| Outcome: SF-36 PCS score over time | ||
| BMI | −3.25 (−4.65 to −1.84) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciaffi, J.; Mancarella, L.; Pederzani, G.; Lisi, L.; Brusi, V.; Pignatti, F.; Ricci, S.; Vitali, G.; Faldini, C.; Ursini, F. Efficacy, Safety, and Tolerability of a Very Low-Calorie Ketogenic Diet in Women with Obesity and Symptomatic Knee Osteoarthritis: A Pilot Interventional Study. Nutrients 2024, 16, 3236. https://doi.org/10.3390/nu16193236
Ciaffi J, Mancarella L, Pederzani G, Lisi L, Brusi V, Pignatti F, Ricci S, Vitali G, Faldini C, Ursini F. Efficacy, Safety, and Tolerability of a Very Low-Calorie Ketogenic Diet in Women with Obesity and Symptomatic Knee Osteoarthritis: A Pilot Interventional Study. Nutrients. 2024; 16(19):3236. https://doi.org/10.3390/nu16193236
Chicago/Turabian StyleCiaffi, Jacopo, Luana Mancarella, Giulia Pederzani, Lucia Lisi, Veronica Brusi, Federica Pignatti, Susanna Ricci, Giorgia Vitali, Cesare Faldini, and Francesco Ursini. 2024. "Efficacy, Safety, and Tolerability of a Very Low-Calorie Ketogenic Diet in Women with Obesity and Symptomatic Knee Osteoarthritis: A Pilot Interventional Study" Nutrients 16, no. 19: 3236. https://doi.org/10.3390/nu16193236
APA StyleCiaffi, J., Mancarella, L., Pederzani, G., Lisi, L., Brusi, V., Pignatti, F., Ricci, S., Vitali, G., Faldini, C., & Ursini, F. (2024). Efficacy, Safety, and Tolerability of a Very Low-Calorie Ketogenic Diet in Women with Obesity and Symptomatic Knee Osteoarthritis: A Pilot Interventional Study. Nutrients, 16(19), 3236. https://doi.org/10.3390/nu16193236







