Isatidis Folium Represses Dextran Sulfate Sodium-Induced Colitis and Suppresses the Inflammatory Response by Inhibiting Inflammasome Activation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of EIF
2.2. Animal Studies
2.2.1. Animals
2.2.2. The Intestinal Barrier-Protective Effects of EIF on Ulcerative Colitis
2.3. In Vitro Study
2.3.1. Cell Culture
2.3.2. Cell Viability and Toxicity of EIF
2.3.3. Measurement of Nitic Oxide (NO) and Pro-Inflammatory Cytokine Levels
2.3.4. NF-κB Luciferase Reporter-Based Assay
2.3.5. Western Blotting
2.4. Statistical Analyses
3. Results
3.1. Effects of EIF in DSS-Induced Colitis
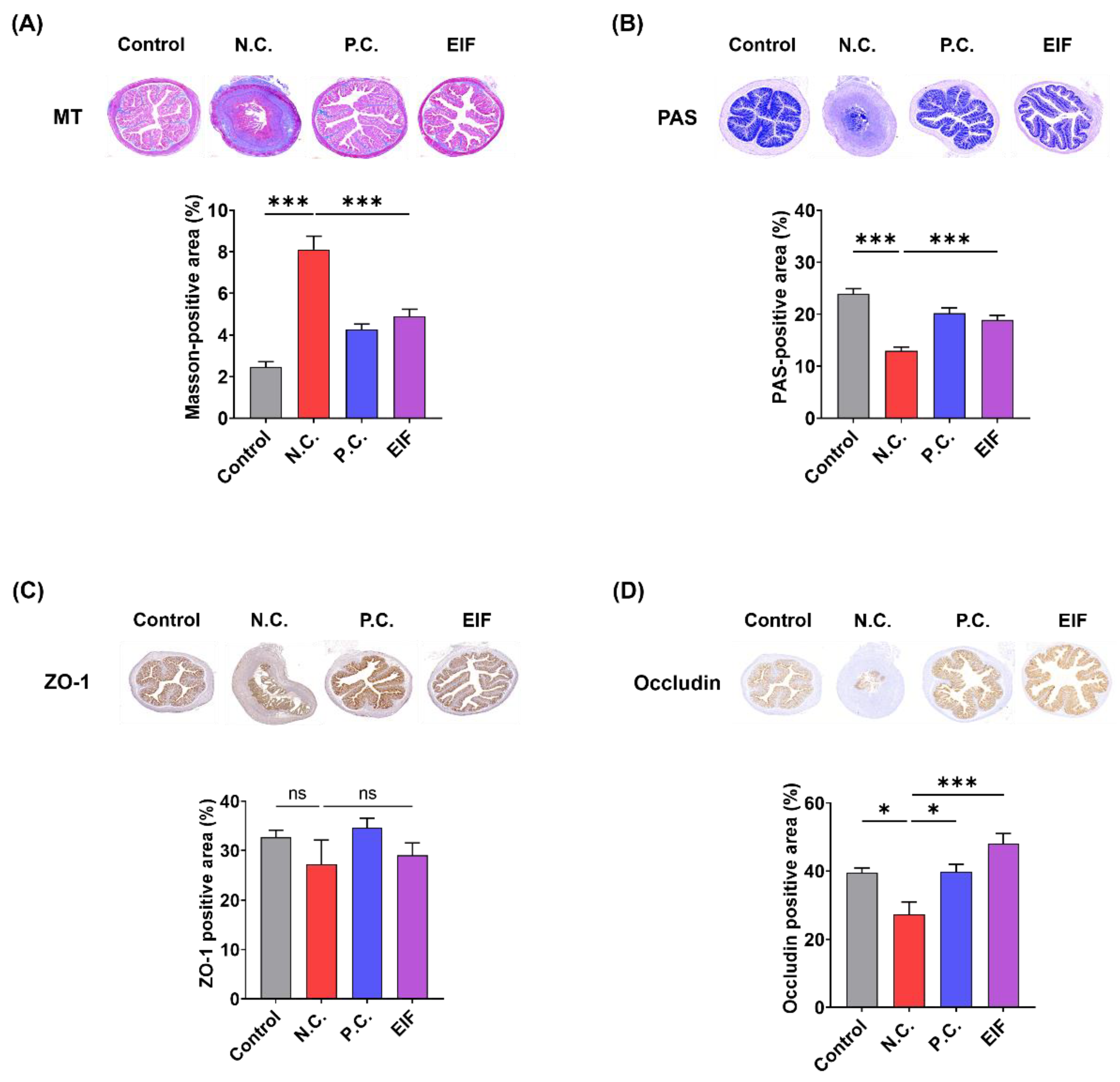
3.2. Modulatory Effects of EIF on Histopathological Changes in DSS-Induced Colitis
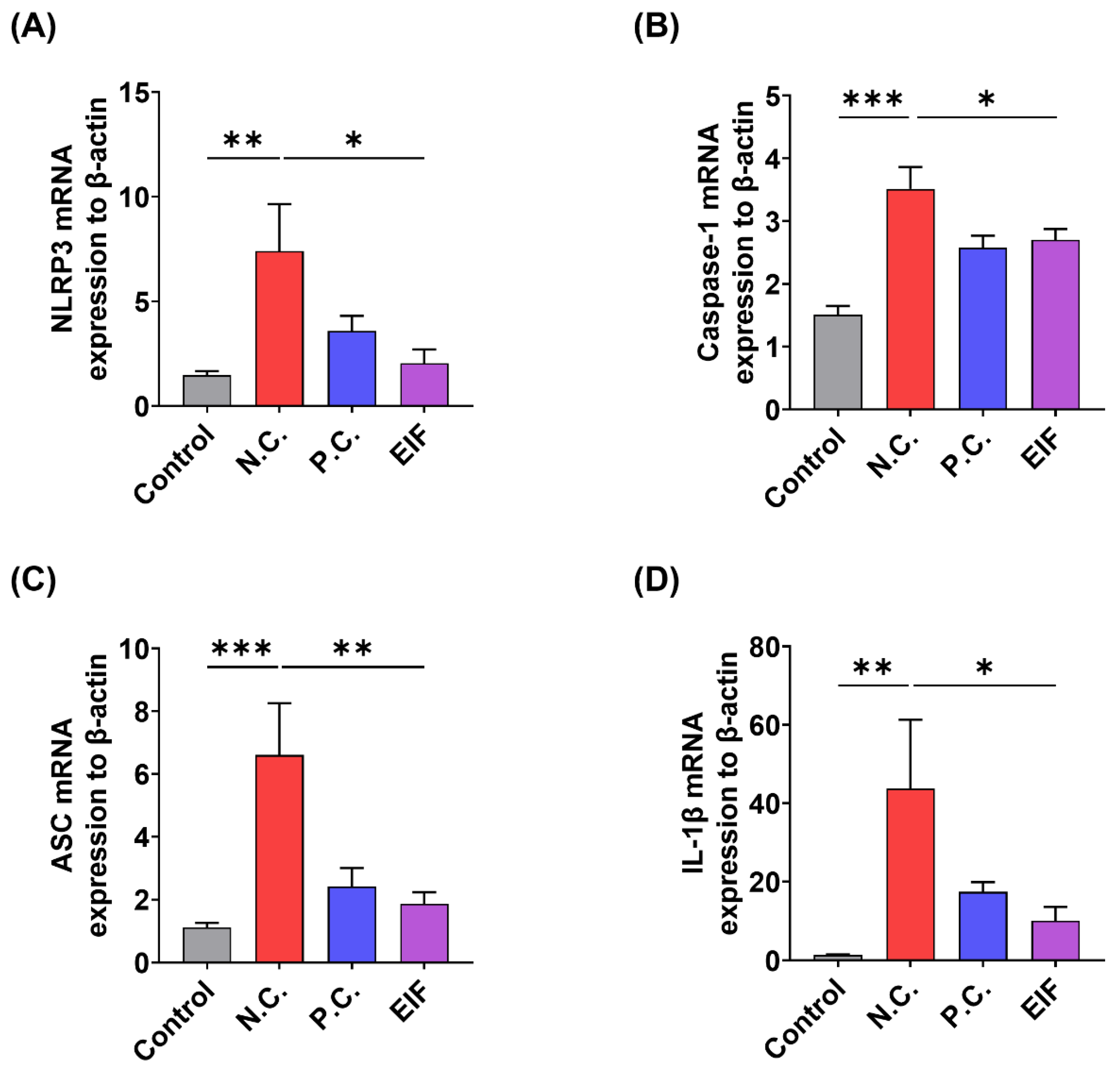
3.3. Modulatory Effects of EIF on Inflammasome Component Levels in DSS-Induced Colitis
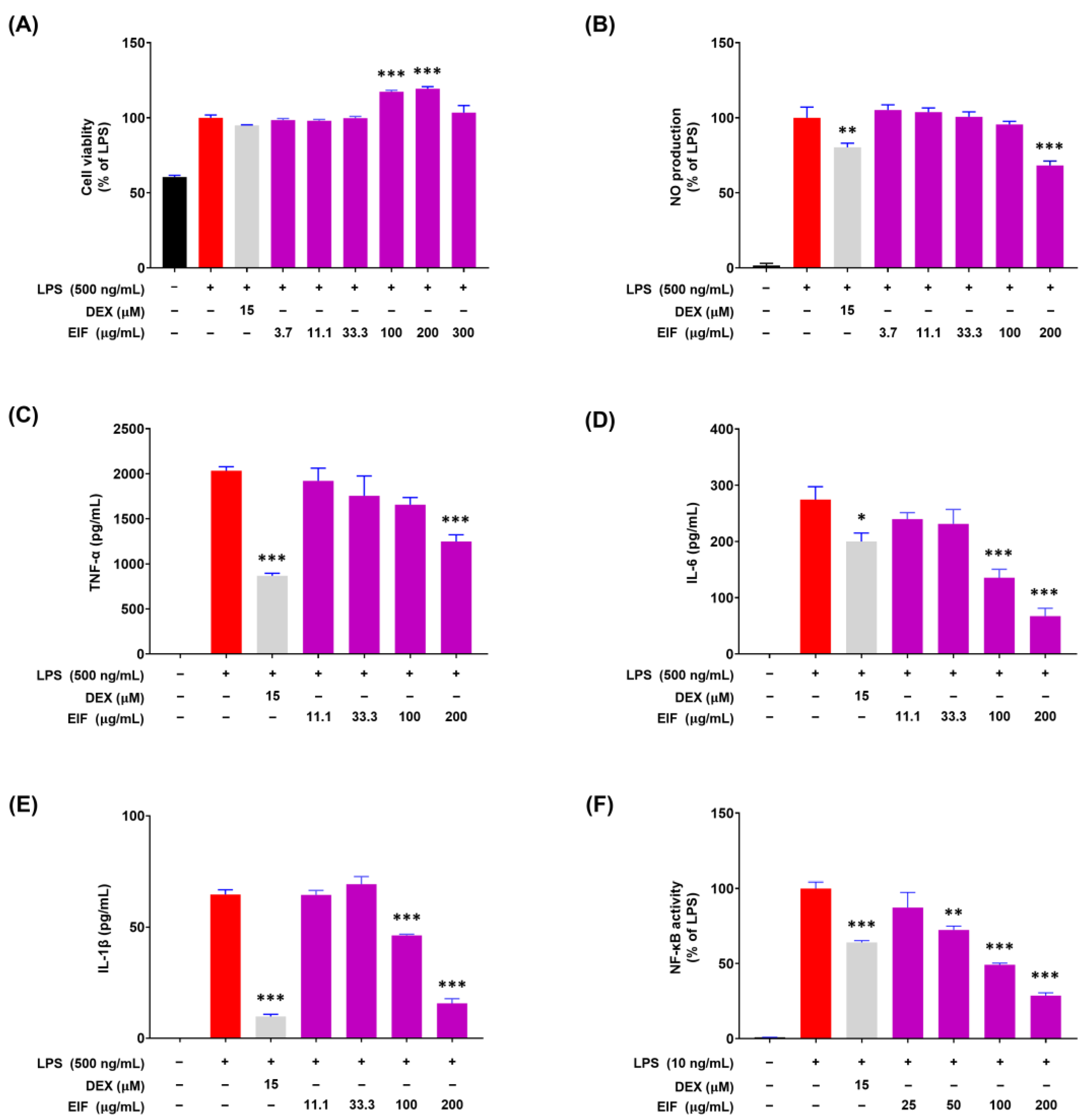
3.4. Modulatory Effects of EIF on LPS-Induced Inflammatory Responses in RAW264.7 Cells
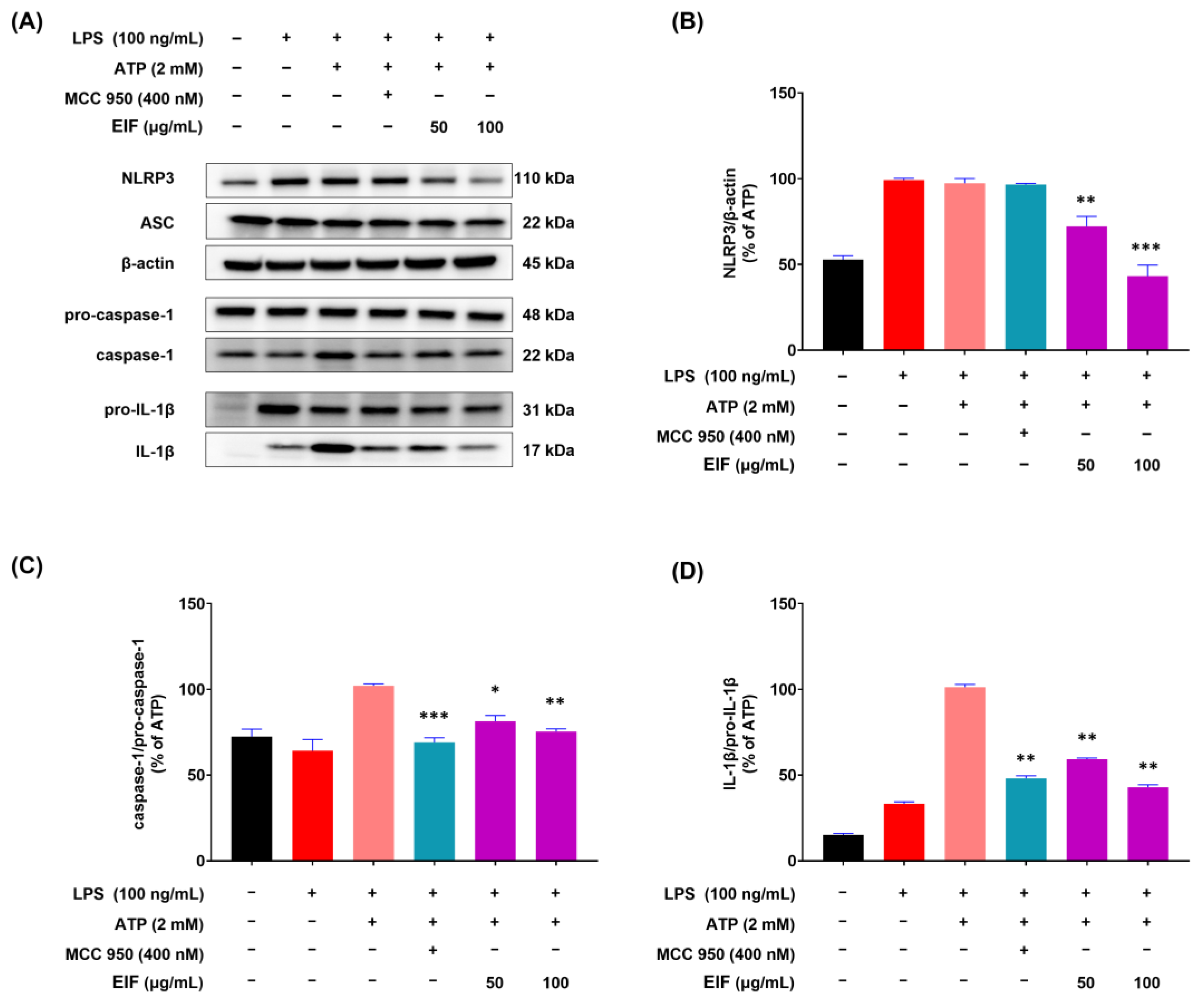
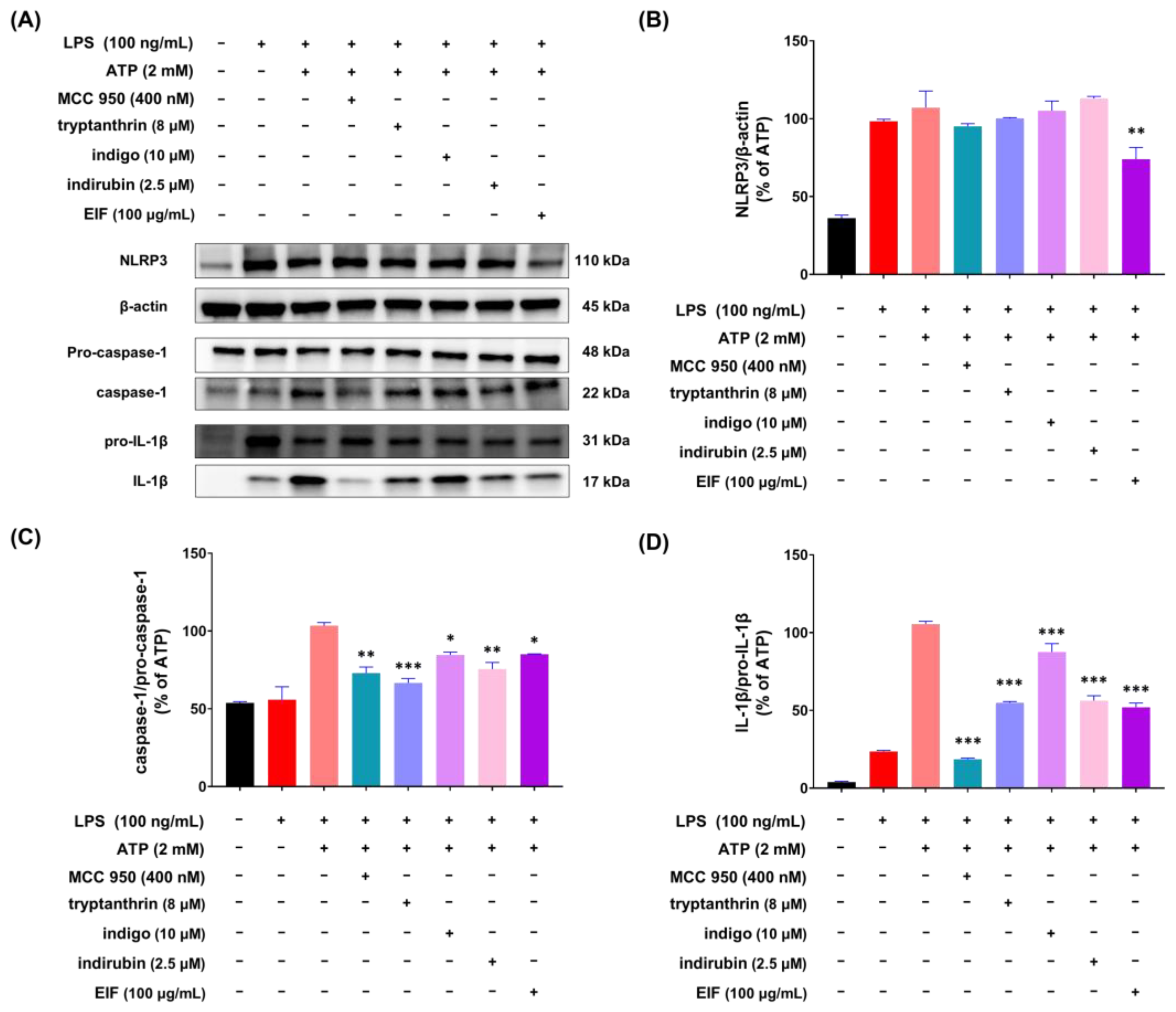
3.5. Effect of EIF on the Inhibition of Proteins Related to LPS/ATP-Induced Inflammasomes
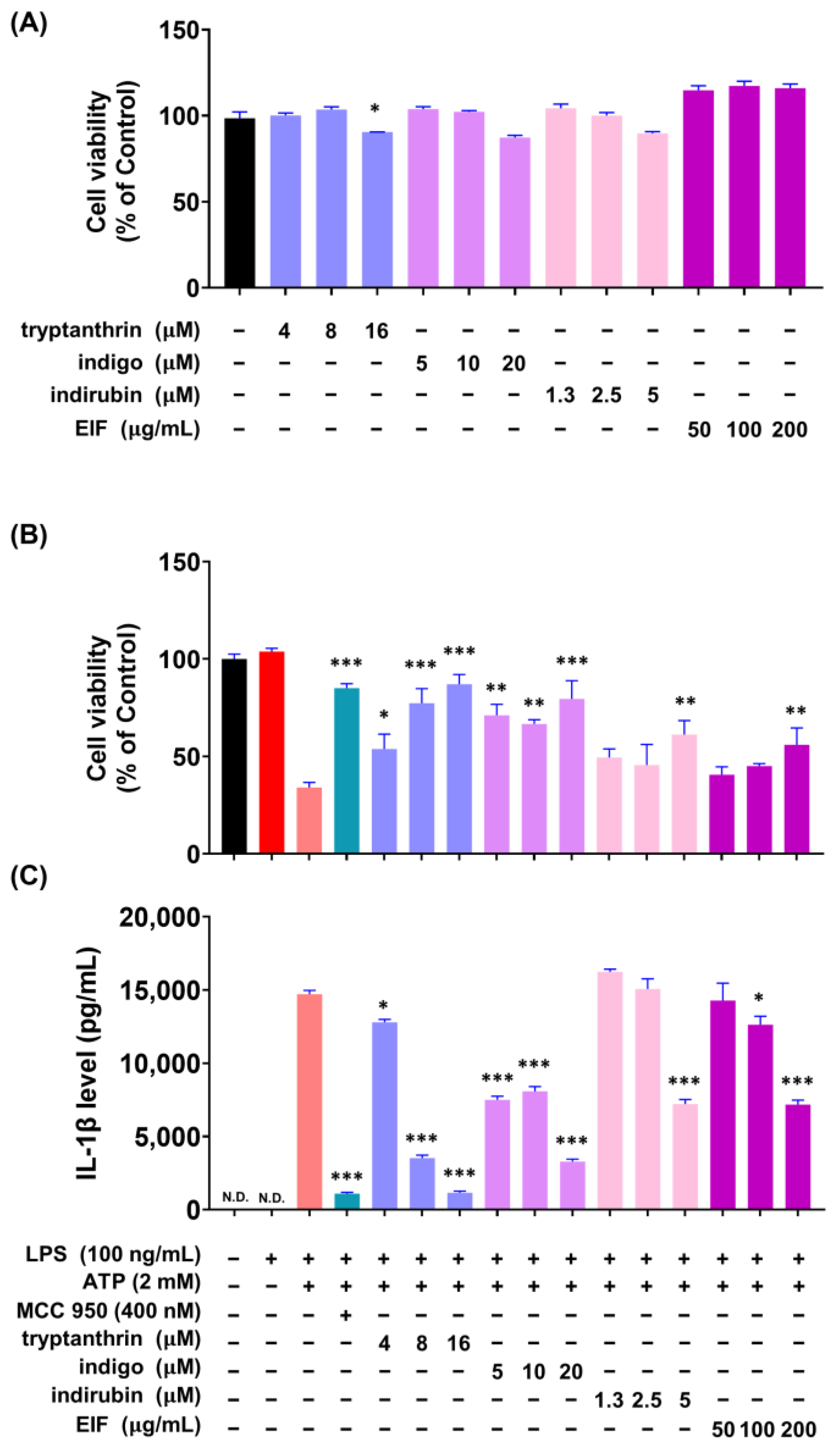
3.6. Effect of EIF Contituents on J774a.1 and BMDM Cells Induced by LPS/ATP
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baumgart, D.C.; Sandborn, W.J. Crohn’s disease. Lancet 2012, 380, 1590–1605. [Google Scholar] [CrossRef]
- Podolsky, D.K. Inflammatory bowel disease. N. Engl. J. Med. 2002, 347, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Abraham, C.; Medzhitov, R. Interactions between the host innate immune system and microbes in inflammatory bowel disease. Gastroenterology 2011, 140, 1729–1737. [Google Scholar] [CrossRef] [PubMed]
- Kaser, A.; Zeissig, S.; Blumberg, R.S. Inflammatory bowel disease. Annu. Rev. Immunol. 2010, 28, 573–621. [Google Scholar] [CrossRef] [PubMed]
- Sartor, R.B. Microbial influences in inflammatory bowel diseases. Gastroenterology 2008, 134, 577–594. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Lee, M.; Chang, E.B. The gut microbiome and inflammatory bowel diseases. Annu. Rev. Med. 2022, 73, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Werts, C.; Rubino, S.; Ling, A.; Girardin, S.E.; Philpott, D.J. Nod-like receptors in intestinal homeostasis, inflammation, and cancer. J. Leukoc. Biol. 2011, 90, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 2009, 27, 519–550. [Google Scholar] [CrossRef]
- Kanneganti, T.D.; Ozören, N.; Body-Malapel, M.; Amer, A.; Park, J.H.; Franchi, L.; Whitfield, J.; Barchet, W.; Colonna, M.; Vandenabeele, P.; et al. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature 2006, 440, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Villani, A.C.; Lemire, M.; Fortin, G.; Louis, E.; Silverberg, M.S.; Collette, C.; Baba, N.; Libioulle, C.; Belaiche, J.; Bitton, A.; et al. Common variants in the NLRP3 region contribute to Crohn’s disease susceptibility. Nat. Genet. 2009, 41, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.S.; Warrington, R.; Watson, W.; Kim, H.L. An introduction to immunology and immunopathology. Allergy Asthma Clin. Immunol. 2018, 14, 49. [Google Scholar] [CrossRef]
- Dzopalic, T.; Rajkovic, I.; Dragicevic, A.; Colic, M. The response of human dendritic cells to co-ligation of pattern-recognition receptors. Immunol. Res. 2012, 52, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Wang, Q.; Wang, T.; Xu, J.; Li, T.; Liu, Q.; Yao, Q.; Wang, P. Pattern recognition receptors (PRRs) in macrophages possess prognosis and immunotherapy potential for melanoma. Front. Immunol. 2021, 12, 765615. [Google Scholar] [CrossRef] [PubMed]
- Wicherska-Pawłowska, K.; Wróbel, T.; Rybka, J. Toll-like receptors (TLRs), NOD-like receptors (NLRs), and RIG-I-like receptors (RLRs) in innate Immunity. TLRs, NLRs, and RLRs ligands as immunotherapeutic agents for hematopoietic diseases. Int. J. Mol. Sci. 2021, 22, 13397. [Google Scholar] [CrossRef] [PubMed]
- Blevins, H.M.; Xu, Y.; Biby, S.; Zhang, S. The NLRP3 inflammasome pathway: A review of mechanisms and inhibitors for the treatment of inflammatory diseases. Front. Aging Neurosci. 2022, 14, 879021. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, H.; Kouadir, M.; Song, H.; Shi, F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 2019, 10, 128. [Google Scholar] [CrossRef] [PubMed]
- Zito, G.; Buscetta, M.; Cimino, M.; Dino, P.; Bucchieri, F.; Cipollina, C. Cellular models and assays to study NLRP3 inflammasome biology. Int. J. Mol. Sci. 2020, 21, 4294. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Luo, H.; Tan, D.; Zhang, S.; Zhoung, Z.; Wang, S.; Vong, C.T.; Wang, Y. A recent update on the use of Chinese medicine in the treatment of inflammatory bowel disease. Phytomedicine 2021, 92, 153709. [Google Scholar] [CrossRef] [PubMed]
- Gravina, A.G.; Pellegrino, R.; Palladino, G.; Coppola, A.; Brandimarte, G.; Tuccillo, C.; Ciardiello, F.; Romano, M.; Federico, A. Hericium erinaceus, in combination with natural flavonoid/alkaloid and B3/B8 vitamins, can improve inflammatory burden in Inflammatory bowel diseases tissue: An ex vivo study. Front. Immunol. 2023, 14, 1215329. [Google Scholar] [CrossRef]
- Gravina, A.G.; Pellegrino, R.; Auletta, S.; Palladino, G.; Brandimarte, G.; D’Onofrio, R.; Arboretto, G.; Imperio, G.; Ventura, A.; Cipullo, M.; et al. Hericium erinaceus, a medicinal fungus with a centuries-old history: Evidence in gastrointestinal diseases. World J. Gastroenterol. 2023, 29, 3048–3065. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, Z.; Gao, T.; Chen, Y.; Yang, Q.; Fu, C.; Zhu, Y.; Wang, F.; Liao, W. Isatidis radix and isatidis folium: A systematic review on ethnopharmacology, phytochemistry and pharmacology. J. Ethnopharmacol. 2022, 283, 114648. [Google Scholar] [CrossRef]
- Lotts, T.; Kabrodt, K.; Hummel, J.; Binder, D.; Schellenberg, I.; Ständer, S.; Agelopoulos, K. Isatis tinctoria L.-derived petroleum ether extract mediates anti-inflammatory effects via inhibition of interleukin-6, interleukin-33 and mast cell degranulation. Acta Derm. Venereol. 2020, 100, adv00131. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, Z.-Q.; Xiao, H. Antiviral activity of the effective monomers from folium isatidis against influenza virus in vivo. Virol. Sinica 2010, 25, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Lu, Y.; Jin, J.; Dong, L.; Xu, F.; Chen, S.; Wang, Z.; Liang, G.; Shan, X. n-Butanol extract from Folium isatidis inhibits lipopolysaccharide-induced inflammatory cytokine production in macrophages and protects mice against lipopolysaccharide-induced endotoxic shock. Drug Des. Dev. Ther. 2015, 9, 5601–5609. [Google Scholar] [CrossRef]
- Gao, D.; Cho, C.W.; Kim, C.T.; Jeong, W.S.; Kang, J.S. Evaluation of the antiwrinkle activity of enriched isatidis folium extract and an HPLC-UV method for the quality control of its cream products. Plants 2020, 9, 1586. [Google Scholar] [CrossRef]
- Min, G.Y.; Kim, T.I.; Kim, J.H.; Cho, W.K.; Yang, J.H.; Ma, J.Y. Anti-atopic effect of isatidis folium water extract in TNF-α/IFN-γ-induced HaCaT cells and DNCB-induced atopic dermatitis mouse model. Molecules 2023, 28, 3960. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Yang, H.; Kim, T.; Ha, H.; Hwang, Y.H. Identification and pharmacokinetics of bioavailable anti-resorptive phytochemicals after oral administration of Psoralea corylifolia L. Biomed. Pharmacother. 2021, 144, 112300. [Google Scholar] [CrossRef]
- Liau, B.C.; Jong, T.T.; Lee, M.R.; Chen, S.S. LC-APCI-MS method for detection and analysis of tryptanthrin, indigo, and indirubin in daqingye and banlangen. J. Pharm. Biomed. Anal. 2007, 43, 346–351. [Google Scholar] [CrossRef]
- Chen, J.K.; Chen, T.T.; Crampton, L. Chinese Medical Herbology and Pharmacology; Art of Medicine Press: City of Industry, CA, USA, 2004. [Google Scholar]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zheng, J.; Sun, G.; Yang, H.; Sun, X.; Yao, X.; Lin, A.; Liu, H. 5-Aminosalicylic acid ameliorates dextran sulfate sodium-induced colitis in mice by modulating gut microbiota and bile acid metabolism. Cell. Mol. Life Sci. 2022, 79, 460. [Google Scholar] [CrossRef] [PubMed]
- Rousseaux, C.; Lefebvre, B.; Dubuquoy, L.; Lefebvre, P.; Romano, O.; Auwerx, J.; Metzger, D.; Wahli, W.; Desvergne, B.; Naccari, G.C.; et al. Intestinal antiinflammatory effect of 5-aminosalicylic acid is dependent on peroxisome proliferator–activated receptor-γ. J. Exp. Med. 2005, 201, 1205–1215. [Google Scholar] [CrossRef]
- Ding, P.; Liu, J.; Li, Q.; Lu, Q.; Li, J.; Shi, R.; Shi, L.; Mao, T.; Ge, D.; Niu, H.; et al. Investigation of the active ingredients and mechanism of Hudi enteric-coated capsules in DSS-induced ulcerative colitis mice based on network pharmacology and experimental verification. Drug Des. Devel. Ther. 2021, 15, 4259–4273. [Google Scholar] [CrossRef] [PubMed]
- Lan, D.; Niu, J.; Miao, J.; Dong, X.; Wang, H.; Yang, G.; Wang, K.; Miao, Y. Expression of guanylate cyclase-C, guanylin and uroguanylin is downregulated proportionally to the ulcerative colitis disease activity index. Sci. Rep. 2016, 6, 25034. [Google Scholar] [CrossRef]
- Xie, J.; Liu, Y.; Chen, B.; Zhang, G.; Ou, S.; Luo, J.; Peng, X. Ganoderma lucidum polysaccharide improves rat DSS-induced colitis by altering cecal microbiota and gene expression of colonic epithelial cells. Food Nutr. Res. 2019, 63, 1559. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Chung, Y.C.; Lee, A.; Ryuk, J.A.; Hwang, Y.H. Isodorsmanin A prevents inflammatory response in LPS-stimulated macrophages by inhibiting the JNK and NF-κB signaling pathways. Curr. Issues Mol. Biol. 2023, 45, 1601–1612. [Google Scholar] [CrossRef] [PubMed]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Immunol. 2014, 104, 15.25.11–15.25.14. [Google Scholar] [CrossRef] [PubMed]
- Okayasu, I.; Hatakeyama, S.; Yamada, M.; Ohkusa, T.; Inagaki, Y.; Nakaya, R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology 1990, 98, 694–702. [Google Scholar] [CrossRef]
- DeRoche, T.C.; Xiao, S.Y.; Liu, X. Histological evaluation in ulcerative colitis. Gastroenterol. Rep. 2014, 2, 178–192. [Google Scholar] [CrossRef]
- Johansson, M.E.; Sjövall, H.; Hansson, G.C. The gastrointestinal mucus system in health and disease. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 352–361. [Google Scholar] [CrossRef]
- Thoo, L.; Noti, M.; Krebs, P. Keep calm: The intestinal barrier at the interface of peace and war. Cell Death Dis. 2019, 10, 849. [Google Scholar] [CrossRef]
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.-K.; Rajendiran, T.M.; Soni, T.; Aring, L.; Muraleedharan, K.M.; Garcia-Hernandez, V.; Kamada, N.; Samuelson, L.C.; Nusrat, A.; Iwase, S.; et al. The manganese transporter SLC39A8 links alkaline ceramidase 1 to inflammatory bowel disease. Nat Commun. 2024, 15, 4775. [Google Scholar] [CrossRef]
- Zhang, Z.; Xue, Z.; Yang, H.; Zhao, F.; Liu, C.; Chen, J.; Lu, S.; Zou, Z.; Zhou, Y.; Zhang, X. Differential effects of EPA and DHA on DSS-induced colitis in mice and possible mechanisms involved. Food Funct. 2021, 12, 1803. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal. Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, C.; Zhang, W.; Wang, Y.; Qian, P.; Huang, H. Inflammation and aging: Signaling pathways and intervention therapies. Signal. Transduct. Target. Ther. 2023, 8, 239. [Google Scholar] [CrossRef]
- Swanson, K.V.; Deng, M.; Ting, J.P.Y. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Yang, H.; Wang, H.; Qin, S.; Zhan, X.; Li, H.; Wei, Z.; Fang, Z.; Li, Q.; Liu, T.; et al. Tryptanthrin suppresses multiple inflammasome activation to regulate NASH progression by targeting ASC protein. Phytomedicine 2024, 131, 155758. [Google Scholar] [CrossRef]
- Liu, N.; Zhang, G.X.; Niu, Y.T.; Wang, Q.; Zheng, J.; Yang, J.M.; Sun, T.; Niu, J.G.; Yu, J.Q. Anti-inflammatory and analgesic activities of indigo through regulating the IKKβ/IκB/NF-κB pathway in mice. Food Funct. 2020, 11, 8537–8546. [Google Scholar] [CrossRef]
- Qi, T.; Li, H.; Li, S. Indirubin improves antioxidant and anti-inflammatory functions in lipopolysaccharide-challenged mice. Oncotarget 2017, 8, 36658–36663. [Google Scholar] [CrossRef] [PubMed]
- Shim, K.S.; Gu, D.R.; Hwang, Y.H.; Yang, H.; Ryuk, J.A.; Ha, H. Water extract of Fritillariae thunbergii Bulbus inhibits RANKL-mediated osteoclastogenesis and ovariectomy-induced trabecular bone loss. Molecules 2021, 27, 169. [Google Scholar] [CrossRef] [PubMed]


| Gene Name | Assay ID | NCBI Reference Sequence |
|---|---|---|
| NOD-like receptor protein 3 (NLRP3) | Mm00840904_m1 | NG_007509.2 |
| Caspase-1 Apoptosis-associated speck-like protein (ASC) | Mm00438023_m1 Mm00445747_g1 | NM_001043585.1 NG_029446.1 |
| β-actin | Mm00607939_s1 | NM_007393.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, Y.C.; Lee, A.; Jang, C.H.; Ryuk, J.A.; Ha, H.; Hwang, Y.-H. Isatidis Folium Represses Dextran Sulfate Sodium-Induced Colitis and Suppresses the Inflammatory Response by Inhibiting Inflammasome Activation. Nutrients 2024, 16, 3323. https://doi.org/10.3390/nu16193323
Chung YC, Lee A, Jang CH, Ryuk JA, Ha H, Hwang Y-H. Isatidis Folium Represses Dextran Sulfate Sodium-Induced Colitis and Suppresses the Inflammatory Response by Inhibiting Inflammasome Activation. Nutrients. 2024; 16(19):3323. https://doi.org/10.3390/nu16193323
Chicago/Turabian StyleChung, You Chul, Ami Lee, Chan Ho Jang, Jin Ah Ryuk, Hyunil Ha, and Youn-Hwan Hwang. 2024. "Isatidis Folium Represses Dextran Sulfate Sodium-Induced Colitis and Suppresses the Inflammatory Response by Inhibiting Inflammasome Activation" Nutrients 16, no. 19: 3323. https://doi.org/10.3390/nu16193323







