The Population-Attributable Fractions of Small-for-Gestational-Age Births: Results from the Japan Birth Cohort Consortium
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Outcome Variables
2.3. Risk Factor for PAF and Covariates
2.4. Statistical Analysis
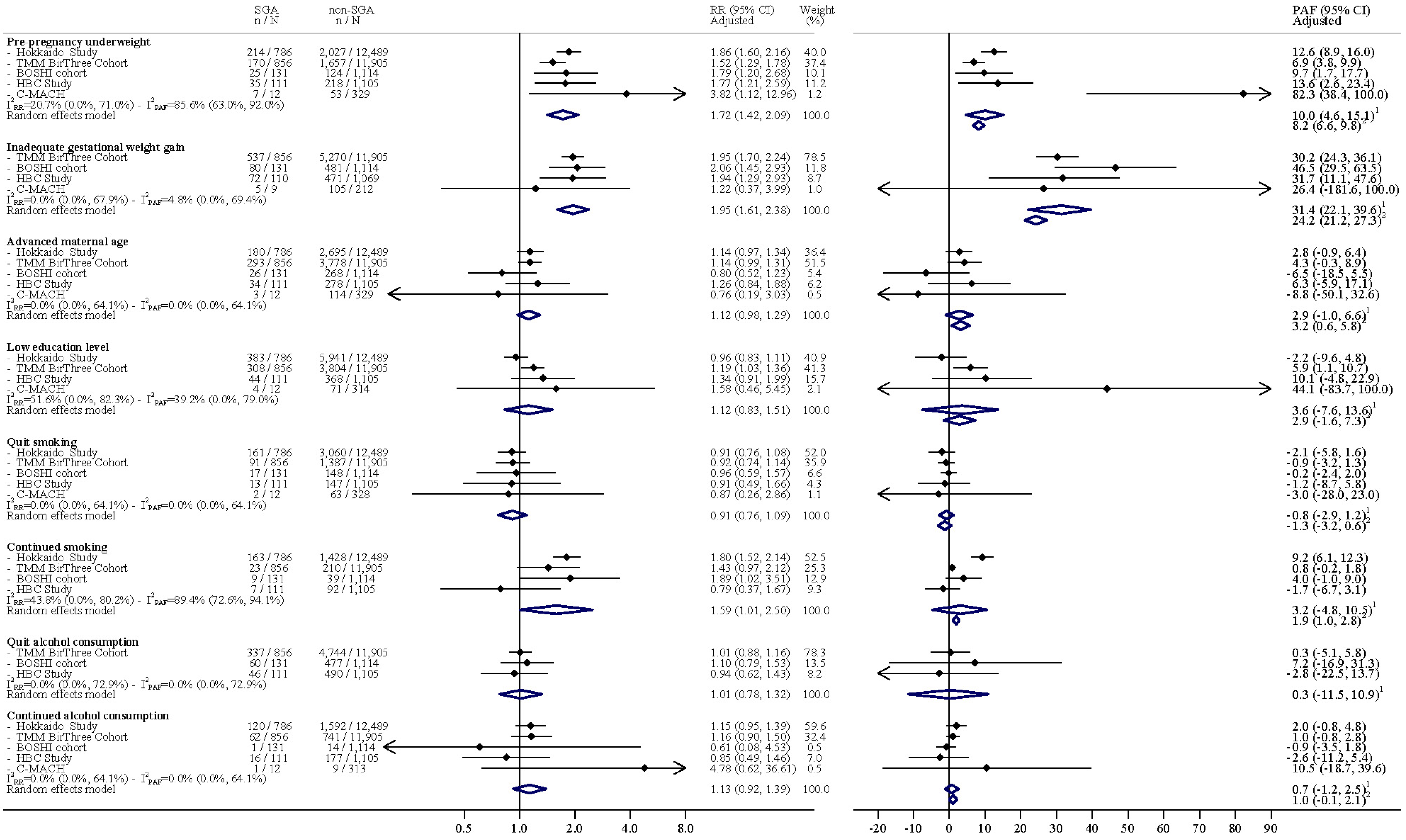
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Nutrition Targets 2025: Low Birth Weight Policy Brief: WHO, 2014. 2014. Available online: https://www.who.int/publications/i/item/WHO-NMH-NHD-14.5 (accessed on 7 December 2023).
- Levine, T.A.; Grunau, R.E.; McAuliffe, F.M.; Pinnamaneni, R.; Foran, A.; Alderdice, F.A. Early childhood neurodevelopment after intrauterine growth restriction: A systematic review. Pediatrics 2015, 135, 126–141. [Google Scholar] [CrossRef] [PubMed]
- Murray, E.; Fernandes, M.; Fazel, M.; Kennedy, S.H.; Villar, J.; Stein, A. Differential effect of intrauterine growth restriction on childhood neurodevelopment: A systematic review. BJOG Int. J. Obstet. Gynaecol. 2015, 122, 1062–1072. [Google Scholar] [CrossRef] [PubMed]
- Longo, S.; Bollani, L.; Decembrino, L.; Di Comite, A.; Angelini, M.; Stronati, M. Short-term and long-term sequelae in intrauterine growth retardation (IUGR). J. Matern. Fetal Neonatal Med. 2013, 26, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, P.; Bo, T.; Luo, K. Cognitive and Behavioral Outcomes of Intrauterine Growth Restriction School-Age Children. Pediatrics 2016, 137, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J. Maternal nutrition, fetal nutrition, and disease in later life. Nutrition 1997, 13, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Petrou, S.; Sach, T.; Davidson, L. The long-term costs of preterm birth and low birth weight: Results of a systematic review. Child Care Health Dev. 2001, 27, 97–115. [Google Scholar] [CrossRef] [PubMed]
- McCowan, L.; Horgan, R.P. Risk factors for small for gestational age infants. Best Pract. Res. Clin. Obstet. Gynaecol. 2009, 23, 779–793. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, R.F.; Abell, S.K.; Ranasinha, S.; Misso, M.; Boyle, J.A.; Black, M.H.; Li, N.; Hu, G.; Corrado, F.; Rode, L.; et al. Association of Gestational Weight Gain With Maternal and Infant Outcomes: A Systematic Review and Meta-analysis. JAMA 2017, 317, 2207–2225. [Google Scholar] [CrossRef]
- Blumenshine, P.; Egerter, S.; Barclay, C.J.; Cubbin, C.; Braveman, P.A. Socioeconomic disparities in adverse birth outcomes: A systematic review. Am. J. Prev. Med. 2010, 39, 263–272. [Google Scholar] [CrossRef]
- Ruiz, M.; Goldblatt, P.; Morrison, J.; Kukla, L.; Švancara, J.; Riitta-Järvelin, M.; Taanila, A.; Saurel-Cubizolles, M.J.; Lioret, S.; Bakoula, C.; et al. Mother’s education and the risk of preterm and small for gestational age birth: A DRIVERS meta-analysis of 12 European cohorts. J. Epidemiol. Community Health 2015, 69, 826–833. [Google Scholar] [CrossRef]
- Gurung, S.; Tong, H.H.; Bryce, E.; Katz, J.; Lee, A.C.; Black, R.E.; Walker, N. A systematic review on estimating population attributable fraction for risk factors for small-for-gestational-age births in 81 low- and middle-income countries. J. Glob. Health 2022, 12, 04024. [Google Scholar] [CrossRef] [PubMed]
- Taylor, L.K.; Lee, Y.Y.; Lim, K.; Simpson, J.M.; Roberts, C.L.; Morris, J. Potential prevention of small for gestational age in Australia: A population-based linkage study. BMC Pregnancy Childbirth 2013, 13, 210. [Google Scholar] [CrossRef] [PubMed]
- Dzakpasu, S.; Fahey, J.; Kirby, R.S.; Tough, S.C.; Chalmers, B.; Heaman, M.I.; Bartholomew, S.; Biringer, A.; Darling, E.K.; Lee, L.S.; et al. Contribution of prepregnancy body mass index and gestational weight gain to adverse neonatal outcomes: Population attributable fractions for Canada. BMC Pregnancy Childbirth 2015, 15, 21. [Google Scholar] [CrossRef] [PubMed]
- Chawanpaiboon, S.; Vogel, J.P.; Moller, A.B.; Lumbiganon, P.; Petzold, M.; Hogan, D.; Landoulsi, S.; Jampathong, N.; Kongwattanakul, K.; Laopaiboon, M.; et al. Global, regional, and national estimates of levels of preterm birth in 2014: A systematic review and modelling analysis. Lancet Glob. Health 2019, 7, e37–e46. [Google Scholar] [CrossRef] [PubMed]
- OECD. Low Birth Weight in: OECD Family Database; OECD: Paris, France, 2020. [Google Scholar]
- Nishihama, Y.; Nakayama, S.F.; Tabuchi, T. Population attributable fraction of risk factors for low birth weight in the Japan Environment and Children’s Study. Environ. Int. 2022, 170, 107560. [Google Scholar] [CrossRef] [PubMed]
- Morisaki, N.; Obara, T.; Piedvache, A.; Kobayashi, S.; Miyashita, C.; Nishimura, T.; Ishikuro, M.; Sata, F.; Horikawa, R.; Mori, C.; et al. Association between smoking and hypertension in pregnancy among Japanese women: A meta-analysis of birth cohort studies in the Japan Birth Cohort Consortium (JBiCC) and JECS. J. Epidemiol. 2022, 33, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Itabashi, K.; Miura, F.; Uehara, R.; Nakamura, Y. New Japanese neonatal anthropometric charts for gestational age at birth. Pediatr. Int. 2014, 56, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Kiserud, T.; Benachi, A.; Hecher, K.; Perez, R.G.; Carvalho, J.; Piaggio, G.; Platt, L.D. The World Health Organization fetal growth charts: Concept, findings, interpretation, and application. Am. J. Obs. Gynecol. 2018, 218, S619–S629. [Google Scholar] [CrossRef]
- Japan Society of Obstetrics and Gynecology. Guideline for gestational weight gain. Acta Obstet. Gynaecol. Jpn. 2021, 73, 642. [Google Scholar]
- Chen, A.; Xu, F.; Xie, C.; Wu, T.; Vuong, A.M.; Miao, M.; Yuan, W.; DeFranco, E.A. Gestational Weight Gain Trend and Population Attributable Risks of Adverse Fetal Growth Outcomes in Ohio. Paediatr. Perinat Epidemiol. 2015, 29, 346–350. [Google Scholar] [CrossRef]
- Santos, S.; Voerman, E.; Amiano, P.; Barros, H.; Beilin, L.J.; Bergström, A.; Charles, M.A.; Chatzi, L.; Chevrier, C.; Chrousos, G.P.; et al. Impact of maternal body mass index and gestational weight gain on pregnancy complications: An individual participant data meta-analysis of European, North American and Australian cohorts. BJOG Int. J. Obstet. Gynaecol. 2019, 126, 984–995. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health, Labour and Welfare. Vital Statistics. 2019. Available online: http://www.mhlw.go.jp/toukei/saikin/hw/jinkou/geppo/nengai11/kekka02.html (accessed on 7 December 2023).
- Ministry of Health, Labour and Welfare. National Nutrition Survey on Preschool Children. 2010. Available online: https://www.mhlw.go.jp/toukei/list/73-22a.html#mokuteki (accessed on 7 December 2023).
- Zou, G. A modified poisson regression approach to prospective studies with binary data. Am. J. Epidemiol. 2004, 159, 702–706. [Google Scholar] [CrossRef] [PubMed]
- Mansournia, M.A.; Altman, D.G. Population attributable fraction. BMJ 2018, 360, k757. [Google Scholar] [CrossRef] [PubMed]
- Biswas, T.; Magalhaes, R.J.S.; Townsend, N.; Das, S.K.; Mamun, A. Double Burden of Underweight and Overweight among Women in South and Southeast Asia: A Systematic Review and Meta-analysis. Adv. Nutr. 2020, 11, 128–143. [Google Scholar] [CrossRef] [PubMed]
- NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 2016, 387, 1377–1396. [Google Scholar] [CrossRef] [PubMed]
- Morisaki, N.; Piedvache, A.; Morokuma, S.; Nakahara, K.; Ogawa, M.; Kato, K.; Sanefuji, M.; Shibata, E.; Tsuji, M.; Shimono, M.; et al. Gestational Weight Gain Growth Charts Adapted to Japanese Pregnancies Using a Bayesian Approach in a Longitudinal Study: The Japan Environment and Children’s Study. J. Epidemiol. 2023, 33, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, K.; Aoki, S.; Toma, R.; Fujiwara, K.; Sakamaki, K.; Hirahara, F. Pregnancy Outcomes Based on Pre-Pregnancy Body Mass Index in Japanese Women. PLoS ONE 2016, 11, e0157081. [Google Scholar] [CrossRef]
- Hayashi, F.; Takimoto, H.; Yoshita, K.; Yoshiike, N. Perceived body size and desire for thinness of young Japanese women: A population-based survey. Br. J. Nutr. 2006, 96, 1154–1162. [Google Scholar] [CrossRef]
- Owen, P.R.; Laurel-Seller, E. Weight and Shape Ideals: Thin Is Dangerously In. J. Appl. Soc. Psychol. 2000, 30, 979–990. [Google Scholar] [CrossRef]
- Swami, V.; Frederick, D.A.; Aavik, T.; Alcalay, L.; Allik, J.; Anderson, D.; Andrianto, S.; Arora, A.; Brännström, A.; Cunningham, J.; et al. The attractive female body weight and female body dissatisfaction in 26 countries across 10 world regions: Results of the international body project I. Pers. Soc. Psychol. Bull. 2010, 36, 309–325. [Google Scholar] [CrossRef]
- Swami, V.; Caprario, C.; Tovée, M.J.; Furnham, A. Female physical attractiveness in Britain and Japan: A cross-cultural study. Eur. J. Personal. 2006, 20, 69–81. [Google Scholar] [CrossRef]
- Parker, J.D.; Schoendorf, K.C.; Kiely, J.L. Associations between measures of socioeconomic status and low birth weight, small for gestational age, and premature delivery in the United States. Ann. Epidemiol. 1994, 4, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Bushnik, T.; Yang, S.; Kaufman, J.S.; Kramer, M.S.; Wilkins, R. Socioeconomic disparities in small-for-gestational-age birth and preterm birth. Health Rep. 2017, 28, 3–10. [Google Scholar] [PubMed]
- Thompson, J.M.; Clark, P.M.; Robinson, E.; Becroft, D.M.; Pattison, N.S.; Glavish, N.; Pryor, J.E.; Wild, C.J.; Rees, K.; Mitchell, E.A. Risk factors for small-for-gestational-age babies: The Auckland Birthweight Collaborative Study. J. Paediatr. Child Health 2001, 37, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Beard, J.R.; Lincoln, D.; Donoghue, D.; Taylor, D.; Summerhayes, R.; Dunn, T.M.; Earnest, A.; Morgan, G. Socioeconomic and maternal determinants of small-for-gestational age births: Patterns of increasing disparity. Acta Obs. Gynecol. Scand. 2009, 88, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Pillas, D.; Marmot, M.; Naicker, K.; Goldblatt, P.; Morrison, J.; Pikhart, H. Social inequalities in early childhood health and development: A European-wide systematic review. Pediatr. Res. 2014, 76, 418–424. [Google Scholar] [CrossRef] [PubMed]
- United Nation. Mean Age of Women at Birth of First Child. 2021. Available online: https://w3.unece.org/PXWeb/en/Table?IndicatorCode=34 (accessed on 7 December 2023).
- Lean, S.C.; Heazell, A.E.P.; Dilworth, M.R.; Mills, T.A.; Jones, R.L. Placental Dysfunction Underlies Increased Risk of Fetal Growth Restriction and Stillbirth in Advanced Maternal Age Women. Sci. Rep. 2017, 7, 9677. [Google Scholar] [CrossRef] [PubMed]
- Lean, S.C.; Derricott, H.; Jones, R.L.; Heazell, A.E.P. Advanced maternal age and adverse pregnancy outcomes: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0186287. [Google Scholar] [CrossRef]
- Newburn-Cook, C.V.; Onyskiw, J.E. Is older maternal age a risk factor for preterm birth and fetal growth restriction? A systematic review. Health Care Women Int. 2005, 26, 852–875. [Google Scholar] [CrossRef]
- World Health Organization. Global Status Report on Alcohol and Health; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Mamluk, L.; Edwards, H.B.; Savović, J.; Leach, V.; Jones, T.; Moore, T.H.M.; Ijaz, S.; Lewis, S.J.; Donovan, J.L.; Lawlor, D.; et al. Low alcohol consumption and pregnancy and childhood outcomes: Time to change guidelines indicating apparently ‘safe’ levels of alcohol during pregnancy? A systematic review and meta-analyses. BMJ Open 2017, 7, e015410. [Google Scholar] [CrossRef]
- Nykjaer, C.; Alwan, N.A.; Greenwood, D.C.; Simpson, N.A.; Hay, A.W.; White, K.L.; Cade, J.E. Maternal alcohol intake prior to and during pregnancy and risk of adverse birth outcomes: Evidence from a British cohort. J. Epidemiol. Community Health 2014, 68, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Lundsberg, L.S.; Illuzzi, J.L.; Belanger, K.; Triche, E.W.; Bracken, M.B. Low-to-moderate prenatal alcohol consumption and the risk of selected birth outcomes: A prospective cohort study. Ann. Epidemiol. 2015, 25, 46–54.e43. [Google Scholar] [CrossRef] [PubMed]
- Gardebjer, E.M.; Cuffe, J.S.; Pantaleon, M.; Wlodek, M.E.; Moritz, K.M. Periconceptional alcohol consumption causes fetal growth restriction and increases glycogen accumulation in the late gestation rat placenta. Placenta 2014, 35, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Pielage, M.; El Marroun, H.; Odendaal, H.J.; Willemsen, S.P.; Hillegers, M.H.J.; Steegers, E.A.P.; Rousian, M. Alcohol exposure before and during pregnancy is associated with reduced fetal growth: The Safe Passage Study. BMC Med. 2023, 21, 318. [Google Scholar] [CrossRef]
- Patra, J.; Bakker, R.; Irving, H.; Jaddoe, V.W.; Malini, S.; Rehm, J. Dose-response relationship between alcohol consumption before and during pregnancy and the risks of low birthweight, preterm birth and small for gestational age (SGA)-a systematic review and meta-analyses. BJOG Int. J. Obstet. Gynaecol. 2011, 118, 1411–1421. [Google Scholar] [CrossRef]
- Kicinski, M.; Springate, D.A.; Kontopantelis, E. Publication bias in meta-analyses from the Cochrane Database of Systematic Reviews. Stat. Med. 2015, 34, 2781–2793. [Google Scholar] [CrossRef]
- Shin, D.; Chung, H.; Weatherspoon, L.; Song, W.O. Validity of prepregnancy weight status estimated from self-reported height and weight. Matern. Child Health J. 2014, 18, 1667–1674. [Google Scholar] [CrossRef]
| Hokkaido Study | TMM BIRTHREE Cohort Study | HBC Study | BOSHI Study | C-MACH Study | Nationwide | ||
|---|---|---|---|---|---|---|---|
| Age | <35 years old | 78.3 | 68.1 | 74.3 | 76.4 | 65.7 | 70.9 |
| ≥35 years old | 21.7 | 31.9 | 25.7 | 23.6 | 34.3 | 29.1 | |
| Parity | Nulliparous | 41.3 | 45.7 | 49.7 | 42.7 | 36.1 | 41.5 |
| Multiparous | 58.7 | 54.3 | 50.3 | 57.3 | 63.9 | 58.5 | |
| Education | High school | 47.6 | 32.2 | 33.9 | NA | 23.0 | 27.0 |
| College and more | 52.4 | 67.8 | 66.1 | NA | 77.0 | 73.0 | |
| Pre-pregnancy BMI | <18.5 kg/m2 | 16.9 | 14.3 | 20.8 | 12.0 | 17.6 | 19.6 |
| ≥18.5 kg/m2 | 83.1 | 85.7 | 79.2 | 88.0 | 82.4 | 80.4 | |
| GWG | Adequate | NA | 54.5 | 53.9 | 54.9 | 50.2 | 50.4 |
| Inadequate | NA | 45.5 | 46.1 | 45.1 | 49.8 | 49.6 | |
| Smoking | Never | 63.8 | 86.6 | 78.7 | 82.9 | 79.4 | 81.1 |
| Quit smoking after pregnancy | 24.3 | 11.6 | 13.2 | 13.3 | 19.1 | 13.7 | |
| Continued smoking | 12.0 | 1.8 | 8.1 | 3.9 | 1.5 | 5.2 | |
| Alcohol consumption | Never | 87.1 | 53.9 | 40.0 | 55.7 | 96.9 | 91.3 |
| Quit alcohol consumption | NA | 39.8 | 44.1 | 43.1 | NA | NA | |
| Continued alcohol consumption | 12.9 | 6.3 | 15.9 | 1.2 | 3.1 | 8.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishitsuka, K.; Piedvache, A.; Kobayashi, S.; Iwama, N.; Nishimura, T.; Watanabe, M.; Metoki, H.; Iwata, H.; Miyashita, C.; Ishikuro, M.; et al. The Population-Attributable Fractions of Small-for-Gestational-Age Births: Results from the Japan Birth Cohort Consortium. Nutrients 2024, 16, 186. https://doi.org/10.3390/nu16020186
Ishitsuka K, Piedvache A, Kobayashi S, Iwama N, Nishimura T, Watanabe M, Metoki H, Iwata H, Miyashita C, Ishikuro M, et al. The Population-Attributable Fractions of Small-for-Gestational-Age Births: Results from the Japan Birth Cohort Consortium. Nutrients. 2024; 16(2):186. https://doi.org/10.3390/nu16020186
Chicago/Turabian StyleIshitsuka, Kazue, Aurélie Piedvache, Sumitaka Kobayashi, Noriyuki Iwama, Tomoko Nishimura, Masahiro Watanabe, Hirohito Metoki, Hiroyoshi Iwata, Chihiro Miyashita, Mami Ishikuro, and et al. 2024. "The Population-Attributable Fractions of Small-for-Gestational-Age Births: Results from the Japan Birth Cohort Consortium" Nutrients 16, no. 2: 186. https://doi.org/10.3390/nu16020186
APA StyleIshitsuka, K., Piedvache, A., Kobayashi, S., Iwama, N., Nishimura, T., Watanabe, M., Metoki, H., Iwata, H., Miyashita, C., Ishikuro, M., Obara, T., Sakurai, K., Rahman, M. S., Tanaka, K., Miyake, Y., Horikawa, R., Kishi, R., Tsuchiya, K. J., Mori, C., ... Morisaki, N. (2024). The Population-Attributable Fractions of Small-for-Gestational-Age Births: Results from the Japan Birth Cohort Consortium. Nutrients, 16(2), 186. https://doi.org/10.3390/nu16020186







