Capsaicin Exerts Antitumor Activity in Mesothelioma Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines and Reagents
2.2. Generation of Cisplatin-Resistant MM Cell Lines
2.3. Capsaicin and Treatment Medium Preparation
2.4. MTS Assay
2.5. Half-Maximal Inhibitory Concentration (IC50) Evaluation
2.6. Cytofluorimetric Analysis of MM Cell Cycle
2.7. Wound-Healing Assay
2.8. Transwell Migration Assay
2.9. Transwell Invasion Assay
2.10. Western Blotting
3. Results
3.1. Capsaicin (CAPS) Reduces Cell Proliferation Both in Parental and Cisplatin-Resistant Mesothelioma Cells
3.2. CAPS Impairs Cell Cycle in Mesothelioma Cells
3.3. CAPS Reduces Migration of Various Mesothelioma Lines
3.3.1. CAPS Impairs Lateral Motility of Various Mesothelioma Lines
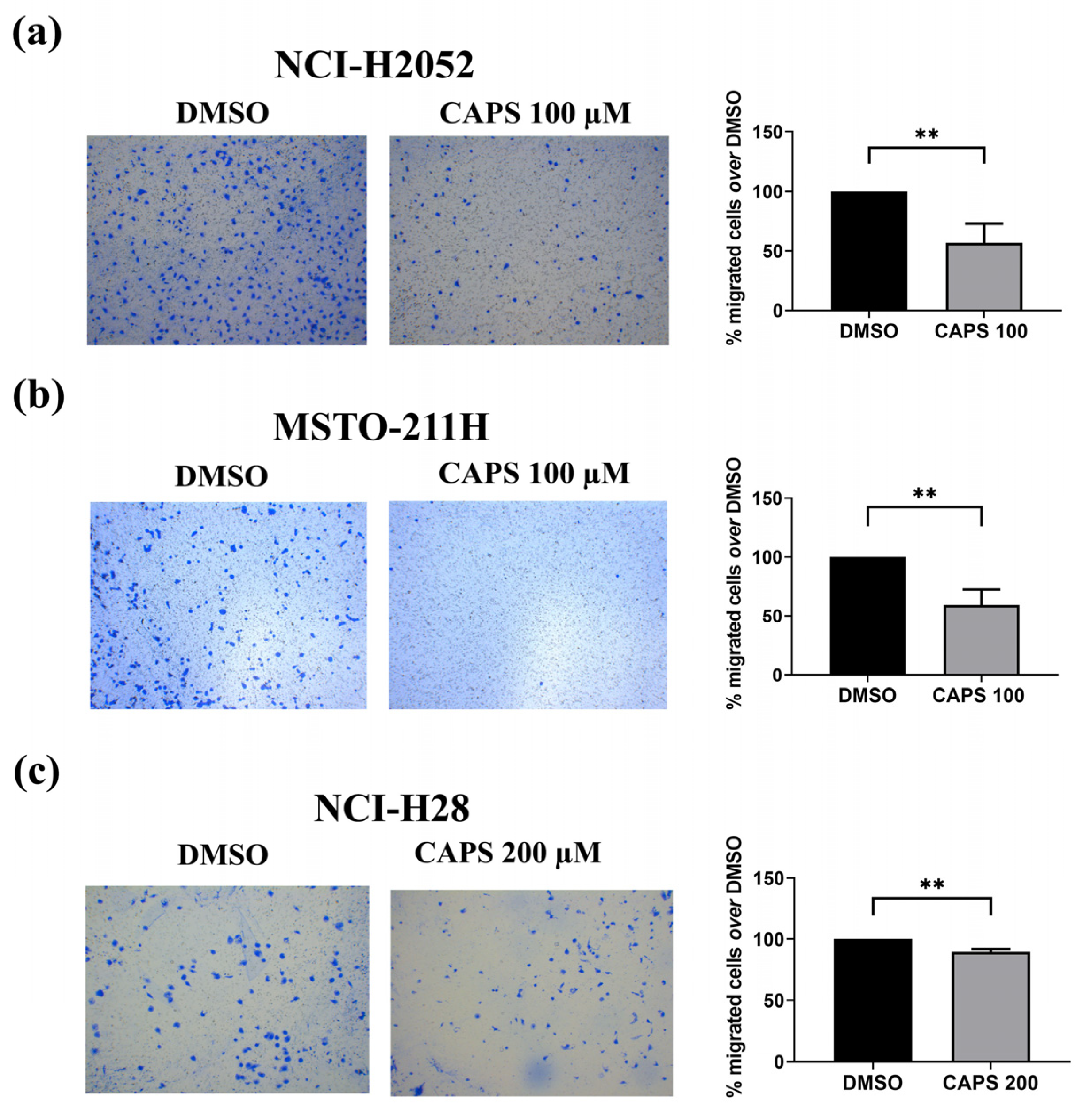
3.3.2. CAPS Reduces Transwell Migration of Various Mesothelioma Cells
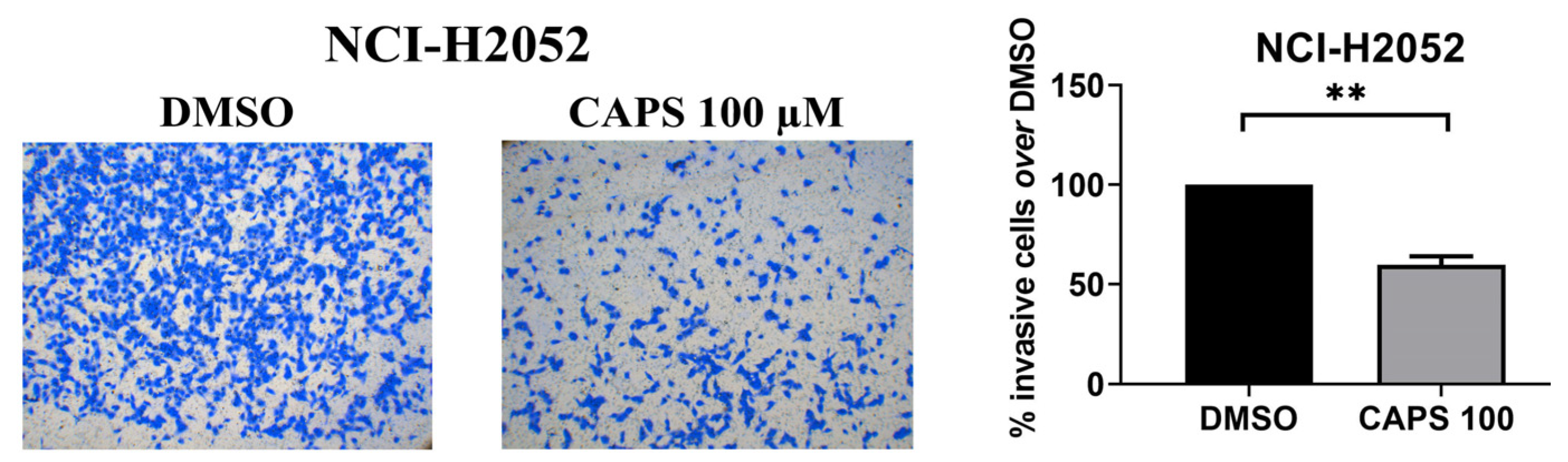
3.4. CAPS Reduces Invasive Ability of Mesothelioma Cells
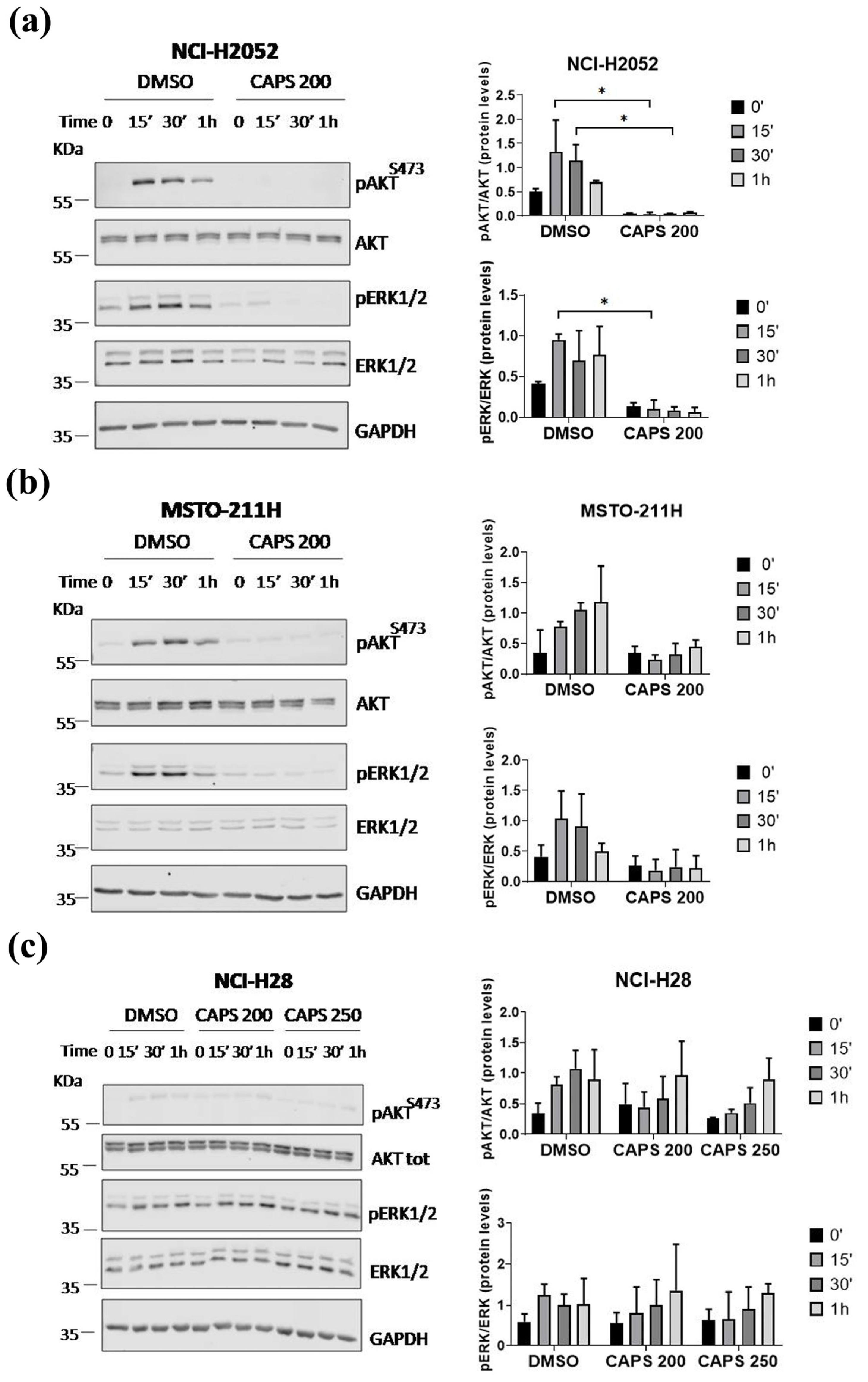
3.5. CAPS Inhibits the Activation of the AKT and ERK1/2 Signaling Pathways in MM Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tipu, S.A.; Ahmed, I.; Ishtiaq, S. Malignant mesothelioma. Pak. J. Med. Sci. 2013, 29, 1433–1438. [Google Scholar] [CrossRef] [PubMed]
- Hajj, G.; Cavarson, C.; Pinto, C.; Venturi, G.; Navarro, J.; Lima, V. Malignant pleural mesothelioma: An update. J. Bras. Pneumol. 2021, 47, e20210129. [Google Scholar] [CrossRef]
- Stevenson, J.; Ettinger, D.; Wood, D.; Aisner, D.; Akerley, W.; Bauman, J.; Bharat, A.; Bruno, D.; Chang, J.; Chirieac, L.; et al. NCCN Guidelines® Insights: Mesothelioma: Pleural, Version 1.2024. J. Natl. Compr. Canc. Netw. 2024, 22, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Park, S.; Yon, D.K.; Lee, S.W.; Woo, W.; Dragioti, E.; Koyanagi, A.; Jacob, L.; Kostev, K.; Radua, J.; et al. Global, Regional, and National Burden of Mesothelioma 1990–2019 A Systematic Analysis of the Global Burden of Disease Study 2019. Ann. Am. Thorac. Soc. 2023, 20, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Wadowski, B.; De Rienzo, A.; Bueno, R. The Molecular Basis of Malignant Pleural Mesothelioma. Thorac. Surg. Clin. 2020, 30, 383–393. [Google Scholar] [CrossRef]
- Mangone, L.; Storchi, C.; Pinto, C.; Giorgi Rossi, P.; Bisceglia, I.; Romanelli, A. Incidence of malignant mesothelioma and asbestos exposure in the Emilia-Romagna region, Italy. Med. Lav. 2022, 113, e2022047. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Dell’Anno, I.; Lapidot, M.; Sekido, Y.; Chan, M.-L.; Kohno, M.; Serre-Beinier, V.; Felley-Bosco, E.; de Perrot, M. Progress of malignant mesothelioma research in basic science: A review of the 14th international conference of the international mesothelioma interest group (iMig2018). Lung Cancer 2019, 127, 138–145. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. ASBESTOS (CHRYSOTILE, AMOSITE, CROCIDOLITE, TREMOLITE, ACTINOLITE AND ANTHOPHYLLITE). In Arsenic, Metals, Fibres and Dusts. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, No. 100C; International Agency for Research on Cancer: Lyon, France, 2012; pp. 219–309. [Google Scholar]
- Aryal, A.; Morley, C. Call for a global ban policy on and scientific management of asbestos to eliminate asbestos-related diseases. J. Public Health Policy 2020, 41, 279–285. [Google Scholar] [CrossRef]
- Carbone, M.; Pass, H.; Ak, G.; Alexander, H.J.; Baas, P.; Baumann, F.; Blakely, A.; Bueno, R.; Bzura, A.; Cardillo, G.; et al. Medical and Surgical Care of Patients With Mesothelioma and Their Relatives Carrying Germline BAP1 Mutations. J. Thorac. Oncol. 2022, 17, 873–889. [Google Scholar] [CrossRef]
- Carbone, M.; Adusumilli, P.S.; Alexander, H.R.; Baas, P.; Bardelli, F.; Bononi, A.; Bueno, R.; Felley-Bosco, E.; Galateau-Salle, F.; Jablons, D.; et al. Mesothelioma: Scientific clues for prevention, diagnosis, and therapy. CA Cancer J. Clin. 2019, 69, 402–429. [Google Scholar] [CrossRef]
- Asciak, R.; George, V.; Rahman, N.M. Update on biology and management of mesothelioma. Eur. Respir. Rev. 2021, 30, 200226. [Google Scholar] [CrossRef] [PubMed]
- Brcic, L.; Kern, I. Clinical significance of histologic subtyping of malignant pleural mesothelioma. Transl. Lung Cancer Res. 2020, 9, 924–933. [Google Scholar] [CrossRef] [PubMed]
- Dacic, S. Pleural mesothelioma classification—Update and challenges. Mod. Pathol. 2022, 35, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Roca, E.; Aujayeb, A.; Astoul, P. Diagnosis of Pleural Mesothelioma: Is Everything Solved at the Present Time? Curr. Oncol. 2024, 31, 4968–4983. [Google Scholar] [CrossRef] [PubMed]
- Karpes, J.B.; Shamavonian, R.; Dewhurst, S.; Cheng, E.; Wijayawardana, R.; Ahmadi, N.; Morris, D.L. Malignant Peritoneal Mesothelioma: An In-Depth and Up-to-Date Review of Pathogenesis, Diagnosis, Management and Future Directions. Cancers 2023, 15, 4704. [Google Scholar] [CrossRef]
- Sun, H.H.; Vaynblat, A.; Pass, H.I. Diagnosis and prognosis—Review of biomarkers for mesothelioma. Ann. Transl. Med. 2017, 5, 244. [Google Scholar] [CrossRef]
- Hazarika, M.; White, R.M.; Johnson, J.R.; Pazdur, R. FDA Drug Approval Summaries: Pemetrexed (Alimta®). Oncologist 2004, 9, 482–488. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. FDA Approves Drug Combination for Treating Mesothelioma. First Approval in 16 Years for Mesothelioma, a Type of Cancer Caused by Inhaling Asbestos Fibers. 2020. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-drug-combination-treating-mesothelioma (accessed on 26 April 2024).
- Sinn, K.; Mosleh, B.; Hoda, M.A. Malignant pleural mesothelioma: Recent developments. Curr. Opin. Oncol. 2021, 33, 80–86. [Google Scholar] [CrossRef]
- Hu, Z.I.; Ghafoor, A.; Sengupta, M.; Hassan, R. Malignant mesothelioma: Advances in immune checkpoint inhibitor and mesothelin-targeted therapies. Cancer 2021, 127, 1010–1020. [Google Scholar] [CrossRef]
- Vogelzang, N.J.; Rusthoven, J.J.; Symanowski, J.; Denham, C.; Kaukel, E.; Ruffie, P.; Gatzemeier, U.; Boyer, M.; Emri, S.; Manegold, C.; et al. Phase III Study of Pemetrexed in Combination With Cisplatin Versus Cisplatin Alone in Patients With Malignant Pleural Mesothelioma. J. Clin. Oncol. 2003, 21, 2636–2644. [Google Scholar] [CrossRef]
- Santoro, A.; O’Brien, M.E.; Stahel, R.A.; Nackaerts, K.; Baas, P.; Karthaus, M.; Eberhardt, W.; Paz-Ares, L.; Sundstrom, S.; Liu, Y.; et al. Pemetrexed Plus Cisplatin or Pemetrexed Plus Carboplatin for Chemonaïve Patients with Malignant Pleural Mesothelioma: Results of the International Expanded Access Program. J. Thorac. Oncol. 2008, 3, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Oehl, K.; Vrugt, B.; Wagner, U.; Kirschner, M.B.; Meerang, M.; Weder, W.; Felley-Bosco, E.; Wollscheid, B.; Bankov, K.; Demes, M.C.; et al. Alterations in BAP1 Are Associated with Cisplatin Resistance through Inhibition of Apoptosis in Malignant Pleural Mesothelioma. Clin. Cancer Res. 2021, 27, 2277–2291. [Google Scholar] [CrossRef] [PubMed]
- Baas, P.; Scherpereel, A.; Nowak, A.K.; Fujimoto, N.; Peters, S.; Tsao, A.S.; Mansfield, A.S.; Popat, S.; Jahan, T.; Antonia, S.; et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): A multicentre, randomised, open-label, phase 3 trial. Lancet 2021, 397, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Barbier, M.C.; Fengler, A.; Pardo, E.; Bhadhuri, A.; Meier, N.; Gautschi, O. Cost Effectiveness and Budget Impact of Nivolumab Plus Ipilimumab Versus Platinum Plus Pemetrexed (with and Without Bevacizumab) in Patients with Unresectable Malignant Pleural Mesothelioma in Switzerland. Pharmacoeconomics 2023, 41, 1641–1655. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Song, X.; Zeng, W.; Zheng, Z.; Lin, W. First-line nivolumab plus ipilimumab for unresectable MPM in China: A cost-effectiveness analysis. Orphanet J. Rare Dis. 2023, 18, 326. [Google Scholar] [CrossRef]
- Ye, Z.; Tang, Z.-Q.; Xu, Z.; Zhou, Q.; Li, H. Cost-effectiveness of nivolumab plus ipilimumab as first-line treatment for American patients with unresectable malignant pleural mesothelioma. Front. Public Health 2022, 10, 947375. [Google Scholar] [CrossRef]
- Andretta, E.; Costa, C.; Longobardi, C.; Damiano, S.; Giordano, A.; Pagnini, F.; Montagnaro, S.; Quintiliani, M.; Lauritano, C.; Ciarcia, R. Potential Approaches Versus Approved or Developing Chronic Myeloid Leukemia Therapy. Front. Oncol. 2021, 11, 801779. [Google Scholar] [CrossRef]
- Ciarcia, R.; Longobardi, C.; Ferrara, G.; Montagnaro, S.; Andretta, E.; Pagnini, F.; Florio, S.; Maruccio, L.; Lauritano, C.; Damiano, S. The Microalga Skeletonema marinoi Induces Apoptosis and DNA Damage in K562 Cell Line by Modulating NADPH Oxidase. Molecules 2022, 27, 8270. [Google Scholar] [CrossRef]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, M.S.; et al. Role of Phenolic Compounds in Human Disease: Current Knowledge and Future Prospects. Molecules 2021, 27, 233. [Google Scholar] [CrossRef]
- Maiuolo, J.; Gliozzi, M.; Carresi, C.; Musolino, V.; Oppedisano, F.; Scarano, F.; Nucera, S.; Scicchitano, M.; Bosco, F.; Macri, R.; et al. Nutraceuticals and Cancer: Potential for Natural Polyphenols. Nutrients 2021, 13, 3834. [Google Scholar] [CrossRef]
- Adaszek, Ł.; Gadomska, D.; Mazurek, Ł.; Łyp, P.; Madany, J.; Winiarczyk, S. Properties of capsaicin and its utility in veterinary and human medicine. Res. Vet. Sci. 2019, 123, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.-R.; Jang, S.H.; Kim, C.J.; Kim, A.-R.; Yoon, D.-J.; Park, N.-H.; Han, I.-S. Capsaicin suppresses the migration of cholangiocarcinoma cells by down-regulating matrix metalloproteinase-9 expression via the AMPK–NF-κB signaling pathway. Clin. Exp. Metastasis 2014, 31, 897–907. [Google Scholar] [CrossRef]
- Que, T.; Ren, B.; Fan, Y.; Liu, T.; Hou, T.; Dan, W.; Liu, B.; Wei, Y.; Lei, Y.; Zeng, J.; et al. Capsaicin inhibits the migration, invasion and EMT of renal cancer cells by inducing AMPK/mTOR-mediated autophagy. Chem. Biol. Interact. 2022, 366, 110043. [Google Scholar] [CrossRef]
- Chen, M.; Xiao, C.; Jiang, W.; Yang, W.; Qin, Q.; Tan, Q.; Lian, B.; Liang, Z.; Wei, C. Capsaicin Inhibits Proliferation and Induces Apoptosis in Breast Cancer by Down-Regulating FBI-1-Mediated NF-κB Pathway. Drug Des. Dev. Ther. 2021, 15, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.C.; Witte, T.R.; Hardman, W.E.; Luo, H.; Chen, Y.C.; Carpenter, A.B.; Lau, J.K.; Dasgupta, P. Capsaicin Displays Anti-Proliferative Activity against Human Small Cell Lung Cancer in Cell Culture and Nude Mice Models via the E2F Pathway. PLoS ONE 2010, 5, e10243. [Google Scholar] [CrossRef]
- Qian, K.; Wang, G.; Cao, R.; Liu, T.; Qian, G.; Guan, X.; Guo, Z.; Xiao, Y.; Wang, X. Capsaicin Suppresses Cell Proliferation, Induces Cell Cycle Arrest and ROS Production in Bladder Cancer Cells through FOXO3a-Mediated Pathways. Molecules 2016, 21, 1406. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Deng, X.; Lei, T.; Yu, C.; Wang, Y.; Zhao, G.; Luo, X.; Tang, K.; Quan, Z.; Jiang, D. Capsaicin inhibits proliferation and induces apoptosis in osteosarcoma cell lines via the mitogen-activated protein kinase pathway. Oncol. Rep. 2017, 38, 2685–2696. [Google Scholar] [CrossRef]
- Zhang, J.-H.; Lai, F.-J.; Chen, H.; Luo, J.; Zhang, R.-Y.; Bu, H.-Q.; Wang, Z.-H.; Lin, H.-H.; Lin, S.-Z. Involvement of the phosphoinositide 3-kinase/Akt pathway in apoptosis induced by capsaicin in the human pancreatic cancer cell line PANC-1. Oncol. Lett. 2013, 5, 43–48. [Google Scholar] [CrossRef]
- Revathidevi, S.; Munirajan, A.K. Akt in cancer: Mediator and more. Semin. Cancer Biol. 2019, 59, 80–91. [Google Scholar] [CrossRef]
- Roskoski, R. ERK1/2 MAP kinases: Structure, function, and regulation. Pharmacol. Res. 2012, 66, 105–143. [Google Scholar] [CrossRef]
- Wu, D.; Jia, H.; Zhang, Z.; Li, S. Capsaicin suppresses breast cancer cell viability by regulating the CDK8/PI3K/Akt/Wnt/β-catenin signaling pathway. Mol. Med. Rep. 2020, 22, 4868–4876. [Google Scholar] [CrossRef] [PubMed]
- Chapa-Oliver, A.; Mejía-Teniente, L. Capsaicin: From Plants to a Cancer-Suppressing Agent. Molecules 2016, 21, 931. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Chen, S.; Chien, S.; Kuo, S.; Tsai, H.; Chen, D. Capsaicin may induce breast cancer cell death through apoptosis-inducing factor involving mitochondrial dysfunction. Hum. Exp. Toxicol. 2011, 30, 1657–1665. [Google Scholar] [CrossRef]
- Thoennissen, N.H.; O’Kelly, J.; Lu, D.; Iwanski, G.B.; La, D.T.; Abbassi, S.; Leiter, A.; Karlan, B.; Mehta, R.; Koeffler, H.P. Capsaicin causes cell-cycle arrest and apoptosis in ER-positive and -negative breast cancer cells by modulating the EGFR/HER-2 pathway. Oncogene 2010, 29, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Lu, W.-C.; Wang, C.-W.; Chan, Y.-C.; Chen, M.-K. Capsaicin induces cell cycle arrest and apoptosis in human KB cancer cells. BMC Complement. Altern. Med. 2013, 13, 46. [Google Scholar] [CrossRef]
- Venier, N.A.; Colquhoun, A.J.; Sasaki, H.; Kiss, A.; Sugar, L.; Adomat, H.; Fleshner, N.E.; Klotz, L.H.; Venkateswaran, V. Capsaicin: A novel radio-sensitizing agent for prostate cancer. Prostate 2015, 75, 113–125. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, D.; Huang, J.; Hu, Y.; Xu, Y. Application of capsaicin as a potential new therapeutic drug in human cancers. J. Clin. Pharm. Ther. 2020, 45, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Catanzaro, D.; Vianello, C.; Ragazzi, E.; Caparrotta, L.; Montopoli, M. Cell Cycle Control by Natural Phenols in Cisplatin-Resistant Cell Lines. Nat. Prod. Commun. 2014, 9, 1934578X1400901. [Google Scholar] [CrossRef]
- Huh, H.-C.; Lee, S.-Y.; Lee, S.-K.; Park, N.H.; Han, I.-S. Capsaicin Induces Apoptosis of Cisplatin-Resistant Stomach Cancer Cells by Causing Degradation of Cisplatin-Inducible Aurora-A Protein. Nutr. Cancer 2011, 63, 1095–1103. [Google Scholar] [CrossRef]
- Costa, A.; Forte, I.; Pentimalli, F.; Iannuzzi, C.; Alfano, L.; Capone, F.; Camerlingo, R.; Calabrese, A.; von Arx, C.; Benot Dominguez, R.; et al. Pharmacological inhibition of CDK4/6 impairs diffuse pleural mesothelioma 3D spheroid growth and reduces viability of cisplatin-resistant cells. Front. Oncol. 2024, 14, 1418951. [Google Scholar] [CrossRef]
- Di Marzo, D.; Forte, I.M.; Indovina, P.; Di Gennaro, E.; Rizzo, V.; Giorgi, F.; Mattioli, E.; Iannuzzi, C.A.; Budillon, A.; Giordano, A.; et al. Pharmacological targeting of p53 through RITA is an effective antitumoral strategy for malignant pleural mesothelioma. Cell Cycle 2014, 13, 652–665. [Google Scholar] [CrossRef] [PubMed]
- Ventura, E.; Iannuzzi, C.A.; Pentimalli, F.; Giordano, A.; Morrione, A. RBL1/p107 Expression Levels Are Modulated by Multiple Signaling Pathways. Cancers 2021, 13, 5025. [Google Scholar] [CrossRef] [PubMed]
- Arnedo Alejandra Wound Healing Size Tool. Available online: https://github.com/AlejandraArnedo/Wound-healing-size-tool/wiki#reference (accessed on 6 November 2022).
- Suarez-Arnedo, A.; Torres Figueroa, F.; Clavijo, C.; Arbeláez, P.; Cruz, J.C.; Muñoz-Camargo, C. An image J plugin for the high throughput image analysis of in vitro scratch wound healing assays. PLoS ONE 2020, 15, e0232565. [Google Scholar] [CrossRef] [PubMed]
- Ventura, E.; Xie, C.; Buraschi, S.; Belfiore, A.; Iozzo, R.; Giordano, A.; Morrione, A. Complexity of progranulin mechanisms of action in mesothelioma. J. Exp. Clin. Cancer Res. 2022, 41, 333. [Google Scholar] [CrossRef] [PubMed]
- Facchetti, G.; Petrella, F.; Spaggiari, L.; Rimoldi, I. Malignant Pleural Mesothelioma: State of the art and advanced cell therapy. Eur. J. Med. Chem. 2017, 142, 266–270. [Google Scholar] [CrossRef]
- Ocak, B. The Importance of Platinum Sensitivity in Metastatic Malignant Pleural Mesothelioma Patients. Eurasian J. Med. Investig. 2021, 5, 89–94. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Wyckoff, J.; Condeelis, J. Cell migration in tumors. Curr. Opin. Cell Biol. 2005, 17, 559–564. [Google Scholar] [CrossRef]
- Novikov, N.M.; Zolotaryova, S.Y.; Gautreau, A.M.; Denisov, E.V. Mutational drivers of cancer cell migration and invasion. Br. J. Cancer 2021, 124, 102–114. [Google Scholar] [CrossRef]
- Meirson, T.; Gil-Henn, H.; Samson, A.O. Invasion and metastasis: The elusive hallmark of cancer. Oncogene 2020, 39, 2024–2026. [Google Scholar] [CrossRef]
- Wu, J.; Jiang, J.; Chen, B.; Wang, K.; Tang, Y.; Liang, X. Plasticity of cancer cell invasion: Patterns and mechanisms. Transl. Oncol. 2021, 14, 100899. [Google Scholar] [CrossRef]
- Chen, K.-B.; Huang, Y.-J.; Huang, Y.; Wu, Z.-W.; Jin, X.-L.; Zhang, H.; Xiang, X.-P.; Chen, L.; Chen, L. Metastasis of Sarcomatoid Malignant Mesothelioma With p16/CDKN2A Deletion Manifested as a Subcutaneous Mass in the Back: A Case Report and Review of Literature. Int. J. Surg. Pathol. 2021, 29, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, J.; Ueta, K.; Takenaka, M.; Takahashi, M.; Nishizawa, S. Sarcomatoid Malignant Mesothelioma Presenting with Intramedullary Spinal Cord Metastasis: A Case Report and Literature Review. Glob. Spine J. 2014, 4, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Nishikubo, M.; Kin, Y.; Tane, S.; Nakamura, K.; Miyagi, Y.; Miura, A.; Nishio, W.; Senzaki, H.; Uchino, K. Cellular cannibalism and consequent thrombocytopenia in a patient with bone marrow metastasis of malignant pleural mesothelioma: A case report. Mol. Clin. Oncol. 2021, 15, 163. [Google Scholar] [CrossRef] [PubMed]
- Sternbach, D.S.; Henderson, D.L.; Mehrotra, D.B.; Fantasia, D.J. Oral metastasis of pleural sarcomatoid mesothelioma, a rare aggressive variant of mesothelioma and a diagnostic challenge. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2022, 133, e153. [Google Scholar] [CrossRef]
- van der Bij, S.; Koffijberg, H.; Burgers, J.A.; Baas, P.; van de Vijver, M.J.; de Mol, B.A.J.M.; Moons, K.G.M. Prognosis and prognostic factors of patients with mesothelioma: A population-based study. Br. J. Cancer 2012, 107, 161–164. [Google Scholar] [CrossRef]
- Clark, R.; Lee, S.-H. Anticancer Properties of Capsaicin Against Human Cancer. Anticancer Res. 2016, 36, 837–843. [Google Scholar]
- Li, H.; Krstin, S.; Wang, S.; Wink, M. Capsaicin and Piperine Can Overcome Multidrug Resistance in Cancer Cells to Doxorubicin. Molecules 2018, 23, 557. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, M.; Yu, H.; Yuan, G.; Luo, L.; Xu, X.; Xu, Y.; Sui, X.; Leung, E.L.-H.; Wu, Q. The Role and Mechanisms of Action of Natural Compounds in the Prevention and Treatment of Cancer and Cancer Metastasis. Front. Biosci. 2022, 27, 192. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Wang, D.; Gong, X.; Zhou, Q.; Yue, X.; Li, C.; Li, Z.; Tian, G.; Zhang, B.; Wang, Q.; et al. Co-Delivery of Curcumin and Capsaicin by Dual-Targeting Liposomes for Inhibition of aHSC-Induced Drug Resistance and Metastasis. ACS Appl. Mater. Interfaces 2021, 13, 16019–16035. [Google Scholar] [CrossRef]
- Jin, J.; Lin, G.; Huang, H.; Xu, D.; Yu, H.; Ma, X.; Zhu, L.; Ma, D.; Jiang, H. Capsaicin Mediates Cell Cycle Arrest and Apoptosis in Human Colon Cancer Cells via Stabilizing and Activating p53. Int. J. Biol. Sci. 2014, 10, 285–295. [Google Scholar] [CrossRef]
- Yoon, J.-H.; Ahn, S.-G.; Lee, B.-H.; Jung, S.-H.; Oh, S.-H. Role of autophagy in chemoresistance: Regulation of the ATM-mediated DNA-damage signaling pathway through activation of DNA–PKcs and PARP-1. Biochem. Pharmacol. 2012, 83, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.K.; Tavares, M.T.; Pasqualoto, K.F.M.; de Azevedo, R.A.; Teixeira, S.F.; Ferreira-Junior, W.A.; Bertin, A.M.; De-Sá-Junior, P.L.; Barbuto, J.A.M.; Figueiredo, C.R.; et al. RPF151, a novel capsaicin-like analogue: In vitro studies and in vivo preclinical antitumor evaluation in a breast cancer model. Tumor Biol. 2015, 36, 7251–7267. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Naaz, F.; Basit, R.; Das, D.; Bisht, P.; Shaikh, M.; Lone, B.A.; Pokharel, Y.R.; Ahmed, Q.N.; Parveen, S.; et al. 1,2,3-Triazole Tethered Hybrid Capsaicinoids as Antiproliferative Agents Active against Lung Cancer Cells (A549). ACS Omega 2022, 7, 32078–32100. [Google Scholar] [CrossRef]
- Li, B.-H.; Yuan, L. Inhibitory effects of capsaicin on migration and invasion of breast cancer MDA-MB-231 cells and its mechanism. Sheng Li Xue Bao 2017, 69, 183–188. [Google Scholar]
- Hwang, Y.P.; Yun, H.J.; Choi, J.H.; Han, E.H.; Kim, H.G.; Song, G.Y.; Kwon, K.; Jeong, T.C.; Jeong, H.G. Suppression of EGF-induced tumor cell migration and matrix metalloproteinase-9 expression by capsaicin via the inhibition of EGFR-mediated FAK/Akt, PKC/Raf/ERK, p38 MAPK, and AP-1 signaling. Mol. Nutr. Food Res. 2011, 55, 594–605. [Google Scholar] [CrossRef]
- Islam, A.; Yang, Y.-T.; Wu, W.-H.; Chueh, P.J.; Lin, M.-H. Capsaicin attenuates cell migration via SIRT1 targeting and inhibition to enhance cortactin and β-catenin acetylation in bladder cancer cells. Am. J. Cancer Res. 2019, 9, 1172–1182. [Google Scholar] [PubMed]
- Justus, C.R.; Leffler, N.; Ruiz-Echevarria, M.; Yang, L.V. In vitro cell migration and invasion assays. J. Vis. Exp. 2014, 88, 51046. [Google Scholar] [CrossRef]
- Kramer, N.; Walzl, A.; Unger, C.; Rosner, M.; Krupitza, G.; Hengstschläger, M.; Dolznig, H. In vitro cell migration and invasion assays. Mutat. Res. Mutat. Res. 2013, 752, 10–24. [Google Scholar] [CrossRef]
- Liang, C.-C.; Park, A.Y.; Guan, J.-L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef]
- Quijano Moreno, S.L.; García de Lacoba, M. Metastasis of malignant pleural mesothelioma to the scalp following chemotherapy: A case report and review of the literature. Rev. Española Patol. 2022, 55, S27–S31. [Google Scholar] [CrossRef]
- Naldi, G.; Bergomi, S.; Visca, P.; Cecere, F. Ovarian metastasis from malignant pleural mesothelioma. Tumori J. 2020, 106, NP49–NP51. [Google Scholar] [CrossRef] [PubMed]
- Meyerhoff, R.R.; Yang, C.F.J.; Speicher, P.J.; Gulack, B.C.; Hartwig, M.G.; D’Amico, T.A.; Harpole, D.H.; Berry, M.F. Impact of mesothelioma histologic subtype on outcomes in the Surveillance, Epidemiology, and End Results database. J. Surg. Res. 2015, 196, 23–32. [Google Scholar] [CrossRef]
- Verma, V.; Ahern, C.A.; Berlind, C.G.; Lindsay, W.D.; Shabason, J.; Sharma, S.; Culligan, M.J.; Grover, S.; Friedberg, J.S.; Simone, C.B. Survival by Histologic Subtype of Malignant Pleural Mesothelioma and the Impact of Surgical Resection on Overall Survival. Clin. Lung Cancer 2018, 19, e901–e912. [Google Scholar] [CrossRef]
- Yang, H.; Testa, J.R.; Carbone, M. Mesothelioma epidemiology, carcinogenesis, and pathogenesis. Curr. Treat. Options Oncol. 2008, 9, 147–157. [Google Scholar] [CrossRef]
- Li, C.; Rezov, V.; Joensuu, E.; Vartiainen, V.; Rönty, M.; Yin, M.; Myllärniemi, M.; Koli, K. Pirfenidone decreases mesothelioma cell proliferation and migration via inhibition of ERK and AKT and regulates mesothelioma tumor microenvironment in vivo. Sci. Rep. 2018, 8, 10070. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.-F.; Liu, F.; Ma, Y.-C.; Qian, Z.-R.; Shi, L.; Mu, H.; Ding, F.; Fu, X.-Q.; Li, X.-H. Baicalin Regulates Proliferation, Apoptosis, Migration, and Invasion in Mesothelioma. Med. Sci. Monit. 2019, 25, 8172–8180. [Google Scholar] [CrossRef] [PubMed]
- Laszlo, V.; Valko, Z.; Kovacs, I.; Ozsvar, J.; Hoda, M.A.; Klikovits, T.; Lakatos, D.; Czirok, A.; Garay, T.; Stiglbauer, A.; et al. Nintedanib is active in malignant pleural mesothelioma cell models and inhibits angiogenesis and tumor growth in vivo. Clin. Cancer Res. 2018, 24, 3729–3740. [Google Scholar] [CrossRef]
- Shin, D.-H.; Kim, O.-H.; Jun, H.-S.; Kang, M.-K. Inhibitory effect of capsaicin on B16-F10 melanoma cell migration via the phosphatidylinositol 3-kinase/Akt/Rac1 signal pathway. Exp. Mol. Med. 2008, 40, 486. [Google Scholar] [CrossRef]
- Xu, Y.; Kong, W.; Zhao, S.; Xiong, D.; Wang, Y. Capsaicin enhances cisplatin-induced anti-metastasis of nasopharyngeal carcinoma by inhibiting EMT and ERK signaling via serpin family B member 2. Carcinogenesis 2024, 45, 556–568. [Google Scholar] [CrossRef]
- Dai, N.; Ye, R.; He, Q.; Guo, P.; Chen, H.; Zhang, Q. Capsaicin and sorafenib combination treatment exerts synergistic anti-hepatocellular carcinoma activity by suppressing EGFR and PI3K/Akt/mTOR signaling. Oncol. Rep. 2018, 40, 3235–3248. [Google Scholar] [CrossRef]
- Chen, J.-C.; Ko, J.-C.; Yen, T.-C.; Chen, T.-Y.; Lin, Y.-C.; Ma, P.-F.; Lin, Y.-W. Capsaicin enhances erlotinib-induced cytotoxicity via AKT inactivation and excision repair cross-complementary 1 (ERCC1) down-regulation in human lung cancer cells. Toxicol. Res. 2019, 8, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhao, Q.; Liu, X.; Zhou, X.; Wang, Y.; Zhao, M.; Wu, F.; Zhao, G.; Guo, X. Capsaicin combined with cisplatin inhibits TGF-β1-induced EMT and TSCC cells migration via the Claudin-1/PI3K/AKT/mTOR signaling pathway. Cancer Cell Int. 2024, 24, 300. [Google Scholar] [CrossRef] [PubMed]
- YANG, J.; LI, T.Z.; XU, G.H.; LUO, B.B.; CHEN, Y.X.; ZHANG, T. Low-concentration capsaicin promotes colorectal cancer metastasis by triggering ROS production and modulating Akt/mTOR and STAT-3 pathways. Neoplasma 2013, 60, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Luján-Méndez, F.; Roldán-Padrón, O.; Castro-Ruíz, J.; López-Martínez, J.; García-Gasca, T. Capsaicinoids and Their Effects on Cancer: The “Double-Edged Sword” Postulate from the Molecular Scale. Cells 2023, 12, 2573. [Google Scholar] [CrossRef]
- Liu, N.-C.; Hsieh, P.-F.; Hsieh, M.-K.; Zeng, Z.-M.; Cheng, H.-L.; Liao, J.-W.; Chueh, P.J. Capsaicin-Mediated tNOX (ENOX2) Up-regulation Enhances Cell Proliferation and Migration in Vitro and in Vivo. J. Agric. Food Chem. 2012, 60, 2758–2765. [Google Scholar] [CrossRef] [PubMed]
- Hatono, M.; Ikeda, H.; Suzuki, Y.; Kajiwara, Y.; Kawada, K.; Tsukioki, T.; Kochi, M.; Suzawa, K.; Iwamoto, T.; Yamamoto, H.; et al. Effect of isoflavones on breast cancer cell development and their impact on breast cancer treatments. Breast Cancer Res. Treat. 2021, 185, 307–316. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, H.; Zheng, Q.; Li, H.; You, H.; Feng, Y.; Feng, W. Promotion of tumor progression induced by continuous low-dose administration of antineoplastic agent gemcitabine or gemcitabine combined with cisplatin. Life Sci. 2022, 306, 120826. [Google Scholar] [CrossRef]
- Zhang, R.; Humphreys, I.; Sahu, R.P.; Shi, Y.; Srivastava, S.K. In vitro and in vivo induction of apoptosis by capsaicin in pancreatic cancer cells is mediated through ROS generation and mitochondrial death pathway. Apoptosis 2008, 13, 1465–1478. [Google Scholar] [CrossRef]
- Popescu, G.D.A.; Scheau, C.; Badarau, I.A.; Dumitrache, M.-D.; Caruntu, A.; Scheau, A.-E.; Costache, D.O.; Costache, R.S.; Constantin, C.; Neagu, M.; et al. The Effects of Capsaicin on Gastrointestinal Cancers. Molecules 2020, 26, 94. [Google Scholar] [CrossRef]
- Liu, T.; Wang, G.; Tao, H.; Yang, Z.; Wang, Y.; Meng, Z.; Cao, R.; Xiao, Y.; Wang, X.; Zhou, J. Capsaicin mediates caspases activation and induces apoptosis through P38 and JNK MAPK pathways in human renal carcinoma. BMC Cancer 2016, 16, 790. [Google Scholar] [CrossRef]
- Jung, M.-Y.; Kang, H.-J.; Moon, A. Capsaicin-induced apoptosis in SK-Hep-1 hepatocarcinoma cells involves Bcl-2 downregulation and caspase-3 activation. Cancer Lett. 2001, 165, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Cömertpay, S.; Demirbanka, F.G. Lowered Cyclin E levels increase the efficiency and the specificity of capsaicin against cancerous cells of mesothelium. Cell. Mol. Biol. 2020, 66, 98–104. [Google Scholar] [CrossRef]
- Chou, C.; Wu, Y.; Wang, Y.; Chou, M.; Kuo, S.; Chen, D. Capsaicin-induced apoptosis in human breast cancer MCF-7 cells through caspase-independent pathway. Oncol. Rep. 2009, 21, 665–671. [Google Scholar] [CrossRef]
- Lee, M.-J.; Kee, K.-H.; Suh, C.-H.; Lim, S.-C.; Oh, S.-H. Capsaicin-induced apoptosis is regulated by endoplasmic reticulum stress- and calpain-mediated mitochondrial cell death pathways. Toxicology 2009, 264, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Acklin, S.; Xia, F. The Role of Nucleotide Excision Repair in Cisplatin-Induced Peripheral Neuropathy: Mechanism, Prevention, and Treatment. Int. J. Mol. Sci. 2021, 22, 1975. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.; Kim, B.; Kim, W. Cisplatin-Induced Nausea and Vomiting: Effect of Herbal Medicines. Plants 2022, 11, 3395. [Google Scholar] [CrossRef]
- Bright, H.; Singh, A.; Joel, A.; Georgy, J.; John, A.; Rajkumar, P.; Jiji, H.; Stehno-Bittel, L.; Samuel, P.; Chandy, S. Randomized Placebo-Controlled Trial of Topical Capsaicin for Delayed Chemotherapy-Induced Nausea and Vomiting. JCO Glob. Oncol. 2024, 10, e2400130. [Google Scholar] [CrossRef]
- Moreno-Alonso, D.; Llorens-Torromé, S.; Corcoy de Febrer, B.; Amandi García, M.; Serrano-Bermúdez, G.; Trelis-Navarro, J.; Mayoral-Rojals, V.; Serrano-Afonso, A. Adhesive capsaicin 8% patch for improved control of pain caused by chemotherapy-induced peripheral neuropathy in patients with multiple myeloma: A single-centre, seven-case series. J. Oncol. Pharm. Pract. 2024, 30, 752–758. [Google Scholar] [CrossRef]
- Al-Samydai, A.; Alshaer, W.; Al-Dujaili, E.A.S.; Azzam, H.; Aburjai, T. Preparation, Characterization, and Anticancer Effects of Capsaicin-Loaded Nanoliposomes. Nutrients 2021, 13, 3995. [Google Scholar] [CrossRef]
- Rollyson, W.D.; Stover, C.A.; Brown, K.C.; Perry, H.E.; Stevenson, C.D.; McNees, C.A.; Ball, J.G.; Valentovic, M.A.; Dasgupta, P. Bioavailability of capsaicin and its implications for drug delivery. J. Control. Release 2014, 196, 96–105. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, J.; Zheng, Q.; Wang, M.; Deng, W.; Li, Q.; Firempong, C.K.; Wang, S.; Tong, S.; Xu, X.; et al. In vitro and in vivo evaluation of capsaicin-loaded microemulsion for enhanced oral bioavailability. J. Sci. Food Agric. 2015, 95, 2678–2685. [Google Scholar] [CrossRef] [PubMed]
- Chanda, S.; Bashir, M.; Babbar, S.; Koganti, A.; Bley, K. In Vitro Hepatic and Skin Metabolism of Capsaicin. Drug Metab. Dispos. 2008, 36, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Chaiyasit, K.; Khovidhunkit, W.; Wittayalertpanya, S. Pharmacokinetic and the effect of capsaicin in Capsicum frutescens on decreasing plasma glucose level. J. Med. Assoc. Thai 2009, 92, 108–113. [Google Scholar]
- Harada, N.; Okajima, K.; Arai, M.; Kurihara, H.; Nakagata, N. Administration of capsaicin and isoflavone promotes hair growth by increasing insulin-like growth factor-I production in mice and in humans with alopecia. Growth Horm. IGF Res. 2007, 17, 408–415. [Google Scholar] [CrossRef] [PubMed]
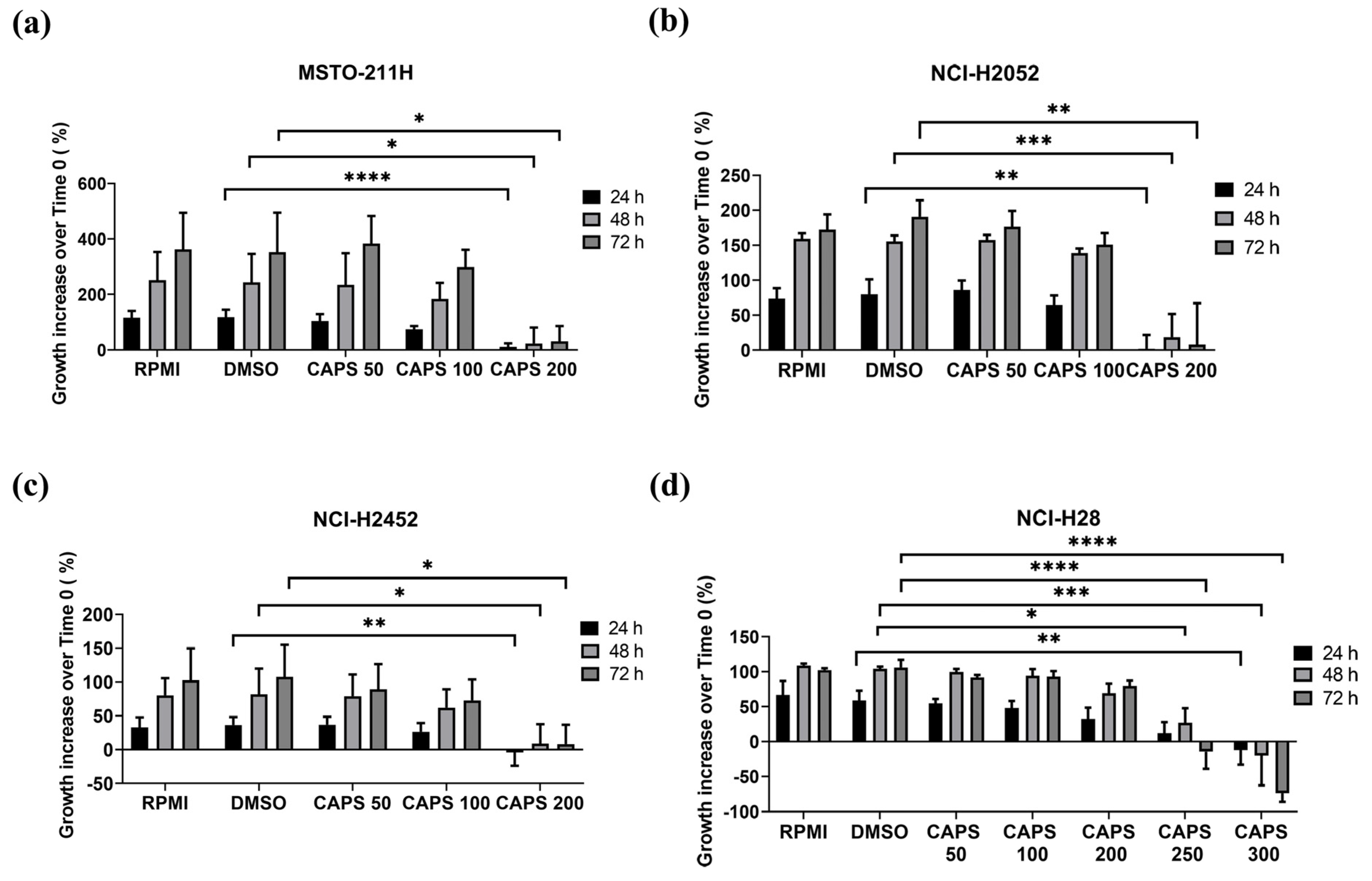



| MM Cell Line | IC50 (24 h Treatment) | IC50 (48 h Treatment) | IC50 (72 h Treatment) |
|---|---|---|---|
| MSTO-211H | 193.6 | 192.8 | 189.5 |
| NCI-H2052 | 216.1 | 198.1 | 173.0 |
| NCI-H2452 | 231.4 | 215.0 | 191.6 |
| NCI-H28 | 325.2 | 275.9 | 242.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andretta, E.; Costa, A.; Ventura, E.; Quintiliani, M.; Damiano, S.; Giordano, A.; Morrione, A.; Ciarcia, R. Capsaicin Exerts Antitumor Activity in Mesothelioma Cells. Nutrients 2024, 16, 3758. https://doi.org/10.3390/nu16213758
Andretta E, Costa A, Ventura E, Quintiliani M, Damiano S, Giordano A, Morrione A, Ciarcia R. Capsaicin Exerts Antitumor Activity in Mesothelioma Cells. Nutrients. 2024; 16(21):3758. https://doi.org/10.3390/nu16213758
Chicago/Turabian StyleAndretta, Emanuela, Aurora Costa, Elisa Ventura, Massimiliano Quintiliani, Sara Damiano, Antonio Giordano, Andrea Morrione, and Roberto Ciarcia. 2024. "Capsaicin Exerts Antitumor Activity in Mesothelioma Cells" Nutrients 16, no. 21: 3758. https://doi.org/10.3390/nu16213758
APA StyleAndretta, E., Costa, A., Ventura, E., Quintiliani, M., Damiano, S., Giordano, A., Morrione, A., & Ciarcia, R. (2024). Capsaicin Exerts Antitumor Activity in Mesothelioma Cells. Nutrients, 16(21), 3758. https://doi.org/10.3390/nu16213758








