Beneficial Effects of a Freeze-Dried Kale Bar on Type 2 Diabetes Patients: A Randomized, Double-Blinded, Placebo-Controlled Clinical Trial
Highlights
- Freeze-dried kale powder incorporated into a snack bar has surprisingly positive effects on patients with type 2 diabetes;
- To measure changes in body composition (fat/muscle), a whole-body dual-energy X-ray absorptiometry (DEXA) scan test was conducted, showing the effect of increased intake of freeze-dried kale powder;
- The consumption of freeze-dried kale powder has an appetite-regulating effect on type 2 diabetes patients, possibly due to the increased secretion of GLP-1 from the gut.
Abstract
1. Introduction
2. Materials and Methods
2.1. Recruitment
2.2. Kale Bar and Placebo Bar Formulation
2.3. Biochemical Analysis
2.4. Data Analysis
3. Results
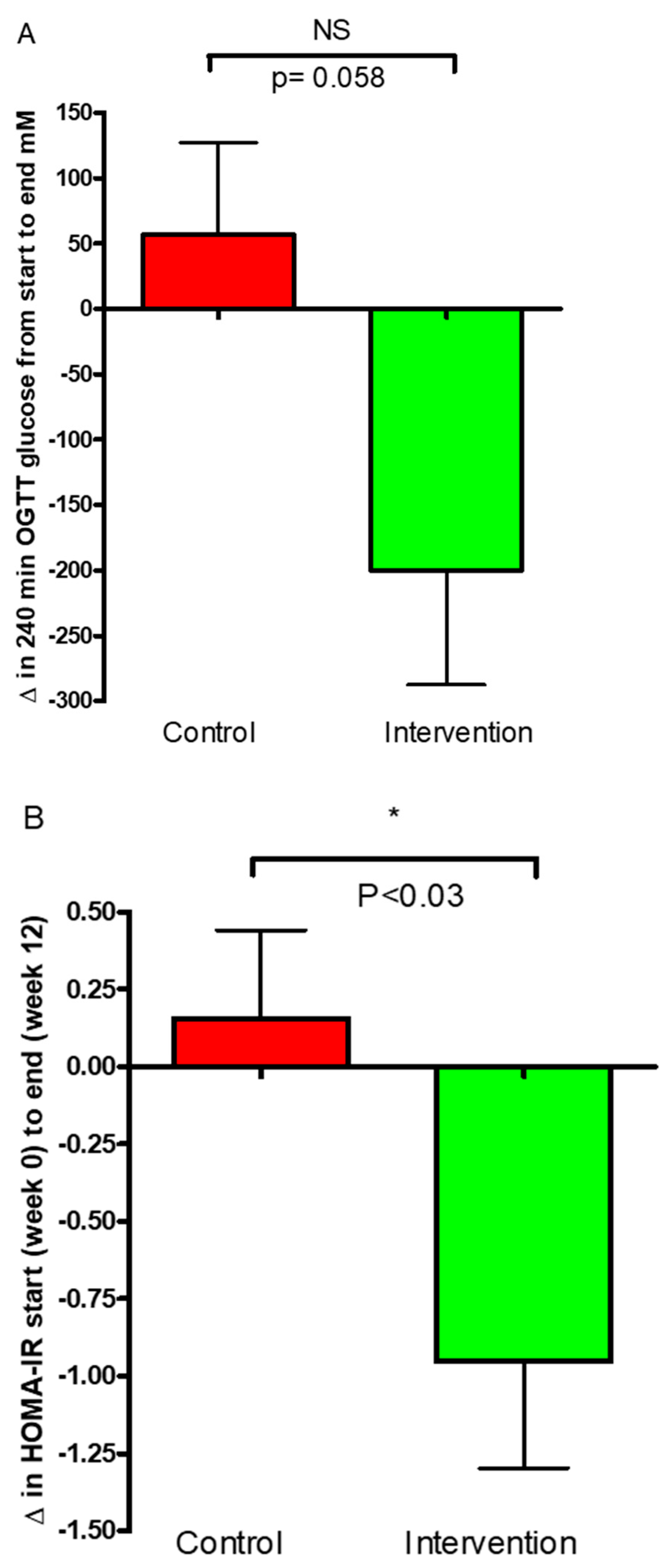
3.1. Glucose Regulation
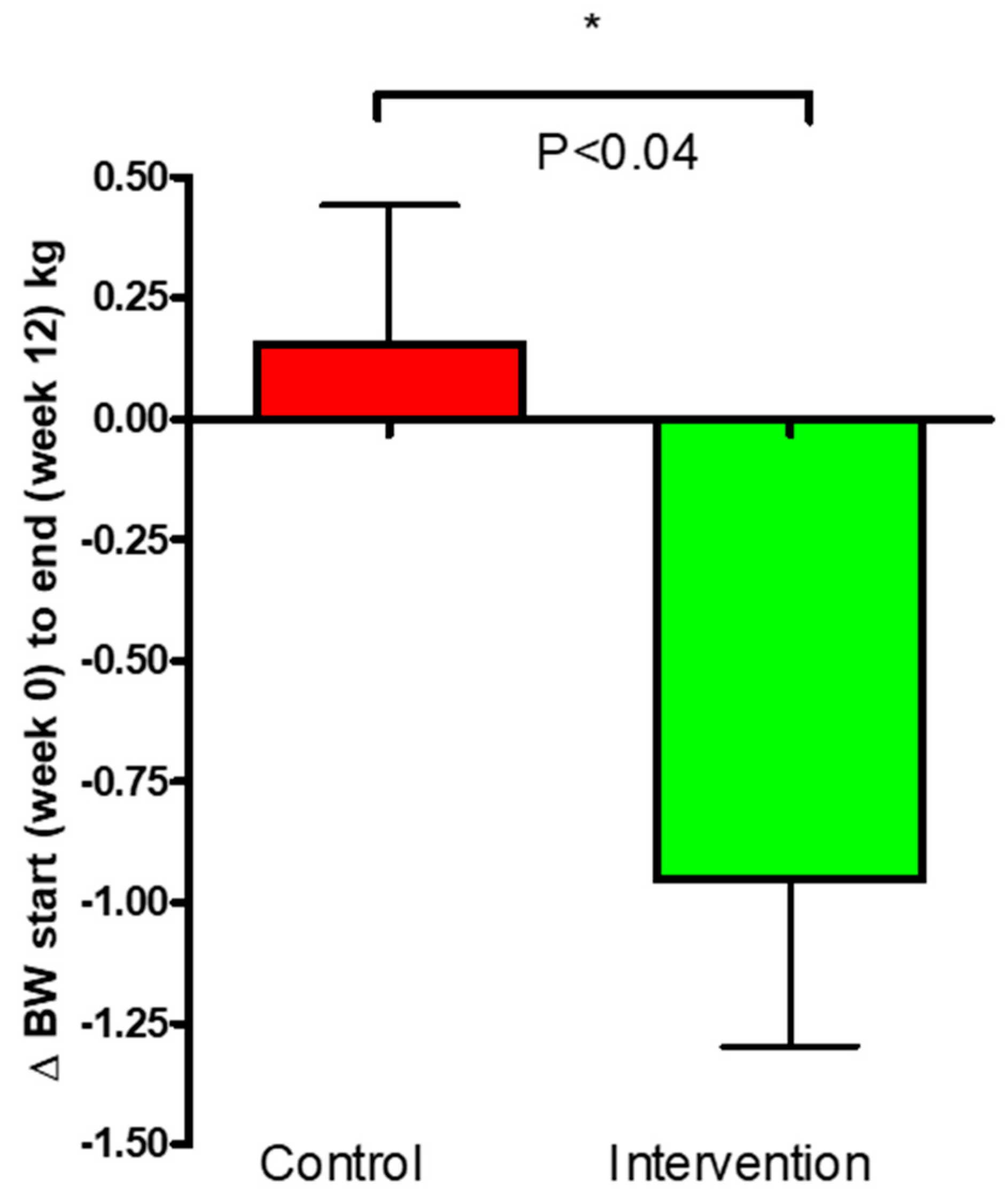
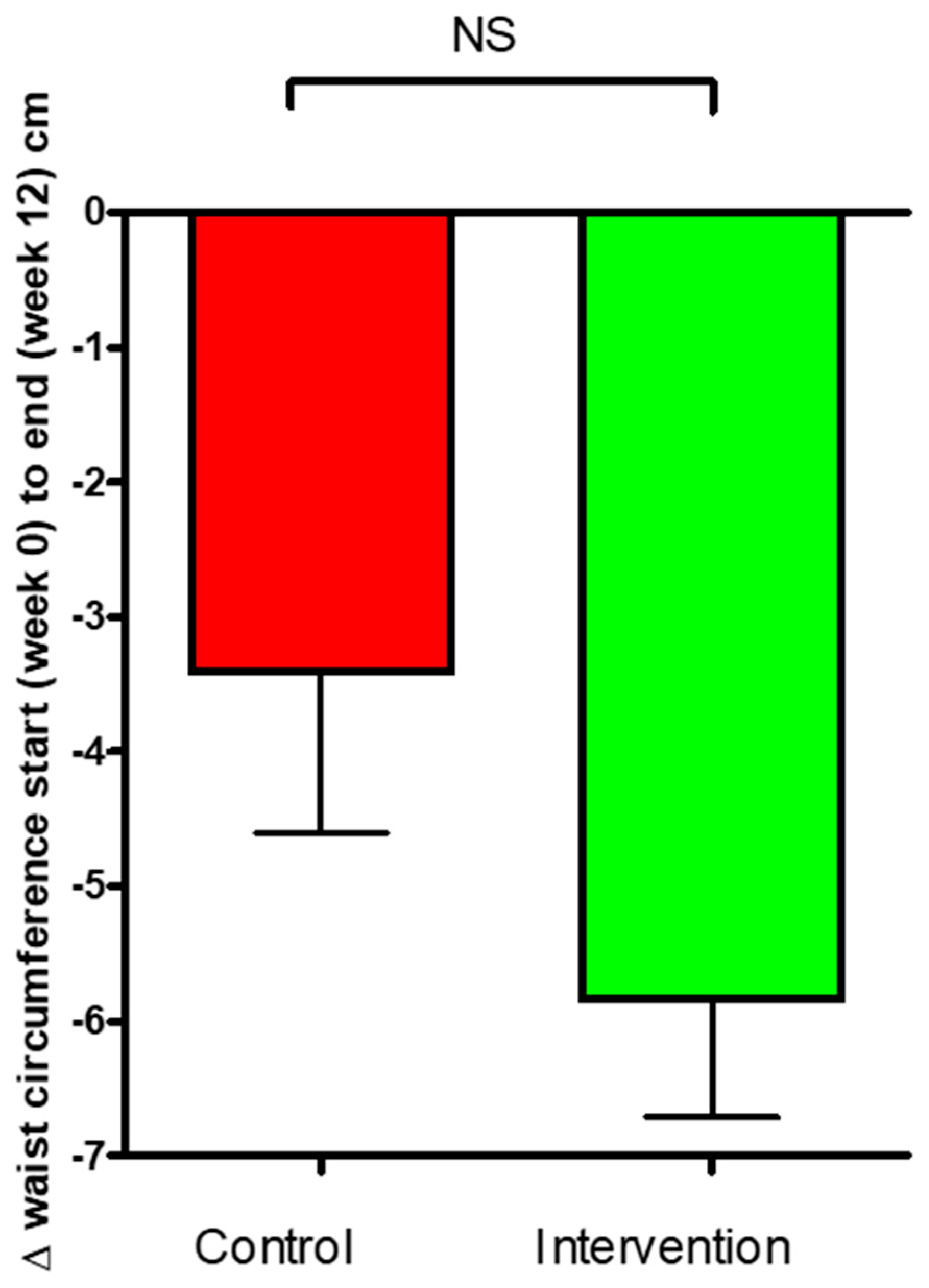
3.2. Body Weight and Composition
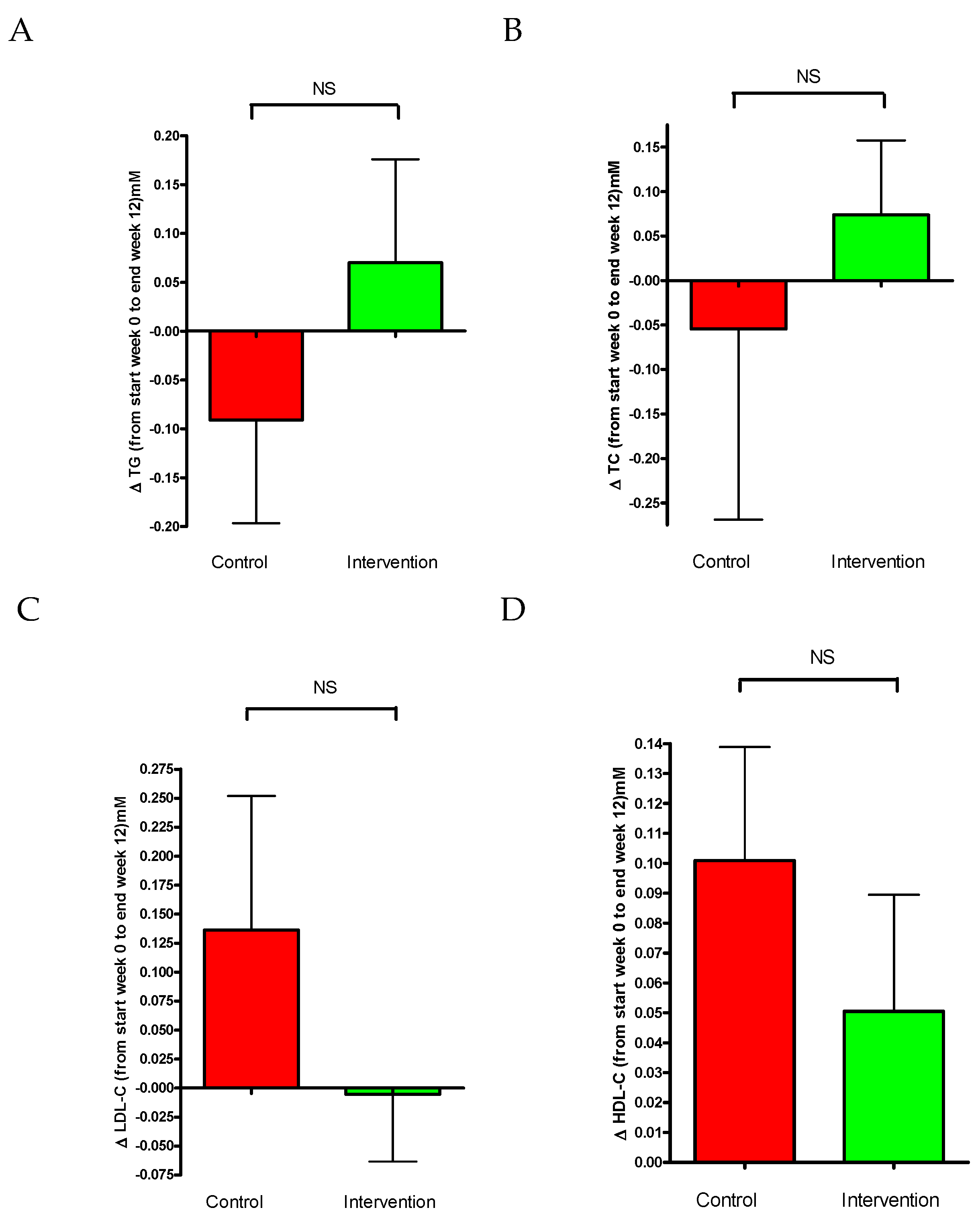
3.3. Plasma Lipid Levels
3.4. Blood Pressure
3.5. Caloric Intake
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Magliano, D.J.; Boyko, E.J. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- Emerging Risk Factors Collaboration; Sarwar, N.; Gao, P.; Seshasai, S.R.; Gobin, R.; Kaptoge, S.; Di Angelantonio, E.; Ingelsson, E.; Lawlor, D.A.; Selvin, E.; et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet 2010, 375, 2215–2222. [Google Scholar] [CrossRef] [PubMed]
- Solis-Herrera, C.; Triplitt, C.; Cersosimo, E.; deFronzo, R.A. Pathogenesis of Type 2 Diabetes Millitus. In Endotext; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Bergman, M.; Buysschaert, M.; Schwarz, P.E.; Albright, A.; Narayan, K.V.; Yach, D. Diabetes prevention: Global health policy and perspectives from the ground. Diabetes Manag. 2012, 2, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Satheesh, N.; Workneh Fanta, S. Kale: Review on nutritional composition, bio-active compounds, anti-nutritional factors, health beneficial properties and value-added products. Cogent Food Agric. 2020, 6, 1811048. [Google Scholar] [CrossRef]
- Fahey, J.W. Brassica. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Benjamin, C., Ed.; Academic Press: Cambridge, MA, USA, 2003; pp. 606–615. [Google Scholar]
- Velasco, P.; Cartea, M.E.; González, C.; Vilar, M.; Ordás, A. Factors Affecting the Glucosinolate Content of Kale (Brassica oleracea acephala Group). J. Agric. Food Chem. 2007, 55, 955–962. [Google Scholar] [CrossRef]
- Ayaz, F.A.; Glew, R.H.; Millson, M.; Huang, H.S.; Chuang, L.T.; Sanz, C.; Hayırlıoglu-Ayaz, S. Nutrient contents of kale (Brassica oleraceae L. var. acephala DC.). Food Chem. 2006, 96, 572–579. [Google Scholar] [CrossRef]
- Lee, J. Quality Characteristics of Cookies added with Kale Powder. Culin. Sci. Hosp. Res. 2015, 21, 40–52. [Google Scholar]
- Choi, S. Quality Characteristics of Muffins added with Kale Powder. Culin. Sci. Hosp. Res. 2015, 21, 187–200. [Google Scholar]
- Goyal, R.; Jialal, I. Diabetes Mellitus Type 2. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK513253/ (accessed on 27 April 2021).
- Eckstein, M.L.; Williams, D.M.; O’Neil, L.K.; Hayes, J.; Stephens, J.W.; Bracken, R.M. Physical exercise and non-insulin glucose-lowering therapies in the management of Type 2 diabetes mellitus: A clinical review. Diabet. Med. 2018, 36, 349–358. [Google Scholar] [CrossRef]
- Gray, A.; Threlkeld, R.J. Nutritional Recommendations for Individuals with Diabetes. In Endotext [Internet]; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK279012/ (accessed on 22 April 2021).
- Wang, P.Y.; Fang, J.C.; Gao, Z.H.; Zhang, C.; Xie, S.Y. Higher intake of fruits, vegetables or their fiber reduces the risk of type 2 diabetes: A meta-analysis. J. Diabetes Investig. 2016, 7, 56–69. [Google Scholar] [CrossRef]
- Aborehab, N.M.; El Bishbishy, M.H.; Waly, N.E. Resistin mediates tomato and broccoli extract effects on glucose homeostasis in high fat diet-induced obesity in rats. BMC Complement. Alternetive Med. 2016, 18, 225. [Google Scholar] [CrossRef]
- Jung, U.J.; Baek, N.I.; Chung, H.G.; Bang, M.H.; Jeong, T.S.; Lee, K.T.; Kang, Y.J.; Lee, M.K.; Kim, H.J.; Yeo, J.; et al. Effects of the ethanol extract of the roots of Brassica rapa on glucose and lipid metabolism in C57BL/KsJ-db/db mice. Clin. Nutr. 2008, 27, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Schloesser, A.; Esatbeyoglu, T.; Schultheiß, G.; Vollert, H.; Lüersen, K.; Fischer, A.; Rimbach, G. Antidiabetic Properties of an Apple/Kale Extract In Vitro, In Situ, and in Mice Fed a Western-Type Diet. J. Med. Food 2017, 20, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Yossef, H.; Mansour, E.; Ahmed, A.; Gwad, A. The Therapeutic effect of Red cabbage on Diabetic Rats. J. Home Econ. 2012, 22, 1–11. [Google Scholar]
- Bahadoran, Z.; Mirmiran, P.; Hosseinpanah, F.; Rajab, A.; Asghari, G.; Azizi, F. Broccoli sprouts powder could improve serum triglyceride and oxidized LDL/LDL-cholesterol ratio in type 2 diabetic patients: A randomized double-blind placebo-controlled clinical trial. Diabetes Res. Clin. Pract. 2012, 96, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Bahadoran, Z.; Tohidi, M.; Nazeri, P.; Mehran, M.; Azizi, F.; Mirmiran, P. Effect of broccoli sprouts on insulin resistance in type 2 diabetic patients: A randomized double-blind clinical trial. Int. J. Food Sci. Nutr. 2012, 63, 767–771. [Google Scholar] [CrossRef]
- Thorup, A.C.; Kristensen, H.L.; Kidmose, U.; Lambert, M.N.T.; Christensen, L.P.; Fretté, X.; Clausen, M.R.; Hansen, S.M.; Jeppesen, P.B. Strong and Bitter Vegetables from Traditional Cultivars and Cropping Methods Improve the Health Status of Type 2 Diabetics: A Randomized Control Trial. Nutrients 2021, 13, 1813. [Google Scholar] [CrossRef]
- Kapusta-Duch, J.; Kopeć, A.; Piatkowska, E.; Borczak, B.; Leszczyńska, T. The beneficial effects of Brassica vegetables on human health. Rocz. Panstw. Zakl. Hig. 2012, 63, 389–395. [Google Scholar]
- Prakash, S.; Hinata, K. Taxonomy, cytogenetics, and origin of crop Brassica, a review. Opera Bot. 1980, 55, 1–57. [Google Scholar]
- Šamec, D.; Urlić, B.; Salopek-Sondi, B. Kale (Brassica oleracea var. acephala) as a superfood: Review of the scientific evidence behind the statement. Crit. Rev. Food Sci. Nutr. 2018, 59, 2411–2422. [Google Scholar] [CrossRef]
- Lisiewska, Z.; Kmiecik, W.; Korus, A. The amino acid composition of kale (Brassica oleracea L. var. acephala), fresh and after culinary and technological processing. Food Chem. 2008, 108, 642–648. [Google Scholar] [CrossRef]
- Becerra-Moreno, A.; Alanís-Garza, P.A.; Mora-Nieves, J.L.; Mora-Mora, J.P.; Jacobo-Velázquez, D.A. Kale: An excellent source of vitamin C, pro-vitamin A, lutein and glucosinolates. CyTA-J. Food 2013, 12, 298–303. [Google Scholar] [CrossRef]
- Emebu, P.K.; Anyika, J.U. Proximate and Mineral composition of Kale (Brassica oleracea), grown in Delta State, Nigeria. Pak. J. Nutr. 2012, 10, 190–194. [Google Scholar] [CrossRef]
- Thavarajah, D.; Thavarajah, P.; Abare, A.; Basnagala, S.; Lacher, C.; Smith, P.; Combs, G.F. Mineral micronutrient and prebiotic carbohydrate profiles of USA-grown kale (Brassica oleracea L. var. acephala). J. Food Compos. Anal. 2016, 52, 9–15. [Google Scholar] [CrossRef]
- Halkier, B.A.; Gershenzon, J. Biology and Biochemistry of Glucosinolates. Annu. Rev. Plant Biol. 2006, 57, 303–333. [Google Scholar] [CrossRef]
- Hwang, E.-S.; Bornhorst, G.; Oteiza, P.I.; Mitchell, A.E. Assessing the fate and bioavailability of glucosinolates in kale (Brassica oleracea) using simulated human digestion and Caco-2 cell uptake models. J. Agric. Food Chem. 2019, 67, 9492–9500. [Google Scholar] [CrossRef]
- Traka, M.H. Health Benefits of Glucosinolates. In Glucosinolates; Kopriva, S., Ed.; Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2016; Volume 80, pp. 247–279. [Google Scholar]
- Nagraj, G.S.; Chouksey, A.; Jaiswal, S.; Jaiswal, A.K. Broccoli. In Nutritional Composition and Antioxidant Properties of Fruits and Vegetables, 1st ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 5–17. [Google Scholar]
- Sharma, A.; Sharma, A.; Yadav, P.; Singh, D. Isothiocyanates in Brassica: Potential Anti Cancer Agents. Asian Pac. J. Cancer Prev. 2016, 17, 4507–4510. [Google Scholar]
- Kondo, S.; Suzuki, A.; Kurokawa, M.; Hasumi, K. Intake of kale suppresses postprandial increases in plasma glucose: A randomized, double-blind, placebo-controlled, crossover study. Biomed. Rep. 2016, 5, 553–558. [Google Scholar] [CrossRef]
- Chung, E.; Shim, E.; Kim, S.Y. Effect of Kale Green Juice on Serum Lipid Level and Phospholipid Fatty Acid Composition in Adult Men with Hypercholesterolemia. J. Life Sci. 2012, 22, 1538–1544. [Google Scholar] [CrossRef]
- Vitakost.dk. Professional Diet Calculator. Available online: https://www.vitakost.dk/da/hjem (accessed on 5 January 2020).
- Han, J.H.; Lee, H.J.; Kim, T.S.; Kang, M.H. The effect of glutathione S-transferase M1 and T1 polymorphisms on blood pressure, blood glucose, and lipid profiles following the supplementation of kale (Brassica oleracea acephala) juice in South Korean subclinical hypertensive patients. Nutr. Res. Pract. 2015, 9, 49. [Google Scholar] [CrossRef]
- World Health Organization. BMI—Body Mass Index. Available online: https://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi (accessed on 17 December 2020).
- Williamson, D.F.; Thompson, T.J.; Thun, M.; Flanders, D.; Pamuk, E.; Byers, T. Intentional weight loss and mortality among overweight individuals with diabetes. Diabetes Care 2000, 23, 1499–1504. [Google Scholar] [CrossRef]
- Wing, R.R.; Lang, W.; Wadden, T.A.; Safford, M.; Knowler, W.C.; Bertoni, A.G.; Hill, J.O.; Brancati, F.L.; Peters, A.; Wagenknecht, L. Benefits of Modest Weight Loss in Improving Cardiovascular Risk Factors in Overweight and Obese Individuals with Type 2 Diabetes. Diabetes Care 2011, 34, 1481–1486. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Li, J.; Lian, Y.; Tang, Z.X.; Zen, Z.; Yu, P.; Li, Y. Long-Term Intensive Lifestyle Intervention Promotes Improvement of Stage III Diabetic Nephropathy. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 3061–3068. [Google Scholar] [CrossRef] [PubMed]
- Stomby, A.; Otten, J.; Ryberg, M.; Andrew, R.; Walker, B.R.; Olsson, T. Diet-induced weight loss alters hepatic glucocorticoid metabolism in type 2 diabetes mellitus. Eur. J. Endocrinol. 2020, 182, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Johansen, M.Y.; MacDonald, C.S.; Hansen, K.B.; Karstoft, K.; Christensen, R.; Pedersen, M.; Hansen, L.S.; Zacho, M.; Wedell-Neergaard, A.S.; Nielsen, S.T.; et al. Effect of an Intensive Lifestyle Intervention on Glycemic Control in Patients with Type 2 Diabetes: A Randomized Clinical Trial. J. Am. Med. Assoc. 2017, 318, 637–646. [Google Scholar] [CrossRef]
- Despres, J.-P. Body Fat Distribution and Risk of Cardiovascular Disease: An Update. Circulation 2012, 126, 1301–1313. [Google Scholar] [CrossRef]
- Katzmarzyk, P.T.; Hu, G.; Cefalu, W.T.; Mire, E.; Bouchard, C. The Importance of Waist Circumference and BMI for Mortality Risk in Diabetic Adults. Diabetes Care 2013, 36, 3128–3130. [Google Scholar] [CrossRef]
- Schübel, R.; Nattenmüller, J.; Sookthai, D.; Nonnenmacher, T.; Graf, M.E.; Riedl, L.; Schlett, C.L.; von Stackelberg, O.; Johnson, T.; Nabers, D.; et al. Effects of intermittent and continuous calorie restriction on body weight and metabolism over 50 wk: A randomized controlled trial. Am. J. Clin. Nutr. 2018, 108, 933–945. [Google Scholar] [CrossRef]
- Kristensen, M.; Jensen, M.G. Dietary fibres in the regulation of appetite and food intake. Importance of viscosity. Appetite 2011, 56, 65–70. [Google Scholar] [CrossRef]
- Yue, Y.; Madsen, S.; Hedemann, M.S.; Knudsen, K.E.B.; Sparsø, F.V.; Laursen, A.; Jensen, H.M.; Knudsen, T.A.; Purup, S. Effect of food ingredients on glucagon-like peptide-1 secretion in STC-1 and HuTu-80 cells. Int. J. Food Sci. Technol. 2019, 54, 3149–3155. [Google Scholar] [CrossRef]
- Müller, T.D.; Finan, B.; Bloom, S.R.; D’Alessio, D.; Drucker, D.J.; Flatt, P.R.; Fritsche, A.; Gribble, F.; Grill, H.J.; Habener, J.F.; et al. Glucagon-like peptide 1 (GLP-1). Mol. Metab. 2019, 30, 72–130. [Google Scholar]
- Holst, J.J. The physiology of glucagon-like peptide 1. Physiol. Rev. 2007, 87, 1409–1439. [Google Scholar] [CrossRef] [PubMed]
- Laskey, M. Dual-energy X-ray absorptiometry and body composition. Nutrients 1996, 12, 45–51. [Google Scholar] [CrossRef]
- Svendsen, O.L.; Haarbo, J.; Hassager, C.; Christiansen, C. Accuracy of measurements of body composition by dual-energy x-rayabsorptiometry in vivo. Am. J. Clin. Nutr. 1993, 57, 605–608. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, D.D.C.; Amorim, D.N.P.; Alves, V.P.; ESilva, K.H.C.V.; Stone, W. Reply on “Significant change for body composition data”. Osteoporos Sarcopenia 2022, 8, 132–133. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Standards of Medical Care in Diabetes—2019 Abridged for Primary Care Providers. Clin. Diabetes 2019, 37, 11–34. [Google Scholar] [CrossRef]
- Kishore, P.; Kim, S.H.; Crandall, J.P. Glycemic Control and Cardiovascular Disease: What’s a Doctor to Do? Curr. Diabetes Rep. 2012, 12, 255–264. [Google Scholar] [CrossRef]
- InformedHealth.org. High Cholesterol: Overview; Institute for Quality and Efficiency in Health Care (IQWiG): Cologne, Germany, 2013. Available online: https://www.ncbi.nlm.nih.gov/books/NBK279318/ (accessed on 20 May 2021).
- Joshi, P.; Martin, S.; Blaha, M.; McEvoy, J.; Santos, R.; Cannon, C.; Blumenthal, R.; Jones, S. Seasonal variations in lipid profiles from 2.8 million us adults: The very large database of lipids (VLDL 14). Lipid Therapeutics and Subclinical Disease. J. Am. Coll. Cardiol. 2014, 63, 12. [Google Scholar] [CrossRef]
- Danesi, F.; Govoni, M.; D’Antuono, L.F.; Bordoni, A. The molecular mechanism of the cholesterol-lowering effect of dill and kale: The influence of the food matrix components. Electrophoresis 2016, 37, 1805–1813. [Google Scholar] [CrossRef]
- Kahlon, T.S.; Chapman, M.H.; Smith, G.E. In vitro binding of bile acids by spinach, kale, brussels sprouts, broccoli, mustard greens, green bell pepper, cabbage and collards. Food Chem. 2007, 100, 1531–1536. [Google Scholar] [CrossRef]
- Kim, S.G. Clinical implications of ambulatory and home blood pressure monitoring. Korean Circ. J. 2010, 40, 423–431. [Google Scholar] [CrossRef]
- Spruill, T.M. Chronic psychosocial stress and hypertension. Curr. Hypertens. Rep. 2010, 12, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Musini, V.M.; Wright, J.M. Factors affecting blood pressure variability: Lessons learned from two systematic reviews of randomized controlled trials. PLoS ONE 2009, 4, e5673. [Google Scholar] [CrossRef] [PubMed]
- Cook, N.R.; Cohen, J.; Hebert, P.R.; Taylor, J.O.; Hennekens, C.H. Implications of small reductions in diastolic blood pressure for primary prevention. Arch. Intern. Med. 1995, 155, 701–709. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Diet, Nutrition and the Prevention of Chronic Diseases: Report of a Joint WHO/FAO Expert Consultation; WHO Technical Report Series; World Health Organization: Geneva, Switzerland, 2003; No. 916. [Google Scholar]
- Chelliah, R.; Chandrashekar, S.; Saravanakumar, K.; Ramakrishnan, S.R.; Rubab, M.; Daliri, E.B.; Barathikannan, K.; Tyagi, A.; Kwame Ofosu, F.; Chen, X.; et al. Effect of Rice Processing towards Lower Rapidly Available Glucose (RAG) Favors Idli, a South Indian Fermented Food Suitable for Diabetic Patients. Nutrients 2019, 11, 1497. [Google Scholar] [CrossRef]
- Luang-In, V.; Rossiter, J.T. Stability studies of isothiocyanates and nitriles in aqueous media. Songklanakarin J. Sci. Technol. 2015, 37, 625–630. [Google Scholar]
- Kim, H.-S.; Lee, S.; Kim, J.H. Real-world Evidence versus Randomized Controlled Trial: Clinical Research Based on Electronic Medical Records. J. Korean Med. Sci. 2018, 33, 213. [Google Scholar] [CrossRef]





| Energy/100 g | 312 kcal/1295 kJ |
|---|---|
| Total fat | 7.3 g |
| Saturated fat | 0.8 g |
| Monounsaturated fat | 0.5 g |
| Polyunsaturated fat | 2.0 g |
| Carbohydrates | 16 g |
| Sugars | 5.5 g |
| Fiber | 41 g |
| Proteins | 25 g |
| Amino acids | Valine—1.27 g Isoleucine—0.939 g Leucine—0.139 g Phenylalanine—1.15 g Lysine—0.205 g Histidine—0.145 g Tryptophan—0.386 g Methionine—0.416 g Threonine—1.07 g |
| NaCl | 0.08 g |
| Minerals & Vitamins | Potassium—2900 mg Calcium—1800 mg Phosphate—480 mg Magnesium—170 mg Vitamin C—350 mg Vitamin K1—330 mg |
| Ingredient | Amount in One Bar | Energy |
|---|---|---|
| Natural rice drink, organic, Naturli’ | 19.85 g | 10.75 kcal |
| Rapeseed oil, organic, Änglamark | 4 g | 35.35 kcal |
| Cocoa powder | 5 g | 20.1 kcal |
| Artificial sweetener, ISIS | 3.5 g | 7.45 kcal |
| Coconut, organic, Änglamark | 5 g | 31.05 kcal |
| Green kale powder | 8.75 g | 27.3 kcal |
| Rice porridge, Pama | 1.5 g | 5.4 kcal |
| TOTAL | 47.6 g | 137.5 kcal |
| Ingredient | Amount in One Bar | Energy |
|---|---|---|
| Cornmeal, organic, Urtekram | 2 g | 7.4 kcal |
| Natural rice drink, organic, Naturli’ | 23 g | 12.5 kcal |
| Rapeseed oil, organic, Änglamark | 4 g | 35.35 kcal |
| Cocoa powder | 5 g | 20.1 kcal |
| Artificial sweetener, ISIS | 3.5 g | 7.45 kcal |
| Coconut, organic, Änglamark | 5 g | 31.05 kcal |
| Rice porridge, Pama | 8.75 g | 31.6 kcal |
| TOTAL | 51.5 g | 145.5 kcal |
| Placebo Bar (51.5 g) | Green Kale Bar (47.6 g) | |
|---|---|---|
| Energy | 145.5 kcal | 137.5 kcal |
| Fat | 8.5 g | 9.05 g |
| Saturated fat | 3.855 g | 3.905 g |
| Monounsaturated fat | 2.895 g | 2.865 g |
| Polyunsaturated fat | 1.45 g | 1.395 g |
| Carbohydrate | 12.65 g | 6.45 g |
| Sugar | 2.08 g | 2.325 g |
| Fiber | 5.1 g | 8.6 g |
| Protein | 2.02 g | 3.485 g |
| NaCl | 0.029 g | 0.033 g |
| Alcohol | 0 g | 0 g |
| Control Group | Intervention Group | p-Value | |
|---|---|---|---|
Participants(n)
| 11 5 6 | 19 17 2 | |
| Age (years) | 64.3 | 62.7 | 0.55 |
| Weight (kg) | 5.46 | 90.49 3.63 | 0.88 |
| BMI (kg/m2) | 29.81 | 29.50 | 0.87 |
| Waist circumference (cm) | 3.63 | 99.39 | 0.77 |
| Fasting plasma glucose (mM) | 7.85 0.26 | 0.19 | 0.84 |
| HbA1c (mM) | 48.18 1.8 | 50.79 2.4 | 0.39 |
| Triglycerides (mM) | 1.46 0.19 | 1.29 0.10 | 0.43 |
| Plasma total cholesterol (mM) | 4.34 0.5 | 3.87 0.20 | 0.40 |
| Plasma HDL-cholesterol (mM) | 1.28 | 1.22 | 0.74 |
| Plasma LDL-cholesterol (mM) | 2.55 0.34 | 2.05 | 0.22 |
| Systolic blood pressure (mmHg) | 126.9 | 124.1 | 0.57 |
| Diastolic blood pressure (mmHg) | 77.73 | 74.68 | 0.16 |
| z | Intervention | |||||
|---|---|---|---|---|---|---|
| Change | P | Change | P | P | ||
| Systolic | 2.82 ± 2.20 | 0.23 | −0.95 ± 3.42 | 0.79 | 0.36 | |
| Diastolic | 1.27 ± 1.48 | 0.41 | 0.74 ± 1.32 | 0.59 | 0.79 | |
| Systolic/day | 3.36 ± 2.53 | 0.21 | 0.84 ± 3.22 | 0.8 | 0.54 | |
| Diastolic/day | 1.09 ± 1.73 | 0.54 | 1.42 ± 1.23 | 0.29 | 0.88 | |
| Systolic/night | 1.09 ± 2.33 | 0.65 | −3.94 ± 3.27 | 0.24 | 0.22 | |
| Diastolic/night | −1.36 ± 2.6 | 0.61 | −3.26 ± 1.55 | 0.05 | 0.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeppesen, P.B.; Dorner, A.; Yue, Y.; Poulsen, N.; Andersen, S.K.; Aalykke, F.B.; Lambert, M.N.T. Beneficial Effects of a Freeze-Dried Kale Bar on Type 2 Diabetes Patients: A Randomized, Double-Blinded, Placebo-Controlled Clinical Trial. Nutrients 2024, 16, 3641. https://doi.org/10.3390/nu16213641
Jeppesen PB, Dorner A, Yue Y, Poulsen N, Andersen SK, Aalykke FB, Lambert MNT. Beneficial Effects of a Freeze-Dried Kale Bar on Type 2 Diabetes Patients: A Randomized, Double-Blinded, Placebo-Controlled Clinical Trial. Nutrients. 2024; 16(21):3641. https://doi.org/10.3390/nu16213641
Chicago/Turabian StyleJeppesen, Per Bendix, Amanda Dorner, Yuan Yue, Nikolaj Poulsen, Sofie Korsgaard Andersen, Fie Breenfeldt Aalykke, and Max Norman Tandrup Lambert. 2024. "Beneficial Effects of a Freeze-Dried Kale Bar on Type 2 Diabetes Patients: A Randomized, Double-Blinded, Placebo-Controlled Clinical Trial" Nutrients 16, no. 21: 3641. https://doi.org/10.3390/nu16213641
APA StyleJeppesen, P. B., Dorner, A., Yue, Y., Poulsen, N., Andersen, S. K., Aalykke, F. B., & Lambert, M. N. T. (2024). Beneficial Effects of a Freeze-Dried Kale Bar on Type 2 Diabetes Patients: A Randomized, Double-Blinded, Placebo-Controlled Clinical Trial. Nutrients, 16(21), 3641. https://doi.org/10.3390/nu16213641





