Macular Pigment Optical Density as a Measurable Modifiable Clinical Biomarker
Abstract
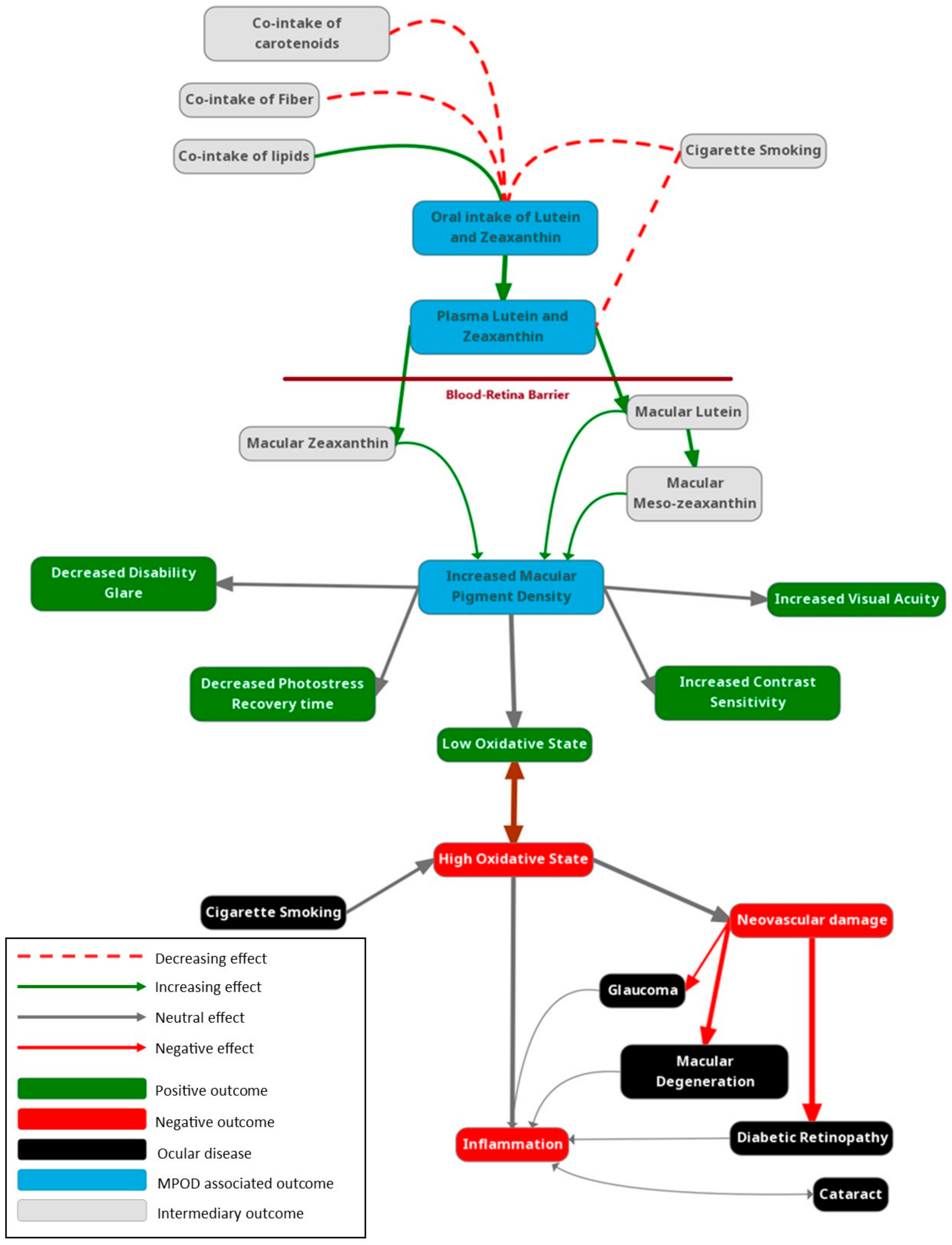
1. Introduction
1.1. Structure
1.2. Nutrition
2. In Vivo Measurement of Carotenoid Status in the Eye
2.1. Macular Pigment Optical Density (MPOD)
2.2. Measurement of MPOD
2.3. Systematic Measurement of Carotenoids—Weakly Associated with MPOD
3. MPOD in Ocular and Systemic Disease
3.1. MPOD in Ocular Disease
3.2. MPOD and Age-Related Macular Degeneration
3.3. Glaucoma
3.4. Systemic Disease
Diabetic Retinopathy
3.5. Visual Performance
3.6. MPOD and Cognitive Function
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bone, R.A.; Landrum, J.T.; Hime, G.W.; Cains, A.; Zamor, J. Stereochemistry of the human macular carotenoids. Investig. Ophthalmol. Vis. Sci. 1993, 34, 2033–2040. [Google Scholar]
- Bone, R.A.; Landrum, J.T.; Tarsis, S.L. Preliminary identification of the human macular pigment. Vision Res. 1985, 25, 1531–1535. [Google Scholar] [CrossRef] [PubMed]
- Handelman, G.J.; Dratz, E.A.; Reay, C.C.; van Kuijk, J.G. Carotenoids in the human macula and whole retina. Investig. Ophthalmol. Vis. Sci. 1988, 29, 850–855. [Google Scholar]
- Bone, R.A.; Landrum, J.T.; Friedes, L.M.; Gomez, C.M.; Kilburn, M.D.; Menendez, E.; Vidal, I.; Wang, W. Distribution of lutein and zeaxanthin stereoisomers in the human retina. Exp. Eye Res. 1997, 64, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Dorey, C.K.; Gierhart, D.; Fitch, K.A.; Crandell, I.; Craft, N.E. Low Xanthophylls, Retinol, Lycopene, and Tocopherols in Grey and White Matter of Brains with Alzheimer’s Disease. J. Alzheimer’s Dis. 2023, 94, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Krinsky, N.I. Carotenoid protection against oxidation. Pure Appl. Chem. 1979, 51, 649–660. [Google Scholar] [CrossRef]
- Brown, M.J.; Ferruzzi, M.G.; Nguyen, M.L.; Cooper, D.A.; Eldridge, A.L.; Schwartz, S.J.; White, W.S. Carotenoid bioavailability is higher from salads ingested with full-fat than with fat-reduced salad dressings as measured with electrochemical detection. Am. J. Clin. Nutr. 2004, 80, 396–403. [Google Scholar] [CrossRef]
- Reboul, E.; Thap, S.; Tourniaire, F.; André, M.; Juhel, C.; Morange, S.; Amiot, M.J.; Lairon, D.; Borel, P. Differential effect of dietary antioxidant classes (carotenoids, polyphenols, vitamins C and E) on lutein absorption. Br. J. Nutr. 2007, 97, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Hornero-Méndez, D.; Mínguez-Mosquera, M.I. Bioaccessibility of carotenes from carrots: Effect of cooking and addition of oil. Innov. Food Sci. Emerg. Technol. 2007, 8, 407–412. [Google Scholar] [CrossRef]
- Riedl, J.; Linseisen, J.; Hoffmann, J.; Wolfram, G. Some dietary fibers reduce the absorption of carotenoids in women. J. Nutr. 1999, 129, 2170–2176. [Google Scholar] [CrossRef] [PubMed]
- Perry, A.; Rasmussen, H.; Johnson, E.J. Xanthophyll (lutein, zeaxanthin) content in fruits, vegetables and corn and egg products. J. Food Compos. Anal. 2009, 22, 9–15. [Google Scholar] [CrossRef]
- Sanabria, J.C.; Bass, J.; Spors, F.; Gierhart, D.L.; Davey, P.G. Measurement of Carotenoids in Perifovea using the Macular Pigment Reflectometer. J. Vis. Exp. 2020, 155, e60429. [Google Scholar] [CrossRef]
- Davey, P.G.; Rosen, R.B.; Gierhart, D.L. Macular Pigment Reflectometry: Developing Clinical Protocols, Comparison with Heterochromatic Flicker Photometry and Individual Carotenoid Levels. Nutrients 2021, 13, 2553. [Google Scholar] [CrossRef] [PubMed]
- Goodrow, E.F.; Wilson, T.A.; Houde, S.C.; Vishwanathan, R.; Scollin, P.A.; Handelman, G.; Nicolosi, R.J. Consumption of one egg per day increases serum lutein and zeaxanthin concentrations in older adults without altering serum lipid and lipoprotein cholesterol concentrations. J. Nutr. 2006, 136, 2519–2524. [Google Scholar] [CrossRef] [PubMed]
- Müller, H. [Daily intake of carotenoids (carotenes and xanthophylls) from total diet and the carotenoid content of selected vegetables and fuit]. Z. Ernahrungswiss 1996, 35, 45–50. [Google Scholar] [CrossRef]
- Bone, R.A.; Landrum, J.T. Heterochromatic flicker photometry. Arch. Biochem. Biophys. 2004, 430, 137–142. [Google Scholar] [CrossRef]
- Stringham, J.M.; Hammond, B.R.; Nolan, J.M.; Wooten, B.R.; Mammen, A.; Smollon, W.; Snodderly, D.M. The utility of using customized heterochromatic flicker photometry (cHFP) to measure macular pigment in patients with age-related macular degeneration. Exp. Eye Res. 2008, 87, 445–453. [Google Scholar] [CrossRef] [PubMed]
- van de Kraats, J.; Berendschot, T.T.; Valen, S.; van Norren, D. Fast assessment of the central macular pigment density with natural pupil using the macular pigment reflectometer. J. Biomed. Opt. 2006, 11, 064031. [Google Scholar] [CrossRef]
- Bone, R.A.; Brener, B.; Gibert, J.C. Macular pigment, photopigments, and melanin: Distributions in young subjects determined by four-wavelength reflectometry. Vision Res. 2007, 47, 3259–3268. [Google Scholar] [CrossRef]
- Morita, H.; Matsushita, I.; Fujino, Y.; Obana, A.; Kondo, H. Measuring macular pigment optical density using reflective images of confocal scanning laser system. Jpn. J. Ophthalmol. 2024, 68, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Wooten, B.R.; Hammond, B.R. Spectral absorbance and spatial distribution of macular pigment using heterochromatic flicker photometry. Optom. Vis. Sci. 2005, 82, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, P.S.; Zhao, D.Y.; Wintch, S.W.; Ermakov, I.V.; McClane, R.W.; Gellermann, W. Resonance Raman measurement of macular carotenoids in normal subjects and in age-related macular degeneration patients. Ophthalmology 2002, 109, 1780–1787. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, P.S.; Yoshida, M.D.; Katz, N.B.; McClane, R.W.; Gellermann, W. Raman detection of macular carotenoid pigments in intact human retina. Investig. Ophthalmol. Vis. Sci. 1998, 39, 2003–2011. [Google Scholar]
- Delori, F.C. Spectrophotometer for noninvasive measurement of intrinsic fluorescence and reflectance of the ocular fundus. Appl. Opt. 1994, 33, 7439–7452. [Google Scholar] [CrossRef]
- Davey, P.G.; Alvarez, S.D.; Lee, J.Y. Macular pigment optical density: Repeatability, intereye correlation, and effect of ocular dominance. Clin. Ophthalmol. 2016, 10, 1671–1678. [Google Scholar] [CrossRef]
- Berendschot, T.T.J.M.; van Norren, D. Objective determination of the macular pigment optical density using fundus reflectance spectroscopy. Arch. Biochem. Biophys. 2004, 430, 149–155. [Google Scholar] [CrossRef]
- Van De Kraats, J.; Kanis, M.J.; Genders, S.W.; van Norren, D. Lutein and zeaxanthin measured separately in the living human retina with fundus reflectometry. Investig. Opthalmol. Vis. Sci. 2008, 49, 5568. [Google Scholar] [CrossRef]
- Van der Veen, R.; Berendschot, T.; Makridaki, M.; Hendrikse, F.; Carden, D.; Murray, I. Correspondence between retinal reflectometry and a flicker-based technique in the measurement of macular pigment spatial profiles. J. Biomed. Opt. 2009, 14, 064046. [Google Scholar] [CrossRef][Green Version]
- Van de Kraats, J.; van Norren, D. Directional and nondirectional spectral reflection from the human fovea. J. Biomed. Opt. 2008, 13, 024010. [Google Scholar] [CrossRef]
- Radtke, M.D.; Poe, M.; Stookey, J.; Jilcott Pitts, S.; Moran, N.E.; Landry, M.J.; Rubin, L.P.; Stage, V.C.; Scherr, R.E. Recommendations for the Use of the Veggie Meter® for Spectroscopy-Based Skin Carotenoid Measurements in the Research Setting. Curr. Dev. Nutr. 2021, 5, nzab104. [Google Scholar] [CrossRef]
- Obana, A.; Asaoka, R.; Takayanagi, Y.; Gohto, Y. Inter-device concordance of Veggie Meter—A reflection spectroscopy to measure skin carotenoids. J. Biophotonics 2023, 16, e202300071. [Google Scholar] [CrossRef] [PubMed]
- Scarmo, S.; Cartmel, B.; Lin, H.; Leffell, D.J.; Welch, E.; Bhosale, P.; Bernstein, P.S.; Mayne, S.T. Significant correlations of dermal total carotenoids and dermal lycopene with their respective plasma levels in healthy adults. Arch. Biochem. Biophys. 2010, 504, 34–39. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rosok, L.M.; Cannavale, C.N.; Keye, S.A.; Holscher, H.D.; Renzi-Hammond, L.; Khan, N.A. Skin and macular carotenoids and relations to academic achievement among school-aged children. Nutr. Neurosci. 2024. [Google Scholar] [CrossRef]
- Hrvolová, B.; Martínez-Huélamo, M.; Colmán-Martínez, M.; Hurtado-Barroso, S.; Lamuela-Raventós, R.M.; Kalina, J. Development of an Advanced HPLC-MS/MS Method for the Determination of Carotenoids and Fat-Soluble Vitamins in Human Plasma. Int. J. Mol. Sci. 2016, 17, 1719. [Google Scholar] [CrossRef]
- Bone, R.A.; Davey, P.G.; Roman, B.O.; Evans, D.W. Efficacy of Commercially Available Nutritional Supplements: Analysis of Serum Uptake, Macular Pigment Optical Density and Visual Functional Response. Nutrients 2020, 12, 1321. [Google Scholar] [CrossRef]
- National Society to Prevent Blindness Vision Problems in the US: Data Analyses; National Society to Prevent Blindness: New York, NY, USA, 1980; pp. 1–46.
- Klein, R.; Klein, B.E.; Tomany, S.C.; Meuer, S.M.; Huang, G.H. Ten-year incidence and progression of age-related maculopathy: The Beaver Dam eye study. Ophthalmology 2002, 109, 1767–1779. [Google Scholar] [CrossRef]
- Lem, D.W.; Davey, P.G.; Gierhart, D.L.; Rosen, R.B. A Systematic Review of Carotenoids in the Management of Age-Related Macular Degeneration. Antioxidants 2021, 10, 1255. [Google Scholar] [CrossRef]
- Age-Related Eye Disease Study 2 Research Group. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: The Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA 2013, 309, 2005–2015. [Google Scholar] [CrossRef]
- AREDS2 Research Group; Chew, E.Y.; Clemons, T.E.; Agrón, E.; Domalpally, A.; Keenan, T.D.L.; Vitale, S.; Weber, C.; Smith, D.C.; Christen, W. Long-term Outcomes of Adding Lutein/Zeaxanthin and ω-3 Fatty Acids to the AREDS Supplements on Age-Related Macular Degeneration Progression: AREDS2 Report 28. JAMA Ophthalmol. 2022, 140, 692–698. [Google Scholar] [CrossRef]
- Age-Related Eye Disease Study 2 (AREDS2) Research Group; Chew, E.Y.; SanGiovanni, J.P.; Ferris, F.L.; Wong, W.T.; Agron, E.; Clemons, T.E.; Sperduto, R.; Danis, R.; Chandra, S.R.; et al. Lutein/zeaxanthin for the treatment of age-related cataract: AREDS2 randomized trial report no. 4. JAMA Ophthalmol. 2013, 131, 843–850. [Google Scholar] [CrossRef]
- Beatty, S.; Boulton, M.; Henson, D.; Koh, H.H.; Murray, I.J. Macular pigment and age related macular degeneration. Br. J. Ophthalmol. 1999, 83, 867–877. [Google Scholar] [CrossRef] [PubMed]
- Tsika, C.; Tsilimbaris, M.K.; Makridaki, M.; Kontadakis, G.; Plainis, S.; Moschandreas, J. Assessment of macular pigment optical density (MPOD) in patients with unilateral wet age-related macular degeneration (AMD). Acta Ophthalmol. 2011, 89, e573–e578. [Google Scholar] [CrossRef] [PubMed]
- Obana, A.; Hiramitsu, T.; Gohto, Y.; Ohira, A.; Mizuno, S.; Hirano, T.; Bernstein, P.S.; Fujii, H.; Iseki, K.; Tanito, M.; et al. Macular carotenoid levels of normal subjects and age-related maculopathy patients in a Japanese population. Ophthalmology 2008, 115, 147–157. [Google Scholar] [CrossRef]
- Bone, R.A.; Landrum, J.T.; Mayne, S.T.; Gomez, C.M.; Tibor, S.E.; Twaroska, E.E. Macular pigment in donor eyes with and without AMD: A case-control study. Investig. Ophthalmol. Vis. Sci. 2001, 42, 235–240. [Google Scholar]
- Beatty, S.; Chakravarthy, U.; Nolan, J.M.; Muldrew, K.A.; Woodside, J.V.; Denny, F.; Stevenson, M.R. Secondary outcomes in a clinical trial of carotenoids with coantioxidants versus placebo in early age-related macular degeneration. Ophthalmology 2013, 120, 600–606. [Google Scholar] [CrossRef]
- Dawczynski, J.; Jentsch, S.; Schweitzer, D.; Hammer, M.; Lang, G.E.; Strobel, J. Long term effects of lutein, zeaxanthin and omega-3-LCPUFAs supplementation on optical density of macular pigment in AMD patients: The LUTEGA study. Graefes Arch. Clin. Exp. Ophthalmol. 2013, 251, 2711–2723. [Google Scholar] [CrossRef]
- Murray, I.J.; Makridaki, M.; van der Veen, R.L.; Carden, D.; Parry, N.R.; Berendschot, T.T. Lutein supplementation over a one-year period in early AMD might have a mild beneficial effect on visual acuity: The CLEAR study. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1781–1788. [Google Scholar] [CrossRef]
- Richer, S.; Stiles, W.; Statkute, L.; Pulido, J.; Frankowski, J.; Rudy, D.; Pei, K.; Tsipursky, M.; Nyland, J. Double-masked, placebo-controlled, randomized trial of lutein and antioxidant supplementation in the intervention of atrophic age-related macular degeneration: The Veterans LAST study (Lutein Antioxidant Supplementation Trial). Optometry 2004, 75, 216–230. [Google Scholar] [CrossRef]
- Trieschmann, M.; Beatty, S.; Nolan, J.M.; Hense, H.W.; Heimes, B.; Austermann, U.; Fobker, M.; Pauleikhoff, D. Changes in macular pigment optical density and serum concentrations of its constituent carotenoids following supplemental lutein and zeaxanthin: The LUNA study. Exp. Eye Res. 2007, 84, 718–728. [Google Scholar] [CrossRef]
- Richer, S.P.; Stiles, W.; Graham-Hoffman, K.; Levin, M.; Ruskin, D.; Wrobel, J.; Park, D.W.; Thomas, C. Randomized, double-blind, placebo-controlled study of zeaxanthin and visual function in patients with atrophic age-related macular degeneration: The Zeaxanthin and Visual Function Study (ZVF) FDA IND #78, 973. Optometry 2011, 82, 667–680.e6. [Google Scholar] [CrossRef]
- Weigert, G.; Kaya, S.; Pemp, B.; Sacu, S.; Lasta, M.; Werkmeister, R.M.; Dragostinoff, N.; Simader, C.; Garhöfer, G.; Schmidt-Erfurth, U.; et al. Effects of lutein supplementation on macular pigment optical density and visual acuity in patients with age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8174–8178. [Google Scholar] [CrossRef] [PubMed]
- Sabour-Pickett, S.; Beatty, S.; Connolly, E.; Loughman, J.; Stack, J.; Howard, A.; Klein, R.; Klein, B.E.; Meuer, S.M.; Myers, C.E.; et al. Supplementation with three different macular carotenoid formulations in patients with early age-related macular degeneration. Retina 2014, 34, 1757–1766. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.M.; Dou, H.L.; Huang, F.F.; Xu, X.R.; Zou, Z.Y.; Lin, X.M. Effect of supplemental lutein and zeaxanthin on serum, macular pigmentation, and visual performance in patients with early age-related macular degeneration. Biomed. Res. Int. 2015, 2015, 564738. [Google Scholar] [CrossRef] [PubMed]
- Davey, P.G.; Henderson, T.; Lem, D.W.; Weis, R.; Amonoo-Monney, S.; Evans, D.W. Visual Function and Macular Carotenoid Changes in Eyes with Retinal Drusen—An Open Label Randomized Controlled Trial to Compare a Micronized Lipid-Based Carotenoid Liquid Supplementation and AREDS-2 Formula. Nutrients 2020, 12, 3271. [Google Scholar] [CrossRef]
- Ma, L.; Yan, S.F.; Huang, Y.M.; Lu, X.R.; Qian, F.; Pang, H.L.; Xu, X.R.; Zou, Z.Y.; Dong, P.C.; Xiao, X.; et al. Effect of lutein and zeaxanthin on macular pigment and visual function in patients with early age-related macular degeneration. Ophthalmology 2012, 119, 2290–2297. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The pathophysiology and treatment of glaucoma: A review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef]
- Kumar, D.M.; Agarwal, N. Oxidative stress in glaucoma: A burden of evidence. J. Glaucoma 2007, 16, 334–343. [Google Scholar] [CrossRef]
- Choi, J.S.; Kim, D.; Hong, Y.M.; Mizuno, S.; Joo, C.K. Inhibition of nNOS and COX-2 expression by lutein in acute retinal ischemia. Nutrition 2006, 22, 668–671. [Google Scholar] [CrossRef]
- Fung, F.K.; Law, B.Y.; Lo, A.C. Lutein Attenuates Both Apoptosis and Autophagy upon Cobalt (II) Chloride-Induced Hypoxia in Rat Műller Cells. PLoS ONE 2016, 11, e0167828. [Google Scholar] [CrossRef]
- Dilsiz, N.; Sahaboglu, A.; Yildiz, M.Z.; Reichenbach, A. Protective effects of various antioxidants during ischemia-reperfusion in the rat retina. Graefes Arch. Clin. Exp. Ophthalmol. 2006, 244, 627–633. [Google Scholar] [CrossRef]
- Li, S.Y.; Fu, Z.J.; Ma, H.; Jang, W.C.; So, K.F.; Wong, D.; Lo, A.C. Effect of lutein on retinal neurons and oxidative stress in a model of acute retinal ischemia/reperfusion. Investig. Ophthalmol. Vis. Sci. 2009, 50, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Bignami, A.; Dahl, D. The radial glia of Müller in the rat retina and their response to injury. An immunofluorescence study with antibodies to the glial fibrillary acidic (GFA) protein. Exp. Eye Res. 1979, 28, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Bringmann, A.; Pannicke, T.; Grosche, J.; Francke, M.; Wiedemann, P.; Skatchkov, S.N.; Osborne, N.N.; Reichenbach, A. Müller cells in the healthy and diseased retina. Prog. Retin. Eye Res. 2006, 25, 397–424. [Google Scholar] [CrossRef] [PubMed]
- Siah, W.F.; Loughman, J.; O’Brien, C. Lower Macular Pigment Optical Density in Foveal-Involved Glaucoma. Ophthalmology 2015, 122, 2029–2037. [Google Scholar] [CrossRef]
- Lem, D.W.; Gierhart, D.L.; Davey, P.G. Carotenoids in the Management of Glaucoma: A Systematic Review of the Evidence. Nutrients 2021, 13, 1949. [Google Scholar] [CrossRef] [PubMed]
- Zeki Fikret, C.; Ucgun, N.I. Macular pigment optical density change analysis in primary open-angle glaucoma and pseudoexfoliation glaucoma. Int. Ophthalmol. 2021, 41, 2235–2240. [Google Scholar] [CrossRef]
- Bruns, Y.; Junker, B.; Boehringer, D.; Framme, C.; Pielen, A. Comparison of Macular Pigment Optical Density in Glaucoma Patients and Healthy Subjects—A Prospective Diagnostic Study. Clin. Ophthalmol. 2020, 14, 1011–1017. [Google Scholar] [CrossRef]
- Loughman, J.; Loskutova, E.; Butler, J.S.; Siah, W.F.; O’Brien, C. Macular Pigment Response to Lutein, Zeaxanthin, and Meso-zeaxanthin Supplementation in Open-Angle Glaucoma: A Randomized Controlled Trial. Ophthalmol. Sci. 2021, 1, 100039. [Google Scholar] [CrossRef]
- Ji, Y.; Zuo, C.; Lin, M.; Zhang, X.; Li, M.; Mi, L.; Liu, B.; Wen, F. Macular Pigment Optical Density in Chinese Primary Open Angle Glaucoma Using the One-Wavelength Reflectometry Method. J. Ophthalmol. 2016, 2016, 2792103. [Google Scholar] [CrossRef]
- Arnould, L.; Seydou, A.; Binquet, C.; Gabrielle, P.H.; Chamard, C.; Bretillon, L.; Bron, A.M.; Acar, N.; Creuzot-Garcher, C. Macular Pigment and Open-Angle Glaucoma in the Elderly: The Montrachet Population-Based Study. J. Clin. Med. 2022, 11, 1830. [Google Scholar] [CrossRef]
- Daga, F.B.; Ogata, N.G.; Medeiros, F.A.; Moran, R.; Morris, J.; Zangwill, L.M.; Weinreb, R.N.; Nolan, J.M. Macular Pigment and Visual Function in Patients with Glaucoma: The San Diego Macular Pigment Study. Investig. Ophthalmol. Vis. Sci. 2018, 59, 4471–4476. [Google Scholar] [CrossRef]
- Carotenoids in Age-Related Eye Disease Study Investigators; Second Carotenoids in Age-Related Eye Disease Study Research Group; Lawler, T.; Mares, J.A.; Liu, Z.; Thuruthumaly, C.; Etheridge, T.; Vajaranant, T.S.; Domalpally, A.; Hammond, B.R.; et al. Association of macular pigment optical density with retinal layer thicknesses in eyes with and without manifest primary open-angle glaucoma. BMJ Open Ophthalmol. 2023, 8, e001331. [Google Scholar] [CrossRef] [PubMed]
- Igras, E.; Loughman, J.; Ratzlaff, M.; O’Caoimh, R.; O’Brien, C. Evidence of lower macular pigment optical density in chronic open angle glaucoma. Br. J. Ophthalmol. 2013, 97, 994–998. [Google Scholar] [CrossRef] [PubMed]
- Siah, W.F.; O’Brien, C.; Loughman, J.J. Macular pigment is associated with glare-affected visual function and central visual field loss in glaucoma. Br. J. Ophthalmol. 2018, 102, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lawler, T.; Liu, Z.; Thuruthumaly, C.; Vajaranant, T.; Wallace, R.; Tinker, L.; Nalbandyan, M.; Mares, J. Low Macular Pigment Optical Density Is Associated with Manifest Primary Open- Angle Glaucoma in Older Women. Curr. Dev. Nutr. 2024, 8, 103789. [Google Scholar] [CrossRef]
- Eraslan, N.; Yilmaz, M.; Celikay, O. Assessment of macular pigment optical density of primary open-angle glaucoma patients under topical medication. Photodiagnosis Photodyn. Ther. 2023, 42, 103585. [Google Scholar] [CrossRef]
- Lem, D.W.; Gierhart, D.L.; Davey, P.G. A Systematic Review of Carotenoids in the Management of Diabetic Retinopathy. Nutrients 2021, 13, 2441. [Google Scholar] [CrossRef]
- Scanlon, G.; Loughman, J.; Farrell, D.; McCartney, D. A review of the putative causal mechanisms associated with lower macular pigment in diabetes mellitus. Nutr. Res. Rev. 2019, 32, 247–264. [Google Scholar] [CrossRef]
- Lima, V.C.; Rosen, R.B.; Maia, M.; Prata, T.S.; Dorairaj, S.; Farah, M.E.; Sallum, J. Macular pigment optical density measured by dual-wavelength autofluorescence imaging in diabetic and nondiabetic patients: A comparative study. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5840–5845. [Google Scholar] [CrossRef]
- Dong, L.Y.; Jin, J.; Lu, G.; Kang, X.L. Astaxanthin attenuates the apoptosis of retinal ganglion cells in db/db mice by inhibition of oxidative stress. Mar. Drugs 2013, 11, 960–974. [Google Scholar] [CrossRef]
- Lima, V.C.; Rosen, R.B.; Farah, M. Macular pigment in retinal health and disease. Int. J. Retin. Vitr. 2016, 2, 19. [Google Scholar] [CrossRef] [PubMed]
- Scanlon, G.; McCartney, D.; Butler, J.S.; Loskutova, E.; Loughman, J. Identification of Surrogate Biomarkers for the Prediction of Patients at Risk of Low Macular Pigment in Type 2 Diabetes. Curr. Eye Res. 2019, 44, 1369–1380. [Google Scholar] [CrossRef] [PubMed]
- Bikbov, M.M.; Fayzrakhmanov, R.R.; Yarmuhametova, A.L.; Zainullin, R.M. Analysis of the central zone of the retina in patients with diabetic macular edema. Diabetes Mellit. 2015, 18, 99–104. [Google Scholar] [CrossRef][Green Version]
- Scanlon, G.; Connell, P.; Ratzlaff, M.; Foerg, B.; McCartney, D.; Murphy, A.; OʼConnor, K.; Loughman, J. Macular pigment optical density is lower in type 2 diabetes, compared with type 1 diabetes and normal controls. Retina 2015, 35, 1808–1816. [Google Scholar] [CrossRef]
- She, C.Y.; Gu, H.; Xu, J.; Yang, X.F.; Ren, X.T.; Liu, N.P. Association of macular pigment optical density with early stage of non-proliferative diabetic retinopathy in Chinese patients with type 2 diabetes mellitus. Int. J. Ophthalmol. 2016, 9, 1433–1438. [Google Scholar] [CrossRef] [PubMed]
- Bikbov, M.; Zainullin, R.; Faizrakhmanov, R. Macular pigment optical density alteration as an indicator of diabetic macular Edema development. Coвpeмeнныe Texнoлoгии B Meдицинe 2015, 7, 73–75. [Google Scholar] [CrossRef][Green Version]
- Chous, A.P.; Richer, S.P.; Gerson, J.D.; Kowluru, R.A. The Diabetes Visual Function Supplement Study (DiVFuSS). Br. J. Ophthalmol. 2016, 100, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Zagers, N.P.; Pot, M.C.; van Norren, D. Spectral and directional reflectance of the fovea in diabetes mellitus: Photoreceptor integrity, macular pigment and lens. Vision Res. 2005, 45, 1745–1753. [Google Scholar] [CrossRef]
- Varghese, M.; Antony, J. Assessment of Macular Pigment Optical Density Using Fundus Reflectometry in Diabetic Patients. Middle East Afr. J. Ophthalmol. 2019, 26, 2–6. [Google Scholar] [CrossRef]
- Cennamo, G.; Lanni, V.; Abbate, R.; Velotti, N.; Fossataro, F.; Sparnelli, F.; Romano, M.R.; de Crecchio, G.; Cennamo, G. The Relationship between Macular Pigment and Vessel Density in Patients with Type 1 Diabetes Mellitus. Ophthalmic Res. 2019, 61, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.S.; Will, J.C.; Bowman, B.A.; Narayan, K.M. Diabetes mellitus and serum carotenoids: Findings from the Third National Health and Nutrition Examination Survey. Am. J. Epidemiol. 1999, 149, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A.; Menon, B.; Gierhart, D.L. Beneficial effect of zeaxanthin on retinal metabolic abnormalities in diabetic rats. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1645–1651. [Google Scholar] [CrossRef] [PubMed]
- Hammond, B.R.; Fletcher, L.M.; Elliott, J.G. Glare disability, photostress recovery, and chromatic contrast: Relation to macular pigment and serum lutein and zeaxanthin. Investig. Ophthalmol. Vis. Sci. 2013, 54, 476–481. [Google Scholar] [CrossRef]
- Kvansakul, J.; Rodriguez-Carmona, M.; Edgar, D.F.; Barker, F.M.; Köpcke, W.; Schalch, W.; Barbur, J.L. Supplementation with the carotenoids lutein or zeaxanthin improves human visual performance. Ophthalmic Physiol. Opt. 2006, 26, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Loughman, J.; Akkali, M.C.; Beatty, S.; Scanlon, G.; Davison, P.A.; O’Dwyer, V.; Cantwell, T.; Major, P.; Stack, J.; Nolan, J.M. The relationship between macular pigment and visual performance. Vision Res. 2010, 50, 1249–1256. [Google Scholar] [CrossRef]
- Stringham, J.M.; O’Brien, K.J.; Stringham, N.T. Contrast Sensitivity and Lateral Inhibition Are Enhanced with Macular Carotenoid Supplementation. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2291–2295. [Google Scholar] [CrossRef]
- Stringham, J.M.; Garcia, P.V.; Smith, P.A.; McLin, L.N.; Foutch, B.K. Macular pigment and visual performance in glare: Benefits for photostress recovery, disability glare, and visual discomfort. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7406–7415. [Google Scholar] [CrossRef]
- Yao, Y.; Qiu, Q.H.; Wu, X.W.; Cai, Z.Y.; Xu, S.; Liang, X.Q. Lutein supplementation improves visual performance in Chinese drivers: 1-year randomized, double-blind, placebo-controlled study. Nutrition 2013, 29, 958–964. [Google Scholar] [CrossRef]
- Stringham, J.M.; Stringham, N.T.; O’Brien, K.J. Macular Carotenoid Supplementation Improves Visual Performance, Sleep Quality, and Adverse Physical Symptoms in Those with High Screen Time Exposure. Foods 2017, 6, 47. [Google Scholar] [CrossRef]
- Richer, S.; Novil, S.; Gullett, T.; Dervishi, A.; Nassiri, S.; Duong, C.; Davis, R.; Davey, P.G. Night Vision and Carotenoids (NVC): A Randomized Placebo Controlled Clinical Trial on Effects of Carotenoid Supplementation on Night Vision in Older Adults. Nutrients 2021, 13, 3191. [Google Scholar] [CrossRef]
- Engles, M.; Wooten, B.; Hammond, B. Macular pigment: A test of the acuity hypothesis. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2922–2931. [Google Scholar] [CrossRef] [PubMed]
- Tudosescu, R.; Alexandrescu, C.M.; Istrate, S.L.; Vrapciu, A.D.; Ciuluvică, R.C.; Voinea, L. Correlations between internal and external ocular factors and macular pigment optical density. Rom. J. Ophthalmol. 2018, 62, 42–47. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Patryas, L.; Parry, N.R.; Carden, D.; Aslam, T.; Murray, I.J. The association between dark adaptation and macular pigment optical density in healthy subjects. Graefes Arch. Clin. Exp. Ophthalmol. 2014, 252, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Bovier, E.R.; Renzi, L.M.; Hammond, B.R. A double-blind, placebo-controlled study on the effects of lutein and zeaxanthin on neural processing speed and efficiency. PLoS ONE 2014, 9, e108178. [Google Scholar] [CrossRef]
- Putnam, C.M.; Bassi, C.J. Macular pigment spatial distribution effects on glare disability. J. Optom. 2015, 8, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Stringham, J.M.; Hammond, B.R. Macular pigment and visual performance under glare conditions. Optom. Vis. Sci. 2008, 85, 82–88. [Google Scholar] [CrossRef]
- Nolan, J.M.; Power, R.; Stringham, J.; Dennison, J.; Stack, J.; Kelly, D.; Moran, R.; Akuffo, K.O.; Corcoran, L.; Beatty, S. Enrichment of Macular Pigment Enhances Contrast Sensitivity in Subjects Free of Retinal Disease: Central Retinal Enrichment Supplementation Trials—Report 1. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3429–3439. [Google Scholar] [CrossRef] [PubMed]
- Hammond, B.R.; Fletcher, L.M.; Roos, F.; Wittwer, J.; Schalch, W. A double-blind, placebo-controlled study on the effects of lutein and zeaxanthin on photostress recovery, glare disability, and chromatic contrast. Investig. Ophthalmol. Vis. Sci. 2014, 55, 8583–8589. [Google Scholar] [CrossRef]
- Stringham, J.M.; O’Brien, K.J.; Stringham, N.T. Macular carotenoid supplementation improves disability glare performance and dynamics of photostress recovery. Eye Vis. 2016, 3, 30. [Google Scholar] [CrossRef] [PubMed]
- Hammond, B.R.; Wooten, B.R.; Snodderly, D.M. Preservation of visual sensitivity of older subjects: Association with macular pigment density. Investig. Ophthalmol. Vis. Sci. 1998, 39, 397–406. [Google Scholar]
- Estévez-Santiago, R.; Olmedilla-Alonso, B.; Beltrán-de-Miguel, B. Assessment of lutein and zeaxanthin status and dietary markers as predictors of the contrast threshold in 2 age groups of men and women. Nutr. Res. 2016, 36, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Nolan, J.M.; Loughman, J.; Akkali, M.C.; Stack, J.; Scanlon, G.; Davison, P.; Beatty, S. The impact of macular pigment augmentation on visual performance in normal subjects: COMPASS. Vision Res. 2011, 51, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.J.; Vishwanathan, R.; Johnson, M.A.; Hausman, D.B.; Davey, A.; Scott, T.M.; Green, R.C.; Miller, L.S.; Gearing, M.; Woodard, J.; et al. Relationship between Serum and Brain Carotenoids, α-Tocopherol, and Retinol Concentrations and Cognitive Performance in the Oldest Old from the Georgia Centenarian Study. J. Aging Res. 2013, 2013, 951786. [Google Scholar] [CrossRef] [PubMed]
- Feeney, J.; O’Leary, N.; Moran, R.; O’Halloran, A.M.; Nolan, J.M.; Beatty, S.; Young, I.S.; Kenny, R.A. Plasma Lutein and Zeaxanthin Are Associated with Better Cognitive Function Across Multiple Domains in a Large Population-Based Sample of Older Adults: Findings from The Irish Longitudinal Study on Aging. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 1431–1436. [Google Scholar] [CrossRef] [PubMed]
- Power, R.; Coen, R.F.; Beatty, S.; Mulcahy, R.; Moran, R.; Stack, J.; Howard, A.N.; Nolan, J.M. Supplemental Retinal Carotenoids Enhance Memory in Healthy Individuals with Low Levels of Macular Pigment in A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J. Alzheimer’s Dis. 2018, 61, 947–961. [Google Scholar] [CrossRef] [PubMed]
- Lindbergh, C.A.; Mewborn, C.M.; Hammond, B.R.; Renzi-Hammond, L.M.; Curran-Celentano, J.M.; Miller, L.S. Relationship of Lutein and Zeaxanthin Levels to Neurocognitive Functioning: An fMRI Study of Older Adults. J. Int. Neuropsychol. Soc. 2017, 23, 11–22. [Google Scholar] [CrossRef]
- Feeney, J.; Finucane, C.; Savva, G.M.; Cronin, H.; Beatty, S.; Nolan, J.M.; Kenny, R.A. Low macular pigment optical density is associated with lower cognitive performance in a large, population-based sample of older adults. Neurobiol. Aging 2013, 34, 2449–2456. [Google Scholar] [CrossRef]
- Khan, N.A.; Walk, A.M.; Edwards, C.G.; Jones, A.R.; Cannavale, C.N.; Thompson, S.V.; Reeser, G.E.; Holscher, H.D. Macular Xanthophylls Are Related to Intellectual Ability among Adults with Overweight and Obesity. Nutrients 2018, 10, 396. [Google Scholar] [CrossRef]
- Saint, S.E.; Renzi-Hammond, L.M.; Khan, N.A.; Hillman, C.H.; Frick, J.E.; Hammond, B.R. The Macular Carotenoids are Associated with Cognitive Function in Preadolescent Children. Nutrients 2018, 10, 193. [Google Scholar] [CrossRef]
- Renzi-Hammond, L.M.; Bovier, E.R.; Fletcher, L.M.; Miller, L.S.; Mewborn, C.M.; Lindbergh, C.A.; Baxter, J.H.; Hammond, B.R. Effects of a Lutein and Zeaxanthin Intervention on Cognitive Function: A Randomized, Double-Masked, Placebo-Controlled Trial of Younger Healthy Adults. Nutrients 2017, 9, 1246. [Google Scholar] [CrossRef]
- Barnett, S.M.; Khan, N.A.; Walk, A.M.; Raine, L.B.; Moulton, C.; Cohen, N.J.; Kramer, A.F.; Hammond, B.R.; Renzi-Hammond, L.; Hillman, C.H. Macular pigment optical density is positively associated with academic performance among preadolescent children. Nutr. Neurosci. 2018, 21, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Lindbergh, C.A.; Renzi-Hammond, L.M.; Hammond, B.R.; Terry, D.P.; Mewborn, C.M.; Puente, A.N.; Miller, L.S. Lutein and Zeaxanthin Influence Brain Function in Older Adults: A Randomized Controlled Trial. J. Int. Neuropsychol. Soc. 2018, 24, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.; Coen, R.F.; Akuffo, K.O.; Beatty, S.; Dennison, J.; Moran, R.; Stack, J.; Howard, A.N.; Mulcahy, R.; Nolan, J.M. Cognitive Function and Its Relationship with Macular Pigment Optical Density and Serum Concentrations of its Constituent Carotenoids. J. Alzheimer’s Dis. 2015, 48, 261–277. [Google Scholar] [CrossRef] [PubMed]
- Ajana, S.; Weber, D.; Helmer, C.; Merle, B.M.; Stuetz, W.; Dartigues, J.F.; Rougier, M.B.; Korobelnik, J.F.; Grune, T.; Delcourt, C.; et al. Plasma Concentrations of Lutein and Zeaxanthin, Macular Pigment Optical Density, and Their Associations with Cognitive Performances Among Older Adults. Investig. Ophthalmol. Vis. Sci. 2018, 59, 1828–1835. [Google Scholar] [CrossRef] [PubMed]
- Vishwanathan, R.; Iannaccone, A.; Scott, T.M.; Kritchevsky, S.B.; Jennings, B.J.; Carboni, G.; Forma, G.; Satterfield, S.; Harris, T.; Johnson, K.C.; et al. Macular pigment optical density is related to cognitive function in older people. Age Ageing 2014, 43, 271–275. [Google Scholar] [CrossRef]
- Renzi, L.M.; Dengler, M.J.; Puente, A.; Miller, L.S.; Hammond, B.R. Relationships between macular pigment optical density and cognitive function in unimpaired and mildly cognitively impaired older adults. Neurobiol. Aging 2014, 35, 1695–1699. [Google Scholar] [CrossRef]
- Stringham, N.T.; Holmes, P.V.; Stringham, J.M. Effects of macular xanthophyll supplementation on brain-derived neurotrophic factor, pro-inflammatory cytokines, and cognitive performance. Physiol. Behav. 2019, 211, 112650. [Google Scholar] [CrossRef] [PubMed]
- Hassevoort, K.M.; Khazoum, S.E.; Walker, J.A.; Barnett, S.M.; Raine, L.B.; Hammond, B.R.; Renzi-Hammond, L.M.; Kramer, A.F.; Khan, N.A.; Hillman, C.H.; et al. Macular Carotenoids, Aerobic Fitness, and Central Adiposity Are Associated Differentially with Hippocampal-Dependent Relational Memory in Preadolescent Children. J. Pediatr. 2017, 183, 108–114.e1. [Google Scholar] [CrossRef]
- Edwards, C.G.; Walk, A.M.; Cannavale, C.N.; Thompson, S.V.; Reeser, G.E.; Holscher, H.D.; Khan, N.A. Macular Xanthophylls and Event-Related Brain Potentials among Overweight Adults and Those with Obesity. Mol. Nutr. Food Res. 2019, 63, e1801059. [Google Scholar] [CrossRef]
- Mewborn, C.M.; Lindbergh, C.A.; Robinson, T.L.; Gogniat, M.A.; Terry, D.P.; Jean, K.R.; Hammond, B.R.; Renzi-Hammond, L.M.; Miller, L.S. Lutein and Zeaxanthin Are Positively Associated with Visual-Spatial Functioning in Older Adults: An fMRI Study. Nutrients 2018, 10, 458. [Google Scholar] [CrossRef]
- Davey, P.G.; Lievens, C.; Ammono-Monney, S. Differences in macular pigment optical density across four ethnicities: A comparative study. Ther. Adv. Ophthalmol. 2020, 12, 2515841420924167. [Google Scholar] [CrossRef] [PubMed]
| Food | Trans-Lutein (µg per 100 g) | Trans-Zeaxanthin (µg per 100 g) | L/Z Ratio |
|---|---|---|---|
| Asparagus, cooked | 991 | 0 | - |
| Broccoli, cooked | 772 | 0 | - |
| Cucumber | 361 | 0 | - |
| Spinach, cooked | 12,640 | 0 | - |
| Spinach, raw | 6603 | 0 | - |
| Tomato, raw | 32 | 0 | - |
| Lettuce, romaine | 3824 | 0 | - |
| Lettuce, iceberg | 171 | 12 | 14.3 |
| Green beans, cooked from frozen | 306 | 0 | - |
| Kale, cooked | 8884 | 0 | - |
| Pepper, orange | 208 | 1665 | 0.1 |
| Pepper, green | 173 | 0 | - |
| Bread, white | 15 | 0 | - |
| Egg (yolk + white), cooked | 237 | 216 | 1.1 |
| Egg yolk, cooked | 645 | 587 | 1.1 |
| Pistachio, shelled | 1405 | 0 | - |
| Grapes, green | 53 | 6 | 8.8 |
| Cilantro | 7703 | 0 | - |
| Lima beans, cooked | 155 | 0 | - |
| Olive, green | 79 | 0 | - |
| Parsley, raw | 4326 | 0 | - |
| Squash, yellow, cooked | 150 | 0 | - |
| Zucchini, cooked with skin | 1355 | 0 | - |
| Method | Advantages | Disadvantages |
|---|---|---|
| Heterochromatic flicker photometry (HFP) [16,17] |
|
|
| Fundus Reflectometry (FR) [18,19] |
|
|
| Fundus Autofluorescence (FAF) [21,23] |
|
|
| Resonance Raman Spectroscopy (RRS) [24,25] |
|
|
| Veggie Meter [30,31] (Cannot measure MPOD but has weak correlation to MPOD) |
|
|
| High-Performance Liquid Chromatography (HPLC) serum carotenoids [34,35] (Cannot measure MPOD) |
|
|
| Author (Year) | Study Design | Inclusion Criteria | Sample Size | Interventions | Duration | Relation between MPOD and AMD | MPOD Technique |
|---|---|---|---|---|---|---|---|
| Beatty (2013) [46] | RCT | Adults ≥55 years with early- or late-stage AMD. | 433 | Group 1: L and Z, Vitamin C, Vitamin E, Copper, Zinc. Group 2: Placebo. | Minimum 12 months, up to 36 months | Supplementation with L, Z, and antioxidants showed functional and morphologic benefits in early AMD. MPOD increased in the active group and decreased in the placebo group. | RS |
| LUTEGA study (2013) [47] | RCT | Adults 60–80 years with non-exudative AMD. | 172 | Group 1: L, Z, Omega-3, antioxidants. Group 2: Placebo | 12 months | Supplementation resulted in a considerable increase in MPOD and improvement/stabilization in BCVA. There was no difference in MPOD accumulation between dosages. | FA |
| CLEAR study (2013) [48] | RCT | Adults 50–80 years with early AMD. | 72 | Group 1: L (10 mg) Group 2: Placebo | 12 months | Lutein supplementation increased MPOD and may have a mild beneficial effect on visual acuity. No change in MPOD was found in the placebo group. | HFP |
| LAST study (2004) [49] | RCT | Adults 55–80 years with atrophic AMD. | 90 | Group 1: L (10 mg) Group 2: L (10 mg) with antioxidants Group 3: Placebo | 12 months | Lutein alone or with antioxidants improved MPOD, glare recovery, and contrast sensitivity. No significant change was found in the placebo group. | HFP |
| LUNA study (2007) [50] | RCT | Adults ≥ 55 years with or without AMD. | 120 | Group 1: L (6 mg) Group 2: Placebo | 6 months | Lutein supplementation increased MPOD and improved visual function. No change was found in the placebo group. | FA |
| ZVF study (2011) [51] | RCT | Early and moderate AMD retinopathy, symptoms of visual deficits. | 60 | Group 1: Z (8 mg) Group 2: Z (8 mg) + L (9 mg), Group 3: Placebo | 12 months | MPOD increased in the intervention groups compared to the placebo group. | HFP |
| Weigert (2011) [52] | RCT | Adults 50–90 years with AREDS stages 2, 3, and 4. | 126 | Group 1: L (20 mg for first 3 months, then 10 mg) Group 2: Placebo | 6 months | Lutein significantly increased MPOD by 27.9%. No significant effect on macular function or visual acuity was observed. | HFP |
| Sabour-Pickett (2014) [53] | RCT | Adults 50–79 years with early AMD. | 52 | Group 1: L (20 mg) and Z (2 mg) Group 2: MZ (10 mg), L (10 mg), Z (2 mg) Group 3: MZ (17 mg), L (3 mg), Z (2 mg) | 12 months | A statistically significant increase in MPOD was observed in Group 2 and Group 3. Improvements in letter contrast sensitivity were seen in all groups, with the best results in Group 3. | HFP |
| Huang (2015) [54] | RCT | Adults 50–79 years with early AMD. | 112 | Group 1: L (10 mg) Group 2: L (20 mg) Group 3: L (10 mg) and Z (10 mg) | 2 years | All active treatment groups showed a significant increase in MPOD. The 20 mg lutein group was the most effective at increasing MPOD and contrast sensitivity at 3 cycles/degree for the first 48 weeks. | FA |
| Davey (2020) [55] | RCT | Adults 50–79 years with retinal drusen. | 56 | Group 1: Lumega-Z softgel Group 2: PreserVision AREDS2 softgel | 6 months | Both groups demonstrated statistically significant improvements in contrast sensitivity function (CSF) in both eyes at six months. | HFP |
| Ma (2012) [56] | RCT | Ages 50–79, early AMD. | 108 | Group 1: L (10 mg) Group 2: L (20 mg) Group 3: L (10 mg) plus Z (10 mg) | 48 weeks | There was a significant increase in MPOD in the high-dose lutein and lutein-plus-zeaxanthin groups, with improvements in contrast sensitivity at certain spatial frequencies. | FA |
| Author (Year) | Study Design | Inclusion Criteria | Sample Size | Intervention(s) | Duration | Relation between MPOD and Glaucoma | MPOD Technique |
|---|---|---|---|---|---|---|---|
| Fikret (2021) [67] | CS | Age not mentioned. Patients with POAG, PEX, and controls. | 79 | None | N/A | Higher MPOD values in patients with PEX glaucoma; no significant differences in POAG compared to controls. There was no correlation between MPOD values and RNFL or GCL. | FR |
| Bruns (2020) [68] | CS | Adults 34–87 years. Patients with POAG and controls. | 86 | None | N/A | No significant difference in MPOD values between POAG patients and controls. | DWA |
| Loughman (2021) [69] | RCT | Adults > 18 years. Patients with POAG and controls. | 62 | Group 1: L (10 mg) + Z (2 mg) + MZ (10 mg). Group 2: Placebo. | 18 months | Supplementation led to a significant increase in MPOD volume. No clinically meaningful changes were noted in glaucoma parameters. | DWA |
| Siah (2015) [65] | CS | Adults 36–84 years. Patients with POAG and controls. | 88 | None | N/A | Lower MPOD was observed in glaucomatous eyes compared to controls. Worse glaucomatous parameters were observed in patients with lower MPOD. | HFP |
| Ji (2016) [70] | CS | Adults 20–76 years. Patients with POAG and controls. | 82 | None | N/A | MPOD was significantly lower in POAG patients compared to controls and correlated positively with GCC thickness. | FR |
| Arnould (2022) [71] | CS | Adults >75 years. Patients with POAG and controls. | 1153 | None | N/A | No significant differences in MPOD were found between the POAG group and the control group. | DWA |
| Daga (2018) [72] | CS | Adults 20–76 years. Patients with POAG and controls. | 107 | None | N/A | No significant association was found between MPOD volume and glaucoma status. | DWA |
| Lawler (2023) [73] | CS | Adults 55–81 years. Patients with POAG and controls. | 379 | None | N/A | MPOD was positively associated with GCC and GCL, among POAG and controls. | HFP |
| Igras (2013) [74] | CS | Adults 58–80 years. Patients with POAG and controls. | 40 | None | N/A | MPOD was significantly lower in POAG patients compared to controls. | HFP |
| Siah (2018) [75] | CS | Adults 36–84 years. Patients with POAG and controls. | 88 | None | N/A | MPOD was associated with improved glare-affected visual function and less central visual field loss in POAG patients. | HFP |
| Liu 2024 [76] | CS | Adults 69–98 years. Patients with POAG and controls. | 26 | None | N/A | Glaucomatous eyes had 25% lower MPOD compared to non-glaucomatous eyes. | HFP |
| Eraslan (2023) [77] | CS | Adults >55 years. Patients with POAG currently receiving topical medication and controls. | 52 | None | N/A | MPOD levels were higher in POAG patients compared to controls, suggesting a possible protective effect of topical therapies. | FR |
| Author (Year) | Study Design | Inclusion Criteria | Sample Size | Intervention(s) | Duration | Relation between MPOD and DR | MPOD Technique |
|---|---|---|---|---|---|---|---|
| Lima (2010) [82] | CS | Adults 56–63; BCVA ≤20/40. | 43 | None | N/A | MPOD was lower in diabetic patients, with a significant inverse correlation with HbA1C levels. | DWA |
| Scanlon (2019) [83] | CS | Adults 50+; BCVA ≤20/40. | 2782 | None | N/A | MPOD was found to be lower in individuals with T2D compared to healthy controls. | HFP |
| Bikbov (2015) [84] | CS | Adults 55–71; BCVA ≤20/40. | 52 | None | N/A | Significant reduction in MPOD in patients with diabetic macular edema compared to controls. | FR |
| Scanlon (2015) [85] | CS | Adults 36–73; BCVA ≤20/25. | 150 | None | N/A | MPOD was significantly lower in T2D compared to T1D and controls. The diabetes control was not associated with MPOD. | HFP |
| She (2016) [86] | CS | Adults over 55–71; BCVA ≤20/25. | 401 | None | N/A | No significant difference in MPOD levels among groups with or without early-stage non-proliferative DR. | HFP |
| Bikbov (2015) [87] | CS | Adults 54–69; BCVA ≤20/25. | 31 | None | N/A | Significant reduction in MPOD in DME patients and strong inverse correlation between retinal thickness and MPOD. | FR |
| Chous (2016) [88] | RCT | Adults 43–69; BCVA ≥20/30; no or mild-to-moderate DR. | 67 | Group 1: Carotenoid supplement Group 2: Placebo | 6 months | Supplemented group showed significant improvements in visual functions which correlated with increased MPOD compared to the placebo. | HFP |
| Zagers (2005) [89] | CS | Adults 23–61; BCVA ≤20/32. | 14 | None | N/A | No significant difference in MPOD density between diabetic patients and healthy controls. | FR |
| Varghese (2019) [90] | CS | Adults 49–54 years. | 150 | None | N/A | MPOD was similar across diabetic patients with and without DR, suggesting no significant difference due to DR. | FR |
| Cennamo (2019) [91] | CS | Adults 31–38 years; T1D and controls. | 59 | None | N/A | MPOD and vessel density were both significantly lower in diabetic patients compared to controls. There was a moderate correlation between vessel density and MPOD. | FR |
| Author (Year) | Study Design | Demographic | Sample Size | Interventions | Duration | Relation between MPOD and Visual Function | MPOD Technique |
|---|---|---|---|---|---|---|---|
| Stringham (2011) [98] | CS | Adults 23–50; BCVA ≤20/25. | 26 | None | N/A | MPOD was associated with faster photostress recovery, lower disability glare thresholds, and reduced visual discomfort. | HFP |
| Engles (2007) [102] | CS | Adults 18–40; BCVA ≤20/40. | 80 | None | N/A | No significant correlation was found between MPOD and measures of visual acuity. | HFP |
| Tudosescu (2018) [103] | CS | Adults 18–65 years; BCVA ≤20/125. | 83 | None | N/A | No significant correlation between MPOD and blue-light exposure from computers, iris color, refractive errors, or glare sensibility was found. | HFP |
| Patryas (2014) [104] | CS | Adults 18–68 years; BCVA ≤20/32. | 33 | None | N/A | MPOD was weakly associated with rod-mediated recovery, but not with cone-mediated recovery. | HFP |
| Bovier (2014) [105] | RCT | Adults 18–32 years; BCVA ≤20/60. | 92 | Group 1: Z—20 mg Group 2: Mixed (Z—26 mg, L—8 mg, Omega-3—190 mg) Group 3: Placebo | 4 months | MPOD increased with supplementation and led to significant improvements in visual processing speed and motor reaction time. | HFP |
| Kvansakul (2006) [95] | RCT | Adults 18–40 years; BCVA ≤20/60. | 92 | Group 1: L—10 mg Group 2: Z—10 mg Group 3: Combination (L—10 mg, Z—10 mg) Group 4: Placebo | 12 months | Supplementation with L or Z increases MPOD and improved contrast acuity thresholds at high mesopic levels, thus enhancing visual performance at low illumination. | HFP |
| Putnam (2015) [106] | CS | Adults 18–35 years; BCVA ≤20/25. | 33 | None | N/A | Increased MPOD correlates with reduced glare disability, significantly at higher spatial frequencies. | HFP |
| Stringham (2008) [107] | RCT | Adults 17–41 years. | 40 | Group 1: L—10 mg, Z—2 mg Group 2: Placebo | 6 months | Supplementation led to increased MPOD, which significantly improved performance in glare disability and photostress recovery tasks. | HFP |
| Stringham (2017) [97] | RCT | Adults 18–25 years. | 59 | Group 1: L—6 mg and Z—6 mg Group 2: L—12 mg and Z—12 mg Group 3: Placebo | 12 months | Increases in MPOD led to improved contrast sensitivity. | HFP |
| Nolan (2016) [108] | RCT | Adults with a mean age of 21.5 years. | 105 | Group 1: L—10 mg, Z—2 mg, and MZ—10 mg Group 2: Placebo | 12 months | MPOD increased with supplementation and was significantly correlated with improvements in contrast sensitivity in the active group compared to the placebo. | DWA |
| Hammond (2014) [109] | RCT | Adults 20–40 years. | 115 | Group 1: L—10 mg, Z—2 mg Group 2: Placebo | 12 months | Supplementation increased MPOD significantly, improving chromatic contrast and photostress recovery time, but glare disability improvements were not statistically significant. | HFP |
| Hammond (2013) [94] | CS | Adults 20–40 years. | 150 | None | N/A | MPOD density significantly correlated with positive outcomes in glare disability, photostress recovery time, and chromatic contrast thresholds. | HFP |
| Stringham (2016) [110] | RCT | Adults 18–25 years, BCVA ≤20/20. | 59 | Group 1: L—10 mg + Z—2 mg Group 2: L—20 mg + Z—4 mg Group 3: Placebo | 12 months | Supplementation led to significant increases in MPOD, which in turn resulted in improvements in photostress recovery and disability glare. | HFP |
| Hammond (1998) [111] | CS | Adults 60–84 years; ≤20/32 visual acuity. | 37 | None | N/A | A higher MPOD was associated with preserved visual sensitivity in older ages. | HFP |
| Estévez-Santiago (2016) [112] | CS | Adults 20–35 and 45–65 years; BCVA ≤20/20. | 108 | None | N/A | The contrast threshold was inversely correlated with MPOD, particularly in the older group. | HFP |
| Nolan (2011) [113] | RCT | Adults 18–41 years; BCVA ≤20/20. | 121 | Group 1: L—12 mg + Z—1 mg Group 2: Placebo | 12 months | A statistically significant increase in MPOD in the active group was not generally associated with improvement in visual performance. | HFP |
| Loughman (2010) [96] | CS | Adults 18–41 years; BCVA ≤20/20. | 142 | None | N/A | MPOD was positively associated with BCVA and contrast sensitivity, while photostress recovery and glare sensitivity were unrelated to MPOD. | HFP |
| Author (Year) | Study Design | Inclusion Criteria | Sample Size | Intervention(s) | Duration | Relation between MPOD and Cognitive Function | MPOD Technique |
|---|---|---|---|---|---|---|---|
| Khan (2018) [119] | CS | Adults 25–45 years with BMI ≥ 25 kg/m2. | 114 | None | N/A | MPOD positively associated with IQ and fluid intelligence, but not with crystallized intelligence. | HFP |
| Saint (2018) [120] | CS | Children 7–13 years. | 51 | None | N/A | MPOD positively associated with reasoning skills and executive mental processes. | HFP |
| Renzi-Hammond (2017) [121] | RCT | Adults 18–30 years. | 51 | Group 1: L (10 mg) + MZ (2 mg). Group 2: Placebo | 1 year | MPOD positively associated with improvements in spatial memory, reasoning ability, and complex attention tasks. | HFP |
| Barnett (2018) [122] | CS | Preadolescent children 8–9 years. | 56 | None | N/A | MPOD positively associated with overall academic achievement, mathematics, and written language. | HFP |
| Lindbergh (2018) [123] | RCT | Adults 64–86 years. | 44 | Group 1: L (10 mg) + MZ (2 mg). Group 2: Placebo | 1 year | L and Z supplementation increased MPOD and was associated with enhanced signals in prefrontal regions, suggesting a potential mechanism for improved cognitive performance. | HFP |
| Kelly (2015) [124] | CS | Group 1: Adults 35–74 years with low MPOD. Group 2: Adults 35–74 years with early AMD. | 226 | None | N/A | MPOD positively associated with phonemic fluency, attention switching, visual and verbal memory, and learning. | HFP and DWA |
| Power (2018) [116] | RCT | Adults 33–57 years with low MPOD. | 91 | Group 1: L (10 mg) + MZ (10 mg) + Z (2 mg). Group 2: Placebo | 12 months | Supplementation improved MPOD, which was positively associated with episodic memory and overall cognitive function. | DWA |
| Ajana (2018) [125] | CS | Adults 75–93 years with low MPOD. | 184 | None | N/A | Higher MPOD was significantly associated with better global cognitive performance, visual memory, and verbal fluency. | DWA |
| Vishwanathan (2014) [126] | CS | Adults 75–80 years. | 108 | None | N/A | MPOD levels were significantly positively associated with better global cognition, verbal learning and fluency, recall, processing speed, and perceptual speed. | HFP |
| Renzi (2014) [127] | CS | Adults 65–83 years with mild cognitive impairment. | 53 | None | N/A | In unimpaired adults, higher MPOD was associated with better visuospatial and constructional abilities. In mildly impaired adults, higher MPOD was associated with better performance in multiple cognitive domains including memory, language, and attention. | HFP |
| Feeney (2013) [118] | CS | Adults 50+ years. | 4453 | None | N/A | Lower MPOD was associated with poorer performance on the MMSE and MoCA, prospective memory, and executive function. | HFP |
| Stringham (2019) [128] | RCT | Adults 18–25 years. | 59 | Group 1: MZ (13 mg) Group 2: MZ (27 mg) Group 3: Placebo | 6 months | Supplementation improved cognitive performance in composite memory, verbal memory, sustained attention, psychomotor speed, and processing speed. | HFP |
| Hassevoort (2017) [129] | CS | Children 7–10 years. | 40 | None | N/A | MPOD was negatively associated with relational memory errors. | HFP |
| Edwards (2019) [130] | CS | Adults 25–45 years with BMI ≥ 25 kg/m2. | 101 | None | N/A | MPOD was positively associated with improvements attentional resource allocation and information processing speed. | HFP |
| Mewborn (2018) [131] | CS | Adults 64–77 years. | 51 | None | N/A | Higher MPOD was positively associated with better neural efficiency in visual–spatial processing. | HFP |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masri, A.; Armanazi, M.; Inouye, K.; Geierhart, D.L.; Davey, P.G.; Vasudevan, B. Macular Pigment Optical Density as a Measurable Modifiable Clinical Biomarker. Nutrients 2024, 16, 3273. https://doi.org/10.3390/nu16193273
Masri A, Armanazi M, Inouye K, Geierhart DL, Davey PG, Vasudevan B. Macular Pigment Optical Density as a Measurable Modifiable Clinical Biomarker. Nutrients. 2024; 16(19):3273. https://doi.org/10.3390/nu16193273
Chicago/Turabian StyleMasri, Abdul, Mohammed Armanazi, Keiko Inouye, Dennis L. Geierhart, Pinakin Gunvant Davey, and Balamurali Vasudevan. 2024. "Macular Pigment Optical Density as a Measurable Modifiable Clinical Biomarker" Nutrients 16, no. 19: 3273. https://doi.org/10.3390/nu16193273
APA StyleMasri, A., Armanazi, M., Inouye, K., Geierhart, D. L., Davey, P. G., & Vasudevan, B. (2024). Macular Pigment Optical Density as a Measurable Modifiable Clinical Biomarker. Nutrients, 16(19), 3273. https://doi.org/10.3390/nu16193273






