Vitamin D Levels in the United States: Temporal Trends (2011–2018) and Contemporary Associations with Sociodemographic Characteristics (2017–2018)
Abstract
1. Introduction
2. Materials and Methods
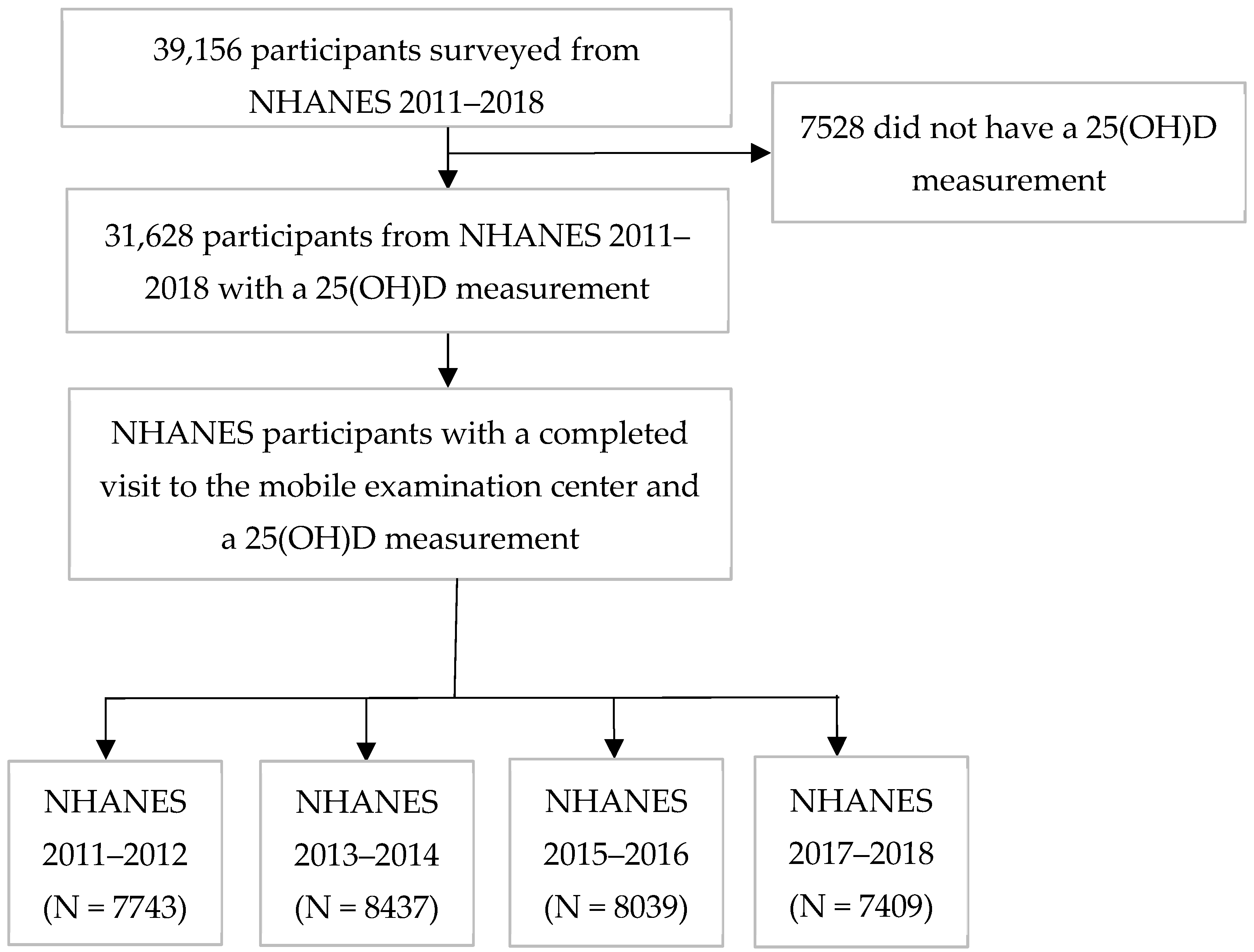
2.1. Study Design
2.2. Laboratory Methods
2.3. 25(OH)D Categories
2.4. Statistical Analysis
3. Results
3.1. Temporal Trends in 25(OH)D in 2011–2018 NHANES
3.2. Factors Associated with 25(OH)D Levels in 2017–2018: Univariate and Bivariate Analyses
3.2.1. Race/Ethnicity
3.2.2. Age
3.2.3. Gender
3.2.4. BMI
3.2.5. Education
3.2.6. Other Factors
3.3. Factors Associated with 25(OH)D Levels in 2017–2018: Multivariate Analyses
3.4. Predictors of 25(OH)D
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef]
- Hossein-nezhad, A.; Holick, M.F. Vitamin D for health: A global perspective. Mayo Clin. Proc. 2013, 88, 720–755. [Google Scholar] [CrossRef]
- Tsiaras, W.G.; Weinstock, M.A. Factors influencing vitamin D status. Acta Derm. Venereol. 2011, 91, 115–124. [Google Scholar] [CrossRef]
- Holick, M.F. Environmental factors that influence the cutaneous production of vitamin D. Am. J. Clin. Nutr. 1995, 61, 638s–645s. [Google Scholar] [CrossRef]
- Kimlin, M.G.; Olds, W.J.; Moore, M.R. Location and vitamin D synthesis: Is the hypothesis validated by geophysical data? J. Photochem. Photobiol. B 2007, 86, 234–239. [Google Scholar] [CrossRef]
- Jukic, A.M.Z.; Harmon, Q.E. Accumulating evidence for vitamin D and conception. Fertil. Steril. 2020, 113, 330–331. [Google Scholar] [CrossRef]
- Jukic, A.M.Z.; Baird, D.D.; Weinberg, C.R.; Wilcox, A.J.; McConnaughey, D.R.; Steiner, A.Z. Pre-conception 25-hydroxyvitamin D (25(OH)D) and fecundability. Hum. Reprod. 2019, 34, 2163–2172. [Google Scholar] [CrossRef]
- Lips, P.; van Schoor, N.M. The effect of vitamin D on bone and osteoporosis. Best. Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 585–591. [Google Scholar] [CrossRef]
- Rosen, C.J.; Adams, J.S.; Bikle, D.D.; Black, D.M.; Demay, M.B.; Manson, J.E.; Murad, M.H.; Kovacs, C.S. The Nonskeletal Effects of Vitamin D: An Endocrine Society Scientific Statement. Endocr. Rev. 2012, 33, 456–492. [Google Scholar] [CrossRef]
- Prietl, B.; Treiber, G.; Pieber, T.R.; Amrein, K. Vitamin D and immune function. Nutrients 2013, 5, 2502–2521. [Google Scholar] [CrossRef]
- Cui, A.; Zhang, T.; Xiao, P.; Fan, Z.; Wang, H.; Zhuang, Y. Global and regional prevalence of vitamin D deficiency in population-based studies from 2000 to 2022: A pooled analysis of 7.9 million participants. Front. Nutr. 2023, 10, 1070808. [Google Scholar] [CrossRef]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef]
- Schleicher, R.L.; Sternberg, M.R.; Lacher, D.A.; Sempos, C.T.; Looker, A.C.; Durazo-Arvizu, R.A.; Yetley, E.A.; Chaudhary-Webb, M.; Maw, K.L.; Pfeiffer, C.M.; et al. The vitamin D status of the US population from 1988 to 2010 using standardized serum concentrations of 25-hydroxyvitamin D shows recent modest increases1, 2, 2. Am. J. Clin. Nutr. 2016, 104, 454–461. [Google Scholar] [CrossRef]
- Schleicher, R.L.; Sternberg, M.R.; Looker, A.C.; Yetley, E.A.; Lacher, D.A.; Sempos, C.T.; Taylor, C.L.; Durazo-Arvizu, R.A.; Maw, K.L.; Chaudhary-Webb, M.; et al. National Estimates of Serum Total 25-Hydroxyvitamin D and Metabolite Concentrations Measured by Liquid Chromatography–Tandem Mass Spectrometry in the US Population during 2007–2010. J. Nutr. 2016, 146, 1051–1061. [Google Scholar] [CrossRef]
- Herrick, K.A.; Storandt, R.J.; Afful, J.; Pfeiffer, C.M.; Schleicher, R.L.; Gahche, J.J.; Potischman, N. Vitamin D status in the United States, 2011–2014. Am. J. Clin. Nutr. 2019, 110, 150–157. [Google Scholar] [CrossRef]
- Cui, A.; Xiao, P.; Ma, Y.; Fan, Z.; Zhou, F.; Zheng, J.; Zhang, L. Prevalence, trend, and predictor analyses of vitamin D deficiency in the US population, 2001–2018. Front. Nutr. 2022, 9, 965376. [Google Scholar] [CrossRef]
- CDC/National Center for Health Statistics. About the National Health and Nutrition Examination Survey. Available online: https://www.cdc.gov/nchs/nhanes/about_nhanes.htm (accessed on 5 April 2024).
- Johnson, C.L.; Dohrmann, S.M.; Burt, V.L.; Mohadjer, L.K. National Health and Nutrition Examination Survey: Sample design, 2011–2014. Vital Health Stat. 2 2014, 162, 1–33. [Google Scholar]
- Chen, T.C.; Clark, J.; Riddles, M.K.; Mohadjer, L.K.; Fakhouri, T.H.I. National Health and Nutrition Examination Survey, 2015–2018: Sample Design and Estimation Procedures. Vital Health Stat. 2 2020, 184, 1–35. [Google Scholar]
- CDC/National Center for Health Statistics. NHANES 2011–2012 Overview. Available online: https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/overview.aspx?BeginYear=2011 (accessed on 26 September 2024).
- CDC/National Center for Health Statistics. NHANES 2013–2014 Overview. Available online: https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?BeginYear=2013 (accessed on 26 September 2024).
- CDC/National Center for Health Statistics. NHANES 2015–2016 Overview. Available online: https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/overview.aspx?BeginYear=2015 (accessed on 26 September 2024).
- CDC/National Center for Health Statistics. NHANES 2017–2018 Overview. Available online: https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/overview.aspx?BeginYear=2017 (accessed on 26 September 2024).
- NHANES. DEMOGRAPHICS INFORMATION—DMQ—SP. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/2017-2018/questionnaires/DMQ_J.pdf (accessed on 26 September 2024).
- National Nutrition Health and Examination Survey (NHANES). MEC In-Person Dietary Interviewers Procedures Manual. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/2017-2018/manuals/2017_MEC_In-Person_Dietary_Interviewers_Manual.pdf (accessed on 26 September 2024).
- National Health and Nutrition Examination Survey (NHANES). Phone Follow-Up Dietary Procedures. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/2017-2018/manuals/NHANES-2018-PhoneFollowUpDietaryManual-508.pdf (accessed on 26 September 2024).
- CDC/National Center for Health Statistics. NHANES Dietary Analyses. Available online: https://wwwn.cdc.gov/nchs/nhanes/tutorials/dietaryanalyses.aspx (accessed on 26 September 2024).
- CDC. Laboratory Procedure Manual. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/2017-2018/labmethods/VID-J-MET-508.pdf (accessed on 26 September 2024).
- Jukic, A.M.Z.; Wilcox, A.J.; McConnaughey, D.R.; Weinberg, C.R.; Steiner, A.Z. 25-Hydroxyvitamin D and Long Menstrual Cycles in a Prospective Cohort Study. Epidemiology 2018, 29, 388–396. [Google Scholar] [CrossRef]
- Pal, L.; Zhang, H.; Williams, J.; Santoro, N.F.; Diamond, M.P.; Schlaff, W.D.; Coutifaris, C.; Carson, S.A.; Steinkampf, M.P.; Carr, B.R.; et al. Vitamin D Status Relates to Reproductive Outcome in Women With Polycystic Ovary Syndrome: Secondary Analysis of a Multicenter Randomized Controlled Trial. J. Clin. Endocrinol. Metab. 2016, 101, 3027–3035. [Google Scholar] [CrossRef]
- Lerchbaum, E.; Obermayer-Pietsch, B. Vitamin D and fertility: A systematic review. Eur. J. Endocrinol. 2012, 166, 765–778. [Google Scholar] [CrossRef]
- Luk, J.; Torrealday, S.; Neal Perry, G.; Pal, L. Relevance of vitamin D in reproduction. Hum. Reprod. 2012, 27, 3015–3027. [Google Scholar] [CrossRef]
- Ganguly, A.; Tamblyn, J.A.; Finn-Sell, S.; Chan, S.Y.; Westwood, M.; Gupta, J.; Kilby, M.D.; Gross, S.R.; Hewison, M. Vitamin D, the placenta and early pregnancy: Effects on trophoblast function. J. Endocrinol. 2018, 236, R93–R103. [Google Scholar] [CrossRef]
- Lumme, J.; Morin-Papunen, L.; Pesonen, P.; Sebert, S.; Hyppönen, E.; Järvelin, M.R.; Herzig, K.H.; Ojaniemi, M.; Niinimäki, M. Vitamin D Status in Women with a History of Infertility and Decreased Fecundability: A Population-Based Study. Nutrients 2023, 15, 2522. [Google Scholar] [CrossRef]
- Jukic, A.M.Z.; Upson, K.; Harmon, Q.E.; Baird, D.D. Increasing serum 25-hydroxyvitamin D is associated with reduced odds of long menstrual cycles in a cross-sectional study of African American women. Fertil. Steril. 2016, 106, 172–179.e172. [Google Scholar] [CrossRef]
- Tamblyn, J.A.; Pilarski, N.S.P.; Markland, A.D.; Marson, E.J.; Devall, A.; Hewison, M.; Morris, R.K.; Coomarasamy, A. Vitamin D and miscarriage: A systematic review and meta-analysis. Fertil. Steril. 2022, 118, 111–122. [Google Scholar] [CrossRef]
- Wang, T.Y.; Wang, H.W.; Jiang, M.Y. Prevalence of vitamin D deficiency and associated risk of all-cause and cause-specific mortality among middle-aged and older adults in the United States. Front. Nutr. 2023, 10, 1163737. [Google Scholar] [CrossRef]
- Schleicher, R.L.; Sternberg, M.R.; Potischman, N.; Gahche, J.J.; Storandt, R.J.; Maw, K.L.; Pfeiffer, C.M. Supplemental Vitamin D Increased Serum Total 25-Hydroxyvitamin D in the US Adult Population During 2007–2014. J. Nutr. 2021, 151, 2446–2454. [Google Scholar] [CrossRef]
- Park, C.Y.; Han, S.N. The Role of Vitamin D in Adipose Tissue Biology: Adipocyte Differentiation, Energy Metabolism, and Inflammation. J. Lipid Atheroscler. 2021, 10, 130–144. [Google Scholar] [CrossRef]
- Abbas, M.A. Physiological functions of Vitamin D in adipose tissue. J. Steroid Biochem. Mol. Biol. 2017, 165, 369–381. [Google Scholar] [CrossRef]
- Webb, A.R.; Kline, L.; Holick, M.F. Influence of season and latitude on the cutaneous synthesis of vitamin D3: Exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J. Clin. Endocrinol. Metab. 1988, 67, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Tjellesen, L.; Christiansen, C. Vitamin D metabolites in normal subjects during one year. A longitudinal study. Scand. J. Clin. Lab. Investig. 1983, 43, 85–89. [Google Scholar] [CrossRef]
- Clemens, T.L.; Adams, J.S.; Henderson, S.L.; Holick, M.F. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet 1982, 1, 74–76. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Chen, T.C.; Lu, Z.; Sauter, E. Vitamin D and skin physiology: A D-lightful story. J. Bone Min. Res. 2007, 22 (Suppl. S2), V28–V33. [Google Scholar] [CrossRef]
- Matsuoka, L.Y.; Wortsman, J.; Haddad, J.G.; Kolm, P.; Hollis, B.W. Racial pigmentation and the cutaneous synthesis of vitamin D. Arch. Dermatol. 1991, 127, 536–538. [Google Scholar] [CrossRef]
- Jones, G.; Kaufmann, M. Vitamin D metabolite profiling using liquid chromatography-tandem mass spectrometry (LC-MS/MS). J. Steroid Biochem. Mol. Biol. 2016, 164, 110–114. [Google Scholar] [CrossRef]
| Weighted Median (25th and 75th Percentiles) | ||||||||
|---|---|---|---|---|---|---|---|---|
| 2011–2012 | 2013–2014 | 2015–2016 | 2017–2018 | |||||
| 25(OH)D (nmol/L) | 67.9 (51.1, 85.0) | 66.5 (51.3, 83.0) | 67.8 (51.8, 85.4) | 68.7 (52.3, 87.8) | ||||
| Weighted Prevalence (%) | ||||||||
| N 2 | % | N 2 | % | N 2 | % | N 2 | % | |
| <30 | 553 | 4.9 | 573 | 5.1 | 488 | 4.4 | 550 | 4.7 |
| 30-<50 | 1902 | 18.5 | 1806 | 18.1 | 1977 | 18.1 | 1540 | 16.6 |
| 50-<75 | 3067 | 37.6 | 3558 | 40.7 | 3339 | 39.1 | 2886 | 38.5 |
| 75-<125 | 2030 | 34.7 | 2297 | 32.6 | 2032 | 33.8 | 2149 | 35.1 |
| ≥125 | 191 | 4.3 | 203 | 3.5 | 203 | 4.6 | 284 | 5.0 |
| 25(OH)D (nmol/L) 2 | |||||||
|---|---|---|---|---|---|---|---|
| N 3 | <30 (%) | 30-<50 (%) | 50-<75 (%) | 75-<125 (%) | ≥125 (%) | Weighted Median (25th and 75th Percentiles) | |
| Race/Ethnicity | |||||||
| Mexican American | 1118 | 8 | 29 | 48 | 14 | 1 | 55.5 (43.1, 68.3) |
| Hispanic non-Mexican | 675 | 4 | 22 | 49 | 23 | 2 | 62.8 (49.2, 74.6) |
| Non-Hispanic White | 2503 | 1 | 10 | 36 | 45 | 7 | 76.1 (61.1, 94.3) |
| Non-Hispanic Black | 1678 | 21 | 32 | 29 | 15 | 2 | 47.7 (32.4, 67.0) |
| Non-Hispanic Asian | 935 | 8 | 24 | 40 | 24 | 4 | 60.0 (45.8, 78.6) |
| Another Race | 500 | 5 | 15 | 48 | 28 | 3 | 65.7 (51.9, 79.1) |
| Age (years) | |||||||
| 0–4 | 471 | 2 | 7 | 37 | 52 | 2 | 76.1 (64.0, 88.0) |
| 5–11 | 940 | 1 | 13 | 54 | 32 | 0 | 67.5 (56.7, 79.4) |
| 12–19 | 1015 | 7 | 23 | 49 | 20 | 2 | 60.9 (46.8, 73.2) |
| 20–39 | 1490 | 7 | 24 | 43 | 23 | 2 | 59.8 (45.7, 75.1) |
| 40–59 | 1576 | 5 | 15 | 37 | 38 | 5 | 70.7 (54.3, 88.2) |
| ≥60 | 1917 | 2 | 10 | 25 | 50 | 13 | 87.0 (63.8, 107.0) |
| Gender | |||||||
| Male | 3610 | 4 | 17 | 42 | 32 | 4 | 67.0 (51.5, 83.1) |
| Female | 3799 | 5 | 16 | 35 | 37 | 7 | 70.7 (53.0, 92.0) |
| BMI (kg/m2) 4 | |||||||
| <18.5 | 1079 | 2 | 13 | 46 | 38 | 1 | 70.3 (57.7, 83.6) |
| 18.5–24.9 | 1988 | 4 | 15 | 41 | 34 | 6 | 69.4 (54.6, 89.0) |
| 25.0–29.9 | 1832 | 5 | 16 | 36 | 38 | 6 | 71.1 (54.5, 89.8) |
| ≥30.0 | 2283 | 7 | 20 | 37 | 31 | 5 | 64.3 (48.1, 85.8) |
| Annual household income (USD) | |||||||
| 0–14,999 | 758 | 8 | 24 | 38 | 28 | 3 | 62.8 (44.9, 82.0) |
| 15,000–34,999 | 1646 | 5 | 21 | 38 | 33 | 4 | 65.9 (49.3, 85.5) |
| 35,000–64,999 | 1671 | 6 | 17 | 38 | 34 | 4 | 67.4 (50.7, 84.9) |
| 65,000 and over | 2345 | 3 | 17 | 40 | 34 | 7 | 68.4 (54.1, 88.7) |
| Educational attainment 5 | |||||||
| <High school | 1352 | 8 | 25 | 40 | 26 | 2 | 60.0 (45.1, 77.6) |
| High school grad/ GED or some college/ AA degree | 4045 | 5 | 17 | 39 | 34 | 5 | 67.8 (51.7, 86.3) |
| ≥College graduate | 1665 | 3 | 12 | 36 | 42 | 8 | 74.4 (58.7, 93.9) |
| Season | |||||||
| Winter months 6 | 3551 | 7 | 22 | 39 | 28 | 4 | 63.5 (47.6, 81.2) |
| Summer months 7 | 3858 | 3 | 12 | 38 | 40 | 6 | 72.9 (57.8, 91.8) |
| Supplement Use 8 | |||||||
| No | 4361 | 7 | 23 | 44 | 25 | 1 | 61.4 (46.6, 75.8) |
| Yes | 2252 | 1 | 7 | 29 | 51 | 11 | 83.6 (67.0, 104.0) |
| Sunscreen Use | |||||||
| Never | 1216 | 13 | 25 | 38 | 23 | 2 | 57.8 (39.2, 74.6) |
| Rarely | 564 | 4 | 18 | 48 | 29 | 2 | 66.7 (52.4, 79.6) |
| Sometimes | 634 | 4 | 20 | 40 | 32 | 3 | 66.5 (50.3, 84.5) |
| Most of the time | 356 | 2 | 14 | 36 | 40 | 8 | 74.3 (56.6, 91.0) |
| Always | 284 | 3 | 16 | 40 | 36 | 6 | 68.7 (54.9, 88.6) |
| Smoking Status 9 | |||||||
| Current | 910 | 7 | 21 | 42 | 27 | 3 | 62.6 (46.9, 78.4) |
| Former | 1212 | 3 | 13 | 37 | 40 | 8 | 73.5 (57.8, 93.3) |
| Never | 3103 | 5 | 17 | 34 | 37 | 7 | 69.7 (51.6, 91.9) |
| Alcohol Use 9 | |||||||
| Yes | 3340 | 5 | 16 | 36 | 37 | 6 | 69.9 (52.4, 89.7) |
| No | 1012 | 3 | 17 | 33 | 37 | 9 | 72.7 (54.0, 95.2) |
| Diabetes | |||||||
| Yes | 814 | 4 | 15 | 30 | 43 | 7 | 75.1 (54.8, 95.3) |
| No | 6591 | 5 | 17 | 39 | 34 | 5 | 68.2 (52.0, 87.0) |
| Kidney Disease 10 | |||||||
| Yes | 207 | 4 | 10 | 20 | 53 | 14 | 89.3 (63.4, 108.3) |
| No | 4768 | 5 | 17 | 36 | 36 | 6 | 69.4 (51.8, 89.7) |
| 25(OH)D (nmol/L) | ||||||
|---|---|---|---|---|---|---|
| Weighted Median (25th and 75th Percentiles) | ||||||
| Race/Ethnicity | ||||||
| Mexican American | Hispanic non-Mexican | Non-Hispanic White | Non-Hispanic Black | Non-Hispanic Asian | Another Race | |
| Age (years) | ||||||
| 0–4 | 63.6 (55.9, 78.3) | 73.7 (64.8, 79.5) | 81.2 (71.5, 91.6) | 61.3 (48.2, 76.1) | 70.1 (56.3, 82.3) | 74.3 (63.3, 103.0) |
| 5–11 | 61.3 (51.9, 70.1) | 65.1 (55.7, 71.2) | 75.7 (67.1, 88.1) | 54.1 (42.8, 76.1) | 58.7 (52.5, 67.1) | 67.6 (57.7, 79.7) |
| 12–19 | 52.0 (39.6, 61.4) | 55.9 (44.3, 61.7) | 69.6 (59.9, 83.1) | 43.6 (30.7, 51.7) | 49.3 (38.8, 60.3) | 61.9 (47.9, 70.2) |
| 20–39 | 49.8 (38.6, 61.6) | 56.8 (46.6, 69.4) | 68.2 (55.3, 82.2) | 39.7 (27.7, 54.7) | 50.8 (38.5, 61.4) | 60.7 (42.6, 77.7) |
| 40–59 | 56.0 (44.1, 68.5) | 68.3 (50.8, 78.8) | 76.4 (62.0, 92.7) | 48.2 (32.1, 70.6) | 66.8 (51.2, 85.3) | 69.4 (53.9, 79.6) |
| ≥60 | 71.3 (54.2, 88.6) | 73.3 (57.1, 90.7) | 91.9 (68.4, 109.0) | 70.4 (43.5, 98.5) | 84.5 (67.2, 104.0) | 73.5 (60.4, 106.0) |
| Gender | ||||||
| Male | 56.1 (44.2, 67.5) | 61.8 (47.2, 73.7) | 73.1 (60.4, 89.6) | 47.4 (33.9, 63.8) | 57.5 (43.4, 74.6) | 65.3 (51.5, 76.3) |
| Female | 54.8 (42.7, 69.0) | 63.8 (52.3, 75.0) | 79.2 (62.1, 99.0) | 48.4 (31.5, 70.8) | 62.0 (47.6, 83.6) | 66.0 (54.3, 81.4) |
| BMI (kg/m2) 2 | ||||||
| <18.5 | 62.8 (53.5, 71.1) | 64.9 (53.6, 73.8) | 76.0 (65.2, 89.9) | 52.9 (40.3, 68.6) | 58.7 (48.7, 69.0) | 73.6 (61.3, 83.1) |
| 18.5–24.9 | 58.3 (51.1, 69.9) | 63.9 (55.7, 73.2) | 76.2 (62.2, 94.6) | 47.1 (34.0, 64.6) | 64.0 (48.7, 85.3) | 63.6 (54.5, 80.9) |
| 25.0–29.9 | 56.0 (43.4, 69.0) | 65.1 (49.5, 75.3) | 79.1 (65.0, 96.9) | 49.5 (34.8, 72.0) | 58.1 (44.0, 76.7) | 71.8 (54.6, 77.2) |
| ≥30.0 | 49.7 (36.9, 62.4) | 59.1 (43.5, 75.4) | 72.8 (57.4, 93.2) | 44.5 (29.0, 66.0) | 55.3 (40.8, 76.4) | 60.7 (43.6, 78.9) |
| 25(OH)D (nmol/L) | |||||
|---|---|---|---|---|---|
| Weighted Median (25th and 75th Percentiles) | |||||
| BMI (kg/m2) 2 | |||||
| N 3 | <18.5 | 18.5–24.9 | 25.0–29.9 | ≥30.0 | |
| Age (years) 4 | |||||
| 20–39 | 1476 | 61.3 (41.4, 70.4) | 63.7 (48.6, 78.4) | 63.2 (48.7, 79.2) | 57.2 (42.8, 71.3) |
| 40–59 | 1557 | 70.8 (44.5, 97.5) | 77.2 (59.5, 98.0) | 72.7 (58.9, 88.1) | 66.7 (50.5, 82.9) |
| ≥60 | 1873 | 59.0 (40.3, 80.5) | 92.7 (69.2, 112.0) | 90.6 (68.9, 110.0) | 84.4 (60.0, 106.0) |
| Gender | |||||
| Male | 3489 | 70.9 (59.9, 83.3) | 65.5 (51.5, 81.3) | 70.6 (55.5, 86.0) | 64.0 (49.1, 82.8) |
| Female | 3693 | 69.6 (55.1, 84.1) | 72.8 (56.8, 93.1) | 76.7 (55.6, 101.0) | 65.6 (47.2, 89.6) |
| 25(OH)D (nmol/L) | ||||||
|---|---|---|---|---|---|---|
| Weighted Median (25th and 75th Percentiles) | ||||||
| Age (years) | ||||||
| 0–4 | 5–11 | 12–19 | 20–39 | 40–59 | ≥60 | |
| Gender | ||||||
| Male | 77.6 (65.3, 87.1) | 68.2 (59.1, 82.4) | 64.1 (51.4, 79.0) | 58.4 (45.5, 71.2) | 69.6 (54.5, 83.8) | 79.0 (60.4, 102.0) |
| Female | 75.4 (63.6, 88.4) | 66.7 (53.7, 78.0) | 62.2 (46.8, 79.6) | 61.7 (46.7, 78.1) | 71.5 (53.8, 91.9) | 92.2 (69.1, 110.0) |
| 25(OH)D (nmol/L) | |||||||
|---|---|---|---|---|---|---|---|
| Weighted Median (25th and 75th Percentiles) | |||||||
| Race/ Ethnicity | Overall | Mexican American | Hispanic non-Mexican | Non-Hispanic White | Non-Hispanic Black | Non-Hispanic Asian | Another Race |
| Male | |||||||
| Age (years) | |||||||
| 0–4 | 77.6 (65.3, 87.1) | 61.1 (55.0, 78.4) | 73.7 (63.1, 77.4) | 81.2 (70.6, 88.7) | 64.7 (47.3, 82.1) | 82.0 (65.3, 87.2) | 74.3 (68.9, 85.5) |
| 5–11 | 68.2 (59.1, 82.4) | 64.4 (57.3, 71.1) | 65.5 (56.1, 69.6) | 76.4 (66.1, 90.2) | 58.7 (45.4, 71.1) | 64.9 (56.5, 70.6) | 67.1 (57.3, 80.7) |
| 12–19 | 61.2 (48.8, 73.6) | 55.4 (41.8, 64.1) | 57.1 (47.9, 67.0) | 69.0 (60.1, 80.4) | 45.7 (36.1, 54.0) | 46.2 (38.8, 60.1) | 60.4 (47.9, 74.0) |
| 20–39 | 58.4 (45.5, 71.2) | 50.7 (38.1, 59.9) | 56.8 (46.2, 70.6) | 64.3 (51.5, 76.1) | 40.2 (28.3, 55.1) | 49.7 (34.7, 58.4) | 59.7 (44.5, 73.0) |
| 40–59 | 69.6 (54.5, 83.8) | 57.7 (42.7, 68.9) | 66.7 (39.7, 78.1) | 75.8 (64.5, 88.5) | 45.8 (33.1, 62.9) | 61.0 (47.3, 76.6) | 64.5 (51.5, 77.2) |
| ≥60 | 79.0 (60.4, 102.0) | 65.2 (48.4, 84.9) | 67.2 (51.8, 82.8) | 85.7 (63.5, 103.0) | 61.3 (41.2, 89.3) | 80.7 (66.7, 96.2) | 73.5 (59.7, 110.0) |
| Female | |||||||
| 0–4 | 75.4 (63.6, 88.4) | 63.6 (59.2, 77.8) | 71.4 (64.9, 82.0) | 82.0 (72.4, 93.4) | 57.2 (48.8, 73.0) | 56.3 (49.1, 70.1) | 77.4 (60.8, 108.0) |
| 5–11 | 66.7 (53.7, 78.0) | 59.1 (50.4, 68.1) | 62.4 (53.9, 73.8) | 75.2 (68.5, 86.8) | 49.5 (39.3, 61.2) | 56.3 (48.4, 62.2) | 67.9 (57.7, 77.7) |
| 12–19 | 60.0 (45.9, 73.0) | 49.6 (37.8, 59.8) | 55.9 (39.7, 59.2) | 70.7 (59.1, 84.1) | 38.5 (26.9, 48.7) | 52.7 (40.4, 62.6) | 63.6 (47.3, 68.3) |
| 20–39 | 61.7 (46.7, 78.1) | 49.3 (39.3, 64.3) | 57.8 (49.1, 69.4) | 71.9 (57.7, 87.7) | 38.9 (27.1, 54.7) | 50.9 (40.5, 64.5) | 60.7 (42.6, 79.2) |
| 40–59 | 71.5 (53.8, 91.9) | 54.8 (44.7, 64.3) | 69.4 (55.1, 78.8) | 77.0 (58.6, 98.0) | 55.5 (30.7, 75.6) | 71.9 (55.9, 93.5) | 72.4 (59.3, 89.7) |
| ≥60 | 92.2 (69.1, 110.0) | 74.3 (57.0, 90.1) | 75.4 (62.1, 99.7) | 93.9 (73.0, 112.0) | 79.1 (47.3, 103.0) | 88.2 (69.9, 112.0) | 71.8 (61.1, 106.0) |
| Fully Adjusted Model 2 N = 3772 | Mexican American 3 N = 498 | Hispanic Non-Mexican 3 N = 341 | Non- Hispanic White 3 N = 1467 | Non- Hispanic Black 3 N = 872 | Non- Hispanic Asian 3 N = 388 | Another Race 3 N = 206 | |
|---|---|---|---|---|---|---|---|
| β (95% CI) (nmol/L) | |||||||
| Age (years) | |||||||
| 20–39 | 0.0 (Ref) | 0.0 (Ref) | 0.0 (Ref) | 0.0 (Ref) | 0.0 (Ref) | 0.0 (Ref) | 0.0 (Ref) |
| 40–59 | 5.9 (2.6, 9.1) | 2.9 (−2.4, 8.1) | 3.5 (−5.0, 12.0) | 5.4 (0.6, 10.2) | 8.1 (3.6, 12.6) | 11.2 (3.6, 18.8) | 8.7 (−2.2, 19.6) |
| 60 and over | 13.0 (9.7, 16.2) | 15.9 (11.8, 20.0) | 9.1 (3.0, 15.2) | 11.9 (7.5, 16.3) | 22.1 (18.3, 25.9) | 19.0 (9.9, 28.2) | 15.3 (6.7, 24.0) |
| Gender | |||||||
| Male | 0.0 (Ref) | 0.0 (Ref) | 0.0 (Ref) | 0.0 (Ref) | 0.0 (Ref) | 0.0 (Ref) | 0.0 (Ref) |
| Female | 2.6 (−0.4, 5.6) | −0.4 (−5.8, 4.9) | 1.3 (−3.8, 6.5) | 2.8 (−1.4, 6.9) | 1.6 (−4.1, 7.2) | 6.4 (3.0, 9.7) | −0.2 (−7.4, 6.9) |
| BMI (kg/m2) 4 | |||||||
| <18.5 | −0.8 (−10.0, 8.3) | NA | −23.1 (−31.9, −14.3) | −0.8 (−13.1, 11.5) | −3.8 (−18.7, 11.1) | 8.8 (−5.9, 23.6) | 39.1 (−11.7, 89.9) |
| 18.5–24.9 | 0.0 (Ref) | 0.0 (Ref) | 0.0 (Ref) | 0.0 (Ref) | 0.0 (Ref) | 0.0 (Ref) | 0.0 (Ref) |
| 25.0–29.9 | −1.2 (−3.5, 1.1) | −3.0 (−10.4, 4.4) | −2.3 (−8.8, 4.3) | −0.8 (−3.7, 2.1) | 4.0 (−1.3, 9.3) | −3.4 (−9.5, 2.7) | −6.9 (−21.8, 8.0) |
| ≥30.0 | −5.4 (−8.6, −2.1) | −8.4 (−16.1, −0.6) | −6.7 (−12.3, −1.0) | −4.1 (−8.4, 0.2) | −3.5 (−6.5, −0.5) | −6.4 (−14.2, 1.4) | −10.7 (−23.2, 1.8) |
| Educational Attainment | |||||||
| <High school | −1.3 (−6.5, 4.0) | −1.6 (−5.8, 2.6) | −2.5 (−10.7, 5.7) | 0.2 (−7.1, 7.4) | −7.3 (−12.6, −2.0) | −3.3 (−12.5, 5.8) | 1.8 (−13.2, 16.8) |
| High school grad/GED or some college/AA degree | 0.0 (Ref) | 0.0 (Ref) | 0.0 (Ref) | 0.0 (Ref) | 0.0 (Ref) | 0.0 (Ref) | 0.0 (Ref) |
| ≥College graduate | 2.2 (−1.8, 6.3) | 6.6 (−1.0, 14.2) | 1.8 (−5.0, 8.5) | 2.7 (−3.2, 8.6) | −0.3 (−4.4, 3.7) | 4.4 (0.4, 8.4) | −7.7 (−16.0, 0.7) |
| Supplement use mcg (IU/day) | |||||||
| <15 mcg (600 IU) | 0.0 (Ref) | 0.0 (Ref) | 0.0 (Ref) | 0.0 (Ref) | 0.0 (Ref) | 0.0 (Ref) | 0.0 (Ref) |
| 16–25 (601–1000) | 11.8 (7.5, 16.1) | 21.6 (10.5, 32.8) | 20.7 (9.7, 31.7) | 8.6 (3.8, 13.4) | 17.3 (13.0, 21.5) | 17.3 (13.1, 21.6) | 23.1 (11.9, 34.3) |
| ≥26 (>1000) | 30.0 (26.7, 33.3) | 29.4 (21.9, 36.8) | 35.1 (30.2, 40.0) | 28.8 (24.8, 32.7) | 33.8 (26.1, 41.5) | 38.3 (28.9, 47.6) | 21.3 (8.6, 34.0) |
| Dietary intake mcg (IU/day) | |||||||
| <15 (600) | 0.0 (Ref) | 0.0 (Ref) | 0.0 (Ref) | 0.0 (Ref) | 0.0 (Ref) | 0.0 (Ref) | 0.0 (Ref) |
| 16–25 (601–1000) | 7.7 (3.9, 11.4) | 4.2 (−2.0, 10.4) | −5.6 (−16.4, 5.2) | 9.3 (5.3, 13.3) | 12.6 (1.7, 23.5) | 10.2 (0.1, 20.2) | −10.6 (−19.8, −1.4) |
| ≥26 (>1000) | 4.1 (−3.0, 11.2) | 17.2 (−3.4, 37.7) | 3.6 (−6.8, 14.1) | −1.5 (−13.0, 10.0) | 9.4 (−4.5, 23.3) | 8.7 (−12.2, 29.6) | 16.1 (0.7, 31.5) |
| Season | |||||||
| 1 November–30 April (Winter) | 0.0 (Ref) | 0.0 (Ref) | 0.0 (Ref) | 0.0 (Ref) | 0.0 (Ref) | 0.0 (Ref) | 0.0 (Ref) |
| 1 May–31 October (Summer) | 8.9 (5.7, 12.1) | 4.2 (−0.8, 9.3) | 6.7 (2.4, 11.0) | 10.5 (6.5, 14.4) | 5.6 (0.3, 10.9) | 2.5 (−0.9, 6.0) | 9.0 (−1.4, 19.5) |
| Smoking Status 5 | |||||||
| Current | −3.7 (−7.0, −0.4) | −2.6 (−10.3, 5.1) | −11.3 (−15.8, −6.8) | −2.6 (−6.4, 1.1) | −7.2 (−11.9, −2.5) | −1.0 (−9.1, 7.1) | −5.9 (−18.1, 6.3) |
| Former | 0.8 (−2.9, 4.6) | 3.6 (−0.6, 7.8) | 5.7 (−1.5, 13.0) | 0.7 (−4.8, 6.2) | 0.0 (−6.7, 6.7) | −3.0 (−10.1, 4.1) | −2.3 (−7.6, 3.0) |
| Never | 0.0 (Ref) | 0.0 (Ref) | 0.0 (Ref) | 0.0 (Ref) | 0.0 (Ref) | 0.0 (Ref) | 0.0 (Ref) |
| Alcohol Use 5 | |||||||
| Yes | −1.2 (−4.5, 2.1) | 1.2 (−5.0, 7.4) | 1.1 (−6.3, 8.5) | −1.2 (−6.2, 3.9) | −4.4 (−9.0, 0.1) | −4.5 (−9.7, 0.6) | 2.0 (−6.2, 10.1) |
| No | 0.0 (Ref) | 0.0 (Ref) | 0.0 (Ref) | 0.0 (Ref) | 0.0 (Ref) | 0.0 (Ref) | 0.0 (Ref) |
| Diabetes | |||||||
| Yes | −1.3 (−5.4, 2.9) | 0.7 (−6.0, 7.5) | −3.0 (−16.3, 10.3) | −3.1 (−8.8, 2.5) | 1.4 (−5.1, 7.8) | 0.6 (−5.6, 6.8) | 2.8 (−8.4, 14.1) |
| No | 0.0 (Ref) | 0.0 (Ref) | 0.0 (Ref) | 0.0 (Ref) | 0.0 (Ref) | 0.0 (Ref) | 0.0 (Ref) |
| Kidney Disease 6 | |||||||
| Yes | 6.5 (1.2, 11.7) | 4.4 (−13.3, 22.1) | 1.7 (−8.6, 12.1) | 4.0 (−2.8, 10.8) | 11.2 (2.4, 20.1) | 10.6 (−9.1, 30.4) | 30.2 (11.4, 48.9) |
| No | 0.0 (Ref) | 0.0 (Ref) | 0.0 (Ref) | 0.0 (Ref) | 0.0 (Ref) | 0.0 (Ref) | 0.0 (Ref) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subramanian, A.; Burrowes, H.B.; Rumph, J.T.; Wilkerson, J.; Jackson, C.L.; Jukic, A.M.Z. Vitamin D Levels in the United States: Temporal Trends (2011–2018) and Contemporary Associations with Sociodemographic Characteristics (2017–2018). Nutrients 2024, 16, 3414. https://doi.org/10.3390/nu16193414
Subramanian A, Burrowes HB, Rumph JT, Wilkerson J, Jackson CL, Jukic AMZ. Vitamin D Levels in the United States: Temporal Trends (2011–2018) and Contemporary Associations with Sociodemographic Characteristics (2017–2018). Nutrients. 2024; 16(19):3414. https://doi.org/10.3390/nu16193414
Chicago/Turabian StyleSubramanian, Anita, Hyacinth B. Burrowes, Jelonia T. Rumph, Jesse Wilkerson, Chandra L. Jackson, and Anne Marie Z. Jukic. 2024. "Vitamin D Levels in the United States: Temporal Trends (2011–2018) and Contemporary Associations with Sociodemographic Characteristics (2017–2018)" Nutrients 16, no. 19: 3414. https://doi.org/10.3390/nu16193414
APA StyleSubramanian, A., Burrowes, H. B., Rumph, J. T., Wilkerson, J., Jackson, C. L., & Jukic, A. M. Z. (2024). Vitamin D Levels in the United States: Temporal Trends (2011–2018) and Contemporary Associations with Sociodemographic Characteristics (2017–2018). Nutrients, 16(19), 3414. https://doi.org/10.3390/nu16193414






