Effects of Soluble and Insoluble Fibre on Glycolipid Metabolism and Gut Microbiota in High-Fat-Diet-Induced Obese Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Analysis of the Nutrient Composition of Barley Leaf Powder
2.2. Extraction and Determination of Dietary Fibres from Barley Leaf Powder
2.3. Scanning Electron Microscopy (SEM) Analysis
2.4. Animal Experiments
2.5. Detection of Serum Biochemical Indexes
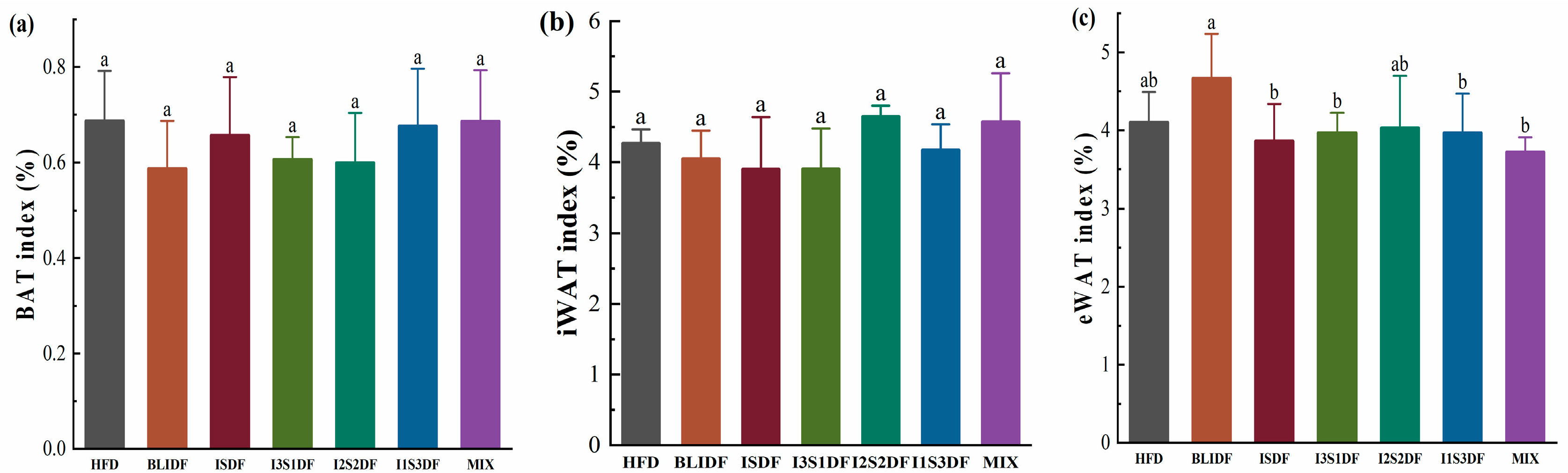
2.6. Analysis of Organ Indexes
2.7. Detection of Liver Biochemical Indexes
2.8. Histological Analysis
2.9. Gut Microbiota Analysis
2.10. Statistical Analysis
3. Results and Discussion
3.1. The Dietary Fibre in Barley Leaf Powder
3.2. Effects of Soluble and Insoluble Dietary Fibre Ratios on Body Weight of Obese Mice
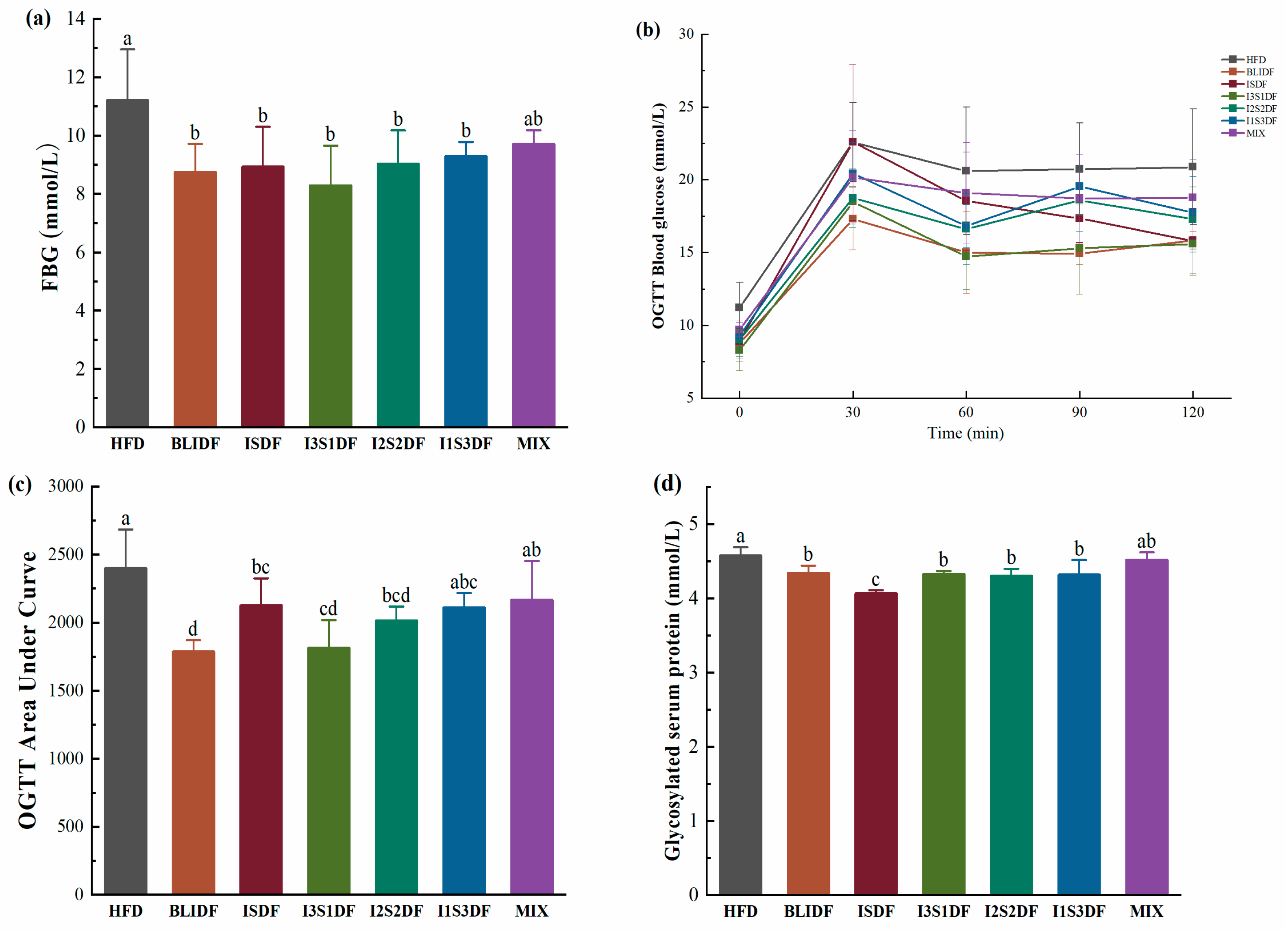
3.3. Effects of Soluble and Insoluble Dietary Fibre Ratios on Glucose Homeostasis of Obese Mice
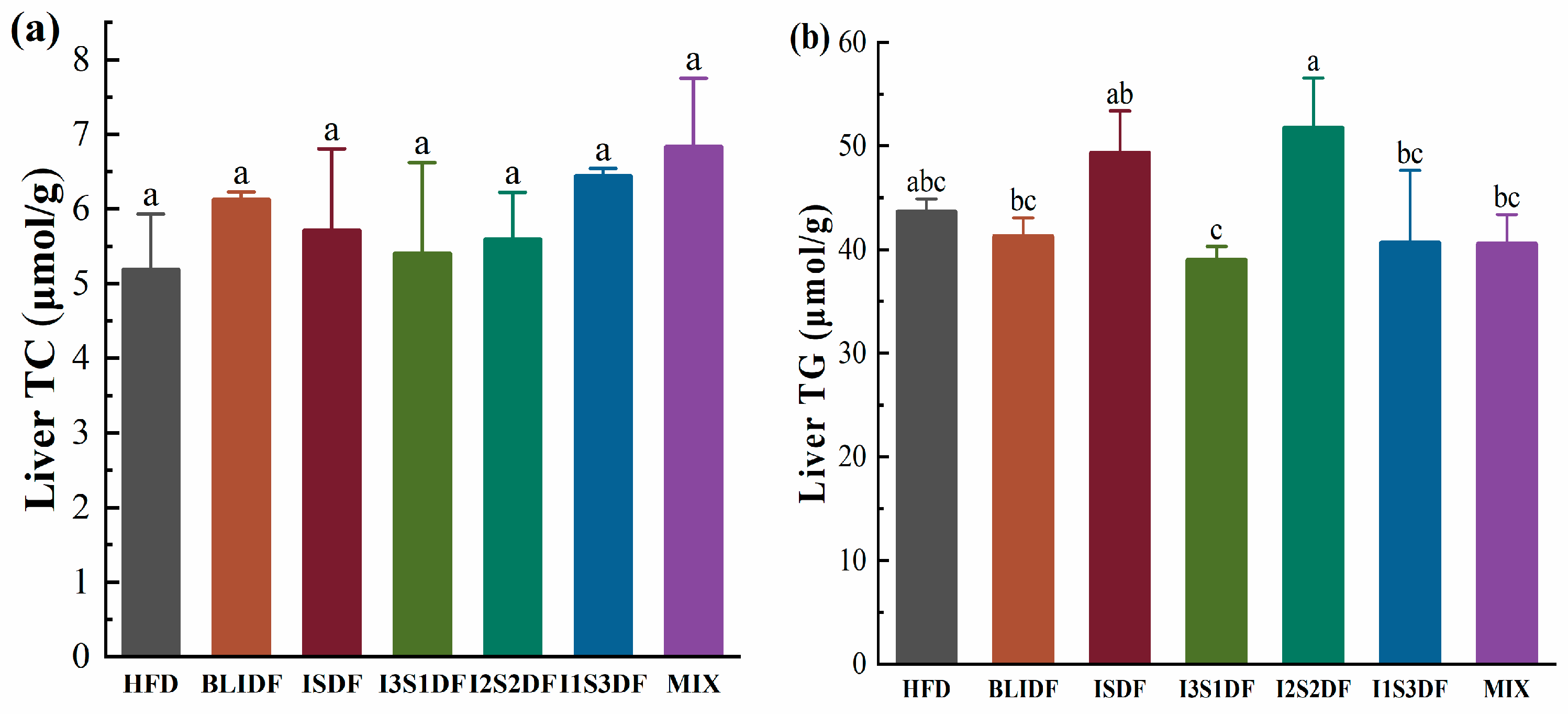
3.4. Effects of Soluble and Insoluble Dietary Fibre Ratios on Fat Accumulation and Lipid Profiles of Obese Mice
3.5. Effects of Soluble and Insoluble Dietary Fibre Ratios on Energy Expenditure and RER of Obese Mice
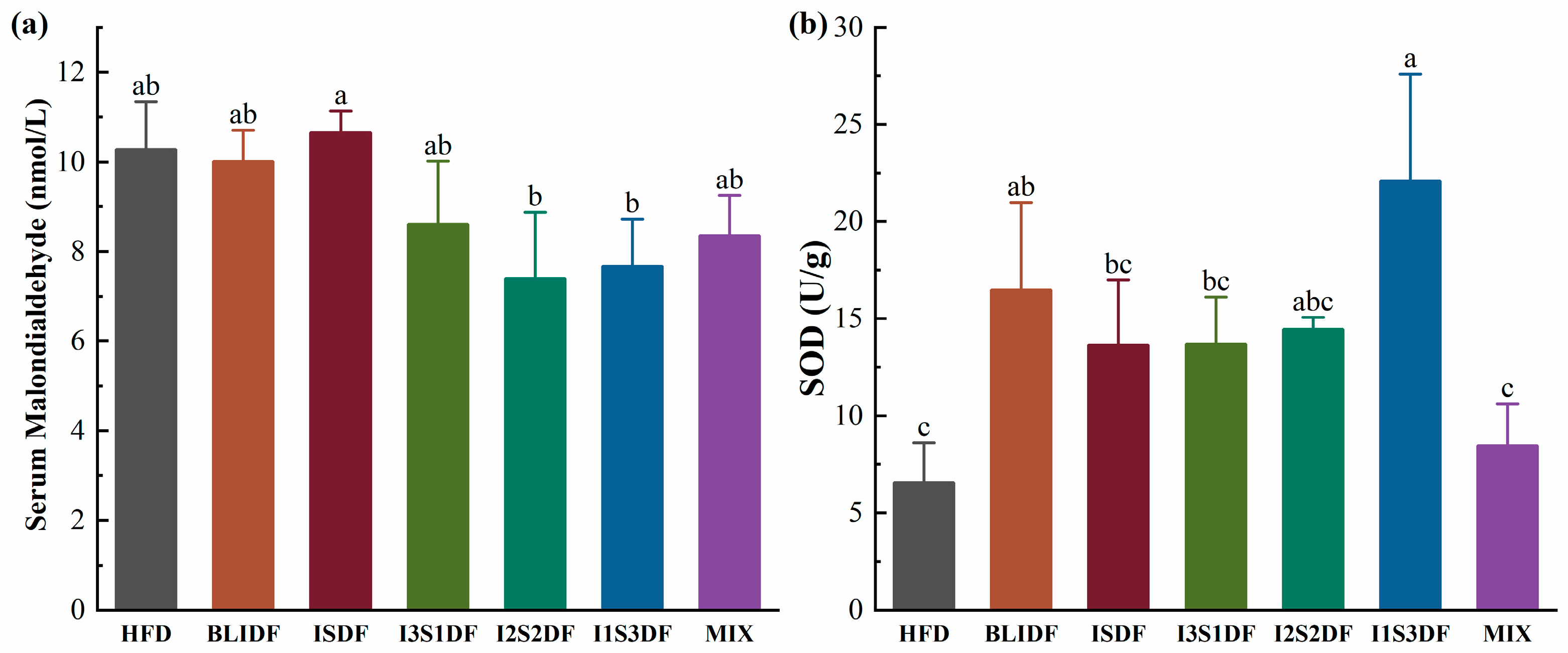
3.6. Effects of Soluble and Insoluble Dietary Fibre Ratios on Inflammation Levels and Antioxidant Ability in Obese Mice
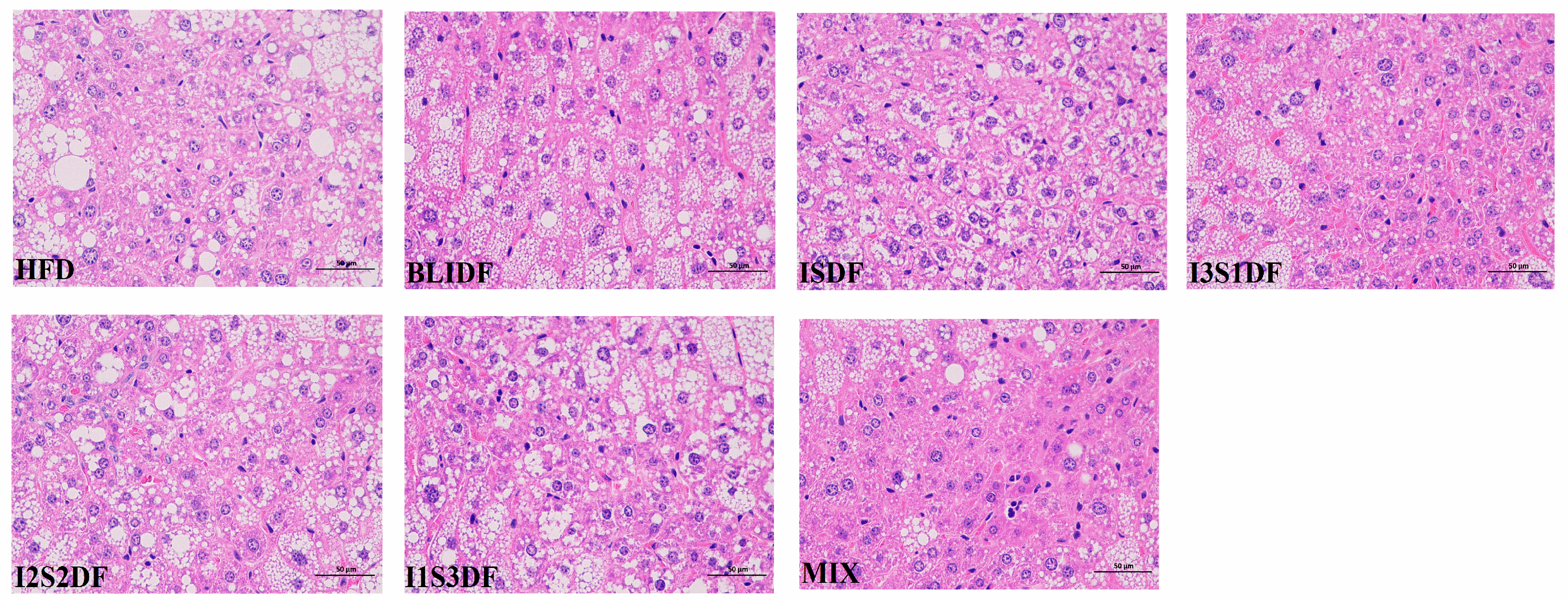
3.7. Effects of Soluble and Insoluble Dietary Fibre Ratios on Liver Histomorphology of Obese Mice
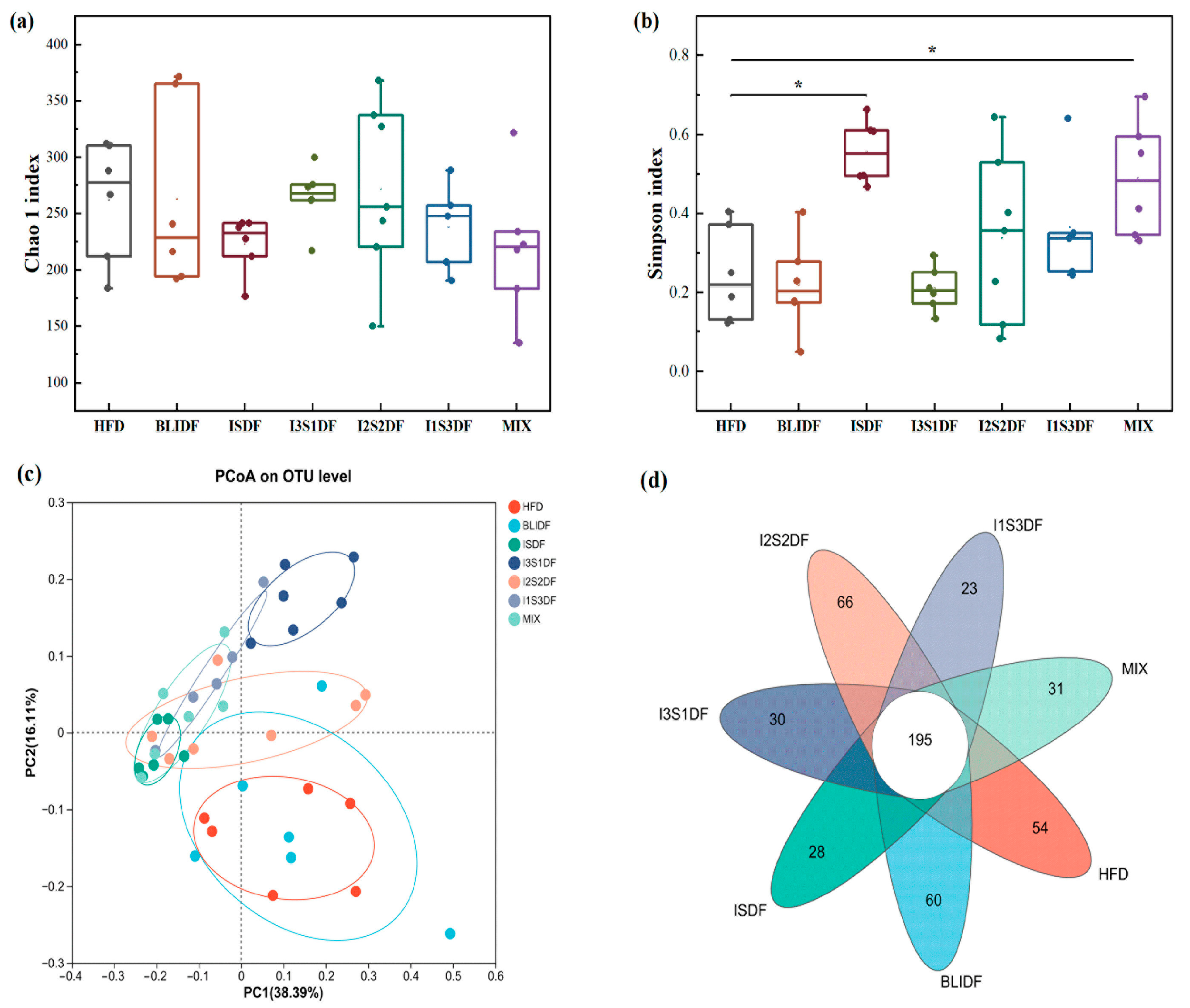
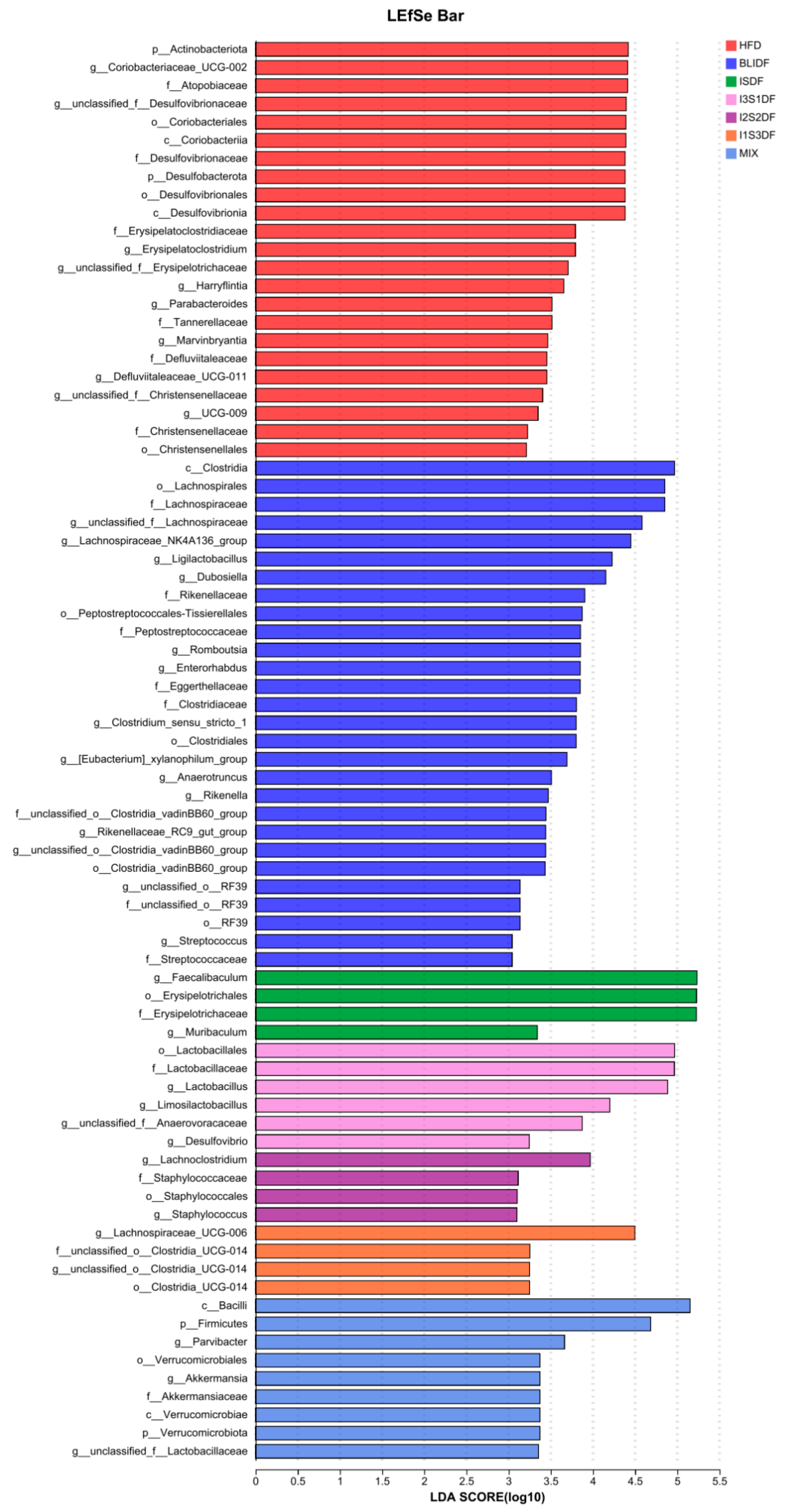
3.8. Effects of Soluble and Insoluble Dietary Fibre Ratios on Gut Microbiota of Obese Mice
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Phelps, N.H.; Singleton, R.K.; Zhou, B.; Heap, R.A.; Lhoste, V.P.; Carrillo-Larco, R.M.; Stevens, G.A.; Rodriguez-Martinez, A.; Bixby, H.; Bentham, J.; et al. Worldwide Trends in Underweight and Obesity from 1990 to 2022: A Pooled Analysis of 3663 Population-Representative Studies with 222 Million Children, Adolescents, and Adults. Lancet 2024, 403, 1027–1050. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.J. Impact of Obesity and Metabolic Syndrome on Immunity. Adv. Nutr. 2016, 7, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Jovanovski, E.; Mazhar, N.; Komishon, A.; Khayyat, R.; Li, D.; Blanco Mejia, S.; Khan, T.; Jenkins, A.L.; Smircic-Duvnjak, L.; Sievenpiper, J.L.; et al. Effect of Viscous Fiber Supplementation on Obesity Indicators in Individuals Consuming Calorie-Restricted Diets: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Eur. J. Nutr. 2021, 60, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hong, T.; Li, N.; Zang, B.; Wu, X. Soluble Dietary Fiber Improves Energy Homeostasis in Obese Mice by Remodeling the Gut Microbiota. Biochem. Biophys. Res. Commun. 2018, 48, 146–151. [Google Scholar] [CrossRef]
- Brown, L.; Rosner, B.; Willett, W.; Sacks, F. Cholesterol-Lowering Effects of Dietary Fiber: A Meta-Analysis. Am. J. Clin. Nutr. 1999, 69, 30–42. [Google Scholar] [CrossRef]
- Peng, F.; Ren, X.; Du, B.; Niu, K.; Yu, Z.; Yang, Y. Insoluble Dietary Fiber of Pear Fruit Pomace (Pyrus ussuriensis Maxim) Consumption Ameliorates Alterations of the Obesity-Related Features and Gut Microbiota Caused by High-Fat Diet. J. Funct. Foods. 2022, 99, 105354. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, B.; Li, L.; Zhao, W. Anti-Obesity Effects of Dietary Fibers Extracted from Flaxseed Cake in Diet-Induced Obese Mice. Nutrients 2023, 15, 1718. [Google Scholar] [CrossRef]
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.-M.; Kennedy, S.; et al. Richness of Human Gut Microbiome Correlates with Metabolic Markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.M.; Clardy, J.; Xavier, R.J. Gut Microbiome Lipid Metabolism and Its Impact on Host Physiology. Cell Host Microbe 2023, 31, 173–186. [Google Scholar] [CrossRef]
- Mariat, D.; Firmesse, O.; Levenez, F.; Guimarăes, V.; Sokol, H.; Doré, J.; Corthier, G.; Furet, J.-P. The Firmicutes/Bacteroidetes Ratio of the Human Microbiota Changes with Age. BMC Microbiol. 2009, 9, 123. [Google Scholar] [CrossRef]
- Li, L.; Yan, S.; Liu, S.; Wang, P.; Li, W.; Yi, Y.; Qin, S. In-Depth Insight into Correlations between Gut Microbiota and Dietary Fiber Elucidates a Dietary Causal Relationship with Host Health. Food Res. Int. 2023, 172, 113133. [Google Scholar] [CrossRef] [PubMed]
- Berger, K.; Falck, P.; Linninge, C.; Nilsson, U.; Axling, U.; Grey, C.; Stålbrand, H.; Nordberg Karlsson, E.; Nyman, M.; Holm, C.; et al. Cereal Byproducts Have Prebiotic Potential in Mice Fed a High-Fat Diet. J. Agric. Food Chem. 2014, 62, 8169–8178. [Google Scholar] [CrossRef] [PubMed]
- Bader Ul Ain, H.; Saeed, F.; Sultan, M.T.; Afzaal, M.; Imran, A.; DeFeo, V.; Cobelschi, C. Effect of Thermally Treated Barley Dietary Fiber against Hypercholesterolemia. Food Sci. Nutr. 2020, 8, 5259–5266. [Google Scholar] [CrossRef]
- GB 5009.5-2016; Food Safety National Standard—Determination of Protein in Foods. Standard Press of China: Beijing, China, 2016.
- GB 5009.6-2016; Food Safety National Standard—Determination of Fat in Foods. Standard Press of China: Beijing, China, 2016.
- GB 5009.3-2016; Food Safety National Standard—Determination of Moisture in Foods. Standard Press of China: Beijing, China, 2016.
- GB 5009.4-2016; Food Safety National Standard—Determination of Ash in Foods. Standard Press of China: Beijing, China, 2016.
- Zhang, Y.; Hu, J.; Zhong, Y.; Liu, S.; Liu, L.; Mu, X.; Chen, C.; Yang, S.; Li, G.; Zhang, D.; et al. Insoluble/Soluble Fraction Ratio Determines Effects of Dietary Fiber on Gut Microbiota and Serum Metabolites in Healthy Mice. Food Funct. 2024, 15, 338–354. [Google Scholar] [CrossRef]
- Suo, M.; Wen, D.; Wang, W.; Zhang, T. Comparative Study on Hemoglobin A1c, Glycated Albumin and Glycosylated Serum Protein in Aplastic Anemia Patients with Type 2 Diabetes Mellitus. Biosci. Rep. 2020, 40, BSR20192300. [Google Scholar] [CrossRef]
- Holst, J.J. Glucagonlike Peptide 1: A Newly Discovered Gastrointestinal Hormone. Gastroenterology 1994, 107, 1848–1855. [Google Scholar] [CrossRef] [PubMed]
- Kruth, H. Lipoprotein Cholesterol and Atherosclerosis. Curr. Mol. Med. 2001, 1, 633–653. [Google Scholar] [CrossRef]
- Kimura, Y.; Tsukui, D.; Kono, H. Uric Acid in Inflammation and the Pathogenesis of Atherosclerosis. Int. J. Mol. Sci. 2021, 22, 12394. [Google Scholar] [CrossRef]
- Marvyn, P.M.; Bradley, R.M.; Mardian, E.B.; Marks, K.A.; Duncan, R.E. Data on Oxygen Consumption Rate, Respiratory Exchange Ratio, and Movement in C57BL/6J Female Mice on the Third Day of Consuming a High-Fat Diet. Data Brief 2016, 7, 472–475. [Google Scholar] [CrossRef]
- Liu, H.; Liang, J.; Liang, C.; Liang, G.; Lai, J.; Zhang, R.; Wang, Q.; Xiao, G. Physicochemical Properties of Dietary Fiber of Bergamot and Its Effect on Diabetic Mice. Front. Nutr. 2022, 9, 1040825. [Google Scholar] [CrossRef]
- Zheng, M.; Liu, Y.; Zhang, G.; Yang, Z.; Xu, W.; Chen, Q. The Applications and Mechanisms of Superoxide Dismutase in Medicine, Food, and Cosmetics. Antioxidants 2023, 12, 1675. [Google Scholar] [CrossRef] [PubMed]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef] [PubMed]
- Munukka, E.; Rintala, A.; Toivonen, R.; Nylund, M.; Yang, B.; Takanen, A.; Hänninen, A.; Vuopio, J.; Huovinen, P.; Jalkanen, S.; et al. Faecalibacterium prausnitzii Treatment Improves Hepatic Health and Reduces Adipose Tissue Inflammation in High-Fat Fed Mice. ISME J. 2017, 11, 1667–1679. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, E.; Corr, S.C. Lactobacillus Spp. for Gastrointestinal Health: Current and Future Perspectives. Front. Immunol. 2022, 13, 840245. [Google Scholar] [CrossRef]







| Nutrients | Content (g/100 g Barley Leaf Powder) |
|---|---|
| Protein | 17.13 ± 0.494 a |
| Fat | 0.32 ± 0.163 |
| Carbohydrate | 14.98 ± 0.424 |
| Insoluble dietary fibre | 55.46 ± 0.989 |
| Soluble dietary fibre | 0.89 ± 0.989 |
| Moisture | 10.13 ± 0.382 |
| Ash | 0.75 ± 0.240 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, H.; Dong, S.; Li, L.; Zhao, W. Effects of Soluble and Insoluble Fibre on Glycolipid Metabolism and Gut Microbiota in High-Fat-Diet-Induced Obese Mice. Nutrients 2024, 16, 3822. https://doi.org/10.3390/nu16223822
Ren H, Dong S, Li L, Zhao W. Effects of Soluble and Insoluble Fibre on Glycolipid Metabolism and Gut Microbiota in High-Fat-Diet-Induced Obese Mice. Nutrients. 2024; 16(22):3822. https://doi.org/10.3390/nu16223822
Chicago/Turabian StyleRen, Han, Sihao Dong, Li Li, and Wei Zhao. 2024. "Effects of Soluble and Insoluble Fibre on Glycolipid Metabolism and Gut Microbiota in High-Fat-Diet-Induced Obese Mice" Nutrients 16, no. 22: 3822. https://doi.org/10.3390/nu16223822
APA StyleRen, H., Dong, S., Li, L., & Zhao, W. (2024). Effects of Soluble and Insoluble Fibre on Glycolipid Metabolism and Gut Microbiota in High-Fat-Diet-Induced Obese Mice. Nutrients, 16(22), 3822. https://doi.org/10.3390/nu16223822








