Investigating Causal Associations between the Gut Microbiota and Dementia: A Mendelian Randomization Study
Abstract
:1. Introduction
2. Methods and Materials
2.1. Study Design
2.2. Data Sources
2.3. Instrumental Variables (IVs)
2.4. Statistical Analyses
2.5. Sensitivity Analyses
2.6. Software
3. Results
3.1. Strength of the IVs
3.2. Two-Sample MR
3.3. MVMR and MR-BMA
3.4. Sensitivity Analyses Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prince, M.; Bryce, R.; Albanese, E.; Wimo, A.; Ribeiro, W.; Ferri, C.P. The global prevalence of dementia: A systematic review and metaanalysis. Alzheimer’s Dement. 2013, 9, 63–75.e2. [Google Scholar] [CrossRef] [PubMed]
- Chiu, M.-J.; Chen, T.-F.; Yip, P.-K.; Hua, M.-S.; Tang, L.-Y. Behavioral and Psychologic Symptoms in Different Types of Dementia. J. Formos. Med. Assoc. 2006, 105, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Arvanitakis, Z.; Shah, R.C.; Bennett, D.A. Diagnosis and Management of Dementia: Review. JAMA 2019, 322, 1589–1599. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Sommerlad, A.; Orgeta, V.; Costafreda, S.G.; Huntley, J.; Ames, D.; Ballard, C.; Banerjee, S.; Burns, A.; Cohen-Mansfield, J.; et al. Dementia prevention, intervention, and care. Lancet 2017, 390, 2673–2734. [Google Scholar] [CrossRef] [PubMed]
- Le, H.H.; Lee, M.-T.; Besler, K.R.; Johnson, E.L. Host hepatic metabolism is modulated by gut microbiota-derived sphingolipids. Cell Host Microbe 2022, 30, 798–808.e7. [Google Scholar] [CrossRef]
- Stacy, A.; Andrade-Oliveira, V.; McCulloch, J.A.; Hild, B.; Oh, J.H.; Perez-Chaparro, P.J.; Sim, C.K.; Lim, A.I.; Link, V.M.; Enamorado, M.; et al. Infection trains the host for microbiota-enhanced resistance to pathogens. Cell 2021, 184, 615–627. [Google Scholar] [CrossRef]
- Donald, K.; Finlay, B.B. Early-life interactions between the microbiota and immune system: Impact on immune system development and atopic disease. Nat. Rev. Immunol. 2023, 23, 735–748. [Google Scholar] [CrossRef]
- Connell, E.; Le Gall, G.; Pontifex, M.G.; Sami, S.; Cryan, J.F.; Clarke, G.; Müller, M.; Vauzour, D. Microbial-derived metabolites as a risk factor of age-related cognitive decline and dementia. Mol. Neurodegener. 2022, 17, 43. [Google Scholar] [CrossRef] [PubMed]
- Alkasir, R.; Li, J.; Li, X.; Jin, M.; Zhu, B. Human gut microbiota: The links with dementia development. Protein Cell 2017, 8, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; O’Riordan, K.J.; Sandhu, K.; Peterson, V.; Dinan, T.G. The gut microbiome in neurological disorders. Lancet Neurol. 2020, 19, 179–194. [Google Scholar] [CrossRef]
- Fang, P.; Kazmi, S.A.; Jameson, K.G.; Hsiao, E.Y. The Microbiome as a Modifier of Neurodegenerative Disease Risk. Cell Host Microbe 2020, 28, 201–222. [Google Scholar] [CrossRef] [PubMed]
- Cammann, D.; Lu, Y.; Cummings, M.J.; Zhang, M.L.; Cue, J.M.; Do, J.; Ebersole, J.; Chen, X.; Oh, E.C.; Cummings, J.L.; et al. Genetic correlations between Alzheimer’s disease and gut microbiome genera. Sci. Rep. 2023, 13, 5258. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Han, Y.; Zheng, Z.; Peng, G.; Liu, P.; Yue, S.; Zhu, S.; Chen, J.; Lv, H.; Shao, L.; et al. Altered Gut Microbial Metabolites in Amnestic Mild Cognitive Impairment and Alzheimer’s Disease: Signals in Host-Microbe Interplay. Nutrients 2021, 13, 228. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.-Q.; Shen, L.-L.; Li, W.-W.; Fu, X.; Zeng, F.; Gui, L.; Lü, Y.; Cai, M.; Zhu, C.; Tan, Y.-L.; et al. Gut Microbiota is Altered in Patients with Alzheimer’s Disease. J. Alzheimers Dis. 2018, 63, 1337–1346. [Google Scholar] [CrossRef]
- Gupta, V.; Walia, G.K.; Sachdeva, M.P. “Mendelian randomization”: An approach for exploring causal relations in epidemiology. Public Health 2017, 145, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.; Yang, R.; Wang, W.; Qi, L.; Huang, T. Associations between gut microbiota and Alzheimer’s disease, major depressive disorder, and schizophrenia. J. Neuroinflamm. 2020, 17, 288. [Google Scholar] [CrossRef] [PubMed]
- Ning, J.; Huang, S.-Y.; Chen, S.-D.; Zhang, Y.-R.; Huang, Y.-Y.; Yu, J.-T. Investigating Casual Associations Among Gut Microbiota, Metabolites, and Neurodegenerative Diseases: A Mendelian Randomization Study. J. Alzheimers Dis. 2022, 87, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Ji, D.; Chen, W.-Z.; Zhang, L.; Zhang, Z.-H.; Chen, L.-J. Gut microbiota, circulating cytokines and dementia: A Mendelian randomization study. J. Neuroinflamm. 2024, 21, 2. [Google Scholar] [CrossRef]
- Li, C.; Chen, J.; Li, S.C. Understanding Horizontal Gene Transfer network in human gut microbiota. Gut Pathog. 2020, 12, 33. [Google Scholar] [CrossRef]
- Sanderson, E. Multivariable Mendelian Randomization and Mediation. Cold Spring Harb. Perspect. Med. 2021, 11, a038984. [Google Scholar] [CrossRef] [PubMed]
- Zuber, V.; Colijn, J.M.; Klaver, C.; Burgess, S. Selecting likely causal risk factors from high-throughput experiments using multivariable Mendelian randomization. Nat. Commun. 2020, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Kurilshikov, A.; Medina-Gomez, C.; Bacigalupe, R.; Radjabzadeh, D.; Wang, J.; Demirkan, A.; Le Roy, C.I.; Raygoza Garay, J.A.; Finnicum, C.T.; Liu, X.; et al. Large-scale association analyses identify host factors influencing human gut microbiome composition. Nat. Genet. 2021, 53, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Kurki MI, Karjalainen J, Palta P et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature 2023, 613, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Mancuso, N.; Spendlove, S.; Pasaniuc, B. Local Genetic Correlation Gives Insights into the Shared Genetic Architecture of Complex Traits. Am. J. Hum. Genet. 2017, 101, 737–751. [Google Scholar] [CrossRef]
- Lawlor, D.A.; Harbord, R.M.; Sterne, J.A.C.; Timpson, N.; Davey Smith, G. Mendelian randomization: Using genes as instruments for making causal inferences in epidemiology. Stat. Med. 2008, 27, 1133–1163. [Google Scholar] [CrossRef] [PubMed]
- Sanna, S.; van Zuydam, N.R.; Mahajan, A.; Kurilshikov, A.; Vich Vila, A.; Võsa, U.; Mujagic, Z.; Masclee, A.A.M.; Jonkers, D.M.A.E.; Oosting, M.; et al. Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat. Genet. 2019, 51, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Larke, L.; Zheng-Bradley, X.; Smith, R.; Kulesha, E.; Xiao, C.; Toneva, I.; Vaughan, B.; Preuss, D.; Leinonen, R.; Shumway, M.; et al. The 1000 Genomes Project: Data management and community access. Nat. Methods 2012, 9, 459–462. [Google Scholar] [CrossRef]
- Shigemizu, D.; Asanomi, Y.; Akiyama, S.; Mitsumori, R.; Niida, S.; Ozaki, K. Whole-genome sequencing reveals novel ethnicity-specific rare variants associated with Alzheimer’s disease. Mol. Psychiatry 2022, 27, 2554–2562. [Google Scholar] [CrossRef]
- Wang, S.; Kang, H. Weak-instrument robust tests in two-sample summary-data Mendelian randomization. Biometrics 2022, 78, 1699–1713. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Dudbridge, F.; Thompson, S.G. Combining information on multiple instrumental variables in Mendelian randomization: Comparison of allele score and summarized data methods. Stat. Med. 2016, 35, 1880–1906. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.; Davey Smith, G.; Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015, 44, 512–525. [Google Scholar] [CrossRef]
- Hartwig, F.P.; Davey Smith, G.; Bowden, J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int. J. Epidemiol. 2017, 46, 1985–1998. [Google Scholar] [CrossRef] [PubMed]
- Verbanck, M.; Chen, C.-Y.; Neale, B.; Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018, 50, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Qin, Y.; Xiao, L.; Dai, X. Causal relationship between gut microflora and dementia: A Mendelian randomization study. Front. Microbiol. 2024, 14, 1306048. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Xing, M.; Liang, S.; Shi, Y.; Li, Z.; Zou, W. Causal relationship of gut microbiota and metabolites on cognitive performance: A mendelian randomization analysis. Neurobiol. Dis. 2024, 191, 106395. [Google Scholar] [CrossRef] [PubMed]
- Geng, S.; Yang, L.; Cheng, F.; Zhang, Z.; Li, J.; Liu, W.; Li, Y.; Chen, Y.; Bao, Y.; Chen, L.; et al. Gut Microbiota Are Associated With Psychological Stress-Induced Defections in Intestinal and Blood-Brain Barriers. Front. Microbiol. 2019, 10, 3067. [Google Scholar] [CrossRef]
- Pei, Y.; Lu, Y.; Li, H.; Jiang, C.; Wang, L. Gut microbiota and intestinal barrier function in subjects with cognitive impairments: A cross-sectional study. Front. Aging Neurosci. 2023, 15, 1174599. [Google Scholar] [CrossRef] [PubMed]
- Garner, M.R.; Gronquist, M.R.; Russell, J.B. Nutritional requirements of Allisonella histaminiformans, a ruminal bacterium that decarboxylates histidine and produces histamine. Curr. Microbiol. 2004, 49, 295–299. [Google Scholar] [CrossRef]
- Jena, P.K.; Sheng, L.; Nguyen, M.; Di Lucente, J.; Hu, Y.; Li, Y.; Maezawa, I.; Jin, L.-W.; Wan, Y.-J.Y. Dysregulated bile acid receptor-mediated signaling and IL-17A induction are implicated in diet-associated hepatic health and cognitive function. Biomark. Res. 2020, 8, 59. [Google Scholar] [CrossRef] [PubMed]
- Fongang, B.; Satizabal, C.; Kautz, T.F.; Wadop, Y.N.; Muhammad, J.A.S.; Vasquez, E.; Mathews, J.; Gireud-Goss, M.; Saklad, A.R.; Himali, J.; et al. Cerebral small vessel disease burden is associated with decreased abundance of gut Barnesiella intestinihominis bacterium in the Framingham Heart Study. Sci. Rep. 2023, 13, 13622. [Google Scholar] [CrossRef]
- Kasim, S.; Moo, L.R.; Zschocke, J.; Jinnah, H.A. Phenylketonuria presenting in adulthood as progressive spastic paraparesis with dementia. J. Neurol. Neurosurg. Psychiatry 2001, 71, 795–797. [Google Scholar] [CrossRef] [PubMed]
- Vogt, N.M.; Kerby, R.L.; Dill-McFarland, K.A.; Harding, S.J.; Merluzzi, A.P.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Zetterberg, H.; Blennow, K.; et al. Gut microbiome alterations in Alzheimer’s disease. Sci. Rep. 2017, 7, 13537. [Google Scholar] [CrossRef] [PubMed]
- Komanduri, M.; Savage, K.; Lea, A.; McPhee, G.; Nolidin, K.; Deleuil, S.; Stough, C.; Gondalia, S. The Relationship between Gut Microbiome and Cognition in Older Australians. Nutrients 2022, 14, 64. [Google Scholar] [CrossRef] [PubMed]

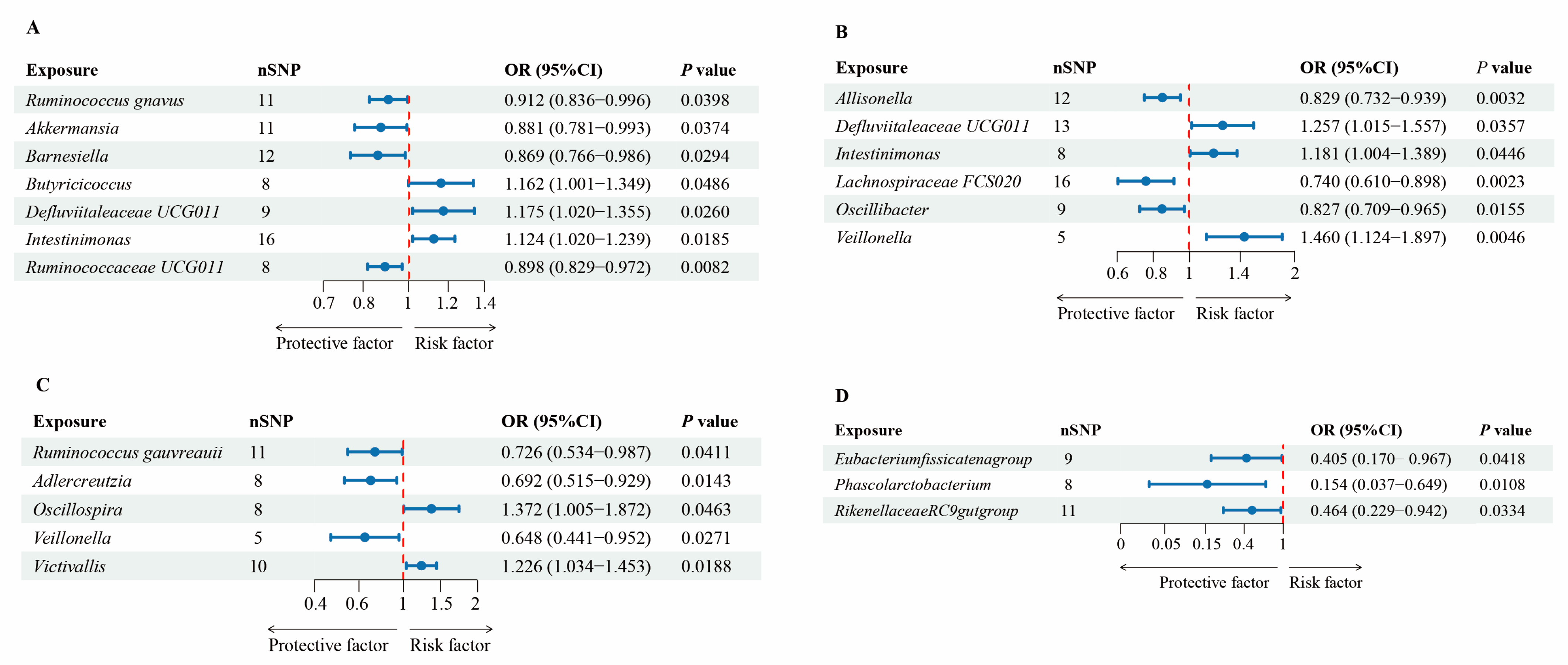
| Exposure | Outcome | Method | OR (95%CI) | Q | Intercept | Outlier |
|---|---|---|---|---|---|---|
| Test | p Value | p Value | ||||
| Ruminococcus gnavus | Dementia | IVW | 0.912 (0.836–0.996) | 0.435 | 0.511 | |
| MR-Egger | 0.779 (0.507–1.198) | 0.393 | 0.481 | |||
| WM | 0.876 (0.778–0.987) | |||||
| Akkermansia | Dementia | IVW | 0.881 (0.781–0.993) | 0.395 | 0.468 | |
| MR-Egger | 0.987 (0.650–1.499) | 0.336 | 0.589 | |||
| WM | 0.891 (0.757–1.048) | |||||
| Barnesiella | Dementia | IVW | 0.869 (0.766–0.986) | 0.836 | 0.790 | |
| MR-Egger | 1.028 (0.613–1.724) | 0.806 | 0.527 | |||
| WM | 0.892 (0.760–1.048) | |||||
| Butyricicoccus | Dementia | IVW | 1.162 (1.001–1.349) | 0.423 | 0.486 | |
| MR-Egger | 1.420 (1.060–1.904) | 0.591 | 0.170 | |||
| WM | 1.137 (0.922–1.402) | |||||
| Defluviitaleaceae UCG011 | Dementia | IVW | 1.175 (1.020–1.355) | 0.147 | 0.193 | |
| MR-Egger | 0.852 (0.522–1.393) | 0.209 | 0.224 | |||
| WM | 1.239 (1.061–1.446) | |||||
| Intestinimonas | Dementia | IVW | 1.124 (1.020–1.239) | 0.722 | 0.771 | |
| MR-Egger | 1.411 (1.076–1.849) | 0.871 | 0.100 | |||
| WM | 1.086 (0.950–1.242) | |||||
| Ruminococcaceae UCG011 | Dementia | IVW | 0.898 (0.829–0.972) | 0.338 | 0.418 | |
| MR-Egger | 1.037 (0.685–1.571) | 0.290 | 0.513 | |||
| WM | 0.901 (0.818–0.993) | |||||
| Allisonella | AD | IVW | 0.829 (0.732–0.939) | 0.351 | 0.382 | |
| MR-Egger | 0.864 (0.347–2.155) | 0.254 | 0.930 | |||
| WM | 0.853 (0.720–1.010) | |||||
| Defluviitaleaceae UCG011 | AD | IVW | 1.257 (1.015–1.557) | 0.276 | 0.353 | |
| MR-Egger | 0.940 (0.424–2.084) | 0.051 | 0.480 | |||
| WM | 1.310 (1.013–1.693) | |||||
| Intestinimonas | AD | IVW | 1.181 (1.004–1.389) | 0.740 | 0.712 | |
| MR-Egger | 1.316 (0.837–2.069) | 0.254 | 0.622 | |||
| WM | 1.158 (0.922–1.455) | |||||
| Lachnospiraceae FCS020 | AD | IVW | 0.740 (0.610–0.898) | 0.508 | 0.507 | |
| MR-Egger | 0.774 (0.459–1.304) | 0.421 | 0.859 | |||
| WM | 0.767 (0.591–0.995) | |||||
| Oscillibacter | AD | IVW | 0.827 (0.709–0.965) | 0.979 | 0.981 | |
| MR-Egger | 1.007 (0.559–1.814) | 0.976 | 0.511 | |||
| WM | 0.801 (0.654–0.980) | |||||
| Veillonella | AD | IVW | 1.460 (1.124–1.897) | 0.560 | 0.673 | |
| MR-Egger | 0.984 (0.087–11.064) | 0.477 | 0.533 | |||
| WM | 1.412 (0.996–2.002) | |||||
| Ruminococcus gauvreauii | VaD | IVW | 0.726 (0.534–0.987) | 0.402 | 0.364 | |
| MR-Egger | 0.376 (0.108–1.311) | 0.411 | 0.315 | |||
| WM | 0.864 (0.561–1.331) | |||||
| Adlercreutzia | VaD | IVW | 0.692 (0.515–0.929) | 0.430 | 0.503 | |
| MR-Egger | 0.719 (0.173–2.989) | 0.322 | 0.959 | |||
| WM | 0.614 (0.417–0.902) | |||||
| Oscillospira | VaD | IVW | 1.372 (1.005–1.872) | 0.431 | 0.444 | |
| MR-Egger | 1.240 (0.302–5.093) | 0.325 | 0.890 | |||
| WM | 1.345 (0.876–2.065) | |||||
| Veillonella | VaD | IVW | 0.648 (0.441–0.952) | 0.808 | 0.878 | 0.774 |
| MR-Egger | 0.984 (0.445–21.928) | |||||
| WM | 0.763 (0.462–1.258) | |||||
| Victivallis | VaD | IVW | 1.226 (1.034–1.453) | 0.843 | 0.822 | |
| MR-Egger | 1.436 (0.391–5.281) | 0.775 | 0.816 | |||
| WM | 1.218 (0.966–1.538) | |||||
| Eubacterium fissicate | FTD | IVW | 0.405 (0.170–0.967) | 0.911 | 0.910 | |
| MR-Egger | 0.265 (0.003–24.273) | 0.856 | 0.857 | |||
| WM | 0.430 (0.144–1.288) | |||||
| Phascolarcto bacterium | FTD | IVW | 0.154 (0.037–0.649) | 0.436 | 0.487 | |
| MR-Egger | 0.044 (0.001–4.161) | 0.367 | 0.563 | |||
| WM | 0.227 (0.033–1.584) | |||||
| Rikenellaceae RC9 | FTD | IVW | 0.464 (0.229–0.942) | 0.994 | 0.991 | |
| MR-Egger | 0.112 (0.001–9.299) | 0.994 | 0.539 | |||
| WM | 0.512 (0.206–1.269) |
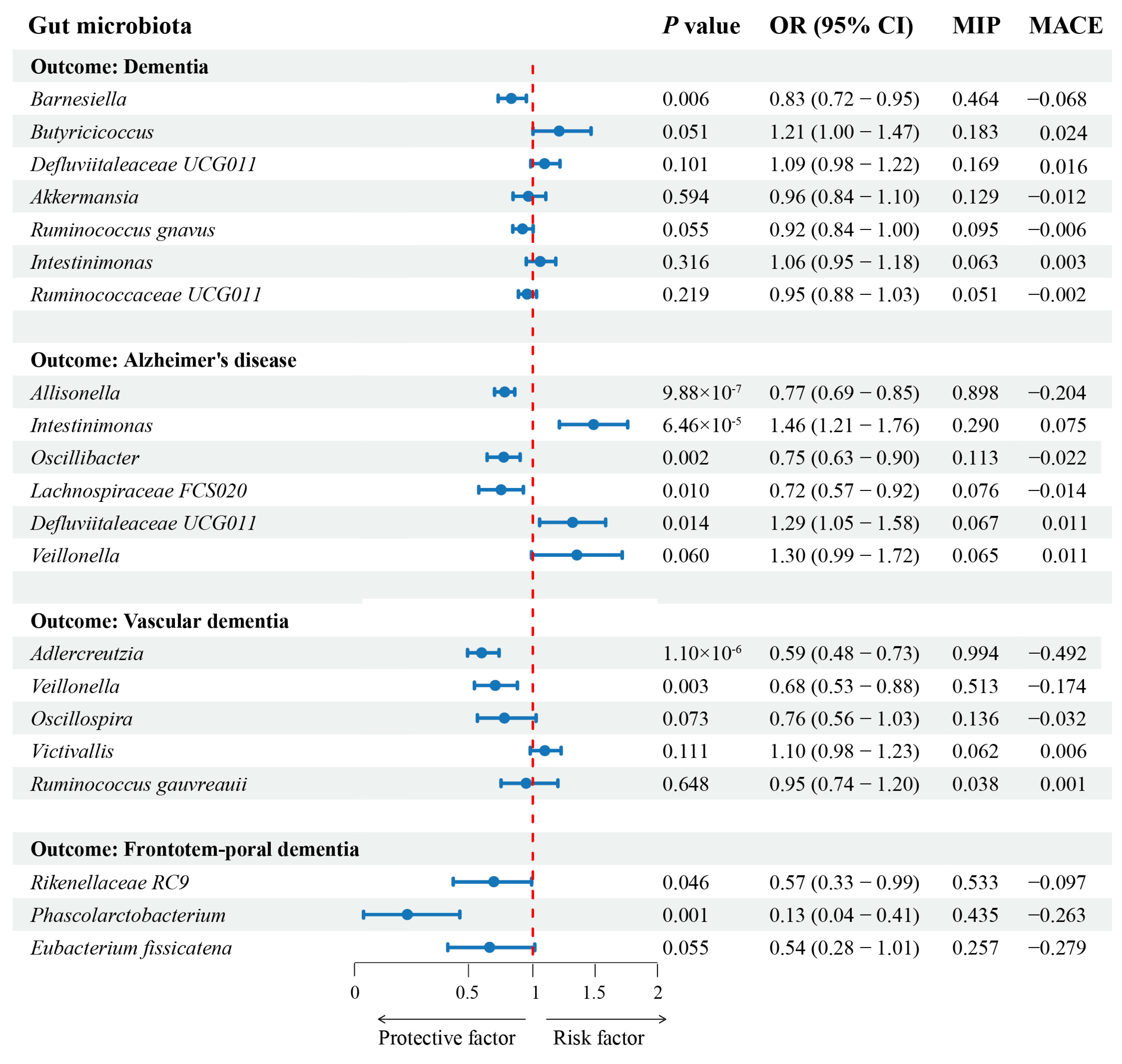
| Gut Microbiota | Rank | MIP | MACE | PP |
|---|---|---|---|---|
| Dementia | ||||
| Barnesiella | 1 | 0.46 | −0.07 | 0.36 |
| Butyricicoccus | 2 | 0.18 | 0.02 | 0.12 |
| Defluviitaleaceae UCG011 | 3 | 0.17 | 0.02 | 0.12 |
| Akkermansia | 4 | 0.13 | −0.01 | 0.1 |
| Ruminococcus gnavus | 5 | 0.1 | −0.01 | 0.07 |
| Intestinimonas | 6 | 0.06 | 0 | 0.05 |
| Ruminococcaceae UCG011 | 7 | 0.05 | 0 | 0.04 |
| AD | ||||
| Allisonella | 1 | 0.9 | −0.2 | 0.53 |
| Intestinimonas | 2 | 0.29 | 0.08 | 0.04 |
| Oscillibacter | 3 | 0.11 | −0.02 | 0.01 |
| Lachnospiraceae FCS020 | 4 | 0.08 | −0.01 | 0.01 |
| Defluviitaleaceae UCG011 | 5 | 0.07 | 0.01 | 0.01 |
| Veillonella | 6 | 0.07 | 0.01 | 0.01 |
| VaD | ||||
| Adlercreutzia | 1 | 0.99 | −0.49 | 0.39 |
| Veillonella | 2 | 0.51 | −0.17 | 0 |
| Oscillospira | 3 | 0.14 | −0.03 | 0 |
| Victivallis | 4 | 0.06 | 0.01 | 0 |
| Ruminococcus gauvreauii | 5 | 0.04 | 0 | 0 |
| FTD | ||||
| Rikenellaceae RC9 | 1 | 0.53 | −0.1 | 0.37 |
| Phascolarctobacterium | 2 | 0.44 | −0.26 | 0.27 |
| Eubacterium fissicatena | 3 | 0.26 | −0.28 | 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, Z.-Y.; Li, H.-M.; Qiu, C.-S.; Tang, X.-L.; Liao, D.-Q.; Du, L.-Y.; Lai, S.-M.; Huang, H.-X.; Zhang, B.-Y.; Kuang, L.; et al. Investigating Causal Associations between the Gut Microbiota and Dementia: A Mendelian Randomization Study. Nutrients 2024, 16, 3312. https://doi.org/10.3390/nu16193312
Xiong Z-Y, Li H-M, Qiu C-S, Tang X-L, Liao D-Q, Du L-Y, Lai S-M, Huang H-X, Zhang B-Y, Kuang L, et al. Investigating Causal Associations between the Gut Microbiota and Dementia: A Mendelian Randomization Study. Nutrients. 2024; 16(19):3312. https://doi.org/10.3390/nu16193312
Chicago/Turabian StyleXiong, Zhi-Yuan, Hong-Min Li, Cheng-Shen Qiu, Xu-Lian Tang, Dan-Qing Liao, Li-Ying Du, Shu-Min Lai, Hong-Xuan Huang, Bing-Yun Zhang, Ling Kuang, and et al. 2024. "Investigating Causal Associations between the Gut Microbiota and Dementia: A Mendelian Randomization Study" Nutrients 16, no. 19: 3312. https://doi.org/10.3390/nu16193312






