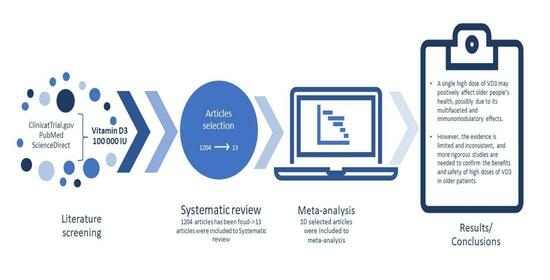
Has a High Dose of Vitamin D3 Impacted Health Conditions in Older Adults?—A Systematic Review and Meta-Analysis Focusing on Dose 100,000 IU
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selection of Studies
2.2. Data Sources and Search Strategy
2.3. Data Extraction and Risk of Bias Assessment
2.4. Statistical Method
3. Results
3.1. Characteristics of the Studies
3.2. Risk of Bias
3.3. Lung function
3.4. Cardiovascular Function
3.5. Immune Function
3.6. Bone Density, Falls and Non-Vertebral Fractures
3.7. Cancer-Free Survival
3.8. Mortality-Associated Outcomes
3.9. Changes in VD3 Serum Levels after High-Dose Treatment
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ARI | acute respiratory infections, |
| BD | bone density |
| BP | blood pressure, |
| COPD | chronic obstructive pulmonary disease, |
| C | control group, |
| CD | cardiovascular disease, |
| DB | double-blind, |
| HDVD3 | high doses of vitamin D3, |
| MVCIP | mechanically ventilated critically ill patients; |
| NA | not available, |
| NVF | non-vertebral fractures, |
| OL | open-label, |
| RA | rheumatoid arthritis, |
| RCT | randomised clinical trial, |
| T | Tested group, |
| VD3 | vitamin D3, |
| 25(OH)D | 25- hydroxyvitamin D3 |
| VICU | ventilated intensive care unit patients, |
| QE | quasi-experimental |
References
- Jones, G. Vitamin D. In Modern Nutrition in Health and Diseases, 11th ed.; Ross, A.C., Caballero, B., Cousins, R.J., Tucker, K.L., Ziegler, T.R., Eds.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2012; pp. 278–292. [Google Scholar]
- Norman, A.W.; Henry, L. Vitamin D. In Present Knowledge in Nutrition, 10th ed.; Erman, J.W., Mcdonald, I.A., Zeisel, S.H., Eds.; Wiley Blackwell: Hoboken, NJ, USA, 2012; p. 207. [Google Scholar]
- Plum, L.A.; DeLuca, H.F. Vitamin D, disease and therapeutic opportunities. Nat. Rev. Drug Discov. 2010, 9, 941–955. [Google Scholar] [CrossRef] [PubMed]
- Vito-D, Vitamin D3 200000 IU (Cholecalciferol), Soft Gel Capsule, Natural. Available online: https://amnasorganics.com/products/vitamin-d-200000-iu-single-monthly-dose (accessed on 10 November 2023).
- SunWhiz-Dt, Vitamin D3 200000 IU, Capsule, NaturaWhiz. Available online: https://w46hizlaboratories.com/product/sunwhiz-d-capsules-vitamin-d3-200000iu/ (accessed on 10 November 2023).
- Higgins, J.P.T. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Han, J.E.; Jones, J.L.; Tangpricha, V.; Brown, M.A.; Brown, L.A.S.; Hao, L.; Hebbar, G.; Lee, M.J.; Liu, S.; Ziegler, T.R.; et al. High dose vitamin D administration in ventilated intensive care unit patients: A pilot double blind randomized controlled trial. J. Clin. Transl. Endocrinol. 2016, 4, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.M.; Jones, J.L.; Han, J.E.; Alvarez, J.A.; Sloan, J.H.; Konrad, R.J.; Zughaier, S.M.; Martin, G.S.; Ziegler, T.R.; Tangpricha, V. High-Dose Vitamin D3 Administration is Associated with Increases in Hemoglobin Concentrations in Mechanically Ventilated Critically Ill Adults: A Pilot Randomized Placebo-Controlled Trial. J. Parenter. Enter. Nutr. 2018, 42, 87–94. [Google Scholar] [CrossRef]
- Johansson, H.; Spadola, G.; Tosti, G.; Mandalà, M.; Minisini, A.M.; Queirolo, P.; Aristarco, V.; Baldini, F.; Cocorocchio, E.; Albertazzi, E.; et al. Vitamin D Supplementation and Disease-Free Survival in Stage II Melanoma: A Randomized Placebo Controlled Trial. Nutrients 2021, 13, 1931. [Google Scholar] [CrossRef] [PubMed]
- Pludowski, P.; Holick, M.F.; Grant, W.B.; Konstantynowicz, J.; Mascarenhas, M.R.; Haq, A.; Povoroznyuk, V.; Balatska, N.; Barbosa, A.P.; Karonova, T.; et al. Vitamin D supplementation guidelines. J. Steroid Biochem. Mol. Biol. 2017, 175, 125–135. [Google Scholar] [CrossRef]
- Munns, C.F.; Shaw, N.; Kiely, M.; Specker, B.L.; Thacher, T.D.; Ozono, K.; Michigami, T.; Tiosano, D.; Mughal, M.Z.; Mäkitie, O.; et al. Global Consensus Recommendations on Prevention and Management of Nutritional Rickets. J. Clin. Endocrinol. Metab. 2016, 101, 394–415. [Google Scholar] [CrossRef]
- Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 29 May 2022).
- Dawson-Hughes, B.; Staten, M.A.; Knowler, W.C.; Nelson, J.; Vickery, E.M.; LeBlanc, E.S.; Neff, L.M.; Park, J.; Pittas, A.G.; D2d Research Group. Intratrial Exposure to Vitamin D and New-Onset Diabetes Among Adults with Prediabetes: A Secondary Analysis from the Vitamin D and Type 2 Diabetes (D2d) Study. Diabetes Care 2020, 43, 2916–2922. [Google Scholar] [CrossRef]
- WHO. World’s Older Population Grows Dramatically NIH-Funded Census Bureau Report Offers Details of Global Aging Phenomenon. 28 March 2016. Available online: https://www.nih.gov/news-events/news-releases/worlds-older-population-grows-dramatically (accessed on 5 November 2023).
- Menon, V.; Kar, S.K.; Suthar, N.; Nebhinani, N. Vitamin D and Depression: A Critical Appraisal of the Evidence and Future Directions. Indian J. Psychol. Med. 2020, 42, 11–21. [Google Scholar] [CrossRef]
- Vellekkatt, F.; Menon, V. Efficacy of vitamin D supplementation in major depression: A meta-analysis of randomized controlled trials. J. Postgrad. Med. 2019, 65, 74–80. [Google Scholar] [CrossRef]
- Ebeling, P.R.; Adler, R.A.; Jones, G.; Liberman, U.A.; Mazziotti, G.; Minisola, S.; Munns, C.F.; Napoli, N.; Pittas, A.G.; Giustina, A.; et al. Management of endocrine diseases: Therapeutics of vitamin D. Eur. J. Endocrinol. 2018, 179, R239–R259. [Google Scholar] [CrossRef]
- Pludowski, P.; Holick, M.F.; Pilz, S.; Wagner, C.L.; Hollis, B.W.; Grant, W.B.; Shoenfeld, Y.; Lerchbaum, E.; Llewellyn, D.J.; Kienreich, K.; et al. Vitamin D effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia and mortality-a review of recent evidence. Autoimmun. Rev. 2013, 12, 976–989. [Google Scholar] [CrossRef] [PubMed]
- Kanno, K.; Akutsu, T.; Ohdaira, H.; Suzuki, Y.; Urashima, M. Effect of Vitamin D Supplements on Relapse or Death in a p53-Immunoreactive Subgroup with Digestive Tract Cancer: Post Hoc Analysis of the AMATERASU Randomized Clinical Trial. JAMA Netw. Open 2023, 6, e2328886. [Google Scholar] [CrossRef] [PubMed]
- Cannata-Andía, J.B.; Díaz-Sottolano, A.; Fernández, P.; Palomo-Antequera, C.; Herrero-Puente, P.; Mouzo, R.; Carrillo-López, N.; Panizo, S.; Ibañez, G.H.; Cusumano, C.A.; et al. A single-oral bolus of 100,000 IU of cholecalciferol at hospital admission did not improve outcomes in the COVID-19 disease: The COVID-VIT-D-a randomised multicentre international clinical trial. BMC Med. 2022, 20, 83. [Google Scholar] [CrossRef] [PubMed]
- De Niet, S.; Trémège, M.; Coffiner, M.; Rousseau, A.-F.; Calmes, D.; Frix, A.-N.; Gester, F.; Delvaux, M.; Dive, A.-F.; Guglielmi, E.; et al. Positive Effects of Vitamin D Supplementation in Patients Hospitalized for COVID-19: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2022, 14, 3048. [Google Scholar] [CrossRef] [PubMed]
- Karonova, T.L.; Golovatyuk, K.A.; Kudryavtsev, I.V.; Chernikova, A.T.; Mikhaylova, A.A.; Aquino, A.D.; Lagutina, D.I.; Zaikova, E.K.; Kalinina, O.V.; Golovkin, A.S.; et al. Effect of Cholecalciferol Supplementation on the Clinical Features and Inflammatory Markers in Hospitalized COVID-19 Patients: A Randomized, Open-Label, Single-Center Study. Nutrients 2022, 14, 2602. [Google Scholar] [CrossRef] [PubMed]
- Slominski, R.M.; Stefan, J.; Athar, M.; Holick, M.F.; Jetten, A.M.; Raman, C.; Slominski, A.T. COVID-19 and Vitamin D: A lesson from the skin. Exp. Dermatol. 2020, 29, 885–890. [Google Scholar] [CrossRef]
- Bertone-Johnson, E.R.; Powers, S.I.; Spangler, L.; Larson, J.; Michael, Y.L.; Millen, A.E.; Bueche, M.N.; Salmoirago-Blotcher, E.; Wassertheil-Smoller, S.; Brunner, R.L.; et al. Vitamin D supplementation and depression in the women’s health initiative calcium and vitamin D trial. Am. J. Epidemiol. 2012, 176, 1–13. [Google Scholar] [CrossRef]
- Ekwaru, J.P.; Zwicker, J.D.; Holick, M.F.; Giovannucci, E.; Veugelers, P.J. The importance of body weight for the dose response relationship of oral vitamin D supplementation and serum 25-hydroxyvitamin D in healthy volunteers. PLoS ONE 2014, 9, e111265. [Google Scholar] [CrossRef]
- Ramasamy, I. Vitamin D Metabolism and Guidelines for Vitamin D Supplementation. Clin. Biochem. Rev. 2020, 41, 103–126. [Google Scholar] [CrossRef]
- Meehan, M.; Penckofer, S. The Role of Vitamin D in the Aging Adult. J. Aging Gerontol. 2014, 2, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Rader, C.P.; Corsten, N.; Rolf, O. Osteomalazie und Vitamin-D-Hypovitaminose [Osteomalacia and vitamin D deficiency]. Orthopade 2015, 44, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Hahn, J.; Cook, N.R.; Alexander, E.K.; Friedman, S.; Walter, J.; Bubes, V.; Kotler, G.; Lee, I.-M.; Manson, J.E.; Costenbader, K.H. Vitamin D and marine omega 3 fatty acid supplementation and incident autoimmune disease: VITAL randomized controlled trial. BMJ 2022, 376, e066452. [Google Scholar] [CrossRef] [PubMed]
- Glueck, C.J.; Jetty, V.; Wang, P.; Shah, P.; Prince, M.; Lee, K.; Goldenberg, M.; Kumar, A. Safety of 50,000–100,000 Units of Vitamin D3/Week in Vitamin D-Deficient, Hypercholesterolemic Patients with Reversible Statin Intolerance. N. Am. J. Med. Sci. 2016, 8, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.P.; Davies, K.M.; Chen, T.C.; Holick, M.F.; Barger-Lux, M.J. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am. J. Clin. Nutr. 2003, 77, 204–210, Erratum in Am. J. Clin. Nutr. 2003, 78, 1047. [Google Scholar] [CrossRef] [PubMed]
- Hathcock, J.N.; Shao, A.; Vieth, R.; Heaney, R. Risk assessment for vitamin D. Am. J. Clin. Nutr. 2007, 85, 6–18. [Google Scholar] [CrossRef]
- Hossein-Nezhad, A.; Holick, M.F. Vitamin D for health: A global perspective. Mayo Clin. Proc. 2013, 88, 720–755. [Google Scholar] [CrossRef]
- Kupisz-Urbanska, M.; Łukaszkiewicz, J.; Marcinkowska-Suchowierska, E. Vitamin D in Elderly. In Vitamin D; Özdemir, Ö., Ed.; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- McCullough, P.J.; Lehrer, D.S.; Amend, J. Daily oral dosing of vitamin D3 using 5000 TO 50,000 international units a day in long-term hospitalized patients: Insights from a seven year experience. J. Steroid Biochem. Mol. Biol. 2019, 189, 228–239. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef]
- Available online: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022337348 (accessed on 17 June 2022).
- Tanner, S.B.; Harwell, S.A. More than healthy bones: A review of vitamin D in muscle health. Adv. Musculoskelet. Dis. 2015, 7, 152–159. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin Trials. 1986, 7, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Cochran, W.G. The Combination of Estimates from Different Experiments. Biometrics 1954, 10, 101–129. [Google Scholar] [CrossRef]
- The Jamovi Project. jamovi, Version 2.3; Computer Software. 2022. Available online: https://www.jamovi.org (accessed on 21 January 2023).
- Scragg, R.; Waayer, D.; Stewart, A.W.; Lawes, C.M.M.; Toop, L.; Murphy, J.; Khaw, K.T.; Camargo, C.A., Jr. The Vitamin D Assessment (ViDA) Study: Design of a randomized controlled trial of vitamin D supplementation for the prevention of cardiovascular disease, acute respiratory infection, falls and non-vertebral fractures. J. Steroid Biochem. Mol. Biol. 2016, 164, 318–325. [Google Scholar] [CrossRef]
- Goncalves-Mendes, N.; Talvas, J.; Dualé, C.; Guttmann, A.; Corbin, V.; Marceau, G.; Sapin, V.; Brachet, P.; Evrard, B.; Laurichesse, H.; et al. IImpact of Vitamin D Supplementation on Influenza Vaccine Response and Immune Functions in Deficient Elderly Persons: A Randomized Placebo-Controlled Trial. Front. Immunol. 2019, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Soubrier, M.; Lambert, C.; Combe, B.; Gaudin, P.; Thomas, T.; Sibilia, J.; Dougados, M.; Dubost, J.-J. A randomised, double-blind, placebo-controlled study assessing the efficacy of high doses of vitamin D on functional disability in patients with rheumatoid arthritis. Clin. Exp. Rheumatol. 2018, 36, 1056–1060. [Google Scholar] [PubMed]
- Sluyter, J.D.; Camargo, J.C.A.; Waayer, D.; Lawes, C.M.M.; Toop, L.; Khaw, K.-T.; Scragg, R. Effect of Monthly, High-Dose, Long-Term Vitamin D on Lung Function: A Randomized Controlled Trial. Nutrients 2017, 9, 1353. [Google Scholar] [CrossRef]
- Rake, C.; Gilham, C.; Bukasa, L.; Ostler, R.; Newton, M.; Wild, J.P.; Aigret, B.; Hill, M.; Gillie, O.; Nazareth, I.; et al. High-dose oral vitamin D supplementation and mortality in people aged 65–84 years: The VIDAL cluster feasibility RCT of open versus double-blind individual randomization. Health Technol. Assess. 2020, 24, 1–54. [Google Scholar] [CrossRef]
- Camargo, C.A., Jr.; Toop, L.; Sluyter, J.; Lawes, C.M.M.; Waayer, D.; Khaw, K.-T.; Martineau, A.R.; Scragg, R. Effect of Monthly Vitamin D Supplementation on Preventing Exacerbations of Asthma or Chronic Obstructive Pulmonary Disease in Older Adults: Post Hoc Analysis of a Randomized Controlled Trial. Nutrients 2021, 13, 521. [Google Scholar] [CrossRef]
- Khaw, K.-T.; Stewart, A.W.; Waayer, D.; Lawes, C.M.M.; Toop, L.; Camargo, C.A., Jr.; Scragg, R. Effect of monthly high-dose vitamin D supplementation on falls and non-vertebral fractures: Secondary and post-hoc outcomes from the randomised, double-blind, placebo-controlled ViDA trial. Lancet Diabetes Endocrinol. 2017, 5, 438–447. [Google Scholar] [CrossRef]
- Reid, I.R.; Horne, A.M.; Mihov, B.; Gamble, G.D.; Al-Abuwsi, F.; Singh, M.; Taylor, L.; Fenwick, S.; Camargo, C.A.; Stewart, A.W.; et al. Effect of monthly high-dose vitamin D on bone density in community-dwelling older adults substudy of a randomized controlled trial. J. Intern. Med. 2017, 282, 452–460. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Janssen, H.C.; Samson, M.M.; Verhaar, H.J. Vitamin D deficiency, muscle function, and falls in elderly people. Am. J. Clin. Nutr. 2002, 75, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Annweiler, C.; Beaudenon, M.; Simon, R.; Guenet, M.; Otekpo, M.; Célarier, T.; Gautier, J. Vitamin D supplementation prior to or during COVID-19 associated with better 3-month survival in geriatric patients: Extension phase of the GERIA-COVID study. J. Steroid Biochem. Mol. Biol. 2021, 213, 105958. [Google Scholar] [CrossRef] [PubMed]
- De Paula, T.P.; Moreira, J.S.R.; Sperb, L.F.; Muller, M.E.P.; Steemburgo, T.; Viana, L.V. Efficacy of single-dose cholecalciferol in the blood pressure of patients with type 2 diabetes, hypertension and hypovitaminoses D. Sci. Rep. 2020, 10, 19611. [Google Scholar] [CrossRef]
- Xu, J.Q.; Murphy, S.L.; Kochanek, K.D.; Arias, E. Mortality in the United States, 2021; NCHS Data Brief, No. 456; National Center for Health Statistics: Hyattsville, MD, USA, 2022. [CrossRef]
- Bruce, B.; Fries, J.F. The Stanford Health Assessment Questionnaire: Dimensions and practical applications. Health Qual. Life Outcomes 2003, 1, 20. [Google Scholar] [CrossRef]
- Laird, E.; Ward, M.; McSorley, E.; Strain, J.J.; Wallace, J. Vitamin D and bone health: Potential mechanisms. Nutrients 2010, 2, 693–724. [Google Scholar] [CrossRef] [PubMed]
- Guzey, M.; Luo, J.; Getzenberg, R.H. Vitamin D3 modulated gene expression patterns in human primary normal and cancer prostate cells. J. Cell. Biochem. 2004, 93, 271–285. [Google Scholar] [CrossRef]
- Pilleron, S.; Sarfati, D.; Janssen-Heijnen, M.; Vignat, J.; Ferlay, J.; Bray, F.; Soerjomataram, I. Global cancer incidence in older adults, 2012 and 2035: A population-based study. Int. J. Cancer 2019, 144, 49–58. [Google Scholar] [CrossRef]
- Berger, N.A.; Savvides, P.; Koroukian, S.M.; Kahana, E.F.; Deimling, G.T.; Rose, J.H.; Bowman, K.F.; Miller, R.H. Cancer in the elderly. Trans. Am. Clin. Clim. Assoc. 2006, 117, 147–155. [Google Scholar]
- Marosi, C.; Köller, M. Challenge of cancer in the elderly. ESMO Open 2016, 1, e000020. [Google Scholar] [CrossRef]
- Available online: https://ourworldindata.org/grapher/deaths-globally-by-age (accessed on 12 November 2023).
- Holick, M.F. Vitamin D: Importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am. J. Clin. Nutr. 2004, 79, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.E.; Bhutiani, N.; Farmer, R.W.; Stromberg, A.J.; Martin, R.C.; Quillo, A.R.; McMasters, K.M.; Scoggins, C.R. Prognostic factors in melanoma patients with tumour-negative sentinel lymph nodes. Surgery 2016, 159, 1412–1421. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Boucher, B.J.; Al Anouti, F.; Pilz, S. Comparing the Evidence from Observational Studies and Randomized Controlled Trials for Nonskeletal Health Effects of Vitamin D. Nutrients 2022, 14, 3811. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Trummer, C.; Theiler-Schwetz, V.; Grübler, M.R.; Verheyen, N.D.; Odler, B.; Karras, S.N.; Zittermann, A.; März, W. Critical Appraisal of Large Vitamin D Randomized Controlled Trials. Nutrients 2022, 14, 303. [Google Scholar] [CrossRef]


| Country | Trials No. | Patients in All Conducted Studies | References |
|---|---|---|---|
| France | 3 | 192 | [8,43,52] |
| UK | 1 | 1901 | [46] |
| USA | 2 | 42 | [7,8] |
| New Zealand | 5 | 5108 | [42,45,47,48,49] |
| Brazil | 1 | 43 | [53] |
| Italy | 1 | 104 | [9] |
| Author, Year | Country | Study Design | Participants (T a/C a) | Age (y) | Baseline VD3 Level (ng/mL) Mean ± SD | Post VD3 Supplementation (ng/mL) Mean ± SD | Study Duration | Dose & Frequency | Results |
|---|---|---|---|---|---|---|---|---|---|
| 100,000 IU every day during 5 days | |||||||||
| Han et al., 2016 [7] | USA | DB a, RCT | 11/10 | 63.1 | C: 21.5 ± 12.2 T: 20.0 ± 7.3 | C: 21 ± 11.2 T: 55 ± 14 | 5 days | 100,000 IU every day | Positive |
| Smith et al., 2018 [8] | USA | RCT | 10/11 | C: 64.8 ± 17.5 T: 68.1 ± 18.6 | C: 21.5 ± 12.2 T: 20.0 ± 7.3 | C: 21.5 ± 12.2 T: 55 ± 14 | 5 days | 100,000 IU for 5 days | Positive |
| 100,000 IU once | |||||||||
| De Paula et al., 2020 [53] | Brazil | RCT | 21/22 | 65 ± 9 | C: 14.5 ± 4.3 T: 14.0 ± 5 | C: 19.0 ± 5 T: 23.0 ± 7 | 8 weeks | 100,000 IU single dose | Positive |
| 100,000 IU once per 15 days | |||||||||
| Goncalves-Mendes et al., 2019 [43] | France | RCT | 19/19 | 64–77 | C: 19.7 ± 5.9 T: 20.7 ± 5.7 | C: 18.1 ± 6.7 T: 44.3 ± 8.6 | 3 months | 100,000 IU per 15 days | Negative-different than expected |
| 100,000 IU per month | |||||||||
| Sluyter et al., 2017 [45] | New Zealand | DB, RCT | 226/216 | 50–84 | C:24.56 ± 9.48 a* T:26.4 ± 9.76 a* | C: 24.56 ± 9.48 * T: 47.6 ± 18.0 * | 1.1 year | 100,000 IU per month | Negative-different than expected |
| Camargo et al., 2021 [47] | New Zealand | RCT | 373/402 | 66.6 ± 8.3 | C: 24.16 * T: 25.8 * | C: 24.16 * T: 54.0 * | 3.3 years | 100,000 IU monthly | Positive in patients who are or have been ever smoker |
| Scragg et al., 2016 [42] | New Zealand | DB a, RCT | 2558/2550 ***a 171/163 ***a | 50–84 | C:24.4 ± 9.6 * T: 24.4 ± 9.6 * | C:26.4 ± 11.6 * T: 54.1 ± 16.0 * | 3.3 years | 100,000 IU per month | Negative-different than expected |
| Khaw et al., 2017 [48] | New Zealand | DB, RCT | 2558/2550 ***a 171/163 ***a | 50–84 | C:24.4 ± 9.6 * T: 24.4 ± 9.6 * | C:26.4 ± 11.6 * T: 54.1 ± 16.0 * | 3.3 years | 100,000 IU per month | Negative-different than expected |
| Rake et al., 2020 [46] | UK | OL a RCT, DB RCT | 372/366 395/392 | 65–84 | C:20.6 ± 5.117 * T: 20.6 ± 5.076 * | C: 20.72 ± 7.647 * T: 43.84 ± 9.435 * | 2 years | 100,000 IU per month ** | Negative-different than expected |
| Reid et al., 2017 [49] | New Zealand | RCT | 228/224 | 50–84 | C:22.4 ± 8.8 * T:22.0 ± 9.2 * | C: 24.0 ± 9.2 * T: 51.6 ± 11.6 * | 2 years | 100,000 IU per month | Negative-different than expected |
| 100,000 IU per 50 days | |||||||||
| Johansson et al., 2021 [9] | Italy | RCT | 52/52 (start study) 25/22 (after 3 years) | 50 | C: 18.48 ± 1.843 T: 17.97 ± 1.983 | C: 22.422 ± 2.15 T: 40.472 ± 2.583 | 3 years | 100,000 IU every 50 days | Positive |
| 100,000 IU per 2 or 3 months | |||||||||
| Annweiler et al., 2021 [52] | France | QE a | 67/28 | 88.0 ± 5.5 | C: 29.56 ± 12.84 * T: 24.64 ± 14.16 * | C: NA T: NA | 2–3 month | 100,000 IU per 2–3 months | Negative-different than expected |
| Bias | Random Sequence Generation (Selection Bias) | Allocation Concealment (Selection Bias) | Blinding Participants and Personnel (Performance Bias) | Incomplete Outcome Data (Attrition Bias) | Selective Reporting (Reporting Bias) | Other Bias | |
|---|---|---|---|---|---|---|---|
| References | |||||||
| Han et al. [7] | + | + | + | + | + | ? | |
| Soubrier et al. [44] | ? | ? | - | + | + | ? | |
| Sluyter et al. [45] | + | + | + | + | + | ? | |
| Annweiler et al. [52] | - | ? | - | + | + | ? | |
| Scragg et al. [42] | + | + | + | + | + | ? | |
| Khaw et al. [48] | + | + | + | + | + | ? | |
| Rake et al. [46] | + | + | + | + | + | ? | |
| Reid et al. [49] | ? | ?/+ | ? | + | + | ? | |
| Goncalves- Mendes et al. [43] | + | + | ? | + | + | ? | |
| Smith et al. [8] | + | + | + | + | + | ? | |
| De Paula et al. [53] | + | + | + | + | + | ? | |
| Camargo et al. [47] | + | ? | ? | + | + | ? | |
| Johansson et al. [9] | + | + | + | + | + | ? | |
| Author, Year | Country | Study Design | Participants (T a/C a) | Age (y) | Baseline 25(OH)D Level (ng/mL) Mean ± SD | Post VD3 Supplementation Level of 25(OH)D (ng/mL) Mean ± SD | Study Duration | Supplementation | Dose & Frequency | Disease/Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Han et al., 2016 [7] | USA | DB a, RCT | 11/10 | 63.1 | C: 21.5 ± 12.2 T: 20.0 ± 7.3 | C: 21 ± 11.2 T: 55 ± 14 | 5 days | VD3 | 100,000 IU every day | VICU a patients |
| Soubrier et al., 2018 [8] | France | DB, RCT | 29/30 | 59.8 ± 10.9 | C: NA a T: NA | C: NA T: NA | 24 weeks | VD3 | 100,000 IU per 4 weeks | RA a |
| Sluyter et al., 2017 [45] | New Zealand | DB, RCT | 226/216 | 50–84 | C: 24.56 ± 9.48 a* T: 26.4 ± 9.76 a* | C: 24.56 ± 9.48 * T: 47.6 ± 18.0 * | 1.1 year | VD3 | 100,000 IU per month | lung function |
| Annweiler et al., 2021 [52] | France | QE a | 67/28 | 88.0 ± 5.5 | C: 29.56 ± 12.84 * T: 24.64 ± 14.16 * | C: NA T: NA | 2-3 month | VD3 | 100,000 IU per 2-3 months | COVID-19 |
| Scragg et al., 2016 [42] | New Zealand | DB a, RCT | 2558/2550 ***a 171/163 ***a | 50–84 | C: 24.4 ± 9.6 * T: 24.4 ± 9.6 * | C:26.4 ± 11.6 * T: 54.1 ± 16.0 * | 3.3 years | VD3 | 100,000 IU per month | CD a |
| Khaw et al., 2017 [48] | New Zealand | DB, RCT | 2558/2550 ***a 171/163 ***a | 50–84 | C: 24.4 ± 9.6 * T: 24.4 ± 9.6 * | C:26.4 ± 11.6 * T: 54.1 ± 16.0 * | 3.3 years | VD3 | 100,000 IU per month | falls, NVF a |
| Rake et al., 2020 [46] | UK | OL a RCT, DB RCT | 372/366 395/392 | 65–84 | C: 20.6 ± 5.117 * T: 20.6 ± 5.076 * | C: 20.72 ± 7.647 * T: 43.84 ± 9.435 * | 2 years | VD3 | 100,000 IU per month ** | mortality in people aged 65-84 years |
| Reid et al., 2017 [49] | New Zealand | RCT | 228/224 | 50–84 | C: 22.4 ± 8.8 * T: 22.0 ± 9.2 * | C:24.0 ± 9.2 * T:51.6 ± 11.6 * | 2 years | VD3 | 100,000 IU per month | BD a |
| Goncalves- Mendes et al., 2019 [43] | France | RCT | 19/19 | 64–77 | C: 19.7 ± 5.9 T: 20.7 ± 5.7 | C: 18.1 ± 6.7 T: 44.3 ± 8.6 | 3 months | VD3 | 100,000 IU per 15 days | influenza vaccine response |
| Smith et al., 2018 [8] | USA | RCT | 10/11 | C:64.8 ± 17.5 T:68.1 ± 18.6 | C: 21.5 ± 12.2 T: 20.0 ± 7.3 | C: 21.5 ± 12.2 T: 55 ± 14 | 5 days | VD3 | 100,000 IU for 5 days | hemoglobin concentration in MVICP a |
| De Paula et al., 2020 [53] | Brazil | RCT | 21/22 | 65 ± 9 | C: 14.5 ± 4.3 T: 14.0 ± 5 | C: 19.0 ± 5 T: 23.0 ± 7 | 8 weeks | VD3 | 100,000 IU single dose | BP a in patients with hypertension, 2 DM a and hypovitaminosis D3 |
| Camargo et al., 2021 [47] | New Zealand | RCT | 373/402 | 66.6 ± 8.3 | C: 24.16 * T: 25.8 * | C: 24.16 * T: 54.0 * | 3.3 years | VD3 | 100,000 IU monthly | asthma and/or COPD a |
| Johansson et al., 2021 [9] | Italy | RCT | 52/52 (start study) 25/22 (after 3 years) | 50 | C: 18.48 ± 1.843 T: 17.97 ± 1.983 | C: 22.422 ± 2.15 T: 40.472 ± 2.583 | 3 years | VD3 | 100,000 IU every 50 days | disease-free survival in stage II melanoma |
| Han, 2016 [7] | USA | DB a, RCT | 11/10 | 63.1 | C: 21.5 ± 12.2 T: 20.0 ± 7.3 | C: 21 ± 11.2 T: 55 ± 14 | 5 days | VD3 | 100,000 IU every day | VICU a patients |
| Soubrier, 2018 [8] | France | DB, RCT | 29/30 | 59.8 ± 10.9 | C: NA a T: NA | C: NA T: NA | 24 weeks | VD3 | 100,000 IU per 4 weeks | RA a |
| Sluyter, 2017 [45] | New Zealand | DB, RCT | 226/216 | 50–84 | C:24.56 ± 9.48 a* T:26.4 ± 9.76 a* | C: 24.56 ± 9.48 * T: 47.6 ± 18.0 * | 1.1 year | VD3 | 100,000 IU per month | lung function |
| Annweiler, 2021 [52] | France | QE a | 67/28 | 88.0 ± 5.5 | C:29.56 ± 12.84 * T:24.64 ± 14.16 * | C: NA T: NA | 2-3 month | VD3 | 100,000 IU per 2-3 months | COVID-19 |
| Scragg, 2016 [42] | New Zealand | DB a, RCT | 2558/2550 ***a 171/163 ***a | 50–84 | C:24.4 ± 9.6 * T: 24.4 ± 9.6 * | C:26.4 ± 11.6 * T: 54.1 ± 16.0 * | 3.3 years | VD3 | 100,000 IU per month | CD a |
| Khaw, 2017 [48] | New Zealand | DB, RCT | 2558/2550 ***a 171/163 ***a | 50–84 | C:24.4 ± 9.6 * T: 24.4 ± 9.6 * | C:26.4 ± 11.6 * T: 54.1 ± 16.0 * | 3.3 years | VD3 | 100,000 IU per month | falls, NVF a |
| Rake, 2020 [46] | UK | OL a RCT, DB RCT | 372/366 395/392 | 65–84 | C:20.6 ± 5.117 * T: 20.6 ± 5.076 * | C: 20.72 ± 7.647 * T: 43.84 ± 9.435 * | 2 years | VD3 | 100,000 IU per month ** | mortality in people aged 65-84 years |
| Reid, 2017 [49] | New Zealand | RCT | 228/224 | 50–84 | C:22.4 ± 8.8 * T:22.0 ± 9.2 * | C:24.0 ± 9.2 * T:51.6 ± 11.6 * | 2 years | VD3 | 100,000 IU per month | BD a |
| Goncalves- Mendes, 2019 [43] | France | RCT | 19/19 | 64–77 | C: 19.7 ± 5.9 T: 20.7 ± 5.7 | C: 18.1 ± 6.7 T: 44.3 ± 8.6 | 3 months | VD3 | 100,000 IU per 15 days | influenza vaccine response |
| Smith, 2018 [8] | USA | RCT | 10/11 | C:64.8 ± 17.5 T:68.1 ± 18.6 | C: 21.5 ± 12.2 T: 20.0 ± 7.3 | C: 21.5 ± 12.2 T: 55 ± 14 | 5 days | VD3 | 100,000 IU for 5 days | hemoglobin concentration in MVCIP a |
| De Paula, 2020 [53] | Brazil | RCT | 21/22 | 65 ± 9 | C: 14.5 ± 4.3 T: 14.0 ± 5 | C: 19.0 ± 5 T: 23.0 ± 7 | 8 weeks | VD3 | 100,000 IU single dose | BP a in patients with hypertension, 2 DM a and hypovitaminosis D3 |
| Camargo, 2021 [47] | New Zealand | RCT | 373/402 | 66.6 ± 8.3 | C: 24.16 * T: 25.8 * | C: 24.16 * T: 54.0 * | 3.3 years | VD3 | 100,000 IU monthly | asthma and/or COPD a |
| Johansson, 2021 [9] | Italy | RCT | 52/52 (start study) 25/22 (after 3 years) | 50 | C: 18.48 ± 1.843 T: 17.97 ± 1.983 | C: 22.422 ± 2.15 T: 40.472 ± 2.583 | 3 years | VD3 | 100,000 IU every 50 days | disease-free survival in stage II melanoma |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Owczarek, B.; Ziomkiewicz, A.; Łukowska-Chojnacka, E. Has a High Dose of Vitamin D3 Impacted Health Conditions in Older Adults?—A Systematic Review and Meta-Analysis Focusing on Dose 100,000 IU. Nutrients 2024, 16, 252. https://doi.org/10.3390/nu16020252
Owczarek B, Ziomkiewicz A, Łukowska-Chojnacka E. Has a High Dose of Vitamin D3 Impacted Health Conditions in Older Adults?—A Systematic Review and Meta-Analysis Focusing on Dose 100,000 IU. Nutrients. 2024; 16(2):252. https://doi.org/10.3390/nu16020252
Chicago/Turabian StyleOwczarek, Barbara, Anna Ziomkiewicz, and Edyta Łukowska-Chojnacka. 2024. "Has a High Dose of Vitamin D3 Impacted Health Conditions in Older Adults?—A Systematic Review and Meta-Analysis Focusing on Dose 100,000 IU" Nutrients 16, no. 2: 252. https://doi.org/10.3390/nu16020252
APA StyleOwczarek, B., Ziomkiewicz, A., & Łukowska-Chojnacka, E. (2024). Has a High Dose of Vitamin D3 Impacted Health Conditions in Older Adults?—A Systematic Review and Meta-Analysis Focusing on Dose 100,000 IU. Nutrients, 16(2), 252. https://doi.org/10.3390/nu16020252