Sodium Benzoate Induces Fat Accumulation and Reduces Lifespan via the SKN-1/Nrf2 Signaling Pathway: Evidence from the Caenorhabditis elegans Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. C. elegans Culture and Strains
2.2. SB Treatment
2.3. Oil Red O (ORO) Staining
2.4. SKN-1::GFP Expression Analysis
2.5. Image Analysis
2.6. Lifespan Analysis
2.7. Pharyngeal Pumping Assay
2.8. Statistical Analysis
3. Results
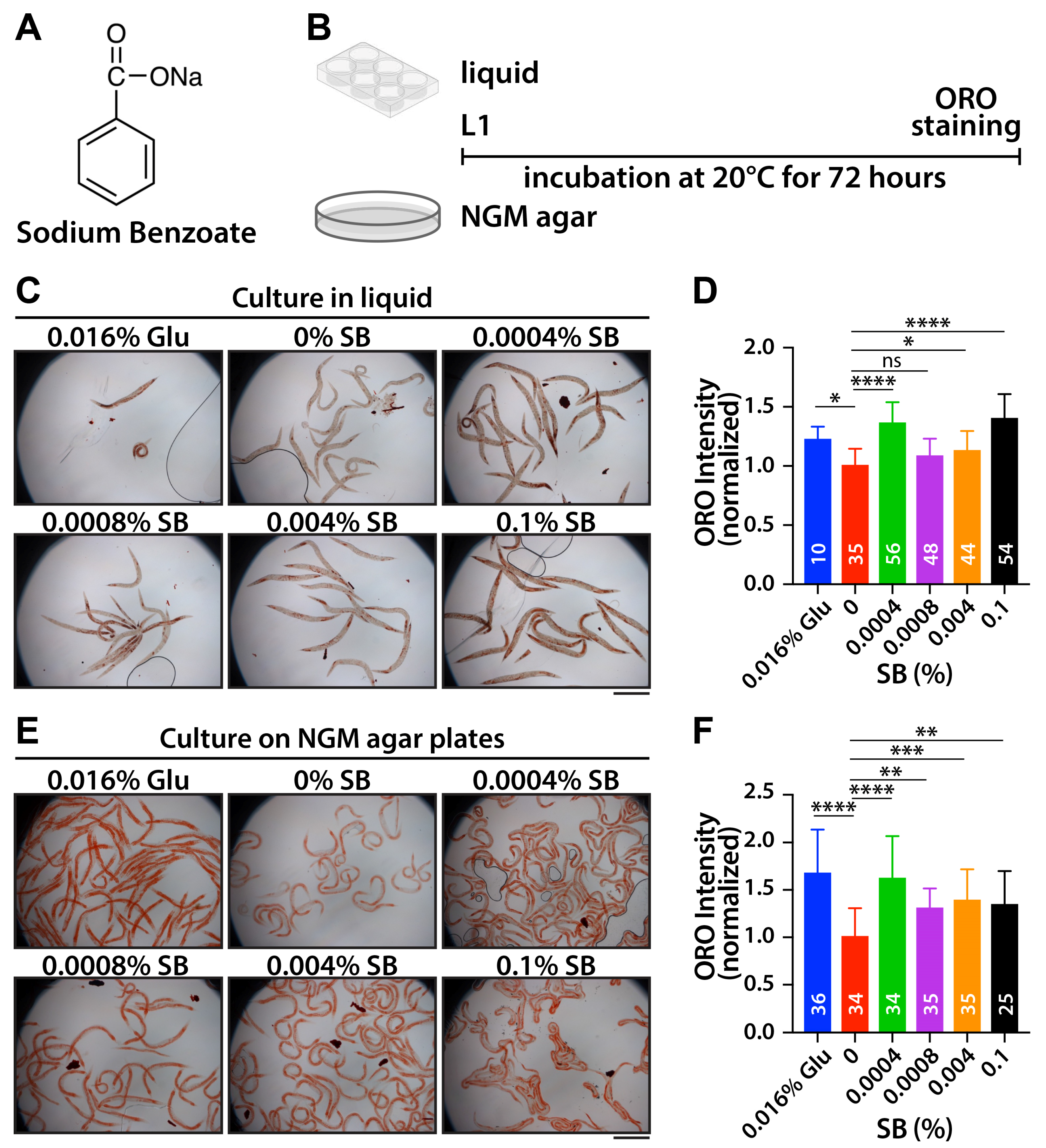
3.1. SB Increases Fat Accumulation in Wild-Type Worms
3.2. SB May Increase Fat Accumulation Through Food Metabolism Pathways
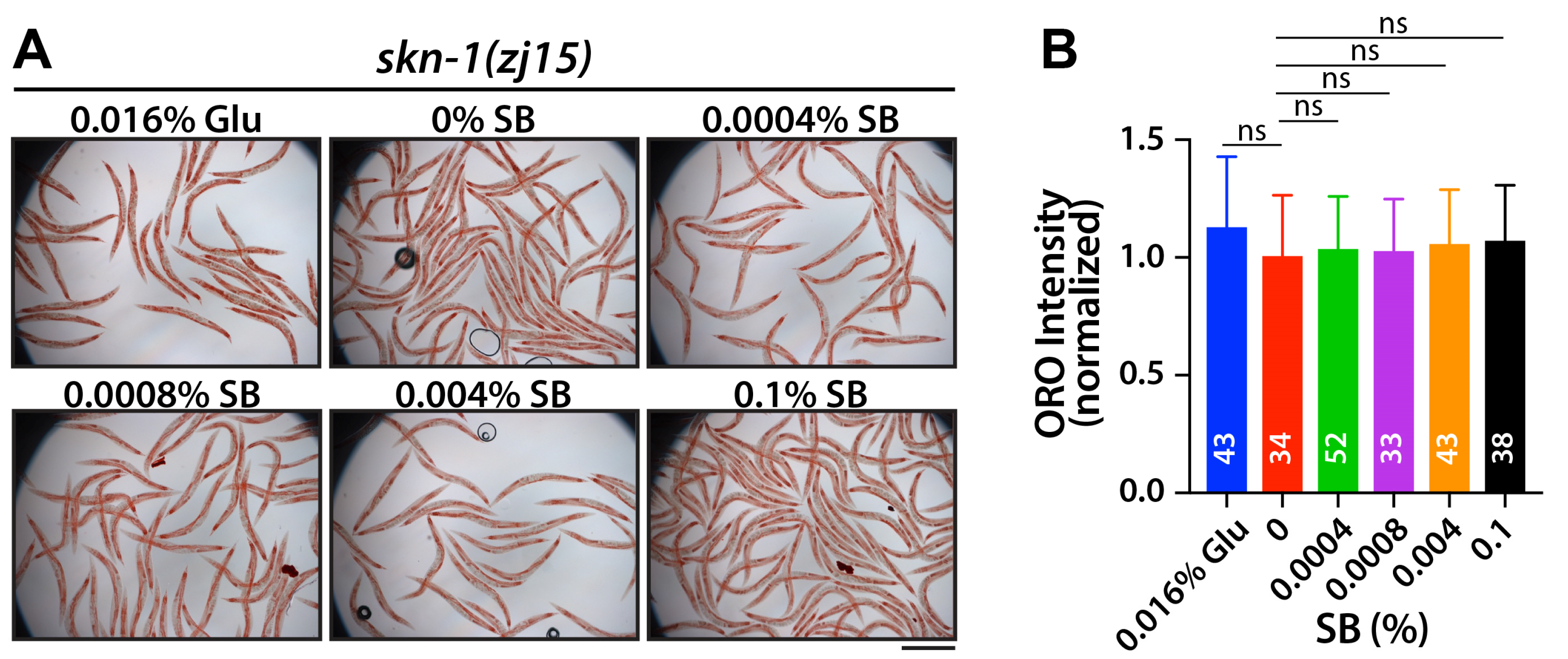
3.3. SB Increases Fat Accumulation, at Least in Part, Through the SKN-1/Nrf2 Signaling Pathway
3.4. SB Treatment Reduces Lifespan Primarily Through the SKN-1/Nrf2 Signaling Pathway
3.5. SB Inhibits SKN-1 Nuclear Localization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bensid, A.; El Abed, N.; Houicher, A.; Regenstein, J.M.; Ozogul, F. Antioxidant and antimicrobial preservatives: Properties, mechanism of action and applications in food—A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 2985–3001. [Google Scholar] [CrossRef] [PubMed]
- Hrncirova, L.; Machova, V.; Trckova, E.; Krejsek, J.; Hrncir, T. Food Preservatives Induce Proteobacteria Dysbiosis in Human-Microbiota Associated Nod2-Deficient Mice. Microorganisms 2019, 7, 383. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, M.; Daly, N.; O’Kelly, R.; Turner, M.J. Time and temperature affect glycolysis in blood samples regardless of fluoride-based preservatives: A potential underestimation of diabetes. Ann. Clin. Biochem. 2017, 54, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Rico, M.; Renwick, S.; Vancuren, S.J.; Robinson, A.V.; Gianetto-Hill, C.; Allen-Vercoe, E.; Barat, J.M. Impact of food preservatives based on immobilized phenolic compounds on an in vitro model of human gut microbiota. Food Chem. 2023, 403, 134363. [Google Scholar] [CrossRef]
- Lennerz, B.S.; Vafai, S.B.; Delaney, N.F.; Clish, C.B.; Deik, A.A.; Pierce, K.A.; Ludwig, D.S.; Mootha, V.K. Effects of sodium benzoate, a widely used food preservative, on glucose homeostasis and metabolic profiles in humans. Mol. Genet. Metab. 2015, 114, 73–79. [Google Scholar] [CrossRef]
- Walczak-Nowicka, L.J.; Herbet, M. Sodium Benzoate-Harmfulness and Potential Use in Therapies for Disorders Related to the Nervous System: A Review. Nutrients 2022, 14, 1497. [Google Scholar] [CrossRef]
- Rangasamy, S.B.; Raha, S.; Dasarathy, S.; Pahan, K. Sodium Benzoate, a Metabolite of Cinnamon and a Food Additive, Improves Cognitive Functions in Mice after Controlled Cortical Impact Injury. Int. J. Mol. Sci. 2021, 23, 192. [Google Scholar] [CrossRef]
- Rangasamy, S.B.; Dasarathi, S.; Nutakki, A.; Mukherjee, S.; Nellivalasa, R.; Pahan, K. Stimulation of Dopamine Production by Sodium Benzoate, a Metabolite of Cinnamon and a Food Additive. J. Alzheimer’s Dis. Rep. 2021, 5, 295–310. [Google Scholar] [CrossRef]
- Khoshnoud, M.J.; Siavashpour, A.; Bakhshizadeh, M.; Rashedinia, M. Effects of sodium benzoate, a commonly used food preservative, on learning, memory, and oxidative stress in brain of mice. J. Biochem. Mol. Toxicol. 2018, 32, e22022. [Google Scholar] [CrossRef]
- Asejeje, F.O.; Alade, T.F.; Oyibo, A.; Abolaji, A.O. Toxicological assessment of sodium benzoate in Drosophila melanogaster. J. Biochem. Mol. Toxicol. 2023, 38, e23586. [Google Scholar] [CrossRef]
- Dong, Y.; Ding, Z.; Song, L.; Zhang, D.; Xie, C.; Zhang, S.; Feng, L.; Liu, H.; Pang, Q. Sodium Benzoate Delays the Development of Drosophila melanogaster Larvae and Alters Commensal Microbiota in Adult Flies. Front. Microbiol. 2022, 13, 911928. [Google Scholar] [CrossRef] [PubMed]
- Gaur, H.; Purushothaman, S.; Pullaguri, N.; Bhargava, Y.; Bhargava, A. Sodium benzoate induced developmental defects, oxidative stress and anxiety-like behaviour in zebrafish larva. Biochem. Biophys. Res. Commun. 2018, 502, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Ciardi, C.; Jenny, M.; Tschoner, A.; Ueberall, F.; Patsch, J.; Pedrini, M.; Ebenbichler, C.; Fuchs, D. Food additives such as sodium sulphite, sodium benzoate and curcumin inhibit leptin release in lipopolysaccharide-treated murine adipocytes in vitro. Br. J. Nutr. 2012, 107, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Hales, C.M.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017–2018; NCHS Data Brief; National Center for Health Statistics: Hyattsville, MD, USA, 2020; pp. 1–8. [Google Scholar]
- Hales, C.M.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Prevalence of Obesity Among Adults and Youth: United States, 2015–2016; NCHS Data Brief; National Center for Health Statistics: Hyattsville, MD, USA, 2017; pp. 1–8. [Google Scholar]
- Simmons, A.L.; Schlezinger, J.J.; Corkey, B.E. What Are We Putting in Our Food That Is Making Us Fat? Food Additives, Contaminants, and Other Putative Contributors to Obesity. Curr. Obes. Rep. 2014, 3, 273–285. [Google Scholar] [CrossRef]
- Kaletta, T.; Hengartner, M.O. Finding function in novel targets: C. elegans as a model organism. Nat. Rev. Drug Discov. 2006, 5, 387–398. [Google Scholar] [CrossRef]
- Pang, S.; Lynn, D.A.; Lo, J.Y.; Paek, J.; Curran, S.P. SKN-1 and Nrf2 couples proline catabolism with lipid metabolism during nutrient deprivation. Nat. Commun. 2014, 5, 5048. [Google Scholar] [CrossRef]
- Shen, P.; Yue, Y.; Park, Y. A living model for obesity and aging research: Caenorhabditis elegans. Crit. Rev. Food Sci. Nutr. 2018, 58, 741–754. [Google Scholar] [CrossRef]
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [CrossRef]
- An, J.H.; Blackwell, T.K. SKN-1 links C. elegans mesendodermal specification to a conserved oxidative stress response. Genes Dev. 2003, 17, 1882–1893. [Google Scholar] [CrossRef]
- Escorcia, W.; Ruter, D.L.; Nhan, J.; Curran, S.P. Quantification of Lipid Abundance and Evaluation of Lipid Distribution in Caenorhabditis elegans by Nile Red and Oil Red O Staining. J. Vis. Exp. JoVE 2018, 133, 57352. [Google Scholar] [CrossRef]
- Steinbaugh, M.J.; Narasimhan, S.D.; Robida-Stubbs, S.; Moronetti Mazzeo, L.E.; Dreyfuss, J.M.; Hourihan, J.M.; Raghavan, P.; Operana, T.N.; Esmaillie, R.; Blackwell, T.K. Lipid-mediated regulation of SKN-1/Nrf in response to germ cell absence. eLife 2015, 4, e07836. [Google Scholar] [CrossRef] [PubMed]
- Ogg, S.; Paradis, S.; Gottlieb, S.; Patterson, G.I.; Lee, L.; Tissenbaum, H.A.; Ruvkun, G. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature 1997, 389, 994–999. [Google Scholar] [CrossRef] [PubMed]
- Tullet, J.M.; Hertweck, M.; An, J.H.; Baker, J.; Hwang, J.Y.; Liu, S.; Oliveira, R.P.; Baumeister, R.; Blackwell, T.K. Direct inhibition of the longevity-promoting factor SKN-1 by insulin-like signaling in C. elegans. Cell 2008, 132, 1025–1038. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.; Hisamoto, N.; An, J.H.; Oliveira, R.P.; Nishida, E.; Blackwell, T.K.; Matsumoto, K. The C. elegans p38 MAPK pathway regulates nuclear localization of the transcription factor SKN-1 in oxidative stress response. Genes Dev. 2005, 19, 2278–2283. [Google Scholar] [CrossRef]
- Li, L.; Chen, Y.; Chenzhao, C.; Fu, S.; Xu, Q.; Zhao, J. Glucose negatively affects Nrf2/SKN-1-mediated innate immunity in C. elegans. Aging 2018, 10, 3089–3103. [Google Scholar] [CrossRef]
- El-Shennawy, L.; Kamel, M.A.E.; Khalaf, A.H.Y.; Yousef, M.I. Dose-dependent reproductive toxicity of sodium benzoate in male rats: Inflammation, oxidative stress and apoptosis. Reprod. Toxicol. 2020, 98, 92–98. [Google Scholar] [CrossRef]
- Olofinnade, A.T.; Onaolapo, A.Y.; Onaolapo, O.J.; Olowe, O.A. The potential toxicity of food-added sodium benzoate in mice is concentration-dependent. Toxicol. Res. 2021, 10, 561–569. [Google Scholar] [CrossRef]
- Nair, B. Final report on the safety assessment of Benzyl Alcohol, Benzoic Acid, and Sodium Benzoate. Int. J. Toxicol. 2001, 20 (Suppl. S3), 23–50. [Google Scholar] [CrossRef]
- Leonard, E.R.; Marques, E.S.; Roy, M.A.; Conlin, S.M.; Ranjan, R.; Timme-Laragy, A.R. Dietary exposure to the food preservative tert-Butylhydroquinone (tBHQ) impairs zebrafish (Danio rerio) survival, growth, organ development, and gene expression in Nrf2a-dependent and independent ways. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2023, 176, 113788. [Google Scholar] [CrossRef]
- Bryan, H.K.; Olayanju, A.; Goldring, C.E.; Park, B.K. The Nrf2 cell defence pathway: Keap1-dependent and -independent mechanisms of regulation. Biochem. Pharmacol. 2013, 85, 705–717. [Google Scholar] [CrossRef]
- Khan, I.S.; Dar, K.B.; Ganie, S.A.; Ali, M.N. Toxicological impact of sodium benzoate on inflammatory cytokines, oxidative stress and biochemical markers in male Wistar rats. Drug Chem. Toxicol. 2022, 45, 1345–1354. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, T.K.; Steinbaugh, M.J.; Hourihan, J.M.; Ewald, C.Y.; Isik, M. SKN-1/Nrf, stress responses, and aging in Caenorhabditis elegans. Free Radic. Biol. Med. 2015, 88, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zheng, Q.; Chen, Z. The Nrf2 Pathway in Liver Diseases. Front. Cell Dev. Biol. 2022, 10, 826204. [Google Scholar] [CrossRef] [PubMed]
- Zeghib, K.; Boutlelis, D.A. Food Additive (Sodium benzoate)-induced Damage on Renal Function and Glomerular Cells in Rats; Modulating Effect of Aqueous Extract of Atriplex halimus L. Iran. J. Pharm. Res. IJPRjwan 2021, 20, 296–306. [Google Scholar] [CrossRef]
- Nezu, M.; Suzuki, N. Roles of Nrf2 in Protecting the Kidney from Oxidative Damage. Int. J. Mol. Sci. 2020, 21, 2951. [Google Scholar] [CrossRef]
- Galicia-Moreno, M.; Lucano-Landeros, S.; Monroy-Ramirez, H.C.; Silva-Gomez, J.; Gutierrez-Cuevas, J.; Santos, A.; Armendariz-Borunda, J. Roles of Nrf2 in Liver Diseases: Molecular, Pharmacological, and Epigenetic Aspects. Antioxidants 2020, 9, 980. [Google Scholar] [CrossRef]
- Hellerer, T.; Axang, C.; Brackmann, C.; Hillertz, P.; Pilon, M.; Enejder, A. Monitoring of lipid storage in Caenorhabditis elegans using coherent anti-Stokes Raman scattering (CARS) microscopy. Proc. Natl. Acad. Sci. USA 2007, 104, 14658–14663. [Google Scholar] [CrossRef]
- Cahill, C.M.; Tzivion, G.; Nasrin, N.; Ogg, S.; Dore, J.; Ruvkun, G.; Alexander-Bridges, M. Phosphatidylinositol 3-kinase signaling inhibits DAF-16 DNA binding and function via 14-3-3-dependent and 14-3-3-independent pathways. J. Biol. Chem. 2001, 276, 13402–13410. [Google Scholar] [CrossRef]
- Hertweck, M.; Gobel, C.; Baumeister, R. C. elegans SGK-1 is the critical component in the Akt/PKB kinase complex to control stress response and life span. Dev. Cell 2004, 6, 577–588. [Google Scholar] [CrossRef]
- Lin, K.; Hsin, H.; Libina, N.; Kenyon, C. Regulation of the Caenorhabditis elegans longevity protein DAF-16 by insulin/IGF-1 and germline signaling. Nat. Genet. 2001, 28, 139–145. [Google Scholar] [CrossRef]
- Xu, A.; Zhang, Z.; Ko, S.H.; Fisher, A.L.; Liu, Z.; Chen, L. Microtubule regulators act in the nervous system to modulate fat metabolism and longevity through DAF-16 in C. elegans. Aging Cell 2019, 18, e12884. [Google Scholar] [CrossRef]
- Deng, J.; Dai, Y.; Tang, H.; Pang, S. SKN-1 Is a Negative Regulator of DAF-16 and Somatic Stress Resistance in Caenorhabditis elegans. G3 2020, 10, 1707–1712. [Google Scholar] [CrossRef]




| Strain | Treatment | Average Lifespan ± (StDv) | p-Value (t-Test) |
|---|---|---|---|
| wild-type | 0.016% Glu | 15.01 ± 3.69 days | 0.0420 |
| 0% SB | 16.23 ± 3.18 days | - | |
| 0.0004% SB | 13.27 ± 2.57 days | <0.0001 | |
| 0.0008% SB | 15.74 ± 3.75 days | 0.3802 | |
| 0.004% SB | 14.92 ± 3.12 days | 0.0167 | |
| 0.1% SB | 12.98 ± 3.23 days | <0.0001 | |
| skn-1(zj15) | 0.016% Glu | 9.93 ± 2.29 days | 0.1667 |
| 0% SB | 10.21 ± 2.50 days | - | |
| 0.0004% SB | 10.03 ± 2.35 days | 0.6873 | |
| 0.0008% SB | 9.79 ± 2.38 days | 0.3673 | |
| 0.004% SB | 9.23 ± 2.22 days | 0.0338 | |
| 0.1% SB | 9.23 ± 1.67 days | 0.0138 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.D.; Lee, J.; Vang, J.; Pan, X. Sodium Benzoate Induces Fat Accumulation and Reduces Lifespan via the SKN-1/Nrf2 Signaling Pathway: Evidence from the Caenorhabditis elegans Model. Nutrients 2024, 16, 3753. https://doi.org/10.3390/nu16213753
Lee JD, Lee J, Vang J, Pan X. Sodium Benzoate Induces Fat Accumulation and Reduces Lifespan via the SKN-1/Nrf2 Signaling Pathway: Evidence from the Caenorhabditis elegans Model. Nutrients. 2024; 16(21):3753. https://doi.org/10.3390/nu16213753
Chicago/Turabian StyleLee, Jiah D., Jiwoo Lee, Jerry Vang, and Xiaoping Pan. 2024. "Sodium Benzoate Induces Fat Accumulation and Reduces Lifespan via the SKN-1/Nrf2 Signaling Pathway: Evidence from the Caenorhabditis elegans Model" Nutrients 16, no. 21: 3753. https://doi.org/10.3390/nu16213753
APA StyleLee, J. D., Lee, J., Vang, J., & Pan, X. (2024). Sodium Benzoate Induces Fat Accumulation and Reduces Lifespan via the SKN-1/Nrf2 Signaling Pathway: Evidence from the Caenorhabditis elegans Model. Nutrients, 16(21), 3753. https://doi.org/10.3390/nu16213753






