Magnesium and Potassium Supplementation for Systolic Blood Pressure Reduction in the General Normotensive Population: A Systematic Review and Subgroup Meta-Analysis for Optimal Dosage and Treatment Length
Abstract
1. Introduction
2. Methods
2.1. Eligibility Criteria
2.2. Information Sources and Search Strategy
2.3. Selection Process
2.4. Data Collection Process and Data Items
2.5. Study Risk-of-Bias Assessment
2.6. Effect Measures and Synthesis Methods
2.7. Reporting Bias Assessment
3. Results
3.1. Study Selection
3.2. Study Characteristics
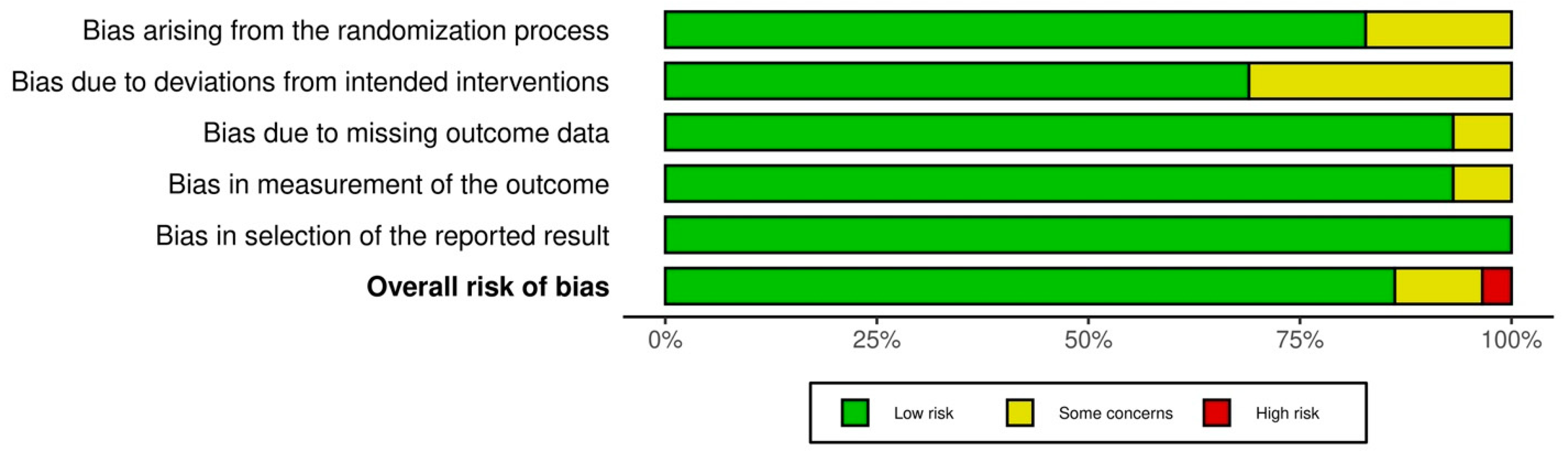
3.3. Risk of Bias in Studies
3.4. Results of Syntheses/Statistical Analysis
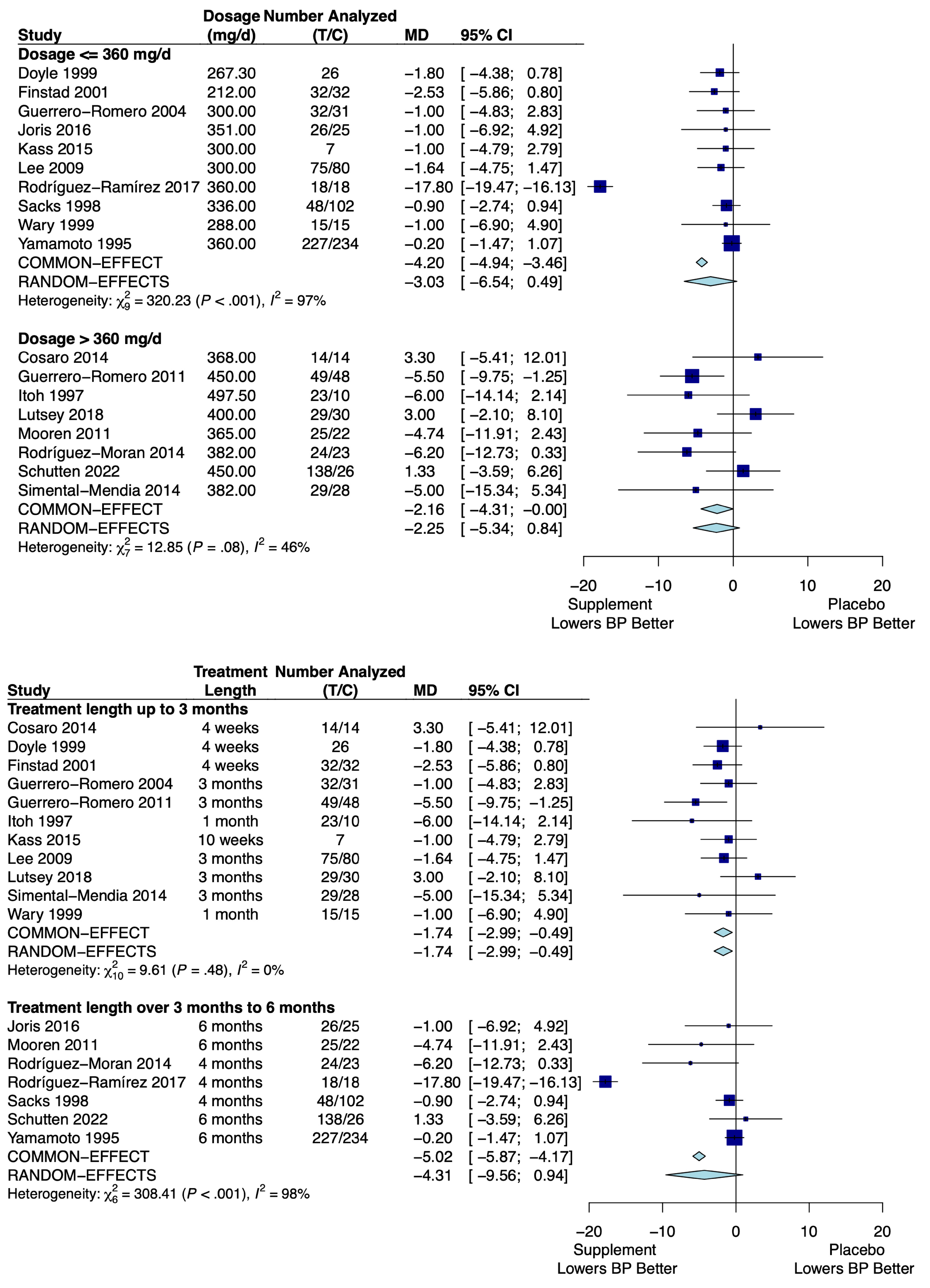
3.4.1. Magnesium
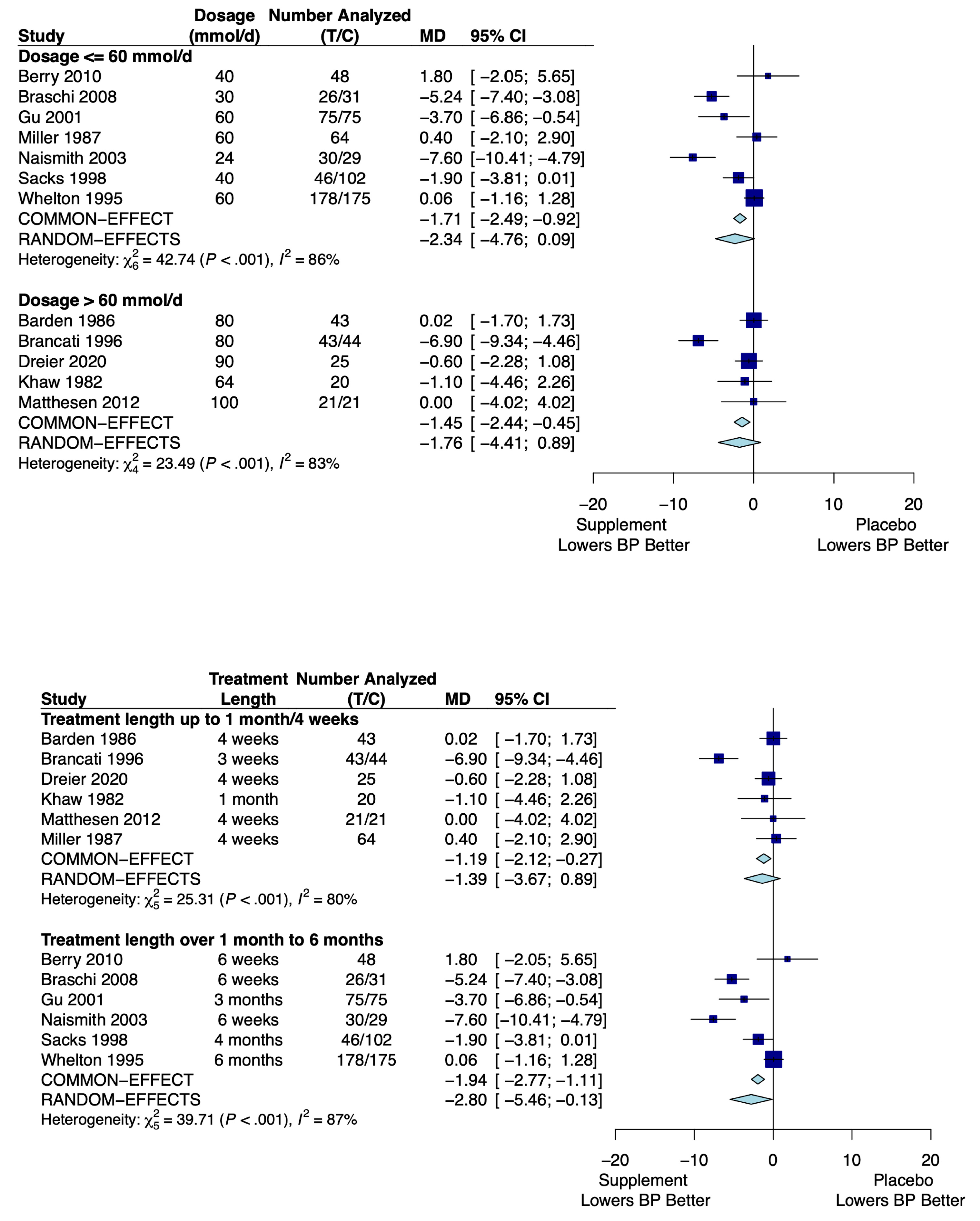
3.4.2. Potassium
3.5. Sensitivity Analysis
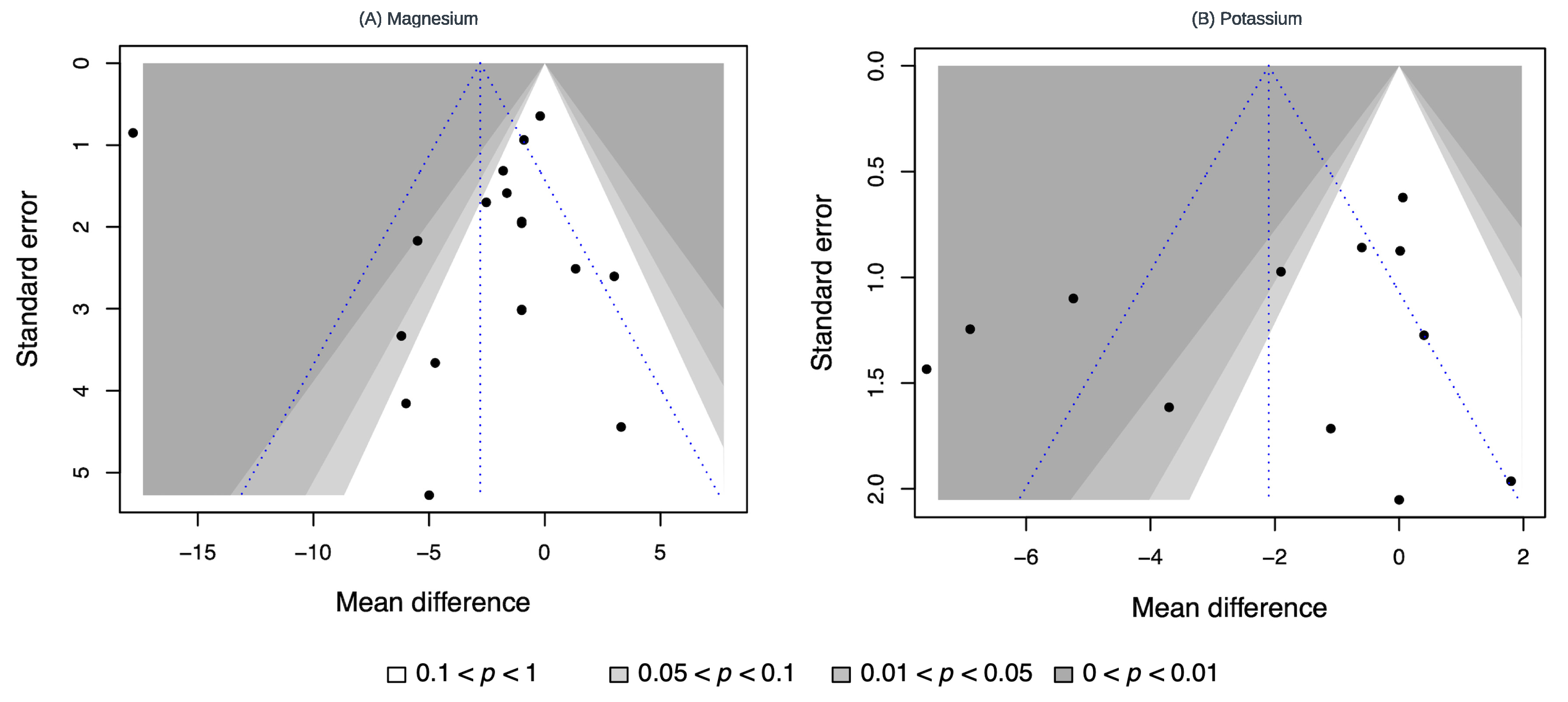
3.6. Publication Bias
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Amini, M.; Zayeri, F.; Salehi, M. Trend analysis of cardiovascular disease mortality, incidence, and mortality-to-incidence ratio: Results from global burden of disease study 2017. BMC Public Health 2021, 21, 401. [Google Scholar] [CrossRef] [PubMed]
- Olvera Lopez, E.; Ballard, B.D.; Jan, A. Cardiovascular Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK535419/ (accessed on 23 March 2024).
- Frąk, W.; Wojtasińska, A.; Lisińska, W.; Młynarska, E.; Franczyk, B.; Rysz, J. Pathophysiology of Cardiovascular Diseases: New Insights into Molecular Mechanisms of Atherosclerosis, Arterial Hypertension, and Coronary Artery Disease. Biomedicines 2022, 10, 1938. [Google Scholar] [CrossRef] [PubMed]
- Laronha, H.; Caldeira, J. Structure and Function of Human Matrix Metalloproteinases. Cells 2020, 9, 1076. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Khalil, R.A. Matrix Metalloproteinases, Vascular Remodeling, and Vascular Disease. Adv. Pharmacol. 2018, 81, 241–330. [Google Scholar] [PubMed]
- Wenzel, P. Monocytes as immune targets in arterial hypertension. Br. J. Pharmacol. 2019, 176, 1966–1977. [Google Scholar] [CrossRef]
- Mirhafez, S.R.; Mohebati, M.; Feiz Disfani, M.; Saberi Karimian, M.; Ebrahimi, M.; Avan, A.; Eslami, S.; Pasdar, A.; Rooki, H.; Esmaeili, H.; et al. An imbalance in serum concentrations of inflammatory and anti-inflammatory cytokines in hypertension. J. Am. Soc. Hypertens. 2014, 8, 614–623. [Google Scholar] [CrossRef]
- Rodrigo, R.; González, J.; Paoletto, F. The role of oxidative stress in the pathophysiology of hypertension. Hypertens. Res. 2011, 34, 431–440. [Google Scholar] [CrossRef]
- Crowley, S.D. The cooperative roles of inflammation and oxidative stress in the pathogenesis of hypertension. Antioxid. Redox Signal 2014, 20, 102–120. [Google Scholar] [CrossRef]
- Camargo, L.L.; Rios, F.J.; Montezano, A.C.; Touyz, R.M. Reactive oxygen species in hypertension. Nat. Rev. Cardiol. 2024. Epub ahead of print. [Google Scholar] [CrossRef]
- Park, J.M.; Do, V.Q.; Seo, Y.S.; Kim, H.J.; Nam, J.H.; Yin, M.Z.; Kim, H.J.; Kim, S.J.; Griendling, K.K.; Lee, M.Y. NADPH Oxidase 1 Mediates Acute Blood Pressure Response to Angiotensin II by Contributing to Calcium Influx in Vascular Smooth Muscle Cells. Arter. Thromb. Vasc. Biol. 2022, 42, e117–e130. [Google Scholar] [CrossRef]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey DEJr Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; MacLaughlin, E.J.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018, 71, e13–e115. [Google Scholar] [PubMed]
- Vidt, D.G.; Borazanian, R.A. Treat high blood pressure sooner: Tougher, simpler JNC 7 guidelines. Cleve Clin. J. Med. 2003, 70, 721–728. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Law, M.R.; Morris, J.K.; Wald, N.J. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: Meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ 2009, 338, b1665. [Google Scholar] [CrossRef] [PubMed]
- Lewington, S.; Clarke, R.; Qizilbash, N.; Peto, R.; Collins, R.; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002, 360, 1903–1913. [Google Scholar]
- Behers, B.J.; Melchor, J.; Behers, B.M.; Meng, Z.; Swanson, P.J.; Paterson, H.I.; Mendez Araque, S.J.; Davis, J.L.; Gerhold, C.J.; Shah, R.S.; et al. Vitamins and Minerals for Blood Pressure Reduction in the General, Normotensive Population: A Systematic Review and Meta-Analysis of Six Supplements. Nutrients 2023, 15, 4223. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar]
- Covidence Systematic Review Software, Veritas Health Innovation, Melbourne, Australia [Internet]. Available online: https://www.covidence.org (accessed on 23 March 2024).
- Higgins, J.P.T.; Savović, J.; Page, M.J.; Elbers, R.G.; Sterne, J.A.C. Assessing Risk of Bias in a Randomized Trial. In Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons, Ltd.: Chichester, UK, 2019; pp. 205–228. [Google Scholar]
- Cochran, W.G. The Combination of Estimates From Different Experiments. Biometrics 1954, 10, 101–129. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Langan, D.; Higgins, J.P.T.; Jackson, D.; Bowden, J.; Veroniki, A.A.; Kontopantelis, E.; Viechtbauer, W.; Simmonds, M. A comparison of heterogeneity variance estimators in simulated random-effects meta-analyses. Res. Synth. Methods 2019, 10, 83–98. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Li, T.; Deeks, J.J. Choosing Effect Measures and Commuting Estimates of Effect. In Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons, Ltd.: Chichester, UK, 2019; pp. 143–176. [Google Scholar]
- Higgins, J.P.T.; Eldridge, S.; Li, T. Including Variants on Randomized Trials. In Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons, Ltd.: Chichester, UK, 2019; pp. 569–593. [Google Scholar]
- Schwarzer, G.; Carpenter, J.R.; Rücker, G. Meta-Analysis with R, 1st ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 1–104. [Google Scholar]
- Song, F.; Gilbody, S. Bias in meta-analysis detected by a simple, graphical test. Increase in studies of publication bias coincided with increasing use of meta-analysis. BMJ 1998, 316, 471. [Google Scholar]
- Light, R.J.; Pillemer, D.B. Summing Up: The Science of Reviewing Research; Harvard University Press: Cambridge, UK, 1984; pp. 1–212. [Google Scholar]
- Meng, Z.; Wu, C.; Lin, L. The Effect Direction Should Be Taken into Account When Assessing Small-Study Effects. J. Evid. Based Dent. Pract. 2023, 23, 101830. [Google Scholar] [CrossRef] [PubMed]
- Sacks, F.M.; Willett, W.C.; Smith, A.; Brown, L.E.; Rosner, B.; Moore, T.J. Effect on blood pressure of potassium, calcium, and magnesium in women with low habitual intake. Hypertension 1998, 31, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Cosaro, E.; Bonafini, S.; Montagnana, M.; Danese, E.; Trettene, M.S.; Minuz, P.; Delva, P.; Fava, C. Effects of magnesium supplements on blood pressure, endothelial function and metabolic parameters in healthy young men with a family history of metabolic syndrome. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.; Flynn, A.; Cashman, K. The effect of magnesium supplementation on biochemical markers of bone metabolism or blood pressure in healthy young adult females. Eur. J. Clin. Nutr. 1999, 53, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Finstad, E.W.; Newhouse, I.J.; Lukaski, H.C.; Mcauliffe, J.E.; Stewart, C.R. The effects of magnesium supplementation on exercise performance. Med. Sci. Sports Exerc. 2001, 33, 493–498. [Google Scholar] [CrossRef]
- Guerrero-Romero, F.; Tamez-Perez, H.E.; González-González, G.; Salinas-Martínez, A.M.; Montes-Villarreal, J.; Treviño-Ortiz, J.H.; Rodríguez-Morán, M. Oral magnesium supplementation improves insulin sensitivity in non-diabetic subjects with insulin resistance. A double-blind placebo-controlled randomized trial. Diabetes Metab. 2004, 30, 253–258. [Google Scholar] [CrossRef]
- Guerrero-Romero, F.; Rodríguez-Morán, M. Magnesium improves the beta-cell function to compensate variation of insulin sensitivity: Double-blind, randomized clinical trial. Eur. J. Clin. Investig. 2011, 41, 405–410. [Google Scholar] [CrossRef]
- Itoh, K.; Kawasaka, T.; Nakamura, M. The effects of high oral magnesium supplementation on blood pressure, serum lipids and related variables in apparently healthy Japanese subjects. Br. J. Nutr. 1997, 78, 737–750. [Google Scholar] [CrossRef]
- Joris, P.J.; Plat, J.; Bakker, S.J.; Mensink, R.P. Long-term magnesium supplementation improves arterial stiffness in overweight and obese adults: Results of a randomized, double-blind, placebo-controlled intervention trial. Am. J. Clin. Nutr. 2016, 103, 1260–1266. [Google Scholar] [CrossRef]
- Kass, L.S.; Poeira, F. The effect of acute vs chronic magnesium supplementation on exercise and recovery on resistance exercise, blood pressure and total peripheral resistance on normotensive adults. J. Int. Soc. Sports Nutr. 2015, 12, 19. [Google Scholar] [CrossRef]
- Lee, S.; Park, H.K.; Son, S.P.; Lee, C.W.; Kim, I.J.; Kim, H.J. Effects of oral magnesium supplementation on insulin sensitivity and blood pressure in normo-magnesemic nondiabetic overweight Korean adults. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Lutsey, P.L.; Chen, L.Y.; Eaton, A.; Jaeb, M.; Rudser, K.D.; Neaton, J.D.; Alonso, A. A Pilot Randomized Trial of Oral Magnesium Supplementation on Supraventricular Arrhythmias. Nutrients 2018, 10, 884. [Google Scholar] [CrossRef] [PubMed]
- Mooren, F.C.; Krüger, K.; Völker, K.; Golf, S.W.; Wadepuhl, M.; Kraus, A. Oral magnesium supplementation reduces insulin resistance in non-diabetic subjects—A double-blind, placebo-controlled, randomized trial. Diabetes Obes. Metab. 2011, 13, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Moran, M.; Guerrero-Romero, F. Oral magnesium supplementation improves the metabolic profile of metabolically obese, normal-weight individuals: A randomized double-blind placebo-controlled trial. Arch. Med. Res. 2014, 45, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Ramírez, M.; Rodríguez-Morán, M.; Reyes-Romero, M.A.; Guerrero-Romero, F. Effect of oral magnesium supplementation on the transcription of TRPM6, TRPM7, and SLC41A1 in individuals newly diagnosed of pre-hypertension. A randomized, double-blind, placebo-controlled trial. Magnes. Res. 2017, 30, 80–87. [Google Scholar] [CrossRef]
- Schutten, J.C.; Joris, P.J.; Groendijk, I.; Eelderink, C.; Groothof, D.; van der Veen, Y.; Westerhuis, R.; Goorman, F.; Danel, R.M.; de Borst, M.H.; et al. Effects of Magnesium Citrate, Magnesium Oxide, and Magnesium Sulfate Supplementation on Arterial Stiffness: A Randomized, Double-Blind, Placebo-Controlled Intervention Trial. J. Am. Heart Assoc. 2022, 11, e021783. [Google Scholar] [CrossRef]
- Simental-Mendía, L.E.; Rodríguez-Morán, M.; Guerrero-Romero, F. Oral magnesium supplementation decreases C-reactive protein levels in subjects with prediabetes and hypomagnesemia: A clinical randomized double-blind placebo-controlled trial. Arch. Med. Res. 2014, 45, 325–330. [Google Scholar] [CrossRef]
- Wary, C.; Brillault-Salvat, C.; Bloch, G.; Leroy-Willig, A.; Roumenov, D.; Grognet, J.M.; Leclerc, J.H.; Carlier, P.G. Effect of chronic magnesium supplementation on magnesium distribution in healthy volunteers evaluated by 31P-NMRS and ion selective electrodes. Br. J. Clin. Pharmacol. 1999, 48, 655–662. [Google Scholar] [CrossRef]
- Yamamoto, M.E.; Applegate, W.B.; Klag, M.J.; Borhani, N.O.; Cohen, J.D.; Kirchner, K.A.; Lakatos, E.; Sacks, F.M.; Taylor, J.O.; Hennekens, C.H. Lack of blood pressure effect with calcium and magnesium supplementation in adults with high-normal blood pressure. Results from Phase I of the Trials of Hypertension Prevention (TOHP). Trials of Hypertension Prevention (TOHP) Collaborative Research Group. Ann. Epidemiol. 1995, 5, 96–107. [Google Scholar] [CrossRef]
- Barden, A.E.; Vandongen, R.; Beilin, L.J.; Margetts, B.; Rogers, P. Potassium supplementation does not lower blood pressure in normotensive women. J. Hypertens. 1986, 4, 339–343. [Google Scholar] [CrossRef]
- Berry, S.E.; Mulla, U.Z.; Chowienczyk, P.J.; Sanders, T.A. Increased potassium intake from fruit and vegetables or supplements does not lower blood pressure or improve vascular function in UK men and women with early hypertension: A randomised controlled trial. Br. J. Nutr. 2010, 104, 1839–1847. [Google Scholar] [CrossRef] [PubMed]
- Brancati, F.L.; Appel, L.J.; Seidler, A.J.; Whelton, P.K. Effect of potassium supplementation on blood pressure in African Americans on a low-potassium diet. A randomized, double-blind, placebo-controlled trial. Arch. Intern. Med. 1996, 156, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Braschi, A.; Naismith, D.J. The effect of a dietary supplement of potassium chloride or potassium citrate on blood pressure in predominantly normotensive volunteers. Br. J. Nutr. 2008, 99, 1284–1292. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dreier, R.; Abdolalizadeh, B.; Asferg, C.L.; Hölmich, L.R.; Buus, N.H.; Forman, J.L.; Andersen, U.B.; Egfjord, M.; Sheykhzade, M.; Jeppesen, J.L. Effect of increased potassium intake on the renin-angiotensin-aldosterone system and subcutaneous resistance arteries: A randomized crossover study. Nephrol. Dial. Transplant. 2020, gfaa114. [Google Scholar]
- Gu, D.; He, J.; Wu, X.; Duan, X.; Whelton, P.K. Effect of potassium supplementation on blood pressure in Chinese: A randomized, placebo-controlled trial. J. Hypertens. 2001, 19, 1325–1331. [Google Scholar] [CrossRef]
- Khaw, K.T.; Thom, S. Randomised double-blind cross-over trial of potassium on blood-pressure in normal subjects. Lancet 1982, 2, 1127–1129. [Google Scholar] [CrossRef]
- Matthesen, S.K.; Larsen, T.; Vase, H.; Lauridsen, T.G.; Pedersen, E.B. Effect of potassium supplementation on renal tubular function, ambulatory blood pressure and pulse wave velocity in healthy humans. Scand. J. Clin. Lab. Investig. 2012, 72, 78–86. [Google Scholar] [CrossRef]
- Miller, J.Z.; Weinberger, M.H.; Christian, J.C. Blood pressure response to potassium supplementation in normotensive adults and children. Hypertension 1987, 10, 437–442. [Google Scholar] [CrossRef]
- Naismith, D.J.; Braschi, A. The effect of low-dose potassium supplementation on blood pressure in apparently healthy volunteers. Br. J. Nutr. 2003, 90, 53–60. [Google Scholar] [CrossRef]
- Whelton, P.K.; Buring, J.; Borhani, N.O.; Cohen, J.D.; Cook, N.; Cutler, J.A.; Kiley, J.E.; Kuller, L.H.; Satterfield, S.; Sacks, F.M.; et al. The effect of potassium supplementation in persons with a high-normal blood pressure. Results from phase I of the Trials of Hypertension Prevention (TOHP). Trials of Hypertension Prevention (TOHP) Collaborative Research Group. Ann. Epidemiol. 1995, 5, 85–95. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Del Gobbo, L.C.; Rosanoff, A.; Wang, J.; Zhang, W.; Song, Y. Effects of Magnesium Supplementation on Blood Pressure: A Meta-Analysis of Randomized Double-Blind Placebo-Controlled Trials. Hypertension 2016, 68, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Jee, S.H.; Miller, E.R., 3rd; Guallar, E.; Singh, V.K.; Appel, L.J.; Klag, M.J. The effect of magnesium supplementation on blood pressure: A meta-analysis of randomized clinical trials. Am. J. Hypertens. 2002, 15, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Filippini, T.; Naska, A.; Kasdagli, M.I.; Torres, D.; Lopes, C.; Carvalho, C.; Moreira, P.; Malavolti, M.; Orsini, N.; Whelton, P.K.; et al. Potassium Intake and Blood Pressure: A Dose-Response Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2020, 9, e015719. [Google Scholar] [CrossRef] [PubMed]
- Poorolajal, J.; Zeraati, F.; Soltanian, A.R.; Sheikh, V.; Hooshmand, E.; Maleki, A. Oral potassium supplementation for management of essential hypertension: A meta-analysis of randomized controlled trials. PLoS ONE 2017, 12, e0174967. [Google Scholar] [CrossRef]
- Schutten, J.C.; Joosten, M.M.; de Borst, M.H.; Bakker, S.J.L. Magnesium and Blood Pressure: A Physiology-Based Approach. Adv. Chronic Kidney Dis. 2018, 25, 244–250. [Google Scholar] [CrossRef]
- Montezano, A.C.; Zimmerman, D.; Yusuf, H.; Burger, D.; Chignalia, A.Z.; Wadhera, V.; van Leeuwen, F.N.; Touyz, R.M. Vascular smooth muscle cell differentiation to an osteogenic phenotype involves TRPM7 modulation by magnesium. Hypertension 2010, 56, 453–462. [Google Scholar] [CrossRef]
- Shimosawa, T.; Takano, K.; Ando, K.; Fujita, T. Magnesium inhibits norepinephrine release by blocking N-type calcium channels at peripheral sympathetic nerve endings. Hypertension 2004, 44, 897–902. [Google Scholar] [CrossRef]
- Pearson, P.J.; Evora, P.R.; Seccombe, J.F.; Schaff, H.V. Hypomagnesemia inhibits nitric oxide release from coronary endothelium: Protective role of magnesium infusion after cardiac operations. Ann. Thorac. Surg. 1998, 65, 967–972. [Google Scholar] [CrossRef]
- Watson, K.V.; Moldow, C.F.; Ogburn, P.L.; Jacob, H.S. Magnesium sulfate: Rationale for its use in preeclampsia. Proc. Natl. Acad. Sci. USA 1986, 83, 1075–1078. [Google Scholar] [CrossRef]
- Wong, N.L.; Hu, D.C.; Wong, E.F. Effect of dietary magnesium on atrial natriuretic peptide release. Am. J. Physiol. 1991, 261 Pt 2, H1353-7. [Google Scholar] [CrossRef]
- Atarashi, K.; Matsuoka, H.; Takagi, M.; Sugimoto, T. Magnesium ion: A possible physiological regulator of aldosterone production. Life Sci. 1989, 44, 1483–1489. [Google Scholar] [CrossRef] [PubMed]
- Haddy, F.J.; Vanhoutte, P.M.; Feletou, M. Role of potassium in regulating blood flow and blood pressure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, R546–R552. [Google Scholar] [CrossRef] [PubMed]
- Ellison, D.H.; Terker, A.S. Why Your Mother Was Right: How Potassium Intake Reduces Blood Pressure. Trans. Am. Clin. Clim. Assoc. 2015, 126, 46–55. [Google Scholar]
- Houston, M.C. The importance of potassium in managing hypertension. Curr. Hypertens. Rep. 2011, 13, 309–317. [Google Scholar] [CrossRef]
- Langan, D. Assessing Heterogeneity in Random-Effects Meta-analysis. Methods Mol. Biol. 2022, 2345, 67–89. [Google Scholar]

| Database | Search String |
|---|---|
| Cochrane Date run: 22 July 2022 Number of results: 4497 | ([mh “ascorbic acid”] OR [mh “vitamin D”] OR [mh “vitamin E”] OR [mh “Vitamin B Complex”] OR “vitamin C”:ti,ab OR “ascorbic acid”:ti,ab OR “vitamin D”:ti,ab OR “vitamin E”:ti,ab OR “vitamin B”:ti,ab OR (“B” NEXT vitamin*):ti,ab OR [mh calcium] OR [mh magnesium] OR [mh potassium] OR calcium:ti,ab OR magnesium:ti,ab OR potassium:ti,ab) AND ([mh “blood pressure”] OR “blood pressure”:ti,ab) “Randomized controlled trial”:pt OR “controlled clinical trial”:pt |
| Embase Date run: 22 July 2022 Number of results: 6585 | (“ascorbic acid”/exp OR “vitamin D”/exp OR “vitamin E”/exp OR “Vitamin B Complex”/exp OR “vitamin C”:ti,ab OR “ascorbic acid”:ti,ab OR “vitamin D”:ti,ab OR “vitamin E”:ti,ab OR “vitamin B”:ti,ab OR “B vitamin*”:ti,ab OR calcium/exp OR magnesium/exp OR potassium/exp OR calcium:ti,ab OR magnesium:ti,ab OR potassium:ti,ab) AND (“blood pressure”/exp OR “blood pressure”:ti,ab) “randomized controlled trial” OR “controlled clinical trial” |
| Medline (PubMed) Date run: 22 July 2022 Number of results: 4579 | (ascorbic acid[MeSH] OR vitamin D[MeSH] OR vitamin E[MeSH] OR Vitamin B Complex[MeSH] OR “vitamin C”[tiab] OR “ascorbic acid”[tiab] OR “vitamin D”[tiab] OR “vitamin E”[tiab] OR “vitamin B”[tiab] OR B vitamin*[tiab] OR calcium[MeSH] OR magnesium[MeSH] OR potassium[MeSH] OR calcium[tiab] OR magnesium[tiab] OR potassium[tiab]) AND (blood pressure[MeSH] OR “blood pressure”[tiab]) Randomized controlled trial[pt] OR controlled clinical trial[pt] |
| Web of Science (Core Collection) Date run: 22 July 2022 Number of results: 537 | (TI = “vitamin C” OR AB = “vitamin C”) OR (TI = “ascorbic acid” OR AB = “ascorbic acid”) OR (TI = “vitamin D” OR AB = “vitamin D”) OR (TI = “vitamin E” OR AB = “vitamin E”) OR (TI = “vitamin B” OR AB = “vitamin B”) OR (TI = “B vitamin*” OR AB = “B vitamin*”) OR (TI = calcium OR AB = calcium) OR (TI = magnesium OR AB = magnesium) OR (TI = potassium OR AB = potassium) AND (TI = “blood pressure” OR AB = “blood pressure”) ALL = “Randomized controlled trial” OR ALL = “controlled clinical trial” |
| Study | Country | Type of Trial | Population (% CVD) | Mean Age (Years) | Baseline BP (mm Hg) | Dosage (mg/Day) | Trial Duration |
|---|---|---|---|---|---|---|---|
| Cosaro 2014 [30] | Italy | Cross-over | Healthy (0%) | 26.3 | 123.7/71.4 | 368 | 4 weeks |
| Doyle 1999 [31] | Ireland | Cross-over | Healthy (0%) | 23 | 112.1/75.9 | 267.3 | 4 weeks |
| Finstad 2001 [32] | Canada | Cross-over | General (0%) | 21.2 | 114.3/69.4 | 212 | 4 weeks |
| Guerrero-Romero 2004 [33] | Mexico | Parallel | Healthy (0%) | 42.6 | 110.5/73 | 300 | 3 months |
| Guerrero-Romero 2011 [34] | Mexico | Parallel | General (0%) | 40.6 | 116.6/73.8 | 450 | 3 months |
| Itoh 1997 [35] | Japan | Parallel | Healthy (0%) | 64.6 | 127.3/76.1 | 497.5 | 1 month |
| Joris 2016 [36] | Netherlands | Parallel | Overweight (0%) | 62 | 128/81.5 | 351 | 6 months |
| Kass 2015 [37] | England | Cross-over | General (0%) | 40.8 | 118.4/81.6 | 300 | 10 weeks |
| Lee 2009 [38] | South Korea | Parallel | Overweight (0%) | 40.1 | 125.7/83.4 | 300 | 3 months |
| Lutsey 2018 [39] | United States of America | Parallel | General (24%) | 61.5 | 119/71 | 400 | 3 months |
| Mooren 2011 [40] | Germany | Parallel | Overweight (0%) | N/R | 136.3/84 | 365 | 6 months |
| Rodriguez-Moran 2014 [41] | Mexico | Parallel | Healthy (0%) | 35.6 | 111.8/71.5 | 382 | 4 months |
| Rodriguez-Ramirez 2017 [42] | Mexico | Parallel | General (0%) | 51.8 | 127.6/77.3 | 360 | 4 months |
| Sacks 1998 [29] | United States of America | Parallel | Healthy (0%) | 38.3 | 115.3/73 * | 336 | 4 months |
| Schutten 2022 [43] | Netherlands | Parallel | Overweight (37.2%) | 63.2 | 130/79 | 450 | 6 months |
| Simental-Mendia 2014 [44] | Mexico | Parallel | General (0%) | 40.4 | 115.2/74.6 | 382 | 3 months |
| Wary 1999 [45] | France | Parallel | Healthy (0%) | 23.7 | 126.5/76.5 | 288 | 1 month |
| Yamamoto 1995 [46] | United States of America | Parallel | Healthy (0%) | 42.5 | 125/84 | 360 | 6 months |
| Study | Country | Type of Trial | Population (% CVD) | Mean Age (Years) | Baseline BP (mm Hg) | Dosage (mmol/Day) | Duration |
| Barden 1986 [47] | Australia | Cross-over | Healthy (0%) | 31.5 | 117.5/71.4 | 80 | 4 weeks |
| Berry 2010 [48] | England | Cross-over | General (0%) | 45.1 | 137/89 * | 40 | 6 weeks |
| Brancati 1996 [49] | United States of America | Parallel | Healthy (0%) | 48.0 | 126.2/77.6 | 80 | 3 weeks |
| Braschi 2008 [50] | England | Parallel | General (0%) | 35.5 | 111.3/68.2 | 30 | 6 weeks |
| Dreier 2020 [51] | Denmark | Cross-over | Healthy (0%) | 26.3 | 119.7/72.6 * | 90 | 4 weeks |
| Gu 2001 [52] | United States of America | Parallel | General (0%) | 56.0 | 135.5/82.3 | 60 | 3 months |
| Khaw 1982 [53] | England | Cross-over | Healthy (0%) | N/R | 118/73.5 | 64 | 1 month |
| Matthesen 2012 [54] | Denmark | Cross-over | Healthy (0%) | 26 | 116/71 | 100 | 4 weeks |
| Miller 1987 [55] | United States of America | Parallel | General (0%) | 42 | 113.2/73.1 | 60 | 4 weeks |
| Naismith 2003 [56] | England | Parallel | General (0%) | 43.1 | 117/73 | 24 | 6 weeks |
| Sacks 1998 [29] | United States of America | Parallel | Healthy (0%) | 38.3 | 116/73 * | 40 | 4 months |
| Whelton 1995 [57] | United States of America | Parallel | Healthy (0%) | 23.7 | 121.6/80.9 | 60 | 6 months |
| Subgroup | SBP Effect |
|---|---|
| Dose ≤ 360 mg/day | −3.03 mm Hg (−6.54, 0.49) |
| Dose > 360 mg/day | −2.25 mm Hg (−5.34, 0.84) |
| Treatment < 3 months | −1.74 mm Hg (−2.99, −0.49) |
| Treatment > 3 months | −4.31 mm Hg (−9.56, 0.94) |
| Subgroup | SBP Effect |
|---|---|
| Dose ≤ 60 mmol/day | −2.34 mm Hg (−4.76, 0.09) |
| Dose > 60 mmol/day | −1.76 mm Hg (−4.41, 0.89) |
| Treatment ≤ 1 month | −1.39 mm Hg (−3.67, 0.89) |
| Treatment > 1 month | −2.80 mm Hg (−5.46, −0.13) |
| Supplement | Baseline-End Corr | Cross-over Corr | I2 (%) | CE (95% CI) | RE (95% CI) | PB |
|---|---|---|---|---|---|---|
| Magnesium (18 studies) | 0.7 | 0.9 | 95 | −3.57 (−4.21, −2.93) | −2.78 (−5.22, −0.34) | N |
| 0.7 | 95 | −3.99 (−4.69, −3.29) | −2.79 (−5.25, −0.34) | N | ||
| 0.5 | 95 | −4.10 (−4.81, −3.38) | −2.81 (−5.28, −0.33) | N | ||
| 0.5 | 0.9 | 95 | −3.64 (−4.29, −2.98) | −2.76 (−5.31, −0.21) | N | |
| 0.7 | 95 | −4.10 (−4.83, −3.38) | −2.77 (−5.34, −0.20) | N | ||
| 0.5 | 95 | −4.23 (−4.97, −3.48) | −2.78 (−5.37, −0.19) | N | ||
| Potassium (12 studies) | 0.7 | 0.9 | 85 | −1.05 (−1.52, −0.57) | −2.03 (−3.71, −0.36) | Y |
| 0.7 | 83 | −1.61 (−2.22, −0.99) | −2.10 (3.81, −0.38) | N | ||
| 0.5 | 83 | −1.83 (−2.50, −1.16) | −2.15 (−3.90, −0.40) | N | ||
| 0.5 | 0.9 | 85 | −1.05 (−1.53, −0.58) | −2.06 (−3.74, −0.37) | Y | |
| 0.7 | 83 | −1.62 (−2.24, −1.00) | −2.12 (−3.85, −0.39) | N | ||
| 0.5 | 82 | −1.85 (−2.52, −1.18) | −2.18 (−3.94, −0.42) | N |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Behers, B.J.; Behers, B.M.; Stephenson-Moe, C.A.; Vargas, I.A.; Meng, Z.; Thompson, A.J.; Melchor, J.; Wojtas, C.N.; Rosario, M.A.; Baker, J.F.; et al. Magnesium and Potassium Supplementation for Systolic Blood Pressure Reduction in the General Normotensive Population: A Systematic Review and Subgroup Meta-Analysis for Optimal Dosage and Treatment Length. Nutrients 2024, 16, 3617. https://doi.org/10.3390/nu16213617
Behers BJ, Behers BM, Stephenson-Moe CA, Vargas IA, Meng Z, Thompson AJ, Melchor J, Wojtas CN, Rosario MA, Baker JF, et al. Magnesium and Potassium Supplementation for Systolic Blood Pressure Reduction in the General Normotensive Population: A Systematic Review and Subgroup Meta-Analysis for Optimal Dosage and Treatment Length. Nutrients. 2024; 16(21):3617. https://doi.org/10.3390/nu16213617
Chicago/Turabian StyleBehers, Benjamin J., Brett M. Behers, Christoph A. Stephenson-Moe, Ian A. Vargas, Zhuo Meng, Anthony J. Thompson, Julian Melchor, Caroline N. Wojtas, Manuel A. Rosario, Joel F. Baker, and et al. 2024. "Magnesium and Potassium Supplementation for Systolic Blood Pressure Reduction in the General Normotensive Population: A Systematic Review and Subgroup Meta-Analysis for Optimal Dosage and Treatment Length" Nutrients 16, no. 21: 3617. https://doi.org/10.3390/nu16213617
APA StyleBehers, B. J., Behers, B. M., Stephenson-Moe, C. A., Vargas, I. A., Meng, Z., Thompson, A. J., Melchor, J., Wojtas, C. N., Rosario, M. A., Baker, J. F., Deevers, A. C., Mouratidis, R. W., & Sweeney, M. J. (2024). Magnesium and Potassium Supplementation for Systolic Blood Pressure Reduction in the General Normotensive Population: A Systematic Review and Subgroup Meta-Analysis for Optimal Dosage and Treatment Length. Nutrients, 16(21), 3617. https://doi.org/10.3390/nu16213617







