Carotenoids Intake and Cardiovascular Prevention: A Systematic Review
Highlights
- Long-term cardiovascular disease risk reduction is associated with elevated levels of carotenoids in the blood, as they alter cardiovascular risk factors and inflammatory markers.
- Increasing consumption of carotenoid-rich foods is more effective than supplements in regard to reducing inflammatory markers and indicators of CVD risk.
- There is insufficient evidence to determine whether one carotenoid is better than others in reducing the risk of cardiovascular disease; in fact, it appears that a combination of several carotenoids is more effective than consuming just one.
- The effects of carotenoids on CVD risk may be amplified or altered by natural sources of carotenoids, which contain a mixture of carotenoids and other phytocompounds.
Abstract
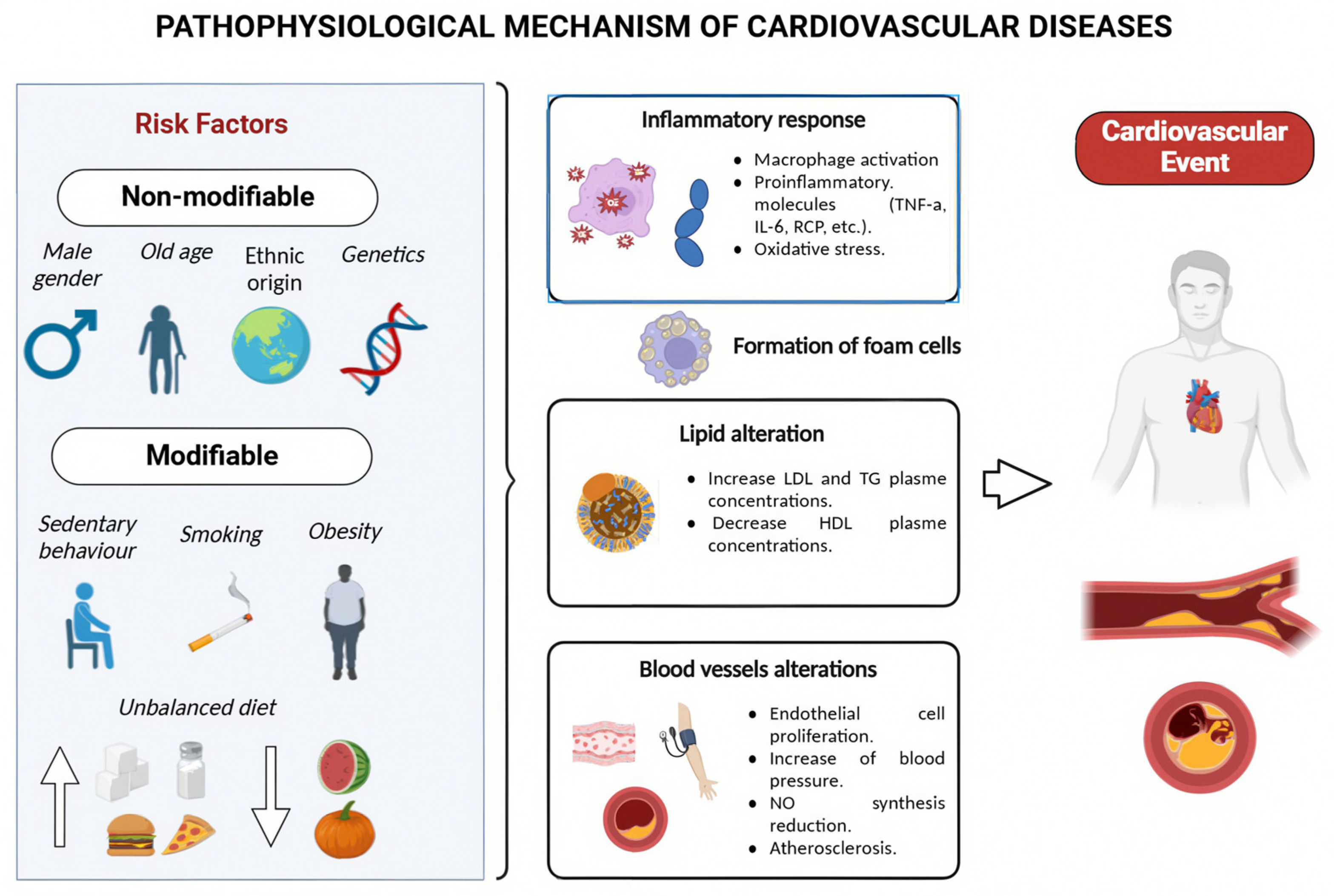
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Search Strategy
2.3. Selection Criteria
2.4. Exclusion Criteria
2.5. Classification of Selected Studies
3. Results
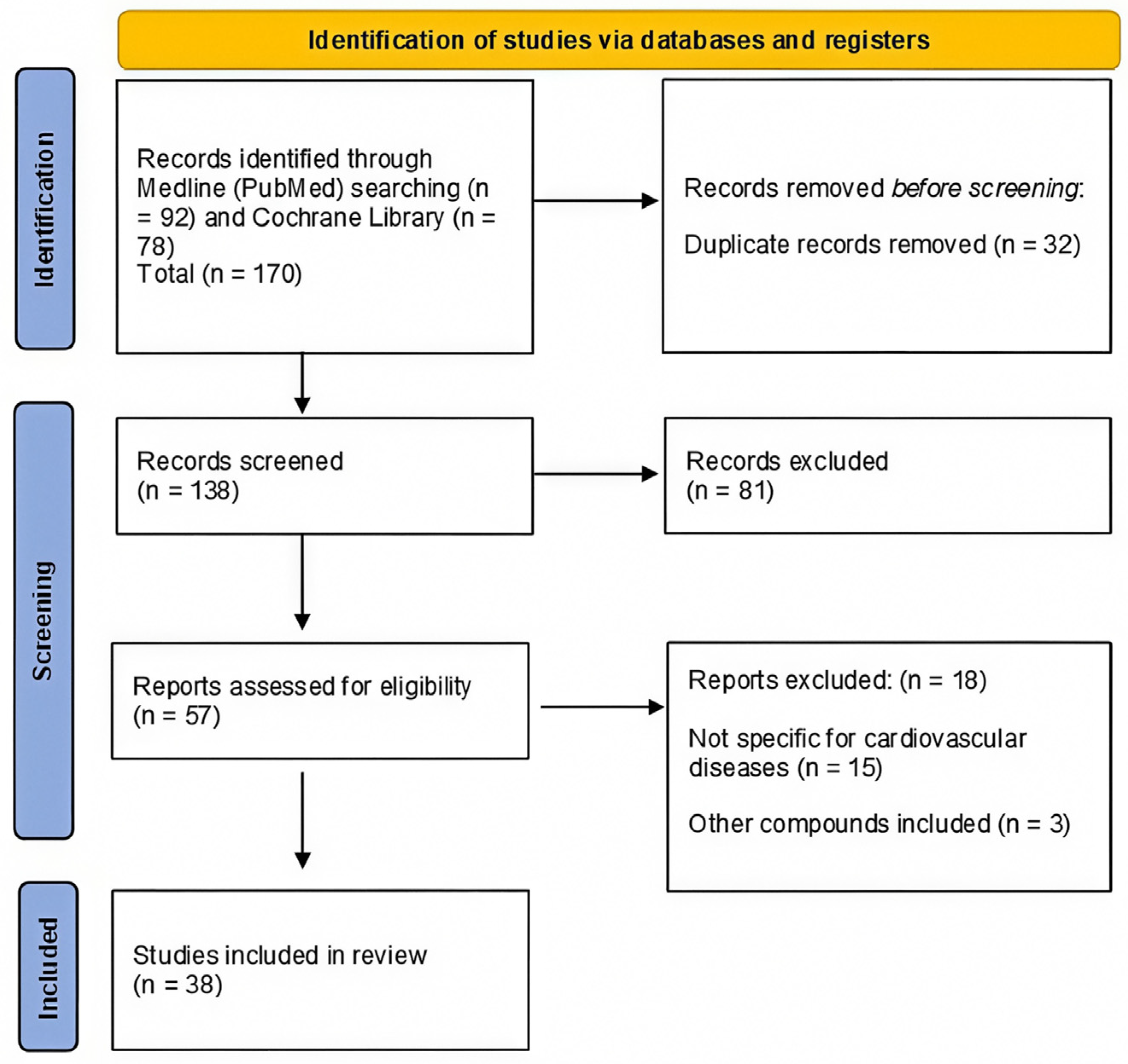
Study Selection
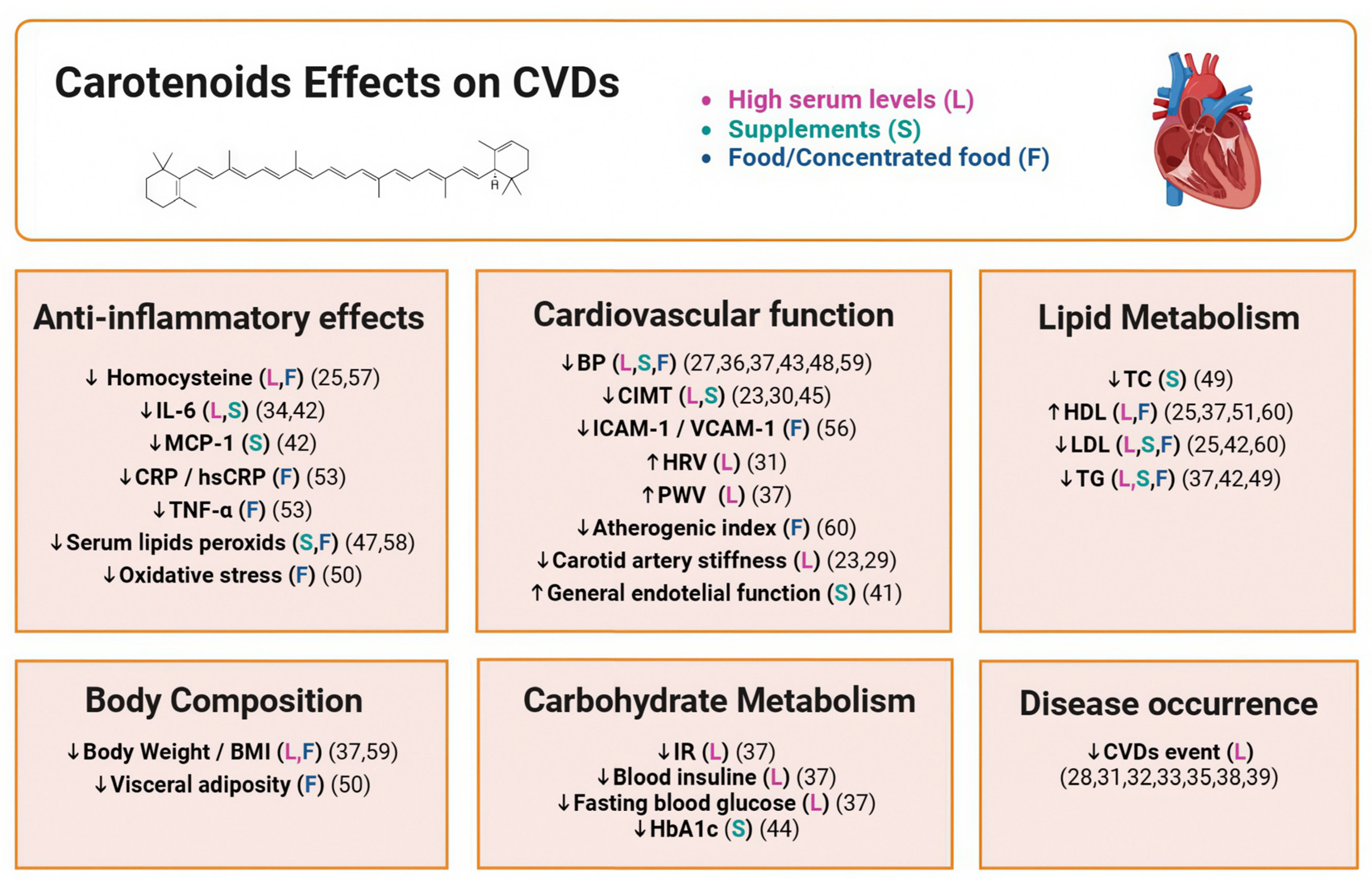
4. Discussion
4.1. Observational Epidemiological Studies
4.2. Intervention Studies with Carotenoid Supplements
4.3. Dietary Intervention Studies
4.3.1. Vegetable and Fruits Juices
4.3.2. Increased Consumption of Fruit and Vegetable
4.4. Final Analysis
4.5. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olvera Lopez, E.; Ballard, B.D.; Jan, A. Cardiovascular Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- GBD 2017 Causes of Death Collaborators Global, Regional, and National Age-Sex-Specific Mortality for 282 Causes of Death in 195 Countries and Territories, 1980–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [CrossRef] [PubMed]
- 2021 ESC Guidelines on Cardiovascular Disease Prevention in Clinical Practice | European Heart Journal | Oxford Academic. Available online: https://academic.oup.com/eurheartj/article/42/34/3227/6358713?login=false (accessed on 22 July 2024).
- Lacey, B.; Herrington, W.G.; Preiss, D.; Lewington, S.; Armitage, J. The Role of Emerging Risk Factors in Cardiovascular Outcomes. Curr. Atheroscler. Rep. 2017, 19, 28. [Google Scholar] [CrossRef]
- Magnussen, C.; Ojeda, F.M.; Leong, D.P.; Alegre-Diaz, J.; Amouyel, P.; Aviles-Santa, L.; De Bacquer, D.; Ballantyne, C.M.; Bernabe-Ortiz, A.; Bobak, M.; et al. Global Impact of Modifiable Risk Factors on Cardiovascular Disease and Mortality. N. Engl. J. Med. 2023, 389, 1273–1285. [Google Scholar] [CrossRef] [PubMed]
- Gammone, M.A.; Riccioni, G.; D’Orazio, N. Carotenoids: Potential Allies of Cardiovascular Health? Food Nutr. Res. 2015, 59, 26762. [Google Scholar] [CrossRef] [PubMed]
- Diet, Nutrition and the Prevention of Chronic Diseases: Report of a Joint WHO/FAO Expert Consultation, Geneva, 28 January–1 February 2002. Available online: https://www.who.int/publications-detail-redirect/924120916X (accessed on 20 December 2023).
- Pagliaro, B.; Santolamazza, C.; Simonelli, F.; Rubattu, S. Phytochemical Compounds and Protection from Cardiovascular Diseases: A State of the Art. BioMed Res. Int. 2015, 2015, 918069. [Google Scholar] [CrossRef]
- Mozos, I.; Stoian, D.; Caraba, A.; Malainer, C.; Horbańczuk, J.O.; Atanasov, A.G. Lycopene and Vascular Health. Front. Pharmacol. 2018, 9, 521. [Google Scholar] [CrossRef]
- Protective Effect of Lycopene on Serum Cholesterol and Blood Pressure: Meta-Analyses of Intervention Trials. In Database of Abstracts of Reviews of Effects (DARE): Quality-Assessed Reviews [Internet]; Centre for Reviews and Dissemination: York, UK, 2011.
- González-Peña, M.A.; Ortega-Regules, A.E.; Anaya de Parrodi, C.; Lozada-Ramírez, J.D. Chemistry, Occurrence, Properties, Applications, and Encapsulation of Carotenoids—A Review. Plants 2023, 12, 313. [Google Scholar] [CrossRef]
- Sandmann, G. Carotenoids and Their Biosynthesis in Fungi. Molecules 2022, 27, 1431. [Google Scholar] [CrossRef]
- Milani, A.; Basirnejad, M.; Shahbazi, S.; Bolhassani, A. Carotenoids: Biochemistry, Pharmacology and Treatment. Br. J. Pharmacol. 2017, 174, 1290–1324. [Google Scholar] [CrossRef]
- Chen, G. The Interactions of Insulin and Vitamin A Signaling Systems for the Regulation of Hepatic Glucose and Lipid Metabolism. Cells 2021, 10, 2160. [Google Scholar] [CrossRef]
- Eggersdorfer, M.; Wyss, A. Carotenoids in Human Nutrition and Health. Arch. Biochem. Biophys. 2018, 652, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Prasad, P.; Lokesh, V.; Shang, X.; Shin, J.; Keum, Y.-S.; Lee, J.-H. Carotenoids: Dietary Sources, Extraction, Encapsulation, Bioavailability, and Health Benefits—A Review of Recent Advancements. Antioxidants 2022, 11, 795. [Google Scholar] [CrossRef]
- Burrows, T.L.; Williams, R.; Rollo, M.; Wood, L.; Garg, M.L.; Jensen, M.; Collins, C.E. Plasma Carotenoid Levels as Biomarkers of Dietary Carotenoid Consumption: A Systematic Review of the Validation Studies. J. Nutr. Intermed. Metab. 2015, 2, 15–64. [Google Scholar] [CrossRef]
- Henning, T.; Wagner, P.; Gedat, E.; Kochlik, B.; Kusch, P.; Sowoidnich, K.; Vastag, M.; Gleim, J.; Braune, M.; Maiwald, M.; et al. Evaluation of Modern Approaches for the Assessment of Dietary Carotenoids as Markers for Fruit and Vegetable Consumption. Nutrients 2023, 15, 1665. [Google Scholar] [CrossRef]
- Fiedor, J.; Burda, K. Potential Role of Carotenoids as Antioxidants in Human Health and Disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef] [PubMed]
- Stringham, N.T.; Green, M.; Roche, W.; Prado-Cabrero, A.; Mulcahy, R.; Nolan, J. Lutein, Zeaxanthin, and Meso-Zeaxanthin Supplementation Attenuates Inflammatory Cytokines and Markers of Oxidative Cardiovascular Processes in Humans. Nutr. Metab. Cardiovasc. Dis. 2024, 34, 1976–1983. [Google Scholar] [CrossRef]
- Przybylska, S.; Tokarczyk, G. Lycopene in the Prevention of Cardiovascular Diseases. Int. J. Mol. Sci. 2022, 23, 1957. [Google Scholar] [CrossRef]
- Ahn, Y.J.; Kim, H. Lutein as a Modulator of Oxidative Stress-Mediated Inflammatory Diseases. Antioxidants 2021, 10, 1448. [Google Scholar] [CrossRef]
- Hajizadeh-Sharafabad, F.; Ghoreishi, Z.; Maleki, V.; Tarighat-Esfanjani, A. Mechanistic Insights into the Effect of Lutein on Atherosclerosis, Vascular Dysfunction, and Related Risk Factors: A Systematic Review of in Vivo, Ex Vivo and in Vitro Studies. Pharmacol. Res. 2019, 149, 104477. [Google Scholar] [CrossRef]
- Murillo, A.G.; Hu, S.; Fernandez, M.L. Zeaxanthin: Metabolism, Properties, and Antioxidant Protection of Eyes, Heart, Liver, and Skin. Antioxidants 2019, 8, 390. [Google Scholar] [CrossRef]
- Zou, Z.; Xu, X.; Huang, Y.; Xiao, X.; Ma, L.; Sun, T.; Dong, P.; Wang, X.; Lin, X. High Serum Level of Lutein May Be Protective against Early Atherosclerosis: The Beijing Atherosclerosis Study. Atherosclerosis 2011, 219, 789–793. [Google Scholar] [CrossRef] [PubMed]
- Crupi, P.; Faienza, M.F.; Naeem, M.Y.; Corbo, F.; Clodoveo, M.L.; Muraglia, M. Overview of the Potential Beneficial Effects of Carotenoids on Consumer Health and Well-Being. Antioxidants 2023, 12, 1069. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chen, H.; Su, Y.; Liu, H.; Hu, J.; Hong, K. Increased Blood Alpha-Carotene, All-Trans-Beta-Carotene and Lycopene Levels Are Associated with Beneficial Changes in Heart Rate Variability: A CVD-Stratified Analysis in an Adult Population-Based Study. Nutr. J. 2021, 20, 43. [Google Scholar] [CrossRef]
- Wang, Y.; Chung, S.-J.; McCullough, M.L.; Song, W.O.; Fernandez, M.L.; Koo, S.I.; Chun, O.K. Dietary Carotenoids Are Associated with Cardiovascular Disease Risk Biomarkers Mediated by Serum Carotenoid Concentrations. J. Nutr. 2014, 144, 1067–1074. [Google Scholar] [CrossRef]
- Wang, L.; Gaziano, J.M.; Norkus, E.P.; Buring, J.E.; Sesso, H.D. Associations of Plasma Carotenoids with Risk Factors and Biomarkers Related to Cardiovascular Disease in Middle-Aged and Older Women. Am. J. Clin. Nutr. 2008, 88, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Hozawa, A.; Jacobs, D.R.; Steffes, M.W.; Gross, M.D.; Steffen, L.M.; Lee, D.-H. Circulating Carotenoid Concentrations and Incident Hypertension: The Coronary Artery Risk Development in Young Adults (CARDIA) Study. J. Hypertens. 2009, 27, 237–242. [Google Scholar] [CrossRef]
- Prentice, R.L.; Pettinger, M.; Neuhouser, M.L.; Tinker, L.F.; Huang, Y.; Zheng, C.; Manson, J.E.; Mossavar-Rahmani, Y.; Anderson, G.L.; Lampe, J.W. Application of Blood Concentration Biomarkers in Nutritional Epidemiology: Example of Carotenoid and Tocopherol Intake in Relation to Chronic Disease Risk. Am. J. Clin. Nutr. 2019, 109, 1189–1196. [Google Scholar] [CrossRef]
- Matos, A.; Gonçalves, V.M.d.S.; Souza, G.; Cruz, S.P.d.; Cruz, S.; Ramalho, A. Vitamin A Nutritional Status in Patients with Coronary Artery Disease and Its Correlation with the Severity of the Disease. Nutr. Hosp. 2018, 35, 1215–1220. [Google Scholar] [CrossRef]
- Wang, C.; Qiu, R.; Cao, Y.; Ouyang, W.-F.; Li, H.-B.; Ling, W.-H.; Chen, Y.-M. Higher Dietary and Serum Carotenoid Levels Are Associated with Lower Carotid Intima-Media Thickness in Middle-Aged and Elderly People. Br. J. Nutr. 2018, 119, 590–598. [Google Scholar] [CrossRef]
- Karppi, J.; Laukkanen, J.A.; Mäkikallio, T.H.; Ronkainen, K.; Kurl, S. Low β-Carotene Concentrations Increase the Risk of Cardiovascular Disease Mortality among Finnish Men with Risk Factors. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 921–928. [Google Scholar] [CrossRef]
- Shardell, M.D.; Alley, D.E.; Hicks, G.E.; El-Kamary, S.S.; Miller, R.R.; Semba, R.D.; Ferrucci, L. Low Serum Carotenoid Concentrations and Carotenoid Interactions Predict Mortality in US Adults: The Third National Health and Nutrition Examination Survey (NHANES III). Nutr. Res. 2011, 31, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Chung, R.W.S.; Leanderson, P.; Lundberg, A.K.; Jonasson, L. Lutein Exerts Anti-Inflammatory Effects in Patients with Coronary Artery Disease. Atherosclerosis 2017, 262, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Weinstein, S.J.; Yu, K.; Männistö, S.; Albanes, D. Serum Beta Carotene and Overall and Cause-Specific Mortality. Circ. Res. 2018, 123, 1339–1349. [Google Scholar] [CrossRef]
- Toh, D.W.K.; Sutanto, C.N.; Loh, W.W.; Lee, W.Y.; Yao, Y.; Ong, C.N.; Kim, J.E. Skin Carotenoids Status as a Potential Surrogate Marker for Cardiovascular Disease Risk Determination in Middle-Aged and Older Adults. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 592–601. [Google Scholar] [CrossRef]
- Matsumoto, M.; Waki, N.; Suganuma, H.; Takahashi, I.; Kurauchi, S.; Sawada, K.; Tokuda, I.; Misawa, M.; Ando, M.; Itoh, K.; et al. Association between Biomarkers of Cardiovascular Diseases and the Blood Concentration of Carotenoids among the General Population without Apparent Illness. Nutrients 2020, 12, E2310. [Google Scholar] [CrossRef]
- Zhu, X.; Cheang, I.; Tang, Y.; Shi, M.; Zhu, Q.; Gao, R.; Liao, S.; Yao, W.; Zhou, Y.; Zhang, H.; et al. Associations of Serum Carotenoids with Risk of All-Cause and Cardiovascular Mortality in Hypertensive Adults. J. Am. Heart Assoc. 2023, 12, e027568. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Tang, R.; Zhou, R.; Qian, Y.; Di, D. The Protective Effect of Serum Carotenoids on Cardiovascular Disease: A Cross-Sectional Study from the General US Adult Population. Front. Nutr. 2023, 10, 1154239. [Google Scholar] [CrossRef]
- Qiu, Z.; Chen, X.; Geng, T.; Wan, Z.; Lu, Q.; Li, L.; Zhu, K.; Zhang, X.; Liu, Y.; Lin, X.; et al. Associations of Serum Carotenoids with Risk of Cardiovascular Mortality Among Individuals with Type 2 Diabetes: Results From NHANES. Diabetes Care 2022, 45, 1453–1461. [Google Scholar] [CrossRef]
- Gajendragadkar, P.R.; Hubsch, A.; Mäki-Petäjä, K.M.; Serg, M.; Wilkinson, I.B.; Cheriyan, J. Effects of Oral Lycopene Supplementation on Vascular Function in Patients with Cardiovascular Disease and Healthy Volunteers: A Randomised Controlled Trial. PLoS ONE 2014, 9, e99070. [Google Scholar] [CrossRef]
- Xu, X.-R.; Zou, Z.-Y.; Xiao, X.; Huang, Y.-M.; Wang, X.; Lin, X.-M. Effects of Lutein Supplement on Serum Inflammatory Cytokines, ApoE and Lipid Profiles in Early Atherosclerosis Population. J. Atheroscler. Thromb. 2013, 20, 170–177. [Google Scholar] [CrossRef]
- Wolak, T.; Sharoni, Y.; Levy, J.; Linnewiel-Hermoni, K.; Stepensky, D.; Paran, E. Effect of Tomato Nutrient Complex on Blood Pressure: A Double Blind, Randomized Dose-Response Study. Nutrients 2019, 11, E950. [Google Scholar] [CrossRef] [PubMed]
- Schwab, S.; Zierer, A.; Heier, M.; Fischer, B.; Huth, C.; Baumert, J.; Meisinger, C.; Peters, A.; Thorand, B. Intake of Vitamin and Mineral Supplements and Longitudinal Association with HbA1c Levels in the General Non-Diabetic Population—Results from the MONICA/KORA S3/F3 Study. PLoS ONE 2015, 10, e0139244. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zou, Z.-Y.; Xu, X.-R.; Lin, X.-M.; Zhang, H.-B.; Xiao, X.; Ouyang, L.; Huang, Y.-M.; Wang, X.; Liu, Y.-Q. Effects of Lutein and Lycopene on Carotid Intima–Media Thickness in Chinese Subjects with Subclinical Atherosclerosis: A Randomised, Double-Blind, Placebo-Controlled Trial. Br. J. Nutr. 2014, 111, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Stonehouse, W.; Brinkworth, G.D.; Thompson, C.H.; Abeywardena, M.Y. Short Term Effects of Palm-Tocotrienol and Palm-Carotenes on Vascular Function and Cardiovascular Disease Risk: A Randomised Controlled Trial. Atherosclerosis 2016, 254, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, A.; Madarame, T.; Koike, H.; Komatsu, Y.; Wise, J.A. Four Week Supplementation with Mixed Fruit and Vegetable Juice Concentrates Increased Protective Serum Antioxidants and Folate and Decreased Plasma Homocysteine in Japanese Subjects. Asia Pac. J. Clin. Nutr. 2007, 16, 411–421. [Google Scholar]
- Engelhard, Y.N.; Gazer, B.; Paran, E. Natural Antioxidants from Tomato Extract Reduce Blood Pressure in Patients with Grade-1 Hypertension: A Double-Blind, Placebo-Controlled Pilot Study. Am. Heart J. 2006, 151, 100. [Google Scholar] [CrossRef]
- Ryu, N.H.; Lim, Y.; Park, J.E.; Kim, J.; Kim, J.Y.; Kwon, S.W.; Kwon, O. Impact of Daily Chlorella Consumption on Serum Lipid and Carotenoid Profiles in Mildly Hypercholesterolemic Adults: A Double-Blinded, Randomized, Placebo-Controlled Study. Nutr. J. 2014, 13, 57. [Google Scholar] [CrossRef]
- Takagi, T.; Hayashi, R.; Nakai, Y.; Okada, S.; Miyashita, R.; Yamada, M.; Mihara, Y.; Mizushima, K.; Morita, M.; Uchiyama, K.; et al. Dietary Intake of Carotenoid-Rich Vegetables Reduces Visceral Adiposity in Obese Japanese Men—A Randomized, Double-Blind Trial. Nutrients 2020, 12, E2342. [Google Scholar] [CrossRef]
- Daniels, J.-A.; Mulligan, C.; McCance, D.; Woodside, J.V.; Patterson, C.; Young, I.S.; McEneny, J. A Randomised Controlled Trial of Increasing Fruit and Vegetable Intake and How This Influences the Carotenoid Concentration and Activities of PON-1 and LCAT in HDL from Subjects with Type 2 Diabetes. Cardiovasc. Diabetol. 2014, 13, 16. [Google Scholar] [CrossRef]
- Wallace, I.R.; McEvoy, C.T.; Hunter, S.J.; Hamill, L.L.; Ennis, C.N.; Bell, P.M.; Patterson, C.C.; Woodside, J.V.; Young, I.S.; McKinley, M.C. Dose-Response Effect of Fruit and Vegetables on Insulin Resistance in People at High Risk of Cardiovascular Disease: A Randomized Controlled Trial. Diabetes Care 2013, 36, 3888–3896. [Google Scholar] [CrossRef]
- Hurtado-Barroso, S.; Martínez-Huélamo, M.; Rinaldi de Alvarenga, J.F.; Quifer-Rada, P.; Vallverdú-Queralt, A.; Pérez-Fernández, S.; Lamuela-Raventós, R.M. Acute Effect of a Single Dose of Tomato Sofrito on Plasmatic Inflammatory Biomarkers in Healthy Men. Nutrients 2019, 11, E851. [Google Scholar] [CrossRef] [PubMed]
- Graydon, R.; Hogg, R.E.; Chakravarthy, U.; Young, I.S.; Woodside, J.V. The Effect of Lutein- and Zeaxanthin-Rich Foods v. Supplements on Macular Pigment Level and Serological Markers of Endothelial Activation, Inflammation and Oxidation: Pilot Studies in Healthy Volunteers. Br. J. Nutr. 2012, 108, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Thies, F.; Masson, L.F.; Rudd, A.; Vaughan, N.; Tsang, C.; Brittenden, J.; Simpson, W.G.; Duthie, S.; Horgan, G.W.; Duthie, G. Effect of a Tomato-Rich Diet on Markers of Cardiovascular Disease Risk in Moderately Overweight, Disease-Free, Middle-Aged Adults: A Randomized Controlled Trial. Am. J. Clin. Nutr. 2012, 95, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Colmán-Martínez, M.; Martínez-Huélamo, M.; Valderas-Martínez, P.; Arranz-Martínez, S.; Almanza-Aguilera, E.; Corella, D.; Estruch, R.; Lamuela-Raventós, R.M. Trans-Lycopene from Tomato Juice Attenuates Inflammatory Biomarkers in Human Plasma Samples: An Intervention Trial. Mol. Nutr. Food Res. 2017, 61, 1600993. [Google Scholar] [CrossRef]
- Paterson, E.; Gordon, M.H.; Niwat, C.; George, T.W.; Parr, L.; Waroonphan, S.; Lovegrove, J.A. Supplementation with Fruit and Vegetable Soups and Beverages Increases Plasma Carotenoid Concentrations but Does Not Alter Markers of Oxidative Stress or Cardiovascular Risk Factors. J. Nutr. 2006, 136, 2849–2855. [Google Scholar] [CrossRef]
- Bub, A.; Watzl, B.; Abrahamse, L.; Delincée, H.; Adam, S.; Wever, J.; Müller, H.; Rechkemmer, G. Moderate Intervention with Carotenoid-Rich Vegetable Products Reduces Lipid Peroxidation in Men. J. Nutr. 2000, 130, 2200–2206. [Google Scholar] [CrossRef]
- Svendsen, M.; Blomhoff, R.; Holme, I.; Tonstad, S. The Effect of an Increased Intake of Vegetables and Fruit on Weight Loss, Blood Pressure and Antioxidant Defense in Subjects with Sleep Related Breathing Disorders. Eur. J. Clin. Nutr. 2007, 61, 1301–1311. [Google Scholar] [CrossRef][Green Version]
- Tomás Luiz, A.; Martín Pozuelo, G.; González Navarro, I.; Elvira Torales, L.; Ponce, H.; González Barrio, R.; García Alonso, J.; Periago, M.J. Influence of dietary carotenoids on biomarkers of cardiometabolic risk in peri- and post-menopausal women. Nutr. Hosp. 2021, 38, 993–1001. [Google Scholar] [CrossRef]
- Biddle, M.J.; Lennie, T.A.; Bricker, G.V.; Kopec, R.E.; Schwartz, S.J.; Moser, D.K. Lycopene Dietary Intervention: A Pilot Study in Patients with Heart Failure. J. Cardiovasc. Nurs. 2015, 30, 205–212. [Google Scholar] [CrossRef]
- Böhm, V.; Lietz, G.; Olmedilla-Alonso, B.; Phelan, D.; Reboul, E.; Bánati, D.; Borel, P.; Corte-Real, J.; de Lera, A.R.; Desmarchelier, C.; et al. From Carotenoid Intake to Carotenoid Blood and Tissue Concentrations—Implications for Dietary Intake Recommendations. Nutr. Rev. 2020, 79, 544–573. [Google Scholar] [CrossRef]

| Parameters | Inclusion Criteria |
| Population | Age 18 years or older |
| Intervention | Carotenoid intake as CVD prevention |
| Comparison | Low carotenoids intake |
| Outcomes | Health and disease markers |
| Study design | Epidemiological observational studies, clinical trials, and RCTs |
| Author, Publication Year | Country/Region | Type of Study/Study Name | Follow-Up Period | Study Size | Carotenoids Evaluated | Findings |
|---|---|---|---|---|---|---|
| Wang, L. et al., 2008 [29] | USA | Case-control studies/Women’s Health Study | 1 year and 6 months | 39,876 | α-carotene, β-carotene, β-cryptoxanthin, lycopene, and lutein/zeaxanthin | Association of ↑ α-carotene, β-carotene, and lycopene with ↑ LDL. ↓ lycopene with ↑ of HDL and ↑ of HbA1c. ↓ β-carotene with ↑ of CRP. |
| Hozawa, A. et al., 2009 [30] | USA | Prospective, multicentre epidemiologic study/ Coronary Artery Risk Development in Young Adults Study | 20 years | 4412 | α-carotene, β-carotene, lutein/zeaxanthin, cryptoxanthin, lycopene | Sum of 4 carotenoids was significantly inversely associated with HT. Lycopene was unrelated to HT in any model. |
| Prentice, RL. et al., 2019 [31] | USA | Randomized controlled Clinical Trial/Nutrition and Physical Activity Assessment Study | 5 years | 5488 | α- and β-carotene, lutein + zeaxanthin (L+Z), and α-tocopherol | ↑ levels of α-carotene, β-carotene with ↓ risk of CVDs. ↑ levels of L+Z shown not to affect CVDs. |
| Matos, A. et al., 2018 [32] | Brazil | Cross-sectional observational study | 1 year | 90 | β-carotene | β-carotene diminished as the extent score rose of CAD, although this was not statistically significant. |
| Zou, Z. et al., 2011 [25] | China | Case-control study | Baseline | 125 | Lutein, zeaxanthin, β-carotene and lycopene | ↓ levels serum lutein with ↑ CIMT. ↓ levels serum Zeaxanthina and β-carotene with ↑ carotid artery stiffness. |
| Wang, C. et al., 2018 [33] | China | Cross-sectional study/ Guangzhou Nutrition and Health Study | Baseline | 2947 | α-carotene, β-carotene, lutein + zeaxanthin, β-cryptoxanthin and lycopene | ↑ carotenoid levels in diet and serum are associated with lower carotid CIMT values |
| Huang, Y. et al., 2021 [27] | USA | Cross-sectional analysis/Midlife in the United States | Baseline | 1074 | Lutein, zeaxanthin, β-cryptoxanthin, 13-cis-β-carotene, α-carotene, all-trans-β-carotene and lycopene | Blood α-carotene, all-trans-β-carotene and lycopene levels were independently associated with higher HRV, reducing the risk of CVDs. |
| Karppi, J. et al., 2012 [34] | Finland | Prospective cohort study/Kuopio Ischaemic Heart Disease Risk Factor | 15.9-year follow-up | 1031 | Lycopene, α-carotene, β-carotene | Low serum concentrations of β-carotene were strongly related to an increased CVDs mortality risk after adjustment for confounders. |
| Shardell, D. et al., 2011 [35] | USA | Observational study, NHANES III | 14.3 years | 13,293 | α-carotene, β-carotene, β-cryptoxanthin, lycopene, and lutein + zeaxanthin | Low α-carotene was associated with ↑ CVDs mortality. Very low serum of total carotenoid, α-carotene, and lycopene concentrations may be risk factors for mortality. |
| Chung, RWS. et al., 2017 [36] | Sweden | Cross-sectional and longitudinal study | 3 months | 193 | Lutein + zeaxanthin, β-cryptoxanthin, lycopene, α- and β-carotene and IL-6 | Inverse association between lutein and IL-6 in CAD patients. |
| Huang, J. et al., 2018 [37] | Finland | Prospective Cohort Study | 5–8 years between 1985 and 1988 | 29,133 | β-carotene | Higher β-carotene biochemical status is associated with lower overall CVDs, heart disease, stroke, and other causes of mortality. |
| Toh, DWK. et al., 2021 [38] | Singapore | Cross-sectional study | 13 months | 108 | β-carotene, α-carotene, lycopene, lutein, zeaxanthin and β-cryptoxanthin | Skin carotenoids and plasma carotenoids were inversely associated with systolic BP and diastolic BP. |
| Matsumoto, M. et al., 2020 [39] | Japan | Resident-based cross-sectional study | Baseline | 1350 | Lutein, zeaxanthin, β-cryptoxanthin, α-carotene, β-carotene, and lycopene | Higher concentration of serum carotenoids in relatively healthy individuals was associated with better CVD markers. |
| Zhu, X et al., 2023 [40] | China | Prospective study NHANES III | 6 years | 13,688 | Lutein/zeaxantine, α-carotene, β-carotene, β-cryptoxanthin, lycopene | Higher concentrations of major serum carotenoids were associated with decreased risk of cardiovascular mortality. |
| Wang, M et al., 2023 [41] | China | Cross-sectional study | 5 years | 12,424 | Lutein/zeaxantine, α-carotene, β-carotene, β-cryptoxanthin, lycopene | Serum carotenoids were negatively associated with the prevalence of CVDs. |
| Wang, Y. et al., 2014 [28] | USA | Cross-sectional study | 3 years | 2856 | Individual dietary carotenoid intake | Significant inverse associations with LDL cholesterol were observed for dietary β-carotene and lutein + zeaxanthin, and with homocysteine for dietary β-carotene, lycopene and total carotenoids. Dietary lutein + zeaxanthin intake was also positively associated with HDL concentrations. |
| Qiu, Z. et al., 2022 [42] | USA | Prospective study | 5 years (2001–2006) | 3107 | α-carotene, β-carotene, β-cryptoxanthin, lutein/zeaxanthin, and lycopene | The action of β-carotene on people with type 2 diabetes is unclear. |
| Author, Publication Year | Country/ Region | Type of Study/Study Name | Follow-Up Period | Study Size | Carotenoids Supplemented | Findings |
|---|---|---|---|---|---|---|
| Gajendragadkar, Pr. et al., 2014 [43] | UK | Randomized, double-blind trial | 2 months | 72 | Lycopene | Lycopene supplementation improves endothelial function in CVDs but not in healthy volunteers. |
| Xu, XR et al., 2013 [44] | China | Randomized, double-blind, placebo-controlled intervention trial | 3 months | 65 | Lutein | After 3 months of supplementation with lutein ↓ IL-6, MCP-1, LDL, and TG levels. |
| Wolak, T. et al., 2019 [45] | Israel | Double-blind, randomized, placebo-controlled study | 2 months | 61 | Tomato nutrient complex (5, 15 and 30 mg lycopene) vs. 15 mg of synthetic lycopene | Carotenoid levels achieved by the tomato nutrient complex (TNC) dose of 15 mg lycopene or higher correlate to a beneficial effect on systolic BP in hypertensive subjects, while lower doses and lycopene alone do not. |
| Schwab, S. et al., 2015 [46] | Germany | Two population-based cohorts/Monitoring of Trends and Determinants in Cardiovascular Diseases and Cooperative Health Research in the Region of Augsburg | 10 years | 2774 | Carotenes | High carotenoid intake could be one strategy for the prevention of cardiovascular complications in non-diabetic people. ↓ HbA1c levels. |
| Zou, Z. et al., 2014 [47] | China | Randomized, double-blind, placebo-controlled trial. | 12 months | 144 | Lutein and lycopene | The mean values of CAIMT decreased significantly in the lutein and combination groups at month 12. The change in CIMT was inversely associated with the increase in serum lutein concentrations in both the active treatment groups and with that in serum lycopene concentrations in the combination group. |
| Stonehouse, W. et al., 2016 [48] | Australia | A randomized, placebo-controlled, double-blind study | 2 months | 90 | Carotenes | Carotenes had no effects on vascular function or CVD risk factors. |
| Kawashima, A. et al., 2007 [49] | USA | Double-blinded placebo controlled randomized study | 1 month | 60 | Juice | Serum lipid peroxides and urine concentrations of 8-OHdG decreased significantly but were not significantly different than a placebo. |
| Engelhard YN. et al., 2006 [50] | Israel | Double-blinded, placebo-controlled pilot study | 8 weeks | 31 | Tomato extract | Reduced systolic and diastolic BP in patients with grade 1 hypertension. No significant changes were found in lipid parameters. |
| Ryu, NH. et al., 2014 [51] | South Korea | Double-blinded, randomized, placebo-controlled study | 4 weeks | 63 | 5 g Chlorella powder a day | Chlorella group exhibited remarkable changes in TC, TG, lutein/zeaxanthin, and α-carotene. |
| Author, Publication Year | Country/Region | Type of Study/Study Name | Follow-Up Period | Study Size | Dietary Intervention | Findings |
|---|---|---|---|---|---|---|
| Takagi, T. et al., 2020 [52] | Japan | Randomized, double-blinded, controlled clinical trial | 8-weeks | 28 | High lycopene + high lutein, high lycopene + low lutein, low lycopene + high lutein, and low lycopene + low lutein by vegetable beverages | Daily beverage-intake significantly decreased the visceral fat level, and CoQ10 oxidation rate was decreased in all the groups. |
| Graydon, R. et al., 2012 [56] | UK | Randomized placebo-controlled trial | 8-week | 52 | Dried spinach powder (lutein and zeaxanthin-rich food) or carrot juice (α and β-carotene rich food) | Lutein and zeaxanthin had no significant effect on MPL or serological markers of endothelial activation, inflammation and oxidation in healthy volunteers. |
| Colmán-Martínez, M. et al., 2017 [58] | Spain | Retrospective, randomized, cross-over, and controlled clinical trial | 4 weeks | 28 | 200 mL (LD) or 400 mL (HD) of tomato juice | Trans-lycopene reduced the concentration of important adhesion molecules ICAM-1, and VCAM-1, related to atherosclerosis. |
| Paterson, E. et al., 2006 [59] | UK | Single blind, randomized, controlled, crossover dietary intervention study. | 4 weeks | 36 | During the test intervention period, the subjects were asked to consume 1 soup (500 mL) plus 1 juice (300 mL) or shot (fruit and vegetable preparation made from concentrated juices and purees) (100 mL) per day | Consumption of the carotenoid-rich soups and beverages only decreased the plasma homocysteine concentration by 8.8%. |
| Bub, A. et al., 2000 [60] | The Netherlands | Clinical trial | 8 weeks | 23 | 330 mL/d of a tomato juice (40 mg lycopene) in addition to their meals or 330 mL carrot juice (15.7 mg a-carotene and 22.3 mg b-carotene) daily | Tomato juice consumption reduced plasma thiobarbituric acid reactive substances (TBARS) and lipoprotein oxidizability in terms of an increased lag time. Carrot juice and spinach powder had no effect on lipid peroxidation. |
| Tomás, A. et al., 2021 [62] | Spain | Clinical trial | 4 weeks | 12 | Orange-carrot juice, tomato juice, and boiled spinach, providing 415 mg of total carotenoids/week (carotenes, cryptoxanthin, lycopene, and lutein + zeaxanthin) | Significant decrease in LDL and atherogenic index, and an increase in HDL were observed. |
| Biddle, MJ. et al., 2015 [63] | USA | Two-group, randomized controlled intervention pilot study | 30 days | 40 | 11.5 ounces of a juice of vegetables (29.4 mg of lycopene; 70 calories; 140 mg of sodium; vitamins A and C; 820 mg of potassium; 2% of the recommended daily allowance for iron and magnesium; and 3 g of fiber | No differences on CRP levels. |
| Author, Publication Year | Country/Region | Type of Study/Study Name | Follow-Up Period | Study Size | Dietary Intervention | Findings |
|---|---|---|---|---|---|---|
| Daniels, JA. et al., 2014 [53] | UK | Randomized, double-blinded, controlled clinical trial | 8-weeks | 80 | Randomized to a 1- or ≥6-portion/day fruit and vegetables diet | ≥6- vs. 1-portion post-intervention comparisons, carotenoids increased in serum, HDL2 and particularly HDL3, as did the activities of PON-1 and LCAT in HDL3. |
| Wallace, I. et al., 2013 [54] | UK | Randomized controlled trial | 12-week | 89 | One to two, four, or seven portions of FandVs | No significant difference was found in measures of whole-body, peripheral, or hepatic IR or adiponectin multimers. |
| Hurtado-Barroso, S. et al., 2019 [55] | Spain | Clinical trial | 1 day | 22 | Single portion of sofrito (240 g/70 kg bodyweight) in a state of fasting | Significant decrease in CRP and TNF-α was observed, but only TNF-α was inversely correlated with an increase in TPE (total polyphenol excretion) and plasma β-carotene. |
| Thies, F. et al., 2012 [57] | UK | Single-blind, randomized controlled trial | 12 weeks | 225 | Diet low in tomato-based foods, a high-tomato-based diet, or a control diet supplemented with lycopene capsules (10 mg/d) | High daily consumption of tomato-based products or lycopene supplements is ineffective at reducing conventional CVD risk markers. |
| Svendsen M. et al., 2007 [61] | Norway | Randomized, controlled trial | 3 months | 138 | Consumption of vegetables to at least 400 g/day, and fruit to at least 300 g/day | Weight reduction and reduced systolic and diastolic BP. No effect on antioxidant defence measured with FRAP. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sumalla-Cano, S.; Eguren-García, I.; Lasarte-García, Á.; Prola, T.A.; Martínez-Díaz, R.; Elío, I. Carotenoids Intake and Cardiovascular Prevention: A Systematic Review. Nutrients 2024, 16, 3859. https://doi.org/10.3390/nu16223859
Sumalla-Cano S, Eguren-García I, Lasarte-García Á, Prola TA, Martínez-Díaz R, Elío I. Carotenoids Intake and Cardiovascular Prevention: A Systematic Review. Nutrients. 2024; 16(22):3859. https://doi.org/10.3390/nu16223859
Chicago/Turabian StyleSumalla-Cano, Sandra, Imanol Eguren-García, Álvaro Lasarte-García, Thomas A. Prola, Raquel Martínez-Díaz, and Iñaki Elío. 2024. "Carotenoids Intake and Cardiovascular Prevention: A Systematic Review" Nutrients 16, no. 22: 3859. https://doi.org/10.3390/nu16223859
APA StyleSumalla-Cano, S., Eguren-García, I., Lasarte-García, Á., Prola, T. A., Martínez-Díaz, R., & Elío, I. (2024). Carotenoids Intake and Cardiovascular Prevention: A Systematic Review. Nutrients, 16(22), 3859. https://doi.org/10.3390/nu16223859











