Equol Nonproducing Status as an Independent Risk Factor for Acute Cardioembolic Stroke and Poor Functional Outcome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. HSs
2.3. Patients with Stroke
2.4. Evaluation via TTE
2.5. Evaluation of Cerebral Small Vessel Disease
2.6. Serum Equol Concentration
2.7. Statistical Analysis
3. Results
3.1. Baseline Characteristics of HS and Patients with Ischemic Stroke
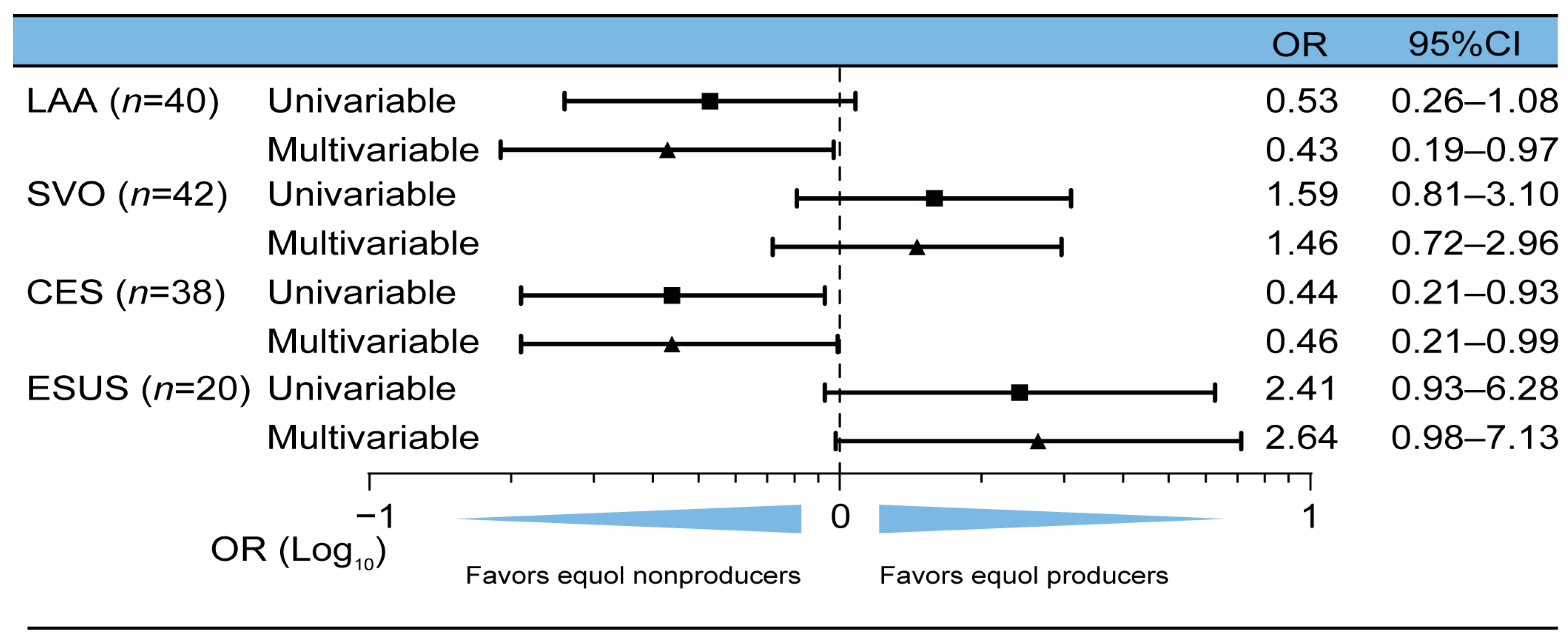
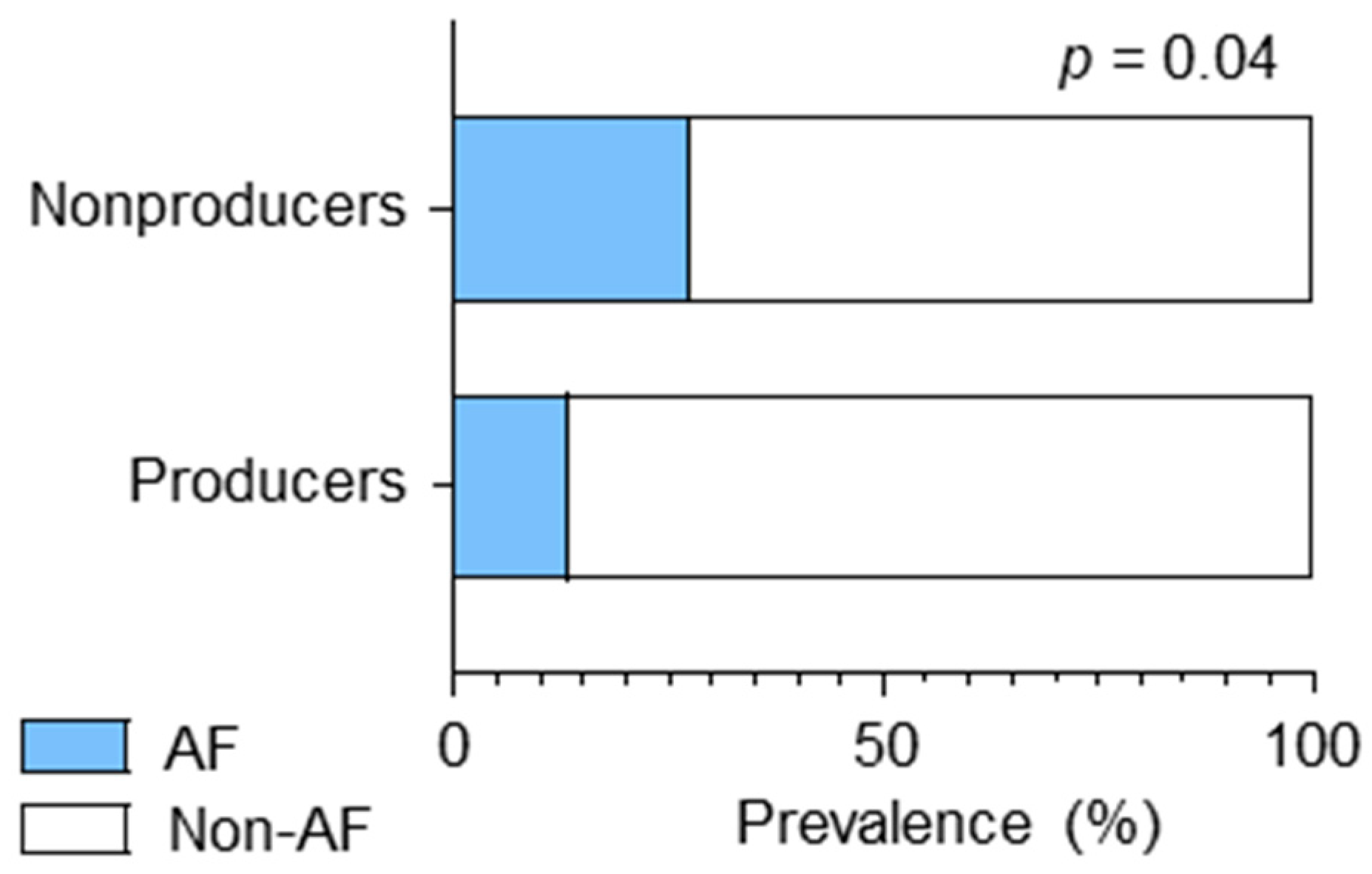
3.2. CES and AF Were Associated with Equol Nonproducing Status
3.3. Equol-Producing Status as an Independent Predictor of Favorable Functional Outcomes
3.4. More Severe SVD in Equol Nonproducers
3.5. No Association between Equol-Producing Status and the Development of ICH
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Křížová, L.; Dadáková, K.; Kašparovská, J.; Kašparovský, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, G.G.J.M.; Lemmen, J.G.; Carlsson, B.; Corton, J.C.; Safe, S.H.; Van Der Saag, P.T.; Van Der Burg, B.; Gustafsson, J.Å. Interaction of Estrogenic Chemicals and Phytoestrogens with Estrogen Receptor β. Endocrinology 1998, 139, 4252–4263. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, G.G.J.M.; Carlsson, B.; Grandien, K.; Enmark, E.; Häggblad, J.; Nilsson, S.; Gustafsson, J.Å. Comparison of the Ligand Binding Specificity and Transcript Tissue Distribution of Estrogen Receptors and α and β. Endocrinology 1997, 138, 863–870. [Google Scholar] [CrossRef]
- Jia, M.; Dahlman-Wright, K.; Gustafsson, J.Å. Estrogen Receptor Alpha and Beta in Health and Disease. Best. Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 557–568. [Google Scholar] [CrossRef]
- Anthony, M.S.; Clarkson, T.B.; Bullock, B.C.; Wagner, J.D. Soy Protein versus Soy Phytoestrogens in the Prevention of Diet-Induced Coronary Artery Atherosclerosis of Male Cynomolgus Monkeys. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 2524–2531. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Larrea, M.B.; Mohan, A.R.; Paganga, G.; Miller, N.J.; Bolwell, G.P.; Rice-Evans, C.A. Antioxidant Activity of Phytoestrogenic Isoflavones. Free. Radic. Res. 1997, 26, 63–70. [Google Scholar] [CrossRef]
- Hirohata, M.; Ono, K.; Takasaki, J.-i.; Takahashi, R.; Ikeda, T.; Morinaga, A.; Yamada, M. Anti-Amyloidogenic Effects of Soybean Isoflavones in Vitro: Fluorescence Spectroscopy Demonstrating Direct Binding to Aβ Monomers, Oligomers and Fibrils. Biochim. Biophys. Acta.—Mol. Basis. Dis. 2012, 1822, 1316–1324. [Google Scholar] [CrossRef]
- Kokubo, Y.; Iso, H.; Ishihara, J.; Okada, K.; Inoue, M.; Tsugane, S. Association of Dietary Intake of Soy, Beans, and Isoflavones with Risk of Cerebral and Myocardial Infarctions in Japanese Populations: The Japan Public Health Center-Based (JPHC) Study Cohort I. Circulation 2007, 116, 2553–2562. [Google Scholar] [CrossRef]
- Zhang, X.; Shu, X.O.; Gao, Y.-T.; Yang, G.; Li, Q.; Li, H.; Jin, F.; Zheng, W. Soy Food Consumption Is Associated with Lower Risk of Coronary Heart Disease in Chinese Women. J. Nutr. 2003, 133, 2874–2878. [Google Scholar] [CrossRef]
- Hodis, H.N.; MacK, W.J.; Kono, N.; Azen, S.P.; Shoupe, D.; Hwang-Levine, J.; Petitti, D.; Whitfield-Maxwell, L.; Yan, M.; Franke, A.A.; et al. Isoflavone Soy Protein Supplementation and Atherosclerosis Progression in Healthy Postmenopausal Women: A Randomized Controlled Trial. Stroke 2011, 42, 3168–3175. [Google Scholar] [CrossRef]
- Mayo, B.; Vázquez, L.; Flórez, A.B. Equol: A Bacterial Metabolite from the Daidzein. Nutrients 2019, 11, 2231. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.R.; Cole, S.J. Method of Defining Equol-Producer Status and Its Frequency among Vegetarians. J. Nutr. 2006, 136, 2188–2193. [Google Scholar] [CrossRef] [PubMed]
- Kurzer, M.S.; Xu, X. Definition of Phytoestrogens. Annu. Rev. Nutr. 1997, 17, 353–381. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, C.; Newton, K.M.; Aiello Bowles, E.J.; Yong, M.; Lampe, J.W. Demographic, Anthropometric, and Lifestyle Factors and Dietary Intakes in Relation to Daidzein-Metabolizing Phenotypes among Premenopausal Women in the United States. Am. J. Clin. Nutr. 2008, 87, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Sekikawa, A.; Ihara, M.; Lopez, O.; Kakuta, C.; Lopresti, B.; Higashiyama, A.; Aizenstein, H.; Chang, Y.-F.; Mathis, C.; Miyamoto, Y.; et al. Effect of S-Equol and Soy Isoflavones on Heart and Brain. Curr. Cardiol. Rev. 2018, 15, 114–135. [Google Scholar] [CrossRef] [PubMed]
- Muthyala, R.S.; Ju, Y.H.; Sheng, S.; Williams, L.D.; Doerge, D.R.; Katzenellenbogen, B.S.; Helferich, W.G.; Katzenellenbogen, J.A. Equol, a Natural Estrogenic Metabolite from Soy Isoflavones: Convenient Preparation and Resolution of R- and S-Equols and Their Differing Binding and Biological Activity through Estrogen Receptors Alpha and Beta. Bioorganic. Med. Chem. 2004, 12, 1559–1567. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, Y.T.; Yang, G.; Li, H.; Cai, Q.; Xiang, Y.B.; Ji, B.T.; Franke, A.A.; Zheng, W.; Shu, X.O. Urinary Isoflavonoids and Risk of Coronary Heart Disease. Int. J. Epidemiol. 2012, 41, 1367–1375. [Google Scholar] [CrossRef]
- Ahuja, V.; Miura, K.; Vishnu, A.; Fujiyoshi, A.; Evans, R.; Zaid, M.; Miyagawa, N.; Hisamatsu, T.; Kadota, A.; Okamura, T.; et al. Significant Inverse Association of Equol-Producer Status with Coronary Artery Calcification but Not Dietary Isoflavones in Healthy Japanese Men. Br. J. Nutr. 2017, 117, 260–266. [Google Scholar] [CrossRef]
- Yu, W.; Wang, Y.; Zhou, D.-X.; Zhao, L.-M.; Li, G.-R.; Deng, X.-L. Equol Is Neuroprotective During Focal Cerebral Ischemia and Reperfusion That Involves P-Src and Gp91phox. Curr. Neurovasc. Res. 2014, 11, 367–377. [Google Scholar] [CrossRef]
- Yulin, M.; Sullivan, J.C.; Schreihofer, D.A. Dietary Genistein and Equol (4′, 7 Isoflavandiol) Reduce Oxidative Stress and Protect Rats against Focal Cerebral Ischemia. Am. J. Physiol.—Regulntegr. Comp. Physiol. 2010, 299, 871–877. [Google Scholar] [CrossRef]
- Kim, J.H.; Lim, C.; Cho, S. Ischemic-Time Associated Reductions in Equol Monosulfate Plasma Levels in a Mouse Model of Ischemic Stroke: Support the Existence of a ‘Brain–Gut Axis’. Neuroreport 2021, 32, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Sekikawa, A.; Higashiyama, A.; Lopresti, B.J.; Ihara, M.; Aizenstein, H.; Watanabe, M.; Chang, Y.; Kakuta, C.; Yu, Z.; Mathis, C.; et al. Associations of Equol-Producing Status with White Matter Lesion and Amyloid-β Deposition in Cognitively Normal Elderly Japanese. Alzheimers. Dement. 2020, 6, e12089. [Google Scholar] [CrossRef] [PubMed]
- Hammond, T.C.; Powell, E.; Green, S.J.; Chlipala, G.; Frank, J.; Yackzan, A.T.; Yanckello, L.M.; Chang, Y.H.; Xing, X.; Heil, S.; et al. Functional Recovery Outcomes Following Acute Stroke Is Associated with Abundance of Gut Microbiota Related to Inflammation, Butyrate and Secondary Bile Acid. Front. Rehabil. Sci. 2022, 3, 1017180. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, Y.; Okamura, T.; Yoshimasa, Y.; Miyamoto, Y.; Kawanishi, K.; Kotani, Y.; Okayama, A.; Tomoike, H. Impact of Metabolic Syndrome Components on the Incidence of Cardiovascular Disease in a General Urban Japanese Population: The Suita Study. Hypertens. Res. 2008, 31, 2027–2035. [Google Scholar] [CrossRef]
- Disorders, I.C.; Material, B.; Evaluation, V.; Neurophysiologic, B.; Cardiovascular, C.; Imaging, D.B.; Vascular, E.; Flow, F.C.B.; Disorders, I.C.; Material, B.; et al. Special Report from the National Institute of Neurological Disorders and Stroke. Classification of Cerebrovascular Diseases III. Stroke 1990, 21, 637–676. [Google Scholar] [CrossRef]
- Védrine, N.; Mathey, J.; Morand, C.; Brandolini, M.; Davicco, M.J.; Guy, L.; Rémésy, C.; Coxam, V.; Manach, C. One-Month Exposure to Soy Isoflavones Did Not Induce the Ability to Produce Equol in Postmenopausal Women. Eur. J. Clin. Nutr. 2006, 60, 1039–1045. [Google Scholar] [CrossRef]
- Setchell, K.D.R.; Brown, N.M.; Summer, S.; King, E.C.; Heubi, J.E.; Cole, S.; Guy, T.; Hokin, B. Dietary Factors Influence Production of the Soy Isoflavone Metabolite S-(-)Equolin Healthy Adults. J. Nutr. 2013, 143, 1950–1958. [Google Scholar] [CrossRef]
- Lip, G.Y.H.; Nieuwlaat, R.; Pisters, R.; Lane, D.A.; Crijns, H.J.G.M. Refining Clinical Risk Stratification for Predicting Stroke and Thromboembolism in Atrial Fibrillation Using a Novel Risk Factor-Based Approach: The Euro Heart Survey on Atrial Fibrillation. Chest 2010, 137, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Wardlaw, J.M.; Smith, E.E.; Biessels, G.J.; Cordonnier, C.; Fazekas, F.; Frayne, R.; Lindley, R.I.; O’Brien, J.T.; Barkhof, F.; Benavente, O.R.; et al. Neuroimaging Standards for Research into Small Vessel Disease and Its Contribution to Ageing and Neurodegeneration. Lancet. Neurol. 2013, 12, 822–838. [Google Scholar] [CrossRef]
- Fazekas, F.; Niederkorn, K.; Schmidt, R.; Offenbacer, H.; Homer, S.; Bertha, G.; Lechner, H. White Matter Signal Abnormalities in Normal Individuals: Correlation with Carotid Ultrasonography, Cerebral Blood Flow Measurements, and Cerebrovascular Risk Factors. Stroke 1988, 19, 1285–1288. [Google Scholar] [CrossRef]
- Hosoki, S.; Saito, S.; Tonomura, S.; Ishiyama, H.; Yoshimoto, T.; Ikeda, S.; Ikenouchi, H.; Yamamoto, Y.; Hattori, Y.; Miwa, K.; et al. Oral Carriage of Streptococcus Mutans Harboring the Cnm Gene Relates to an Increased Incidence of Cerebral Microbleeds. Stroke 2020, 51, 3632–3639. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, Y.-K.; Kim, N.K.; Kim, S.-H.; Kim, O.-J.; Oh, S.-H. Circulating Matrix Metalloproteinase-9 Level Is Associated with Cerebral White Matter Hyperintensities in Non-Stroke Individuals. Eur. Neurol. 2014, 72, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Amin Al Olama, A.; Wason, J.M.S.; Tuladhar, A.M.; Van Leijsen, E.M.C.; Koini, M.; Hofer, E.; Morris, R.G.; Schmidt, R.; De Leeuw, F.E.; Markus, H.S. Simple MRI Score Aids Prediction of Dementia in Cerebral Small Vessel Disease. Neurology 2020, 94, e1294–e1302. [Google Scholar] [CrossRef] [PubMed]
- Arai, Y.; Uehara, M.; Sato, Y.; Kimira, M.; Eboshida, A.; Adlercreutz, H.; Watanabe, S. Comparison of Isoflavones among Dietary Intake, Plasma Concentration and Urinary Excretion for Accurate Estimation of Phytoestrogen Intake. J. Epidemiol. 2000, 10, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Akaza, H.; Miyanaga, N.; Takashima, N.; Naito, S.; Hirao, Y.; Tsukamoto, T.; Mori, M. Is Daidzein Non-Metabolizer a High Risk for Prostate Cancer? A Case-Controlled Study of Serum Soybean Isoflavone Concentration. Jpn. J. Clin. Oncol. 2002, 32, 296–300. [Google Scholar] [CrossRef]
- Morton, M.S.; Arisaka, O.; Miyake, N.; Morgan, L.D.; Evans, B.A.J. Phytoestrogen Concentrations in Serum from Japanese Men and Women over Forty Years of Age. J. Nutr. 2002, 132, 3168–3171. [Google Scholar] [CrossRef]
- Miyanaga, N.; Akaza, H.; Takashima, N.; Nagata, Y.; Sonoda, T.; Mori, M.; Naito, S.; Hirao, Y.; Tsukamoto, T.; Fujioka, T. Higher Consumption of Green Tea May Enhance Equol Production. Asian. Pacific. J. Cancer. Prev. 2003, 4, 297–301. [Google Scholar]
- Ko, K.P.; Kim, C.S.; Ahn, Y.; Park, S.J.; Kim, Y.J.; Park, J.K.; Lim, Y.K.; Yoo, K.Y.; Kim, S.S. Plasma Isoflavone Concentration Is Associated with Decreased Risk of Type 2 Diabetes in Korean Women but Not Men: Results from the Korean Genome and Epidemiology Study. Diabetologia 2015, 58, 726–735. [Google Scholar] [CrossRef]
- Sulter, G.; Steen, C.; De Keyser, J. Use of the Barthel Index and Modified Rankin Scale in Acute Stroke Trials. Stroke 1999, 30, 1538–1541. [Google Scholar] [CrossRef]
- Jordan, K.; Yaghi, S.; Poppas, A.; Chang, A.D.; Grory, B.M.; Cutting, S.; Burton, T.; Jayaraman, M.; Tsivgoulis, G.; Sabeh, M.K.; et al. Left Atrial Volume Index Is Associated with Cardioembolic Stroke and Atrial Fibrillation Detection after Embolic Stroke of Undetermined Source. Stroke 2019, 50, 1997–2001. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Lin, L.-C.; Lin, M.-S.; Lai, L.-P.; Hwang, J.-J.; Tseng, Y.-Z.; Tseng, C.-D.; Lin, J.-L. Identification of Good Responders to Rhythm Control of Paroxysmal and Persistent Atrial Fibrillation by Transthoracic and Transesophageal Echocardiography. Cardiology 2005, 104, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Toufan, M.; Kazemi, B.; Molazadeh, N. The Significance of the Left Atrial Volume Index in Prediction of Atrial Fibrillation Recurrence after Electrical Cardioversion. J. Cardiovasc. Thorac. Res. 2017, 9, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.L.; Wang, Y.; Xiao, G.S. Effects of Equol on Multiple K+ Channels Stably Expressed in HEK 293 Cells. PLoS ONE 2017, 12, e0183708. [Google Scholar] [CrossRef] [PubMed]
- Fedida, D.; Wible, B.; Wang, Z.; Fermini, B.; Faust, F.; Nattel, S.; Brown, A.M. Identity of a Novel Delayed Rectifier Current from Human Heart with a Cloned K+ Channel Current. Circ. Res. 1993, 73, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Remillard, C.V.; Tigno, D.D.; Platoshyn, O.; Burg, E.D.; Brevnova, E.E.; Conger, D.; Nicholson, A.; Rana, B.K.; Channick, R.N.; Rubin, L.J.; et al. Function of Kv1.5 Channels and Genetic Variations of KCNA5 in Patients with Idiopathic Pulmonary Arterial Hypertension. Am. J. Physiol. Cell. Physiol. 2022, 292, 1837–1853. [Google Scholar] [CrossRef] [PubMed]
- Borrego, J.; Feher, A.; Jost, N.; Panyi, G.; Varga, Z.; Papp, F. Peptide Inhibitors of Kv1.5: An Option for the Treatment of Atrial Fibrillation. Pharmaceuticals 2021, 13, 1303. [Google Scholar] [CrossRef]
- Li, G.-R.; Sun, H.; Zhang, X.-H.; Cheng, L.-C.; Chiu, S.-W.; Tse, H.-F.; Lau, C.-P. Omega-3 Polyunsaturated Fatty Acids Inhibit Transient Outward and Ultra-Rapid Delayed Rectifier K 1 Currents and Na 1 Current in Human Atrial Myocytes. Cardiovasc. Res. 2009, 81, 286–293. [Google Scholar] [CrossRef]
- Li, G.-R.; Wang, H.-B.; Qin, G.-W.; Jin, M.-W.; Tang, Q.; Sun, H.-Y.; Du, X.-L.; Deng, X.-L. Acacetin, a Natural Flavone, Selectively Inhibits Human Atrial Repolarization Potassium Currents and Prevents Atrial Fibrillation in Dogs. Stroke 2008, 117, 2449–2457. [Google Scholar] [CrossRef]
- Grammer, J.B.; Bosch, R.F.; Kühlkamp, V.; Seipel, L. Molecular Remodeling of Kv4.3 Potassium Channels in Human Atrial Fibrillation. J. Cardiovasc. Electrophysiol. 2000, 11, 626–633. [Google Scholar] [CrossRef]
- Bartos, D.C.; Anderson, J.B.; Bastiaenen, R.; Johnson, J.N.; Gollob, M.H.; Tester, D.J.; Burgess, D.E.; Homfray, T.; Behr, E.R.; Ackerman, M.J.; et al. A KCNQ1 Mutation Causes a High Penetrance for Familial Atrial Fibrillation. J. Cardiovasc. Electrophysiol. 2013, 24, 562–569. [Google Scholar] [CrossRef]
- Yao, J.; Ma, Y.T.; Xie, X.; Liu, F.; Chen, B.D. Association of KCNE1 Genetic Polymorphisms with Atrial Fibrillation in a Chinese Han Population. Genet. Test. Mol. Biomarkers. 2012, 16, 1343–1346. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Rahim, A.H.; Perez, A.C.; MacIsaac, R.L.; Jhund, P.S.; Claggett, B.L.; Carson, P.E.; Komajda, M.; McKelvie, R.S.; Zile, M.R.; Swedberg, K.; et al. Risk of Stroke in Chronic Heart Failure Patients with Preserved Ejection Fraction, but without Atrial Fibrillation: Analysis of the CHARM-Preserved and I-Preserve Trials. Eur. Heart. J. 2017, 38, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Kim, J.S. Classification/Ischemic Stroke Subtype Classification: An Asian Viewpoint-Small Vessel Disease. J. Stroke 2014, 16, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.R.; Clerici, C. Equol: Pharmacokinetics and Biological Actions. J. Nutr. 2010, 140, 1363–1368. [Google Scholar] [CrossRef] [PubMed]
- Grześk, G.; Rogowicz, D.; Wołowiec, Ł.; Ratajczak, A.; Gilewski, W.; Chudzińska, M.; Sinkiewicz, A.; Banach, J. The Clinical Significance of Drug–Food Interactions of Direct Oral Anticoagulants. Int. J. Mol. Sci. 2021, 22, 8531. [Google Scholar] [CrossRef]
- Cichon, N.; Saluk-bijak, J.; Gorniak, L.; Przyslo, L. Flavonoids as a Natural Enhancer of Neuroplasticity—An Overview of the Mechanism of Neurorestorative Action. Antioxidants 2020, 9, 1035. [Google Scholar] [CrossRef]
- Zhang, X.; Veliky, C.V.; Birru, R.L.; Barinas-Mitchell, E.; Magnani, J.W.; Sekikawa, A. Potential Protective Effects of Equol (Soy Isoflavone Metabolite) on Coronary Heart Diseases—From Molecular Mechanisms to Studies in Humans. Nutrients 2021, 13, 3739. [Google Scholar] [CrossRef]
- Kobayashi, A.; Iguchi, M.; Shimizu, S.; Uchiyama, S. Silent Cerebral Infarcts and Cerebral White Matter Lesions in Patients with Nonvalvular Atrial Fibrillation. J. Stroke. Cerebrovasc. Dis. 2012, 21, 310–317. [Google Scholar] [CrossRef]
- Shimizu, A.; Sakurai, T.; Mitsui, T.; Miyagi, M.; Nomoto, K.; Kokubo, M.; Bando, Y.K.; Murohara, T.; Toba, K. Left Ventricular Diastolic Dysfunction Is Associated with Cerebral White Matter Lesions (Leukoaraiosis) in Elderly Patients without Ischemic Heart Disease and Stroke. Geriatr. Gerontol. Int. 2014, 14, 71–76. [Google Scholar] [CrossRef]
- Athilingam, P.; D’Aoust, R.F.; Miller, L.; Chen, L. Cognitive Profile in Persons With Systolic and Diastolic Heart Failure. Congest. Heart. Fail. 2013, 19, 44–50. [Google Scholar] [CrossRef]
- Papanastasiou, C.A.; Theochari, C.A.; Zareifopoulos, N.; Arfaras-Melainis, A.; Giannakoulas, G.; Karamitsos, T.D.; Palaiodimos, L.; Ntaios, G.; Avgerinos, K.I.; Kapogiannis, D.; et al. Atrial Fibrillation Is Associated with Cognitive Impairment, All-Cause Dementia, Vascular Dementia, and Alzheimer’s Disease: A Systematic Review and Meta-Analysis. J. Gen. Intern. Med. 2021, 36, 3122–3135. [Google Scholar] [CrossRef] [PubMed]
- Igase, M.; Igase, K.; Tabara, Y.; Ohyagi, Y.; Kohara, K. Cross-Sectional Study of Equol Producer Status and Cognitive Impairment in Older Adults. Geriatr. Gerontol. Int. 2017, 17, 2103–2108. [Google Scholar] [CrossRef] [PubMed]
- Rost, N.S.; Rahman, R.M.; Biffi, A.; Smith, E.E.; Kanakis, M.A.; Fitzpatrick, K.; Lima, F.; Worrall, B.B.; Meschia, J.F.; Brown, R.D.; et al. White Matter Hyperintensity Volume Is Increased in Small Vessel Stroke Subtypes. Neurology 2010, 75, 1670–1677. [Google Scholar] [CrossRef] [PubMed]
- Wardlaw, J.M.; Allerhand, M.; Doubal, F.N.; Valdes Hernandez, M.; Morris, Z.; Gow, A.J.; Bastin, M.; Starr, J.M.; Dennis, M.S.; Deary, I.J. Vascular Risk Factors, Large-Artery Atheroma, and Brain White Matter Hyperintensities. Neurology 2014, 82, 1331–1338. [Google Scholar] [CrossRef]
- Ntaios, G.; Baumgartner, H.; Doehner, W.; Donal, E.; Edvardsen, T.; Healey, J.S.; Iung, B.; Kamel, H.; Kasner, S.E.; Korompoki, E.; et al. Embolic Strokes of Undetermined Source: A Clinical Consensus Statement of the ESC Council on Stroke, the European Association of Cardiovascular Imaging and the European Heart Rhythm Association of the ESC. Eur. Heart. J. 2024, 45, 1701–1715. [Google Scholar] [CrossRef]
- Akaza, H.; Miyanaga, N.; Takashima, N.; Naito, S.; Hirao, Y.; Tsukamoto, T.; Fujioka, T.; Mori, M.; Kim, W.J.; Song, J.M.; et al. Comparisons of Percent Equol Producers between Prostate Vancer Patients and Controls: Case-Controlled Studies of Isoflavones in Japanese, Korean and American Residents. Jpn. J. Clin. Oncol. 2004, 34, 86–89. [Google Scholar] [CrossRef]
- Frankenfeld, C.L.; Atkinson, C.; Thomas, W.K.; Gonzalez, A.; Jokela, T.; Wähälä, K.; Schwartz, S.M.; Li, S.S.; Lampe, J.W. High Concordance of Daidzein-Metabolizing Phenotypes in Individuals Measured 1 to 3 Years Apart. Br. J. Nutr. 2005, 94, 873–876. [Google Scholar] [CrossRef]

| Healthy Subjects | Patients with Ischemic Stroke | p Value | |
|---|---|---|---|
| n = 103 | n = 140 | ||
| Age (years) | 81.3 ± 3.3 | 72.2 ± 12.1 | <0.01 |
| Male sex, n (%) | 52 (50.5) | 102 (72.9) | <0.01 |
| BMI (kg/m2) | 22.5 ± 3.1 | 23.1 ± 3.8 | 0.31 |
| Medical history | |||
| Hypertension, n (%) | 56 (54.4) | 110 (78.6) | <0.01 |
| Dyslipidemia, n (%) | 58 (56.3) | 77 (55) | 0.84 |
| Diabetes mellitus, n (%) | 13 (12.6) | 29 (20.7) | 0.1 |
| Coronary artery disease, n (%) | 0 | 8 (5.7) | – |
| Atrial fibrillation, n (%) | 3 (2.9) | 30 (21.4) | <0.01 |
| Laboratory data | |||
| WBC count (×103/μL) | 5.6 ± 1.4 | 6.9 ± 2.1 | <0.01 |
| Platelet count (×103/μL) | 203.2 ± 53.2 | 214.7 ± 62.3 | 0.24 |
| Albumin level (g/dL) | 4.1 ± 0.3 | 4.0 ± 0.5 | 0.09 |
| ALT level (U/L) | 16.3 ± 9.2 | 19.8 ± 11.5 | <0.01 |
| AST level (U/L) | 23.2 ± 7.0 | 24.1 ± 7.5 | 0.22 |
| Creatinine level (mg/dL) | 0.8 ± 0.2 | 1.0 ± 1.8 | <0.01 |
| HDL-C level (mg/dL) | 58.1 ± 14.0 | 52.1 ± 14.9 | <0.01 |
| LDL-C level (mg/dL) | 120.0 ± 23.5 | 117.3 ± 38.4 | 0.33 |
| TG level (mg/dL) | 105.1 ± 56.9 | 128.9 ± 73.7 | 0.01 |
| Patients with SVO n = 42 | Patients with LAA n = 40 | Patients with CES n = 38 | Patients with ESUS n = 20 | |
|---|---|---|---|---|
| Age (years), mean ± SD | 70.9 ± 11.5 | 68.3 ± 12.0 | 78.1 ± 9.0 | 71.6 ± 15.1 |
| Male sex, n (%) | 34 (81.0) | 33 (82.5) | 22 (57.9) | 13 (65.0) |
| BMI (kg/m2) | 23.5 ± 3.7 | 23.4 ± 3.2 | 22.3 ± 4.6 | 23.4 ± 3.8 |
| Medical history | ||||
| Hypertension, n (%) | 31 (73.8) | 36 (90.0) | 27 (71.1) | 16 (80.0) |
| Dyslipidemia, n (%) | 22 (52.4) | 28 (70.0) | 14 (36.8) | 13 (65.0) |
| Diabetes mellitus, n (%) | 7 (16.7) | 14 (35.0) | 5 (13.2) | 3 (15.0) |
| CAD, n (%) | 1 (2.4) | 2 (5.0) | 4 (10.5) | 1 (5.0) |
| CHA2DS2-VASc score, median [IQR] | 2.5 [2–4] | 2.5 [2–4] | 3.5 [3–5] | 3.5 [2.5–4] |
| LDL-C-lowering drugs, n (%) | 29 (69.0) | 38 (95.0) | 18 (47.4) | 16 (80.0) |
| Anticoagulants, n (%) | 3 (7.1) | 4 (10.0) | 36 (94.7) | 2 (10.0) |
| NIHSS score upon admission, mean ± SD | 2.0 ± 2.4 | 3.7 ± 4.4 | 11.7 ± 9.4 | 3.8 ± 5.2 |
| Premorbid mRS score, mean ± SD | 0.5 ± 1.0 | 0.2 ± 0.6 | 1.0 ± 1.6 | 0.5 ± 1.3 |
| Equol producers, n (%) | 23 (54.8) | 13 (32.5) | 11 (29.0) | 13 (65.0) |
| Equol Nonproducers | Equol Producers | p Value | |
|---|---|---|---|
| LVEF < 50% | 5/50 (10.0%) | 5/38 (13.2%) | 0.74 |
| LVDd (mm) | 46.4 ± 5.2 | 47.8 ± 5.5 | 0.53 |
| LVDs (mm) | 30.9 ± 5.0 | 31.8 ± 6.2 | 0.62 |
| LAD (mm) | 39.5 ± 6.4 | 37.5 ± 6.5 | 0.13 |
| LAVI (mL/m2) | 46.3 ± 23.8 | 36.0 ± 11.6 | 0.06 |
| Equol Nonproducers | Equol Producers | p | |
|---|---|---|---|
| PVH (grade 3), n (%) | 13/74 (17.6) | 7/58 (12.1) | 0.38 |
| DSWMH (grade ≥ 2), n (%) | 47/74 (63.5) | 27/58 (46.6) | 0.05 |
| Lacunes ≥ 1, n (%) | 39/74 (52.7) | 26/58 (44.8) | 0.37 |
| CMBs ≥ 1, n (%) | 27/77 (35.1) | 20/60 (33.3) | 0.83 |
| Total SVD severity ≥ 2, n (%) | 42/73 (57.5) | 23/58 (39.7) | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noda, K.; Hattori, Y.; Murata, H.; Kokubo, Y.; Higashiyama, A.; Ihara, M. Equol Nonproducing Status as an Independent Risk Factor for Acute Cardioembolic Stroke and Poor Functional Outcome. Nutrients 2024, 16, 3377. https://doi.org/10.3390/nu16193377
Noda K, Hattori Y, Murata H, Kokubo Y, Higashiyama A, Ihara M. Equol Nonproducing Status as an Independent Risk Factor for Acute Cardioembolic Stroke and Poor Functional Outcome. Nutrients. 2024; 16(19):3377. https://doi.org/10.3390/nu16193377
Chicago/Turabian StyleNoda, Kotaro, Yorito Hattori, Hiroaki Murata, Yoshihiro Kokubo, Aya Higashiyama, and Masafumi Ihara. 2024. "Equol Nonproducing Status as an Independent Risk Factor for Acute Cardioembolic Stroke and Poor Functional Outcome" Nutrients 16, no. 19: 3377. https://doi.org/10.3390/nu16193377






