Zinc Deficiency Promotes Calcification in Vascular Smooth Muscle Cells Independent of Alkaline Phosphatase Action and Partly Impacted by Pit1 Upregulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Cellular Zinc Depletion
2.2. Pit1 Inhibition Experiments
2.3. Cellular Morphology
2.4. Alkaline Phosphatase (ALP) Activity Staining and Enzyme Activity Assay
2.5. Assessment of Calcification
2.5.1. Alizarin Red S Staining
2.5.2. Von Kossa Staining
2.6. Mineralization
2.7. Western Blotting
2.8. Statistical Analysis
3. Results
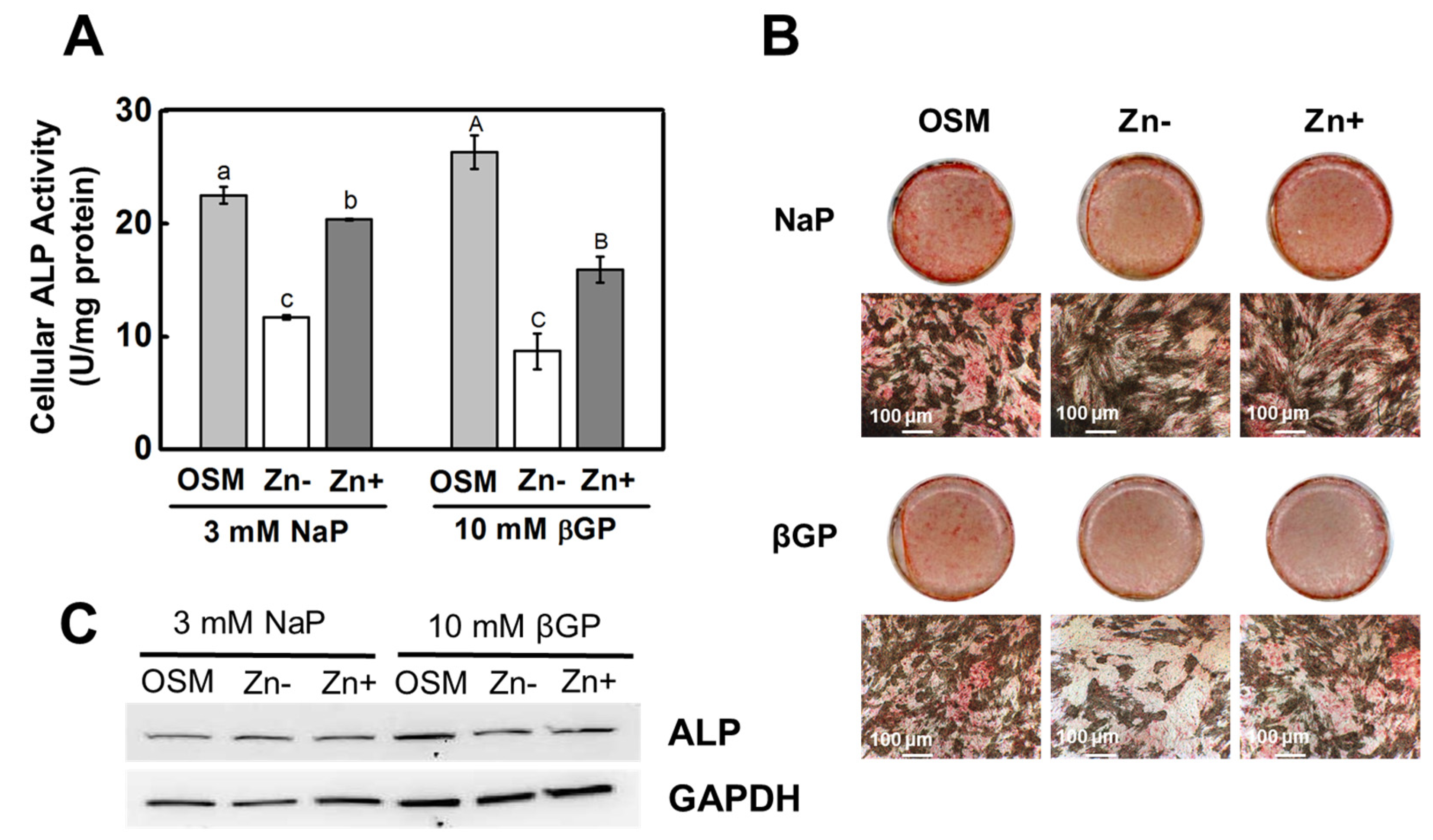
3.1. Zinc Deficiency Results in Diminished ALP Activity in Osteoblastic Cells
3.2. Zinc Deficiency Decreased Ca and P Deposition in Osteoblastic MC3T3-E1 Cells
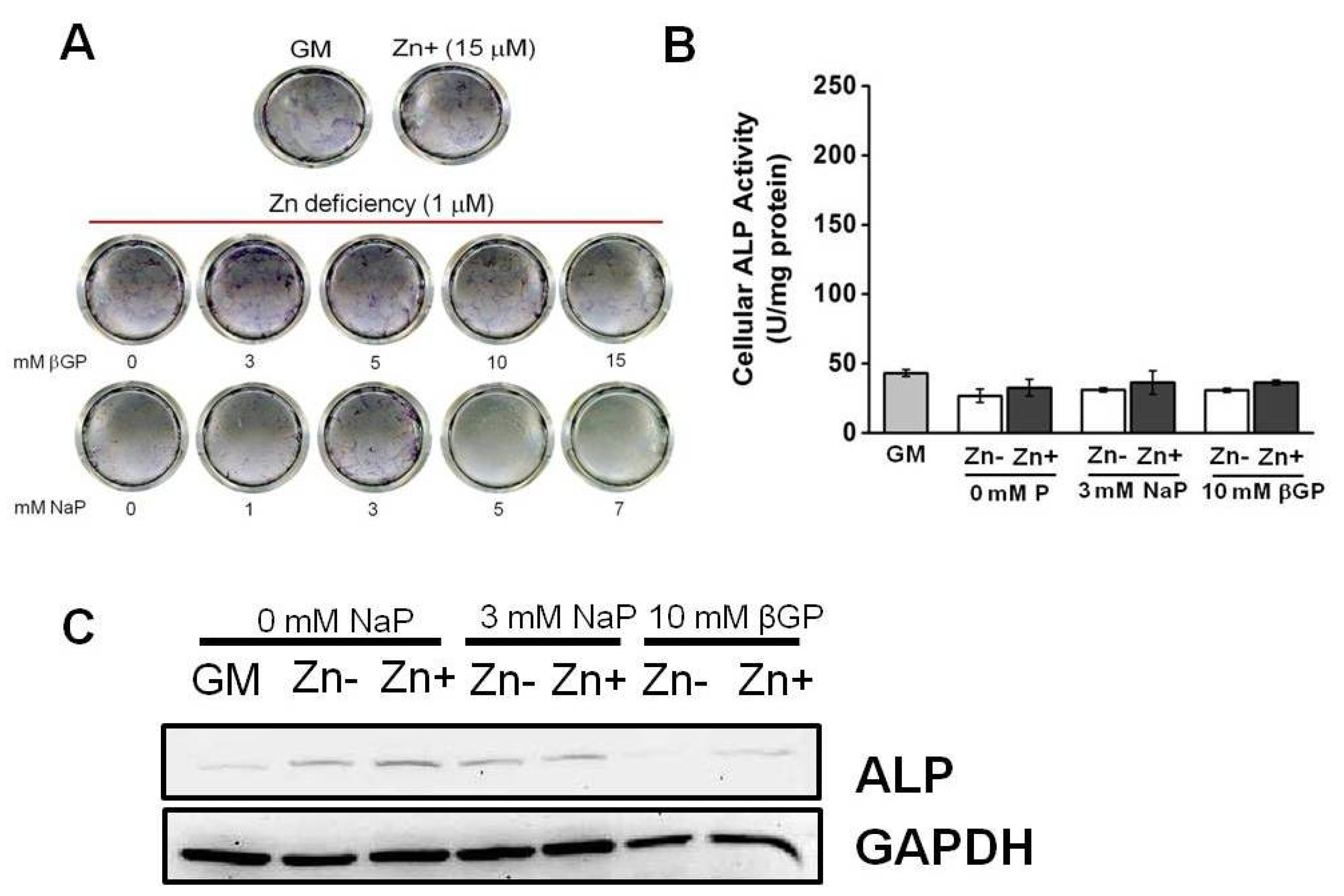
3.3. Zinc Deficiency Did Not Significantly Affect the ALP Activity in Cultured VSMCs
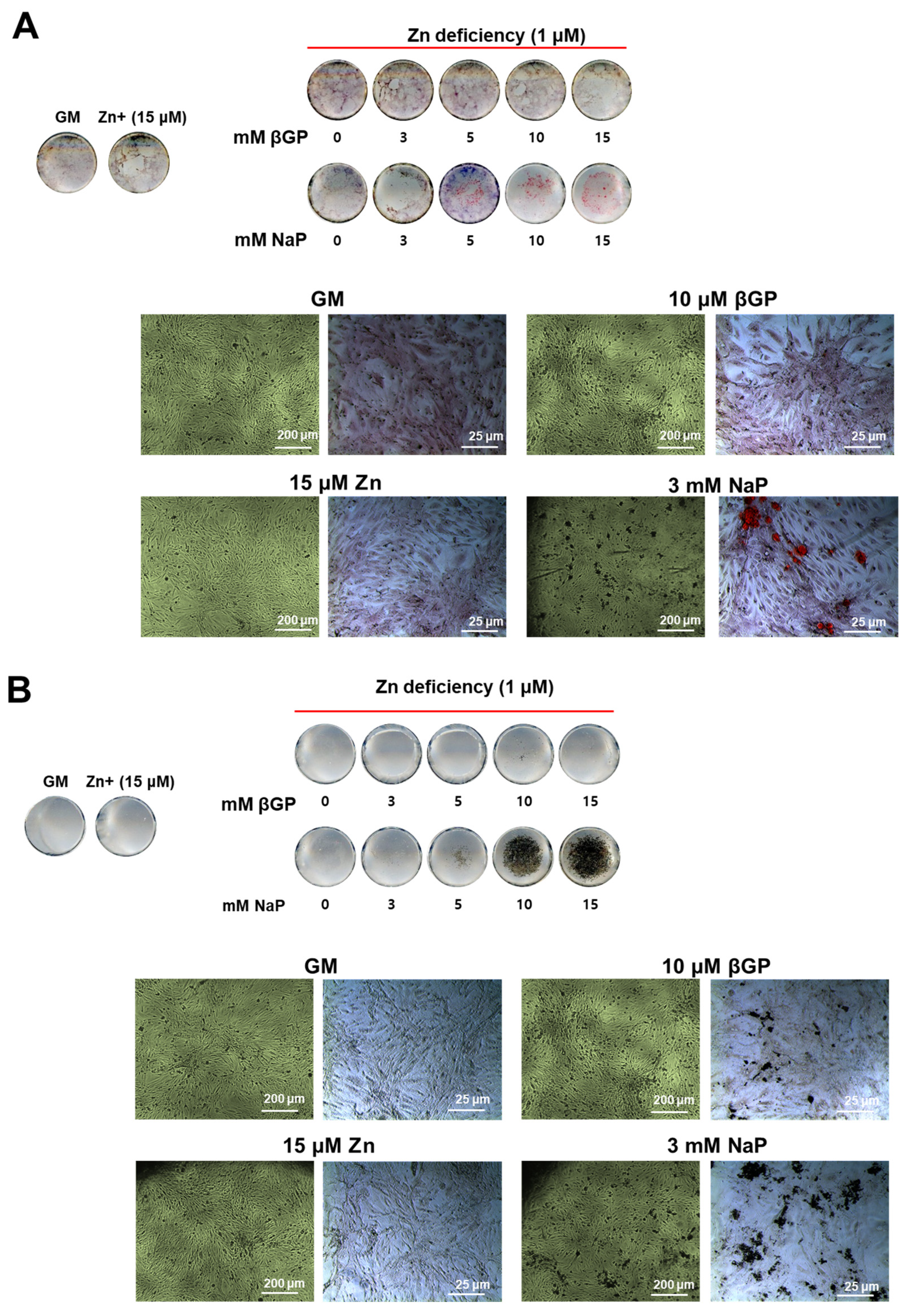
3.4. The Zinc-Deficient VSMCs Accumulates Ca and P with Increasing Doses of NaP but Not with βGP
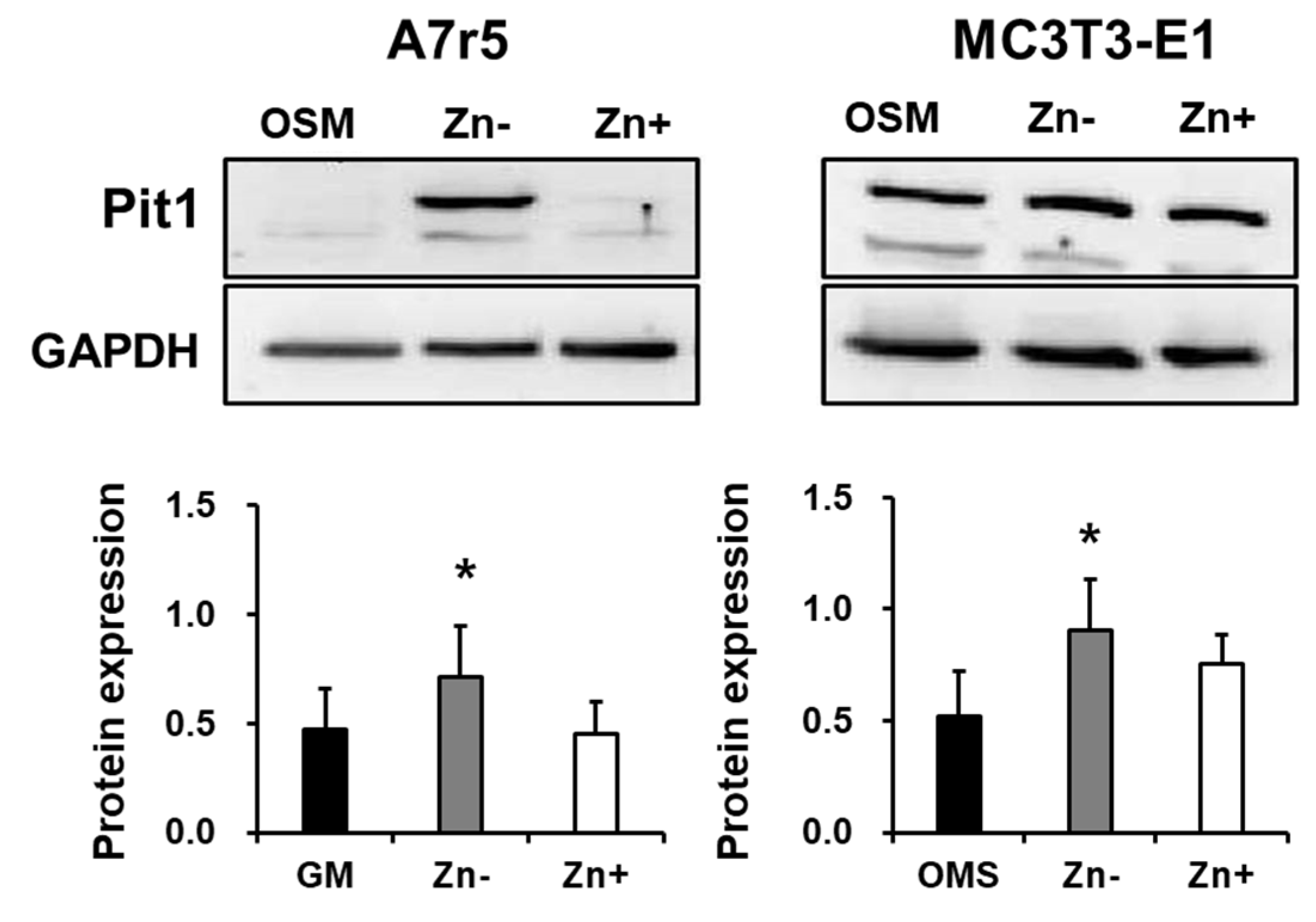
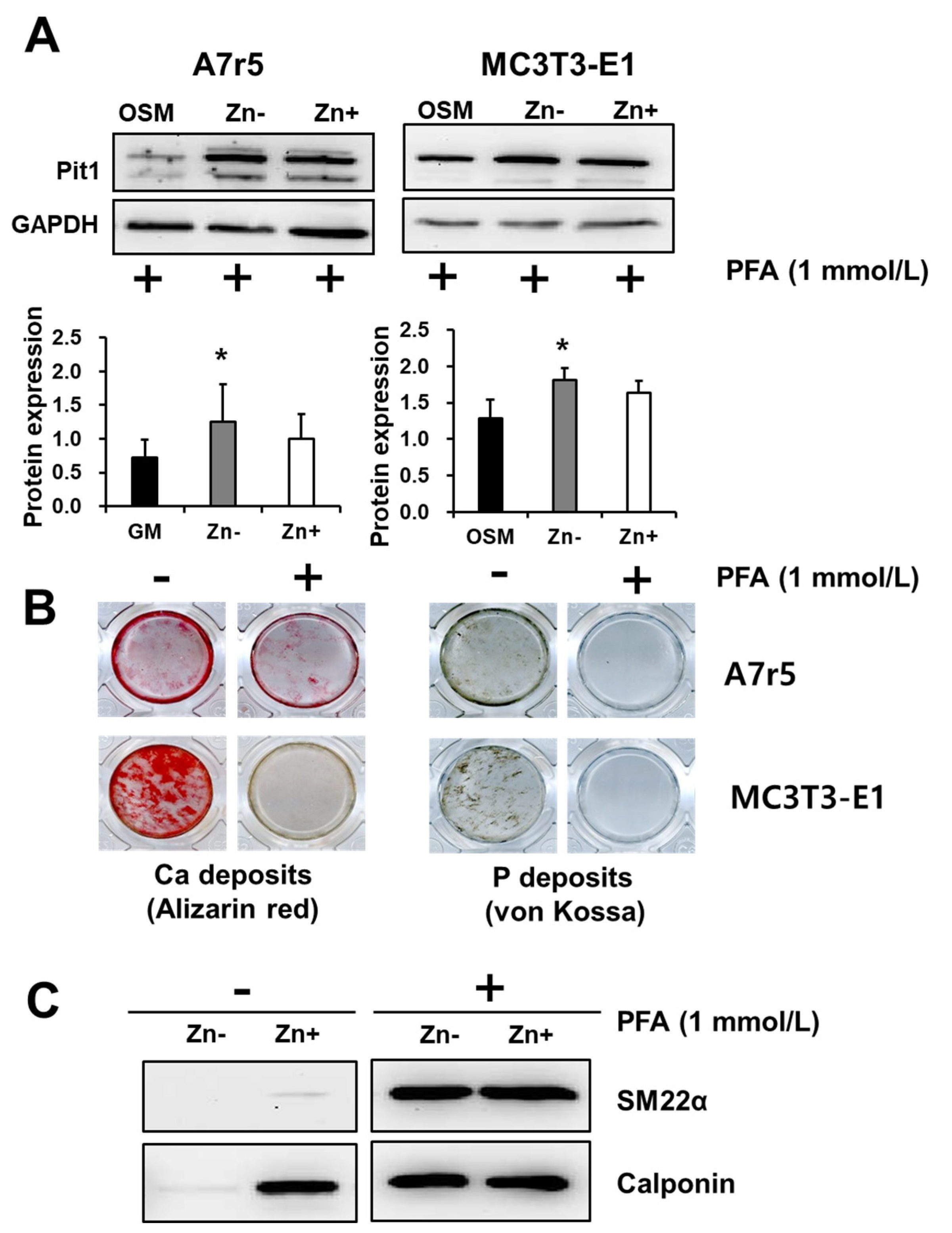
3.5. Zinc Deficiency Upregulates Pit1 Protein Expression in Zinc-Deficient VSMCs but Not in Zinc-Deficient Osteoblastic Cells
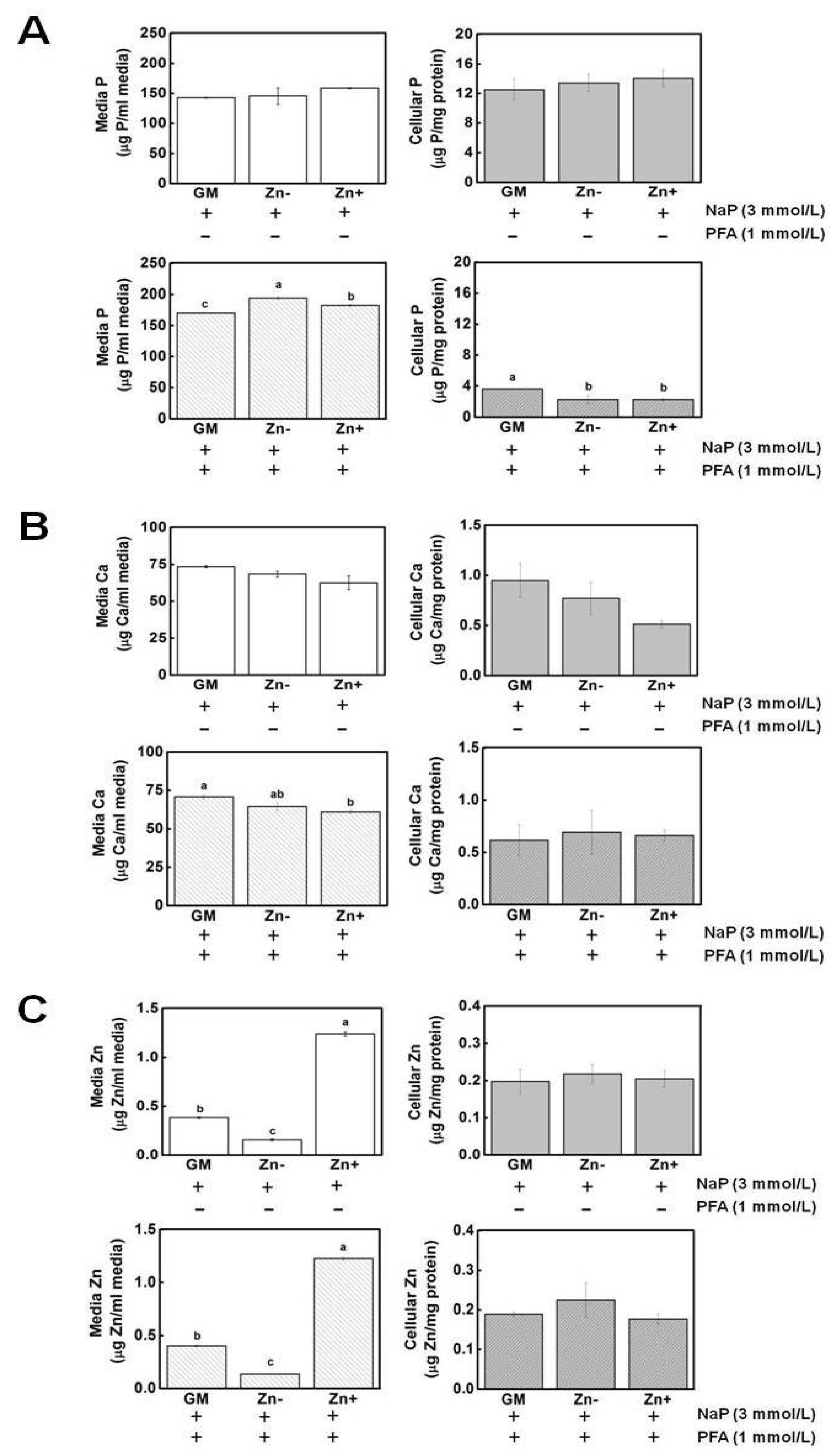
3.6. Zinc Did Not Alter the Concentrations of P and Ca in Media and in Cell Fractions of VSMCs
3.7. Inhibition of Pit1 by PFA Did Not Abrogate Its Expression but Inhibited Mineral Deposition and Restored Marker Expression in Zinc-Deficient VSMCs
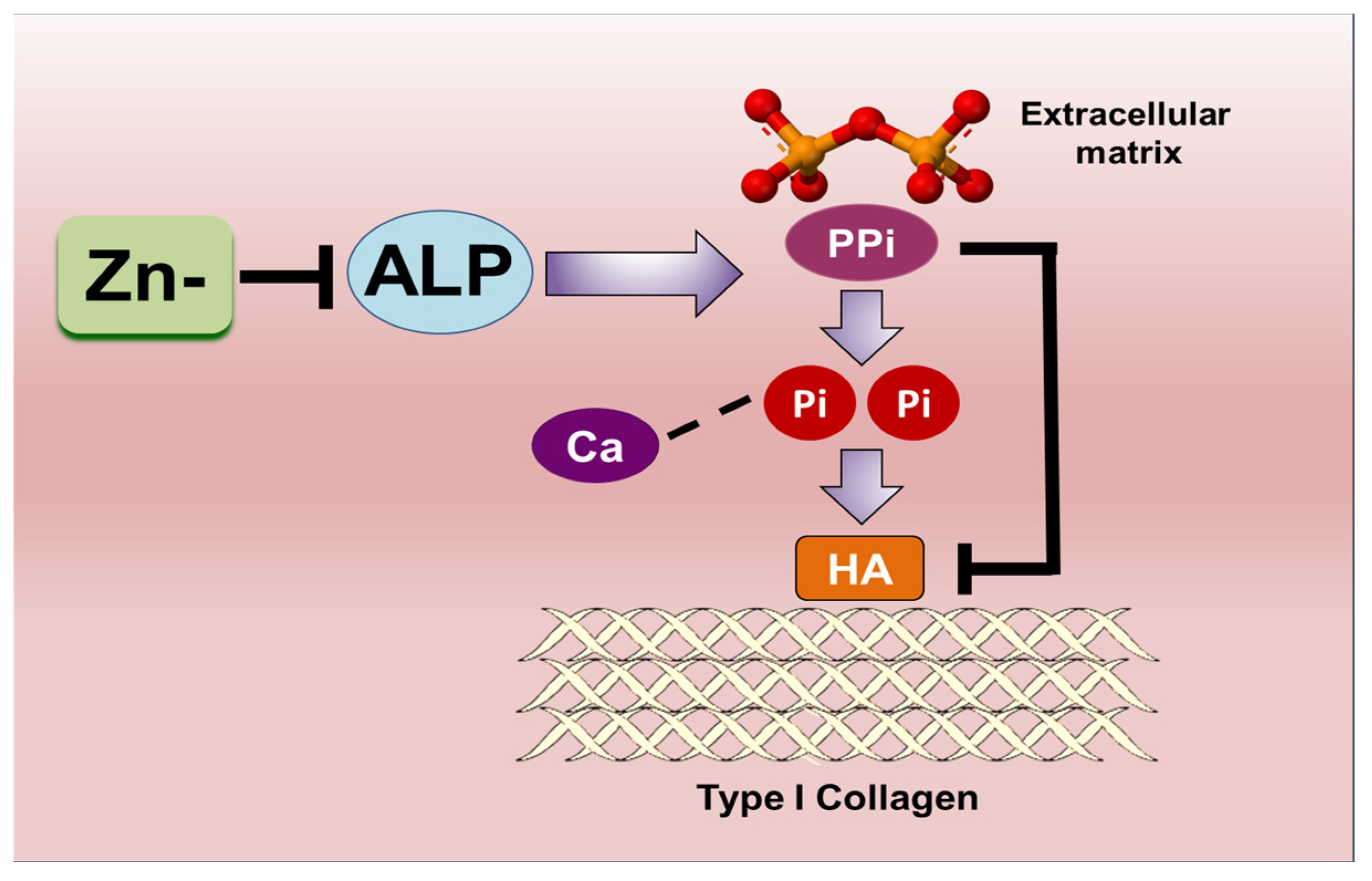
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alexopoulos, N.; Raggi, P. Calcification in atherosclerosis. Nat. Rev. Cardiol. 2009, 6, 681–688. [Google Scholar] [CrossRef]
- Mizobuchi, M.; Ogata, H.; Koiwa, F.; Kinugasa, E.; Akizawa, T. Vitamin D and vascular calcification in chronic kidney disease. Bone 2009, 45, S26–S29. [Google Scholar] [CrossRef]
- Murshed, M.; McKee, M.D. Molecular determinants of extracellular matrix mineralization in bone and blood vessels. Curr. Opin. Nephrol. Hypertens. 2010, 19, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Murshed, M. Mechanism of Bone Mineralization. Cold Spring Harb. Perspect. Med. 2018, 8, a031229. [Google Scholar] [CrossRef]
- Garner, S.C.; Pi, M.; Tu, Q.; Quarles, L.D. Rickets in cation-sensing receptor-deficient mice: An unexpected skeletal phenotype. Endocrinology 2001, 142, 3996–4005. [Google Scholar] [CrossRef]
- Gunther, T.; Chen, Z.F.; Kim, J.; Priemel, M.; Rueger, J.M.; Amling, M.; Moseley, J.M.; Martin, T.J.; Anderson, D.J.; Karsenty, G. Genetic ablation of parathyroid glands reveals another source of parathyroid hormone. Nature 2000, 406, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Pirro, A.E.; Amling, M.; Delling, G.; Baron, R.; Bronson, R.; Demay, M.B. Targeted ablation of the vitamin D receptor: An animal model of vitamin D-dependent rickets type II with alopecia. Proc. Natl. Acad. Sci. USA 1997, 94, 9831–9835. [Google Scholar] [CrossRef]
- Dominguez, L.J.; Farruggia, M.; Veronese, N.; Barbagallo, M. Vitamin D sources, metabolism, and deficiency: Available compounds and guideline for its treatment. Metabolites 2021, 11, 255. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Hashiba, H.; Kogo, H.; Aizawa, S.; Shigematsu, T. [Suppressive effects of bisphosphonates on the vascular calcification in ESRD patients]. Clin. Calcium 2002, 12, 1129–1135. [Google Scholar] [PubMed]
- Terkeltaub, R.A. Inorganic pyrophosphate generation and disposition in pathophysiology. Am. J. Physiol. Cell Physiol. 2001, 281, C1–C11. [Google Scholar] [CrossRef] [PubMed]
- Villa-Bellosta, R.; Wang, X.; Millan, J.L.; Dubyak, G.R.; O’Neill, W.C. Extracellular pyrophosphate metabolism and calcification in vascular smooth muscle. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H61–H68. [Google Scholar] [CrossRef] [PubMed]
- Mornet, E. Hypophosphatasia: The mutations in the tissue-nonspecific alkaline phosphatase gene. Hum. Mutat. 2000, 15, 309–315. [Google Scholar] [CrossRef]
- Donat, A.; Knapstein, P.R.; Jiang, S.; Baranowsky, A.; Ballhause, T.M.; Frosch, K.H.; Keller, J. Glucose metabolism in osteoblasts in healthy and pathophysiological conditions. Int. J. Mol. Sci. 2021, 22, 4120. [Google Scholar] [CrossRef] [PubMed]
- Golub, E.E.; Harrison, G.; Taylor, A.G.; Camper, S.; Shapiro, I.M. The role of alkaline phosphatase in cartilage mineralization. Bone. Miner. 1992, 17, 273–278. [Google Scholar] [CrossRef]
- O’Neill, W.C. Pyrophosphate, alkaline phosphatase, and vascular calcification. Circ. Res. 2006, 99, e2. [Google Scholar] [CrossRef]
- Shioi, A.; Katagi, M.; Okuno, Y.; Mori, K.; Jono, S.; Koyama, H.; Nishizawa, Y. Induction of bone-type alkaline phosphatase in human vascular smooth muscle cells: Roles of tumor necrosis factor-alpha and oncostatin M derived from macrophages. Circ. Res. 2002, 91, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Narisawa, S.; Harmey, D.; Yadav, M.C.; O’Neill, W.C.; Hoylaerts, M.F.; Millan, J.L. Novel inhibitors of alkaline phosphatase suppress vascular smooth muscle cell calcification. J. Bone Miner. Res. 2007, 22, 1700–1710. [Google Scholar] [CrossRef]
- Shantouf, R.; Kovesdy, C.P.; Kim, Y.; Ahmadi, N.; Luna, A.; Luna, C.; Rambod, M.; Nissenson, A.R.; Budoff, M.J.; Kalantar-Zadeh, K. Association of serum alkaline phosphatase with coronary artery calcification in maintenance hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2009, 4, 1106–1114. [Google Scholar] [CrossRef] [PubMed]
- Clark-Greuel, J.N.; Connolly, J.M.; Sorichillo, E.; Narula, N.R.; Ropoport, H.S.; Mohler, E.R., III; Gorman, J.H., III; Gorman, R.C.; Levy, R.J. Transforming growth factor-β1 mechanisms in aortic valve calcification: Increased alkaline phosphatase and related events. Ann. Thorac. Surg. 2007, 83, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Giachelli, C.M. Vascular calcification: In vitro evidence for the role of inorganic phosphate. J. Am. Soc. Nephrol. 2003, 14, S300–S304. [Google Scholar] [CrossRef]
- Giachelli, C.M.; Speer, M.Y.; Li, X.; Rajachar, R.M.; Yang, H. Regulation of vascular calcification: Role of phosphate and osteopontin. Circ. Res. 2005, 96, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Giachelli, C.M. The emerging role of phosphate in vascular calcification. Kidney. Int. 2009, 75, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Jono, S.; McKee, M.D.; Murry, C.E.; Shioi, A.; Nishizawa, Y.; Mori, K.; Morii, H.; Giachelli, C.M. Phosphate regulation of vascular smooth muscle cell calcification. Circ. Res. 2000, 87, E10–E17. [Google Scholar] [CrossRef] [PubMed]
- Shanahan, C.M.; Crouthamel, M.H.; Kapustin, A.; Giachelli, C.M. Arterial calcification in chronic kidney disease: Key roles for calcium and phosphate. Circ. Res. 2011, 109, 697–711. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, H.Y.; Giachelli, C.M. Role of the sodium-dependent phosphate cotransporter, Pit-1, in vascular smooth muscle cell calcification. Circ. Res. 2006, 98, 905–912. [Google Scholar] [CrossRef]
- Li, X.; Giachelli, C.M. Sodium-dependent phosphate cotransporters and vascular calcification. Curr. Opin. Nephrol. Hypertens. 2007, 16, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, H.Y.; Giachelli, C.M. BMP-2 promotes phosphate uptake, phenotypic modulation, and calcification of human vascular smooth muscle cells. Atherosclerosis 2008, 199, 271–277. [Google Scholar] [CrossRef]
- Lee, J.K.; Ha, J.H.; Kim, D.K.; Kwon, J.; Cho, Y.E.; Kwun, I.S. Depletion of zinc causes osteoblast apoptosis with elevation of leptin secretion and phosphorylation of JAK2/STAT3. Nutrients 2022, 15, 77. [Google Scholar] [CrossRef]
- Cho, Y.E.; Choi, S.H.; Kwun, I.S. The Micronutrient Zinc in Human Health and Disease. In Emerging Solutions in Sustainable Food and Nutrition Security; Springer: Cham, Switzerland, 2023; pp. 289–304. Available online: https://link.springer.com/chapter/10.1007/978-3-031-40908-0_11 (accessed on 1 November 2023).
- Bellows, C.G.; Heersche, J.N.; Aubin, J.E. Inorganic phosphate added exogenously or released from beta-glycerophosphate initiates mineralization of osteoid nodules in vitro. Bone Miner. 1992, 17, 15–29. [Google Scholar] [CrossRef]
- Hu, Y.C.; Cheng, H.L.; Hsieh, B.S.; Huang, L.W.; Huang, T.C.; Chang, K.L. Arsenic trioxide affects bone remodeling by effects on osteoblast differentiation and function. Bone 2012, 50, 1406–1415. [Google Scholar] [CrossRef]
- Lau, W.L.; Festing, M.H.; Giachelli, C.M. Phosphate and vascular calcification: Emerging role of the sodium-dependent phosphate co-transporter PiT-1. Thromb. Haemost. 2010, 104, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Hessle, L.; Johnson, K.A.; Anderson, H.C.; Narisawa, S.; Sali, A.; Goding, J.W.; Terkeltaub, R.; Millán, J.L. Tissue-nonspecific alkaline phosphatase and plasma cell membrane glycoprotein-1 are central antagonistic regulators of bone mineralization. Proc. Natl. Acad. Sci. USA 2002, 99, 9445–9449. [Google Scholar] [CrossRef] [PubMed]
- Golub, E.E. Role of matrix vesicles in biomineralization. Biochim. Biophys. Acta 2009, 1790, 1592–1598. [Google Scholar] [CrossRef]
- Demer, L.; Tintut, Y. The bone-vascular axis in chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2010, 19, 349–353. [Google Scholar] [CrossRef]
- Giachelli, C.M.; Jono, S.; Shioi, A.; Nishizawa, Y.; Mori, K.; Morii, H. Vascular calcification and inorganic phosphate. Am. J. Kidney Dis. 2001, 38, S34–S37. [Google Scholar] [CrossRef] [PubMed]
- Yoshiko, Y.; Candeliere, G.A.; Maeda, N.; Aubin, J.E. Osteoblast autonomous Pi regulation via Pit1 plays a role in bone mineralization. Mol. Cell. Biol. 2007, 27, 4465–4474. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alcantara, E.H.; Kwon, J.-H.; Kang, M.-K.; Cho, Y.-E.; Kwun, I.-S. Zinc Deficiency Promotes Calcification in Vascular Smooth Muscle Cells Independent of Alkaline Phosphatase Action and Partly Impacted by Pit1 Upregulation. Nutrients 2024, 16, 291. https://doi.org/10.3390/nu16020291
Alcantara EH, Kwon J-H, Kang M-K, Cho Y-E, Kwun I-S. Zinc Deficiency Promotes Calcification in Vascular Smooth Muscle Cells Independent of Alkaline Phosphatase Action and Partly Impacted by Pit1 Upregulation. Nutrients. 2024; 16(2):291. https://doi.org/10.3390/nu16020291
Chicago/Turabian StyleAlcantara, Ethel H., Jae-Hee Kwon, Min-Kyung Kang, Young-Eun Cho, and In-Sook Kwun. 2024. "Zinc Deficiency Promotes Calcification in Vascular Smooth Muscle Cells Independent of Alkaline Phosphatase Action and Partly Impacted by Pit1 Upregulation" Nutrients 16, no. 2: 291. https://doi.org/10.3390/nu16020291
APA StyleAlcantara, E. H., Kwon, J.-H., Kang, M.-K., Cho, Y.-E., & Kwun, I.-S. (2024). Zinc Deficiency Promotes Calcification in Vascular Smooth Muscle Cells Independent of Alkaline Phosphatase Action and Partly Impacted by Pit1 Upregulation. Nutrients, 16(2), 291. https://doi.org/10.3390/nu16020291






