Olive Leaf Extract Supplementation Improves Postmenopausal Symptoms: A Randomized, Double-Blind, Placebo-Controlled Parallel Study on Postmenopausal Women
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Design
2.3. Intervention
2.3.1. Postmenopausal Symptoms
2.3.2. Body Composition
2.3.3. Handgrip Strength Measurements
2.3.4. Physical Activity
2.3.5. Biochemical Analyses
2.4. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALMI | Appendicular lean mass index |
| BMC | Bone mineral density |
| BMI | Body Mass Index |
| DXA | Dual-energy X-ray absorptiometry |
| E2 | 17-β-oestradiol |
| ERs | Estrogen receptors |
| Erα | Estrogen receptor α |
| Erβ | Estrogen receptor β |
| FFM | Fat-free mass |
| FM | Fat mass |
| HDL-C | High-density lipoproteins |
| HFI | The Hot Flash Interference scale |
| IPAQ-L | International Physical Activity Questionnaire long version |
| LDL-C | Low-density lipoproteins |
| MENQoL | The Menopause-Specific QoL Questionnaire |
| MRUM | Metabolic Research Unit Maastricht |
| OLE | Olive leaf extract |
| PPARγ | Peroxisome proliferator-activated receptor γ |
| QoL | Quality of Life |
| TG | Triacylglycerol |
| VAT | Visceral abdominal tissue |
References
- Graziottin, A.; Leiblum, S.R. Biological and psychosocial pathophysiology of female sexual dysfunction during the menopausal transition. J. Sex. Med. 2005, 2 (Suppl. 3), 133–145. [Google Scholar] [CrossRef] [PubMed]
- Kravitz, H.M.; Kazlauskaite, R.; Joffe, H. Sleep, Health, and Metabolism in Midlife Women and Menopause: Food for Thought. Obstet. Gynecol. Clin. N. Am. 2018, 45, 679–694. [Google Scholar] [CrossRef] [PubMed]
- Tao, M.; Shao, H.; Li, C.; Teng, Y. Correlation between the modified Kupperman Index and the Menopause Rating Scale in Chinese women. Patient Prefer. Adherence 2013, 7, 223–229. [Google Scholar] [PubMed]
- Money, A.; MacKenzie, A.; Norman, G.; Eost-Telling, C.; Harris, D.; McDermott, J.; Todd, C. The impact of physical activity and exercise interventions on symptoms for women experiencing menopause: Overview of reviews. BMC Women’s Health 2024, 24, 399. [Google Scholar] [CrossRef]
- Rossouw, J.E.; Prentice, R.L.; Manson, J.E.; Wu, L.; Barad, D.; Barnabei, V.M.; Ko, M.; LaCroix, A.Z.; Margolis, K.L.; Stefanick, M.L. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA 2007, 297, 1465–1477. [Google Scholar] [CrossRef]
- Beral, V. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet 2003, 362, 419–427. [Google Scholar] [CrossRef]
- Zhao, H.; Zhou, L.; Shangguan, A.J.; Bulun, S.E. Aromatase expression and regulation in breast and endometrial cancer. J. Mol. Endocrinol. 2016, 57, R19–R33. [Google Scholar] [CrossRef]
- Maartens, L.W.; Leusink, G.L.; Knottnerus, J.A.; Pop, V.J. Hormonal substitution during menopause: What are we treating? Maturitas 2000, 34, 113–118. [Google Scholar] [CrossRef]
- Franco, O.H.; Chowdhury, R.; Troup, J.; Voortman, T.; Kunutsor, S.; Kavousi, M.; Oliver-Williams, C.; Muka, T. Use of Plant-Based Therapies and Menopausal Symptoms: A Systematic Review and Meta-analysis. JAMA 2016, 315, 2554–2563. [Google Scholar] [CrossRef]
- Sirotkin, A.V.; Harrath, A.H. Phytoestrogens and their effects. Eur. J. Pharmacol. 2014, 741, 230–236. [Google Scholar] [CrossRef]
- Boss, A.; Bishop, K.S.; Marlow, G.; Barnett, M.P.; Ferguson, L.R. Evidence to Support the Anti-Cancer Effect of Olive Leaf Extract and Future Directions. Nutrients 2016, 8, 513. [Google Scholar] [CrossRef] [PubMed]
- Granados-Principal, S.; Quiles, J.L.; Ramirez-Tortosa, C.L.; Sanchez-Rovira, P.; Ramirez-Tortosa, M.C. Hydroxytyrosol: From laboratory investigations to future clinical trials. Nutr. Rev. 2010, 68, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Filip, R.; Possemiers, S.; Heyerick, A.; Pinheiro, I.; Raszewski, G.; Davicco, M.J.; Coxam, V. Twelve-month consumption of a polyphenol extract from olive (Olea europaea) in a double blind, randomized trial increases serum total osteocalcin levels and improves serum lipid profiles in postmenopausal women with osteopenia. J. Nutr. Health Aging 2015, 19, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, M.N.; Beaumont, M.; Sauvageot, N.; Poquet, L.; Saboundjian, M.; Costes, B.; Verdonk, P.; Brands, G.; Brasseur, J.; Urbin-Choffray, D.; et al. An oleuropein-based dietary supplement may improve joint functional capacity in older people with high knee joint pain: Findings from a multicentre-RCT and post hoc analysis. Ther. Adv. Musculoskelet. Dis. 2022, 14, 1759720x211070205. [Google Scholar] [CrossRef] [PubMed]
- Walston, J.D. Sarcopenia in older adults. Curr. Opin. Rheumatol. 2012, 24, 623–627. [Google Scholar] [CrossRef]
- Priego, T.; Martín, A.I.; González-Hedström, D.; Granado, M.; López-Calderón, A. Role of hormones in sarcopenia. Vitam. Horm. 2021, 115, 535–570. [Google Scholar] [CrossRef]
- Kim, C.; Hwang, J.K. Flavonoids: Nutraceutical potential for counteracting muscle atrophy. Food Sci. Biotechnol. 2020, 29, 1619–1640. [Google Scholar] [CrossRef]
- Mukai, R.; Terao, J. Role of dietary flavonoids in oxidative stress and prevention of muscle atrophy. J. Phys. Fit. Sports Med. 2013, 2, 385–392. [Google Scholar] [CrossRef]
- Nikawa, T.; Ulla, A.; Sakakibara, I. Polyphenols and Their Effects on Muscle Atrophy and Muscle Health. Molecules 2021, 26, 4887. [Google Scholar] [CrossRef]
- González-Hedström, D.; Priego, T.; Amor, S.; de la Fuente-Fernández, M.; Martín, A.I.; López-Calderón, A.; Inarejos-García, A.M.; García-Villalón, Á.L.; Granado, M. Olive Leaf Extract Supplementation to Old Wistar Rats Attenuates Aging-Induced Sarcopenia and Increases Insulin Sensitivity in Adipose Tissue and Skeletal Muscle. Antioxidants 2021, 10, 737. [Google Scholar] [CrossRef]
- Ko, S.H.; Kim, H.S. Menopause-Associated Lipid Metabolic Disorders and Foods Beneficial for Postmenopausal Women. Nutrients 2020, 12, 202. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.L.; Lin, S.Q.; Shen, Y.; Chen, Y.; Zhang, Y.; Chen, F.L. Serum lipid profile changes during the menopausal transition in Chinese women: A community-based cohort study. Menopause 2010, 17, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Thaung Zaw, J.J.; Howe, P.R.C.; Wong, R.H.X. Postmenopausal health interventions: Time to move on from the Women’s Health Initiative? Ageing Res. Rev. 2018, 48, 79–86. [Google Scholar] [CrossRef] [PubMed]
- de Bock, M.; Derraik, J.G.; Brennan, C.M.; Biggs, J.B.; Morgan, P.E.; Hodgkinson, S.C.; Hofman, P.L.; Cutfield, W.S. Olive (Olea europaea L.) leaf polyphenols improve insulin sensitivity in middle-aged overweight men: A randomized, placebo-controlled, crossover trial. PLoS ONE 2013, 8, e57622. [Google Scholar] [CrossRef]
- Perrinjaquet-Moccetti, T.; Busjahn, A.; Schmidlin, C.; Schmidt, A.; Bradl, B.; Aydogan, C. Food supplementation with an olive (Olea europaea L.) leaf extract reduces blood pressure in borderline hypertensive monozygotic twins. Phytother. Res. 2008, 22, 1239–1242. [Google Scholar] [CrossRef]
- Susalit, E.; Agus, N.; Effendi, I.; Tjandrawinata, R.R.; Nofiarny, D.; Perrinjaquet-Moccetti, T.; Verbruggen, M. Olive (Olea europaea) leaf extract effective in patients with stage-1 hypertension: Comparison with Captopril. Phytomedicine 2011, 18, 251–258. [Google Scholar] [CrossRef]
- Radtke, J.V.; Terhorst, L.; Cohen, S.M. The Menopause-Specific Quality of Life Questionnaire: Psychometric evaluation among breast cancer survivors. Menopause 2011, 18, 289–295. [Google Scholar] [CrossRef]
- Hilditch, J.R.; Lewis, J.; Peter, A.; van Maris, B.; Ross, A.; Franssen, E.; Guyatt, G.H.; Norton, P.G.; Dunn, E. A menopause-specific quality of life questionnaire: Development and psychometric properties. Maturitas 1996, 24, 161–175. [Google Scholar] [CrossRef]
- Carpenter, J.S.; Bakoyannis, G.; Otte, J.L.; Chen, C.X.; Rand, K.L.; Woods, N.; Newton, K.; Joffe, H.; Manson, J.E.; Freeman, E.W.; et al. Validity, cut-points, and minimally important differences for two hot flash-related daily interference scales. Menopause 2017, 24, 877–885. [Google Scholar] [CrossRef]
- Guglielmi, G.; Ponti, F.; Agostini, M.; Amadori, M.; Battista, G.; Bazzocchi, A. The role of DXA in sarcopenia. Aging Clin. Exp. Res. 2016, 28, 1047–1060. [Google Scholar] [CrossRef]
- Tosato, M.; Marzetti, E.; Cesari, M.; Savera, G.; Miller, R.R.; Bernabei, R.; Landi, F.; Calvani, R. Measurement of muscle mass in sarcopenia: From imaging to biochemical markers. Aging Clin. Exp. Res. 2017, 29, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, J.; Machado, F.V.C.; Santin, L.C.; Andrello, A.C.; Schneider, L.P.; Fernandes Belo, L.; Rodrigues, A.; Fernandes Rugila, D.; Furlanetto, K.C.; Hernandes, N.A.; et al. Handgrip Strength as a Reflection of General Muscle Strength in Chronic Obstructive Pulmonary Disease. COPD 2021, 18, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [PubMed]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Aubertin-Leheudre, M.; Lord, C.; Khalil, A.; Dionne, I.J. Six months of isoflavone supplement increases fat-free mass in obese-sarcopenic postmenopausal women: A randomized double-blind controlled trial. Eur. J. Clin. Nutr. 2007, 61, 1442–1444. [Google Scholar] [CrossRef]
- Sayahi, M.; Keramat, A.; Nourimand Ph, D.C.F.; Mohammadzadeh, H. Is there a difference between the effects of phytoestrogens and non-phytoestrogens medicinal plants on sexual health? A systematic review and meta-analysis. Int. J. Reprod. Biomed. 2023, 21, 881–900. [Google Scholar] [CrossRef]
- Gençtürk, N.; Bilgiç, F.; Kaban, H.U. The effect of soy isoflavones given to women in the climacteric period on menopausal symptoms and quality of life: Systematic review and meta-analysis of randomized controlled trials. Explore 2024, 20, 103012. [Google Scholar] [CrossRef]
- Rietjens, I.M.; Sotoca, A.M.; Vervoort, J.; Louisse, J. Mechanisms underlying the dualistic mode of action of major soy isoflavones in relation to cell proliferation and cancer risks. Mol. Nutr. Food Res. 2013, 57, 100–113. [Google Scholar] [CrossRef]
- Clusan, L.; Ferrière, F.; Flouriot, G.; Pakdel, F. A Basic Review on Estrogen Receptor Signaling Pathways in Breast Cancer. Int. J. Mol. Sci. 2023, 24, 6834. [Google Scholar] [CrossRef]
- Rietjens, I.; Louisse, J.; Beekmann, K. The potential health effects of dietary phytoestrogens. Br. J. Pharmacol. 2017, 174, 1263–1280. [Google Scholar] [CrossRef]
- Park, Y.; Cho, Y.J.; Sung, N.; Park, M.J.; Guan, X.; Gibbons, W.E.; O’Malley, B.W.; Han, S.J. Oleuropein suppresses endometriosis progression and improves the fertility of mice with endometriosis. J. Biomed. Sci. 2022, 29, 100. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, A.; Brown, J.E.; Hawdon, A.; Faughnan, M.S.; King, L.J.; Millward, J.; Zimmer-Nechemias, L.; Wolfe, B.; Setchell, K.D. Factors affecting the bioavailability of soy isoflavones in humans after ingestion of physiologically relevant levels from different soy foods. J. Nutr. 2006, 136, 45–51. [Google Scholar] [CrossRef] [PubMed]
- García-Martínez, O.; De Luna-Bertos, E.; Ramos-Torrecillas, J.; Ruiz, C.; Milia, E.; Lorenzo, M.L.; Jimenez, B.; Sánchez-Ortiz, A.; Rivas, A. Phenolic Compounds in Extra Virgin Olive Oil Stimulate Human Osteoblastic Cell Proliferation. PLoS ONE 2016, 11, e0150045. [Google Scholar] [CrossRef] [PubMed]
- Haidari, F.; Shayesteh, F.; Mohammad-Shahi, M.; Jalali, M.T.; Ahmadi-Angali, K. Olive Leaf Extract Supplementation Combined with Calorie-Restricted Diet on Reducing Body Weight and Fat Mass in Obese Women: Result of a Randomized Control Trial. Clin. Nutr. Res. 2021, 10, 314–329. [Google Scholar] [CrossRef]
- Binou, P.; Stergiou, A.; Kosta, O.; Tentolouris, N.; Karathanos, V.T. Positive contribution of hydroxytyrosol-enriched wheat bread to HbA(1)c levels, lipid profile, markers of inflammation and body weight in subjects with overweight/obesity and type 2 diabetes mellitus. Eur. J. Nutr. 2023, 62, 2165–2176. [Google Scholar] [CrossRef]
- Puel, C.; Mathey, J.; Agalias, A.; Kati-Coulibaly, S.; Mardon, J.; Obled, C.; Davicco, M.J.; Lebecque, P.; Horcajada, M.N.; Skaltsounis, A.L.; et al. Dose-response study of effect of oleuropein, an olive oil polyphenol, in an ovariectomy/inflammation experimental model of bone loss in the rat. Clin. Nutr. 2006, 25, 859–868. [Google Scholar] [CrossRef]
- Terzic, M.; Micic, J.; Dotlic, J.; Maricic, S.; Mihailovic, T.; Knezevic, N. Impact of Phytoestrogens on Serum Lipids in Postmenopausal Women. Geburtshilfe Frauenheilkd. 2012, 72, 527–531. [Google Scholar] [CrossRef]
- Stevens, Y.; Winkens, B.; Jonkers, D.; Masclee, A. The effect of olive leaf extract on cardiovascular health markers: A randomized placebo-controlled clinical trial. Eur. J. Nutr. 2021, 60, 2111–2120. [Google Scholar] [CrossRef]
- Lockyer, S.; Corona, G.; Yaqoob, P.; Spencer, J.P.E.; Rowland, I. Secoiridoids delivered as olive leaf extract induce acute improvements in human vascular function and reduction of an inflammatory cytokine: A randomised, double-blind, placebo-controlled, cross-over trial. Br. J. Nutr. 2015, 114, 75–83. [Google Scholar] [CrossRef]
- Mesalić, L.; Tupković, E.; Kendić, S.; Balić, D. Correlation between hormonal and lipid status in women in menopause. Bosn. J. Basic Med. Sci. 2008, 8, 188–192. [Google Scholar] [CrossRef]
- Cochran, B.J.; Ong, K.L.; Manandhar, B.; Rye, K.A. APOA1: A Protein with Multiple Therapeutic Functions. Curr. Atheroscler. Rep. 2021, 23, 11. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Mora, R.; Casado-Díaz, A.; De Castro, M.; Quesada-Gómez, J. Oleuropein enhances osteoblastogenesis and inhibits adipogenesis: The effect on differentiation in stem cells derived from bone marrow. Osteoporos. Int. 2011, 22, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Dang, Z.-C.; Audinot, V.; Papapoulos, S.E.; Boutin, J.A.; Löwik, C.W. Peroxisome proliferator-activated receptor γ (PPARγ) as a molecular target for the soy phytoestrogen genistein. J. Biol. Chem. 2003, 278, 962–967. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, C.J.; Kuipers, R.; Rombouts, J.A.; Brouwers, K.; Schrauwen-Hinderling, V.B.; Wildberger, J.E.; Verdijk, L.B.; van Loon, L.J.C. Thigh muscles are more susceptible to age-related muscle loss when compared to lower leg and pelvic muscles. Exp. Gerontol. 2023, 175, 112159. [Google Scholar] [CrossRef]


| Total Population (n = 60) | OLE (n = 29) | Control (n = 31) | |
|---|---|---|---|
| Age (years) | 59.2 ± 5.8 | 58.4 ± 6.2 | 59.8 ± 5.4 |
| Height (cm) | 165 ± 6.7 | 165.5 ± 7.2 | 165.2 ± 6.3 |
| Weight (kg) | 69.7 ± 9.6 | 68.5 ± 8.6 | 70.8 ± 10.5 |
| BMI (kg/m2) | 25.4 ± 2.8 | 24.9 ± 2.1 | 25.9 ± 3.4 |
| OLE | Control | Time × Treatment Interaction | Treatment Effect | Estimated Mean Difference [95% CI] | |||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | After 6 Weeks | After 12 Weeks | Baseline | After 6 Weeks | After 12 Weeks | ||||
| Vasomotor Score | 2.2 ± 1.4 | 2.2 ± 1.6 | 2.2 ± 1.5 | 2.0 ± 1.3 | 2.2 ± 1.4 | 2.3 ± 1.7 | p = 0.607 | p = 0.489 | −0.1 [−0.6 + 0.3] |
| Psychosocial Score | 1.8 ± 0.9 | 1.6 ± 0.9 | 1.6 ± 1.1 | 2.0 ± 1.2 | 1.7 ± 1.0 | 1.7 ± 1.1 | p = 0.753 | p = 0.905 | −0.0 [−0.3 + 0.3] |
| Physical Score | 2.1 ± 0.9 | 1.7 ± 0.6 | 1.8 ± 0.9 | 1.9 ± 0.8 | 1.8 ± 0.8 | 1.7 ± 0.7 | p = 0.058 | p = 0.195 | −0.1 [−0.4 + 0.0] |
| Sexual Score | 2.0 ± 1.5 | 1.4 ± 0.9 | 1.3 ± 0.7 | 1.6 ± 1.3 | 1.6 ± 1.1 | 1.5 ± 1.1 | p = 0.808 | p = 0.116 | −0.3 [−0.6 + 0.7] |
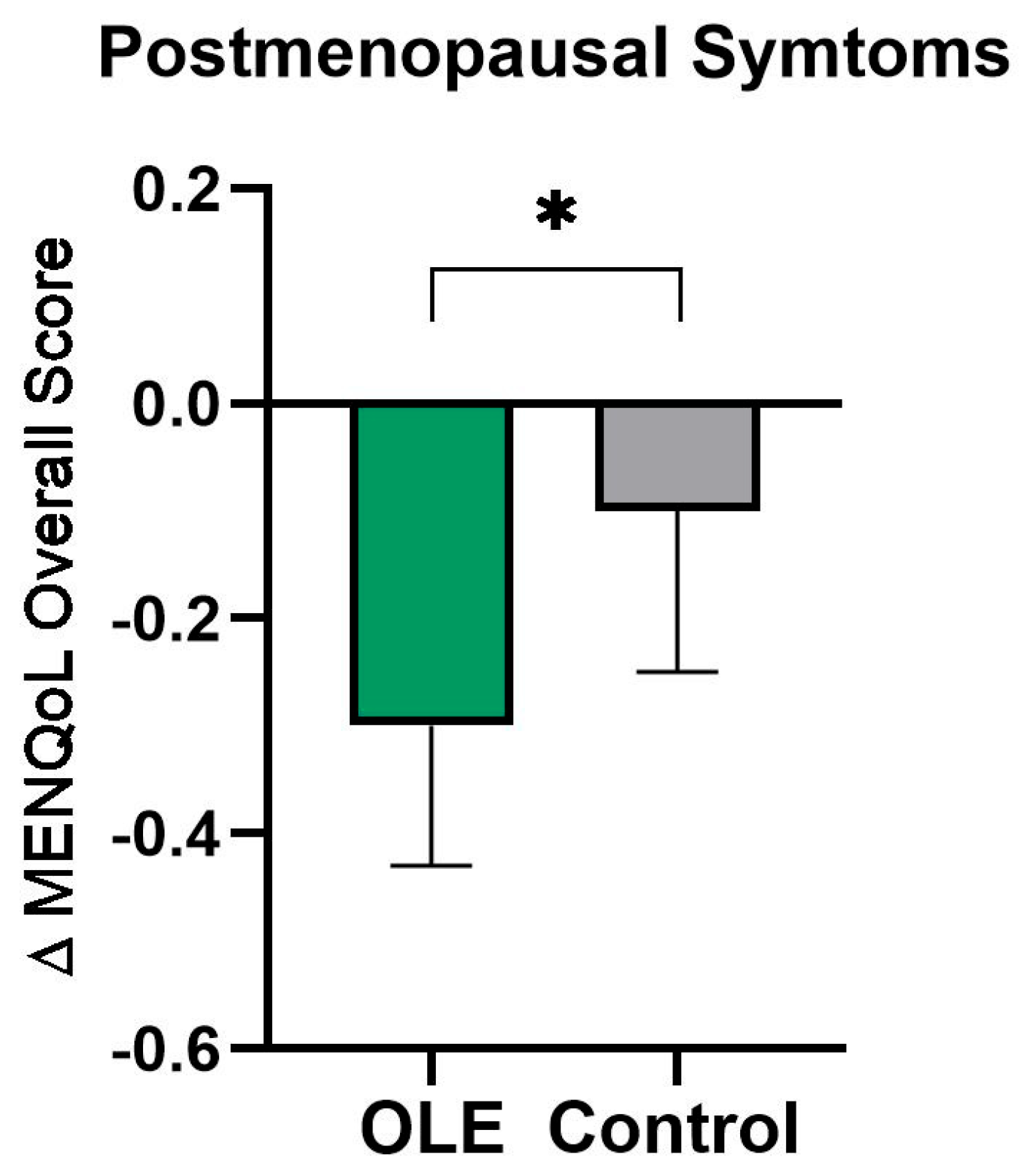
| Overall Score | 2.0 ± 0.7 | 1.7 ± 0.6 | 1.7 ± 0.8 | 1.9 ± 0.9 | 1.8 ± 0.8 | 1.8 ± 0.9 | p = 0.628 | p = 0.027 * | −0.2 [−0.4 − 0.2] |
| OLE | Control | Time × Treatment Interaction | Treatment Effect | Estimated Mean Difference [95% CI] | |||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | After 6 Weeks | After 12 Weeks | Baseline | After 6 Weeks | After 12 Weeks | ||||
| Sleep Score | 1.0 ± 2.1 | 1.5 ± 2.3 | 1.9 ± 2.9 | 1.5 ± 2.8 | 1.5 ± 2.5 | 1.0 ± 2.0 | p = 0.130 | p = 0.136 | +0.5 [−0.1 + 1.2] |
| Mood Score | 0.5 ± 1.0 | 0.8 ± 1.7 | 0.7 ± 1.7 | 1.2 ± 1.9 | 1.2 ± 2.0 | 0.9 ± 1.7 | p = 0.760 | p = 0.733 | +0.1 [−0.5 + 0.7] |
| Concentration Score | 0.7 ± 1.4 | 0.9 ± 1.9 | 1.1 ± 2.1 | 1.1 ± 1.9 | 1.2 ± 2.3 | 1.1 ± 1.8 | p = 0.673 | p = 0.832 | +0.0 [−0.7 + 0.9] |
| OLE | Control | Treatment Effect Estimated Mean Difference [95% CI] | |||
|---|---|---|---|---|---|
| Baseline | After 12 Weeks | Baseline | After 12 Weeks | ||
| Body composition | |||||
| Total Body FM (g) | 24,025 ± 3839 | 24,241 ± 3577 | 25,834 ± 6963 | 25,775 ± 7094 | −366 [−909, 177], p = 0.182 |
| Est. VAT Mass (g) | 384 ± 180 | 382 ± 165 | 444 ± 151 | 436 ± 152 | −5.0 [−30, 20], p = 0.698 |
| Total Body FFM (g) | 45,740 ± 6129 | 46,164 ± 6319 | 46,286 ± 4941 | 46,597 ± 5007 | −52.4 [−729, 624], p = 0.877 |
| Lean body mass/Height2 (kg/m2) | 15.8 ± 1.3 | 15.9 ± 1.4 | 16.2 ± 1.2 | 16.3 ± 1.3 | −0.0 [−0.3, 0.2], p = 0.632 |
| ALM index (kg/m2) | 6.7 ± 0.7 | 6.7 ± 0.7 | 6.7 ± 0.7 | 6.8 ± 0.6 | −0.0 [−0.1, 0.0], p = 0.662 |
| Bone Mineral Density | |||||
| BMD, left arm (g/cm3) | 0.674 ± 0.053 | 0.669 ± 0.051 | 0.689 ± 0.051 | 0.688 ± 0.056 | −0.009 [−0.021, 0.004]; p = 0.185 |
| BMD, right arm (g/cm3) | 0.677 ± 0.048 | 0.690 ± 0.055 | 0.695 ± 0.048 | 0.688 ± 0.055 | +0.017 [0.003, 0.030], p = 0.019 * |
| BMD, left ribs (g/cm3) | 0.615 ± 0.124 | 0.613 ± 0.125 | 0.604 ± 0.078 | 0.600 ± 0.080 | −0.002 [−0.026, 0.023], p = 0.884 |
| BMD, right ribs (g/cm3) | 0.576 ± 0.103 | 0.588 ± 0.131 | 0.574 ± 0.074 | 0.559 ± 0.071 | 0.029 [−0.001, 0.59], p = 0.055 |
| BMD, T spine (g/cm3) | 0.770 ± 0.091 | 0.759 ± 0.114 | 0.767 ± 0.081 | 0.760 ± 0.088 | −0.002 [−0.042, 0.038], p = 0.928 |
| BMD, L spine (g/cm3) | 0.930 ± 0.155 | 0.928 ± 0.138 | 0.989 ± 0.171 | 0.979 ± 0.165 | −0.002 [−0.026, 0.023], p = 0.894 |
| BMD, pelvis (g/cm3) | 1.102 ± 0.148 | 1.115 ± 0.150 | 1.160 ± 0.116 | 1.165 ± 0.129 | 0.001 [−0.020, 0.021], p = 0.926 |
| BMD, left leg (g/cm3) | 1.063 ± 0.088 | 1.067 ± 0.091 | 1.061 ± 0.077 | 1.056 ± 0.079 | −0.002 [−0.019, 0.015], p = 0.830 |
| BMD, right leg (g/cm3) | 1.058 ± 0.089 | 1.064 ± 0.095 | 1.060 ± 0.078 | 1.059 ± 0.080 | −0.005 [−0.025, 0.015], p = 0.643 |
| BMD, total body (g/cm3) | 1.028 ± 0.081 | 1.037 ± 0.086 | 1.031 ± 0.079 | 1.030 ± 0.082 | 0.004 [−0.006, 0.014], p = 0.449 |
| DEXA T-score Total | −1.010 ± 1.060 | −0.900 ± 1.109 | −0.967 ± 1.041 | −0.983 ± 1.083 | 0.050 [−0.089, 0.189], p = 0.476 |
| OLE | Control | Time × Treatment Interaction | Treatment Effect | Estimated Mean Difference [95% CI] | |||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | After 6 Weeks | After 12 Weeks | Baseline | After 6 Weeks | After 12 Weeks | ||||
| Higher strength, left hand (kg) | 30.2 ± 7.3 | 30.0 ± 9.0 | 30.7 ± 7.7 | 29.6 ± 4.4 | 29.3 ± 4.8 | 28.9 ± 4.7 | p = 0.138 | p = 0.478 | +0.4 [−0.8, 1.7] |
| Higher strength, right hand (kg) | 30.9 ± 7.3 | 30.1 ± 8.6 | 30.6 ± 8.4 | 30.9 ± 5.4 | 30.9 ± 5.6 | 30.0 ± 5.8 | p = 0.055 | p = 0.597 | −0.3 [−1.6, 0.9] |
| OLE | Control | Time × Treatment Interaction | Treatment Effect | Estimated Mean Difference [95% CI] | |||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | After 6 Weeks | After 12 Weeks | Baseline | After 6 Weeks | After 12 Weeks | ||||
| TC [mmol/L] | 5.72 ± 1.19 | 5.64 ± 1.19 | 5.40 ± 0.92 | 6.02 ± 0.89 | 6.00 ± 0.73 | 5.92 ± 0.70 | p = 0.688 | p = 0.116 | −0.1 [−0.4, 0.0] |
| HDL [mmol/L] | 1.81 ± 0.41 | 1.80 ± 0.33 | 1.75 ± 0.33 | 1.92 ± 0.43 | 1.87 ± 0.36 | 1.88 ± 0.42 | p = 0.414 | p = 0.530 | −0.0 [−0.0, 0.0] |
| LDL [mmol/L] | 3.48 ± 0.96 | 3.43 ± 0.97 | 3.24 ± 0.74 | 3.65 ± 0.80 | 3.64 ± 0.67 | 3.57 ± 0.66 | p = 0.817 | p = 0.232 | −0.1 [−0.3, 0.0] |
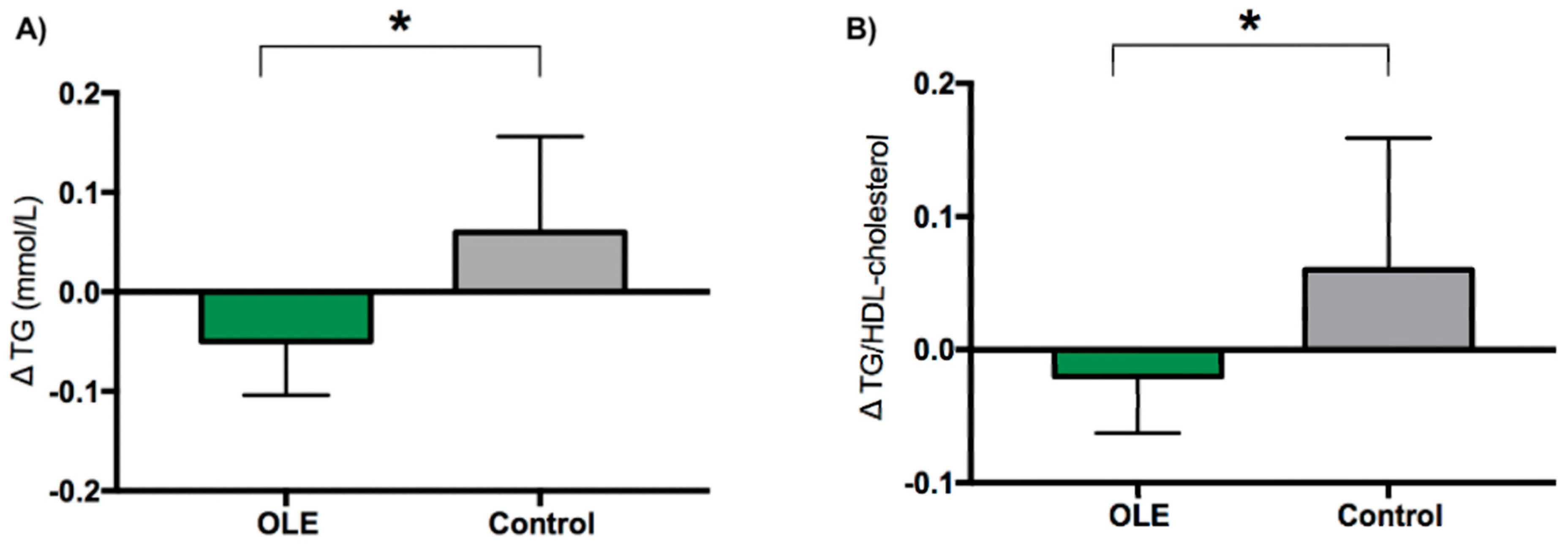
| TG [mmol/L] | 0.98 ± 0.31 | 0.90 ± 0.25 | 0.93 ± 0.27 | 1.09 ± 0.47 | 1.16 ± 0.43 | 1.15 ± 0.60 | p = 0.620 | p = 0.010 * | −0.1 [−0.2, 0.0] |
| TC/HDL-cholesterol | 3.24 ± 0.68 | 3.16 ± 0.58 | 3.14 ± 0.58 | 3.30 ± 0.98 | 3.34 ± 0.93 | 3.34 ± 1.07 | p = 0.983 | p = 0.095 | −0.1 [−0.3, 0.0] |
| TG/HDL-cholesterol | 0.58 ± 0.25 | 0.52 ± 0.19 | 0.56 ± 0.21 | 0.66 ± 0.48 | 0.69 ± 0.43 | 0.72 ± 0.62 | p = 0.912 | p = 0.029 * | −0.1 [−0.2, −0.0] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imperatrice, M.; Lasfar, A.; van Kalkeren, C.A.J.; Troost, F. Olive Leaf Extract Supplementation Improves Postmenopausal Symptoms: A Randomized, Double-Blind, Placebo-Controlled Parallel Study on Postmenopausal Women. Nutrients 2024, 16, 3879. https://doi.org/10.3390/nu16223879
Imperatrice M, Lasfar A, van Kalkeren CAJ, Troost F. Olive Leaf Extract Supplementation Improves Postmenopausal Symptoms: A Randomized, Double-Blind, Placebo-Controlled Parallel Study on Postmenopausal Women. Nutrients. 2024; 16(22):3879. https://doi.org/10.3390/nu16223879
Chicago/Turabian StyleImperatrice, Maria, Anissa Lasfar, Colin A. J. van Kalkeren, and Freddy Troost. 2024. "Olive Leaf Extract Supplementation Improves Postmenopausal Symptoms: A Randomized, Double-Blind, Placebo-Controlled Parallel Study on Postmenopausal Women" Nutrients 16, no. 22: 3879. https://doi.org/10.3390/nu16223879
APA StyleImperatrice, M., Lasfar, A., van Kalkeren, C. A. J., & Troost, F. (2024). Olive Leaf Extract Supplementation Improves Postmenopausal Symptoms: A Randomized, Double-Blind, Placebo-Controlled Parallel Study on Postmenopausal Women. Nutrients, 16(22), 3879. https://doi.org/10.3390/nu16223879






