Genetic Variants Affecting Iron Metabolism in Healthy Adults: A Systematic Review to Support Personalized Nutrition Strategies
Abstract
:1. Introduction
2. Methods
2.1. Eligibility Criteria
2.2. Data Sources and Search Methods
2.3. Study Selection and Data Extraction
2.4. Risk-of-Bias Assessment
3. Results
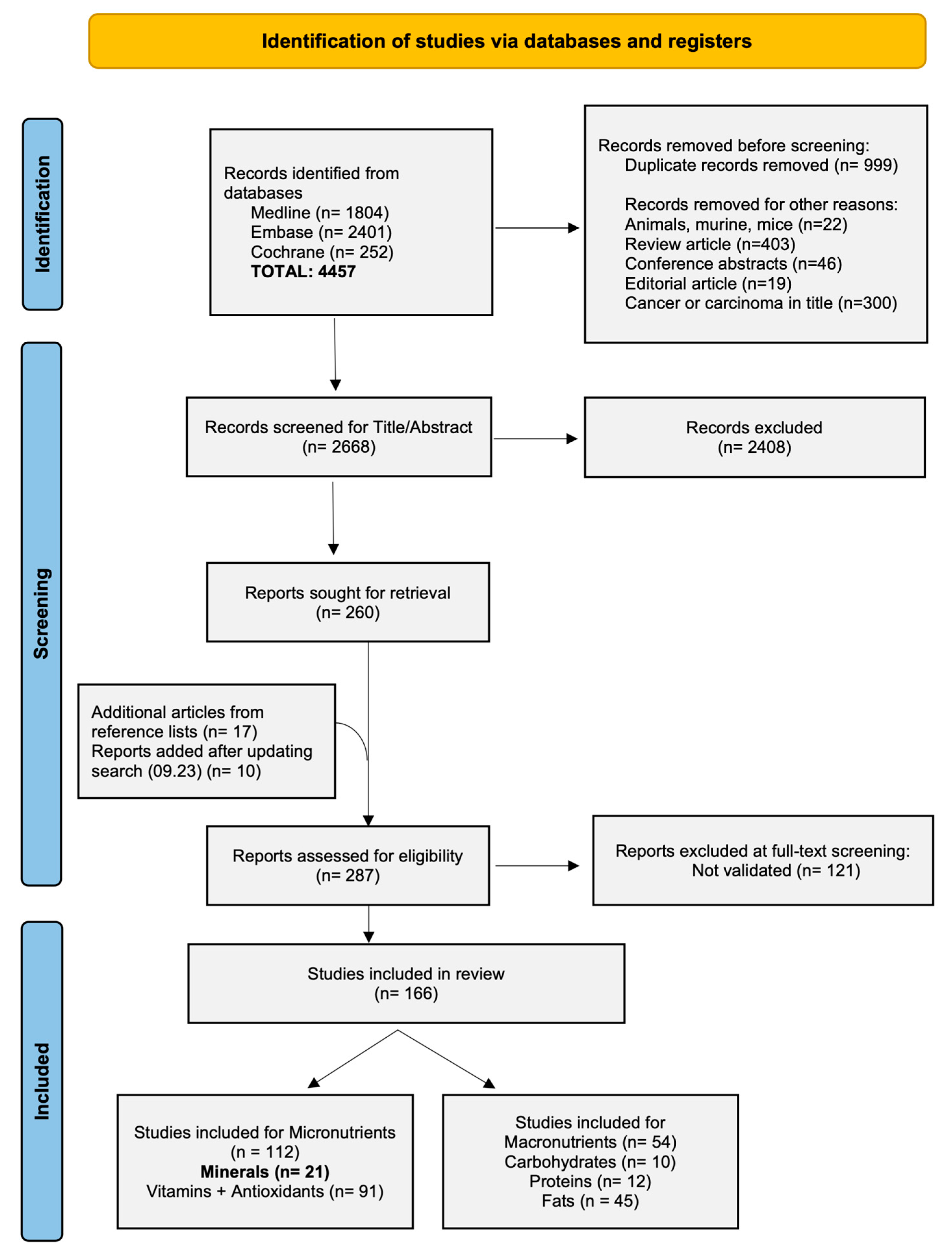
3.1. Study Selection and Characteristics
3.2. Study Quality
3.3. SNPs Associated with Minerals (Specifically Iron)
3.4. Effects of rs855791 in TMPRSS6
3.5. Effects of rs4820268 in TMPRSS6
3.6. Effects of rs2235321 in TMPRSS6
3.7. Effects of rs2235324 in TMPRSS6
3.8. Effects of rs2413450 in TMPRSS6
3.9. Effects of rs1800562 in HFE
3.10. Effects of rs1799945 in HFE
3.11. Effects of rs3811647 in TF
3.12. Effects of rs1799852 in TF
3.13. Effects of rs235756 in BMP2
3.14. Effects of rs2698530 in Chromosome 2
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Swiss Society for Nutrition SSN. Swiss Food Pyramid; Federal Food Safety and Veterinary Office, Ed.; Swiss Society for Nutrition SSN: Bern, Switzerland, 2011. [Google Scholar]
- Mullins, V.A.; Bresette, W.; Johnstone, L.; Hallmark, B.; Chilton, F.H. Genomics in Personalized Nutrition: Can You “Eat for Your Genes”? Nutrients 2020, 12, 3118. [Google Scholar] [CrossRef] [PubMed]
- Bashiardes, S.; Godneva, A.; Elinav, E.; Segal, E. Towards utilization of the human genome and microbiome for personalized nutrition. Curr. Opin. Biotechnol. 2018, 51, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Aruoma, O.I.; Hausman-Cohen, S.; Pizano, J.; Schmidt, M.A.; Minich, D.M.; Joffe, Y.; Brandhorst, S.; Evans, S.J.; Brady, D.M. Personalized Nutrition: Translating the Science of NutriGenomics Into Practice: Proceedings From the 2018 American College of Nutrition Meeting. J. Am. Coll. Nutr. 2019, 38, 287–301. [Google Scholar] [CrossRef] [PubMed]
- Kirk, D.; Catal, C.; Tekinerdogan, B. Precision nutrition: A systematic literature review. Comput. Biol. Med. 2021, 133, 104365. [Google Scholar] [CrossRef] [PubMed]
- W.B. Saunders Co. Nutrigenomics. In Dorland’s Illustrated Medical Dictionary, 32nd ed.; Elsevier: Philadelphia, PA, USA, 2011. [Google Scholar]
- Stevens, G.A.; Paciorek, C.J.; Flores-Urrutia, M.C.; Borghi, E.; Namaste, S.; Wirth, J.P.; Suchdev, P.S.; Ezzati, M.; Rohner, F.; Flaxman, S.R.; et al. National, regional, and global estimates of anaemia by severity in women and children for 2000–2019: A pooled analysis of population-representative data. Lancet Glob. Health 2022, 10, e627–e639. [Google Scholar] [CrossRef]
- Schardt, C.; Adams, M.B.; Owens, T.; Keitz, S.; Fontelo, P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med. Inform. Decis. Mak. 2007, 7, 16. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
- Mourad, O.; Hossam, H.; Zbys, F.; Ahmed, E. Rayyan—A Web and Mobile App for Systematic Reviews. 2016. Available online: https://www.rayyan.ai (accessed on 1 June 2022).
- Institute, J.B. Critical Appraisal Tools. 2017. Available online: https://jbi.global/critical-appraisal-tools (accessed on 1 March 2023).
- Al-Amer, O.; Hawasawi, Y.; Oyouni, A.A.A.; Alshehri, M.; Alasmari, A.; Alzahrani, O.; Aljohani, S.A.S. Study the association of transmembrane serine protease 6 gene polymorphisms with iron deficiency status in Saudi Arabia. Gene 2020, 751, 144767. [Google Scholar] [CrossRef]
- Baeza-Richer, C.; Arroyo-Pardo, E.; Blanco-Rojo, R.; Toxqui, L.; Remacha, A.; Vaquero, M.P.; López-Parra, A.M. Genetic contribution to iron status: SNPs related to iron deficiency anaemia and fine mapping of CACNA2D3 calcium channel subunit. Blood Cells Mol. Dis. 2015, 55, 273–280. [Google Scholar] [CrossRef]
- Meidtner, K.; Podmore, C.; Kroger, J.; van der Schouw, Y.T.; Bendinelli, B.; Agnoli, C.; Arriola, L.; Barricarte, A.; Boeing, H.; Cross, A.J.; et al. Interaction of Dietary and Genetic Factors Influencing Body Iron Status and Risk of Type 2 Diabetes Within the EPIC-InterAct Study. Diabetes Care 2018, 41, 277–285. [Google Scholar] [CrossRef]
- An, P.; Wu, Q.; Wang, H.; Guan, Y.; Mu, M.; Liao, Y.; Zhou, D.; Song, P.; Wang, C.; Meng, L.; et al. TMPRSS6, but not TF, TFR2 or BMP2 variants are associated with increased risk of iron-deficiency anemia. Hum. Mol. Genet. 2012, 21, 2124–2131. [Google Scholar] [CrossRef] [PubMed]
- Jallow, M.W.; Campino, S.; Saidykhan, A.; Prentice, A.M.; Cerami, C. Common Variants in the TMPRSS6 Gene Alter Hepcidin but not Plasma Iron in Response to Oral Iron in Healthy Gambian Adults: A Recall-by-Genotype Study. Curr. Dev. Nutr. 2021, 5, nzab014. [Google Scholar] [CrossRef] [PubMed]
- Poggiali, E.; Andreozzi, F.; Nava, I.; Consonni, D.; Graziadei, G.; Cappellini, M.D. The role of TMPRSS6 polymorphisms in iron deficiency anemia partially responsive to oral iron treatment. Am. J. Hematol. 2015, 90, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Pinto, J.; de Jesus, G.; Anselmo, M.P.; Gonçalves, L.; Brás, D.; Lopes, J.M.; Meneses, J.; Victorino, R.; Faustino, P. Iron refractory iron deficiency anemia in dizygotic twins due to a novel TMPRSS6 gene mutation in addition to polymorphisms associated with high susceptibility to develop ferropenic anemia. J. Investig. Med. High Impact Case Rep. 2017, 5, 2324709617701776. [Google Scholar] [CrossRef] [PubMed]
- Pei, S.N.; Ma, M.C.; You, H.L.; Fu, H.C.; Kuo, C.Y.; Rau, K.M.; Wang, M.C.; Lee, C.T. TMPRSS6 rs855791 polymorphism influences the susceptibility to iron deficiency anemia in women at reproductive age. Int. J. Med. Sci. 2014, 11, 614–619. [Google Scholar] [CrossRef]
- Batar, B.; Bavunoglu, I.; Hacioglu, Y.; Cengiz, M.; Mutlu, T.; Yavuzer, S.; Yavuzer, H.; Cuhadar Ercelebi, D.; Erhan, D.; Unal, S.; et al. The role of TMPRSS6 gene variants in iron-related hematological parameters in Turkish patients with iron deficiency anemia. Gene 2018, 673, 201–205. [Google Scholar] [CrossRef]
- Ji, Y.; Flower, R.; Hyland, C.; Saiepour, N.; Faddy, H. Genetic factors associated with iron storage in Australian blood donors. Blood Transfus. 2018, 16, 123–129. [Google Scholar]
- Lee, P.L.; Barton, J.C.; Khaw, P.L.; Bhattacharjee, S.Y.; Barton, J.C. Common TMPRSS6 mutations and iron, erythrocyte, and pica phenotypes in 48 women with iron deficiency or depletion. Blood Cells Mol. Dis. 2012, 48, 124–127. [Google Scholar] [CrossRef]
- Pagani, A.; Nai, A.; Silvestri, L.; Camaschella, C. Hepcidin and Anemia: A Tight Relationship. Front. Physiol. 2019, 10, 1294. [Google Scholar] [CrossRef]
- Melis, M.A.; Cau, M.; Congiu, R.; Sole, G.; Barella, S.; Cao, A.; Westerman, M.; Cazzola, M.; Galanello, R. A mutation in the TMPRSS6 gene, encoding a transmembrane serine protease that suppresses hepcidin production, in familial iron deficiency anemia refractory to oral iron. Haematologica 2008, 93, 1473–1479. [Google Scholar] [CrossRef]
- NHS. Total Iron-Binding Capacity (TIBC) and Transferrin Test. July 2022. Available online: https://www.nhs.uk/conditions/tibc-test/ (accessed on 3 January 2023).
- NCBI. Reference SNP (rs) Report: rs2413450. 2021. Available online: https://www.ncbi.nlm.nih.gov/snp/rs2413450 (accessed on 6 January 2023).
- Blanco-Rojo, R.; Baeza-Richer, C.; Lápez-Parra, A.M.; Pérez-Granados, A.M.; Brichs, A.; Bertoncini, S.; Buil, A.; Arroyo-Pardo, E.; Soria, J.M.; Vaquero, M.P. Four variants in transferrin and HFE genes as potential markers of iron deficiency anaemia risk: An association study in menstruating women. Nutr. Metab. 2011, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Aranda, N.; Viteri, F.E.; Montserrat, C.; Arija, V. Effects of C282Y, H63D, and S65C HFE gene mutations, diet, and life-style factors on iron status in a general Mediterranean population from Tarragona, Spain. Ann. Hematol. 2010, 89, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Terada, C.T.; Santos, P.C.J.L.; Cançado, R.D.; Rostelato, S.; Lopreato, F.R.; Chiattone, C.S.; Guerra-Shinohara, E.M. Iron deficiency and frequency of HFE C282Y gene mutation in Brazilian blood donors. Transfus. Med. 2009, 19, 245–251. [Google Scholar] [CrossRef]
- NCBI. Reference SNP (rs) Report: rs1800562. 2021. Available online: https://www.ncbi.nlm.nih.gov/snp/rs1800562 (accessed on 20 January 2023).
- Beiranvand, E.; Abediankenari, S.; Rostamian, M.; Beiranvand, B.; Naazeri, S. The study of HFE genotypes and its expression effect on iron status of iranian haemochromatosis, iron deficiency anemia patients, iron-taker and non iron-taker controls. Recent Adv. DNA Gene Seq. 2015, 9, 58–64. [Google Scholar] [CrossRef]
- McLaren, C.E.; Garner, C.P.; Constantine, C.C.; McLachlan, S.; Vulpe, C.D.; Snively, B.M.; Gordeuk, V.R.; Nickerson, D.A.; Cook, J.D.; Leiendecker-Foster, C.; et al. Genome-wide association study identifies genetic loci associated with iron deficiency. PLoS ONE 2011, 6, e17390. [Google Scholar] [CrossRef]
- Mayo-Clinic. Hemochromatosis: Diagnosis and Treatment. 2023. Available online: https://www.mayoclinic.org/diseases-conditions/hemochromatosis/diagnosis-treatment/drc-20351448?p=1 (accessed on 27 January 2023).
- Kutalik, Z.; Benyamin, B.; Bergmann, S.; Mooser, V.; Waeber, G.; Montgomery, G.W.; Martin, N.G.; Madden, P.A.F.; Heath, A.C.; Beckmann, J.S.; et al. Genome-wide association study identifies two loci strongly affecting transferrin glycosylation. Hum. Mol. Genet. 2011, 20, 3710–3717. [Google Scholar] [CrossRef] [PubMed]
- Al-Amer, O.M.; Oyouni, A.A.A.; Alshehri, M.; Alzaheb, R.A. Transferrin (Re3811647) gene polymorphism in iron deficiency anemia in saudi arabia. Indian J. Forensic Med. Toxicol. 2018, 12, 329–334. [Google Scholar] [CrossRef]
- Sarriá, B.; López-Parra, A.M.; Navas-Carretero, S.; Pérez-Granados, A.M.; Baeza, C.; Arroyo-Pardo, E.; Vaquero, M.P. Hepcidin, transferrin (exon 7), and hemochromatosis genotyping suggests that haplotype block analysis is the best strategy for predicting iron deficiency phenotype in women. Nutr. Res. 2007, 27, 672–678. [Google Scholar] [CrossRef]
- Al-Amer, O.M.; Oyouni, A.A.A.; Alshehri, M.A.; Alasmari, A.; Alzahrani, O.R.; Aljohani, S.A.S.; Alasmael, N.; Theyab, A.; Algahtani, M.; Sadoun, H.A.; et al. Association of SNPs within TMPRSS6 and BMP2 genes with iron deficiency status in Saudi Arabia. PLoS ONE 2021, 16, e0257895. [Google Scholar] [CrossRef]
- Zeisel, S.H. Precision (Personalized) Nutrition: Understanding Metabolic Heterogeneity. Annu. Rev. Food Sci. Technol. 2020, 11, 71–92. [Google Scholar] [CrossRef]
- Méplan, C.; Crosley, L.K.; Nicol, F.; Beckett, G.J.; Howie, A.F.; Hill, K.E.; Horgan, G.; Mathers, J.C.; Arthur, J.R.; Hesketh, J.E. Genetic polymorphisms in the human selenoprotein P gene determine the response of selenoprotein markers to selenium supplementation in a gender-specific manner (the SELGEN study). FASEB J. 2007, 21, 3063–3074. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Li, L.; Feng, X.; Cheng, H.; Ge, X.; Bao, Y.; Huang, L.; Wang, F.; Liu, C.; Chen, X.; et al. Genome-wide association and Mendelian randomization study of blood copper levels and 213 deep phenotypes in humans. Commun. Biol. 2022, 5, 405. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Chen, W.; Jin, A.; Wang, M.; Yan, H.; Xiang, X.; Pan, Y. Effects of genetically determined mineral status on life expectancy: A Mendelian randomization study. Chin. Med. J. 2023, 136, 242–244. [Google Scholar] [CrossRef] [PubMed]
| SNP | Gene | Affected Iron Parameter | Odds Ratios for ID/IDA |
|---|---|---|---|
| rs855791 (V736A) | TMPRSS6 | Ferritin, Transferrin, TS, Hemoglobin, MCV/MCH, Hepcidin, TIBC, Serum iron, RBC | 1.78–22.5 |
| rs4820268 (D521D) | TMPRSS6 | Ferritin, TS, Hepcidin, UIBC, TIBC, Serum iron | 1.5/1.7–3.4 |
| rs2235321 (Y739Y) | TMPRSS6 | TS, MCV/MCH, Hepcidin, UIBC, TIBC | 1.9 |
| rs2235324 (K253E) | TMPRSS6 | TS | 8.7 |
| rs2413450 | TMPRSS6 | TIBC | Not reported |
| rs1800562 (C282Y) | HFE | Ferritin, Transferrin, TS, UIBC, TIBC, Serum iron | 0.169 |
| rs1799945 (H63D) | HFE | Ferritin, Transferrin, TS, | Not reported |
| rs3811647 | TF | Ferritin, Transferrin, TS, TIBC, Serum iron | Not reported |
| rs1799852 (L247L) | TF | Transferrin, Haemoglobin | Not reported |
| rs235756 | BMP2 | Ferritin | 29.3 |
| rs2698530 | N/A (Chromosome 2) | UIBC TIBC | Not reported |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bösch, E.S.; Spörri, J.; Scherr, J. Genetic Variants Affecting Iron Metabolism in Healthy Adults: A Systematic Review to Support Personalized Nutrition Strategies. Nutrients 2024, 16, 3793. https://doi.org/10.3390/nu16223793
Bösch ES, Spörri J, Scherr J. Genetic Variants Affecting Iron Metabolism in Healthy Adults: A Systematic Review to Support Personalized Nutrition Strategies. Nutrients. 2024; 16(22):3793. https://doi.org/10.3390/nu16223793
Chicago/Turabian StyleBösch, Elana Sophie, Jörg Spörri, and Johannes Scherr. 2024. "Genetic Variants Affecting Iron Metabolism in Healthy Adults: A Systematic Review to Support Personalized Nutrition Strategies" Nutrients 16, no. 22: 3793. https://doi.org/10.3390/nu16223793
APA StyleBösch, E. S., Spörri, J., & Scherr, J. (2024). Genetic Variants Affecting Iron Metabolism in Healthy Adults: A Systematic Review to Support Personalized Nutrition Strategies. Nutrients, 16(22), 3793. https://doi.org/10.3390/nu16223793








