Agathisflavone Modulates Reactive Gliosis After Trauma and Increases the Neuroblast Population at the Subventricular Zone
Abstract
:1. Introduction
2. Materials and Methods
2.1. Agathisflavone Extraction
2.2. Animals and Treatments
2.3. Immunostaining
2.4. Imaging and Analysis
2.5. Statistics
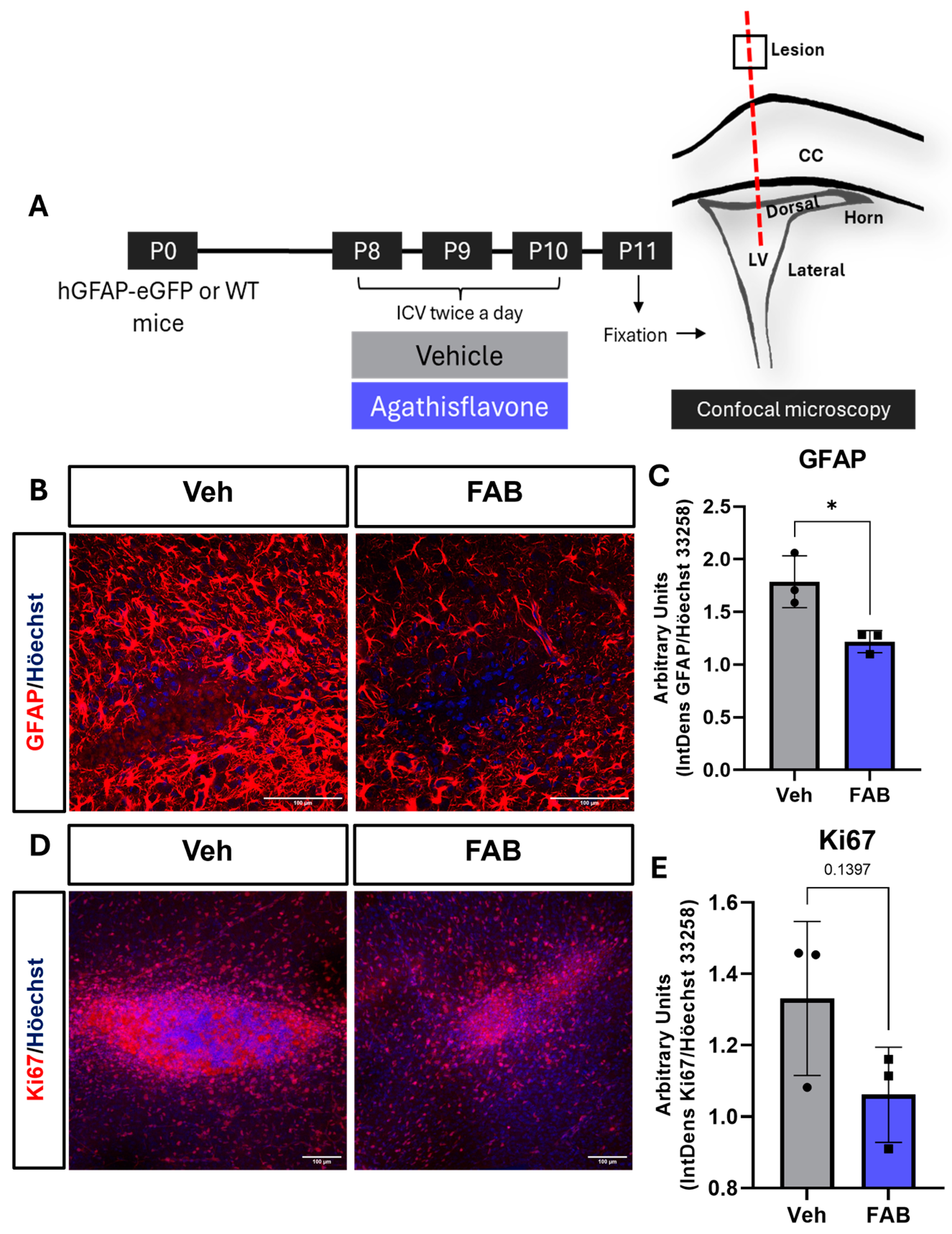
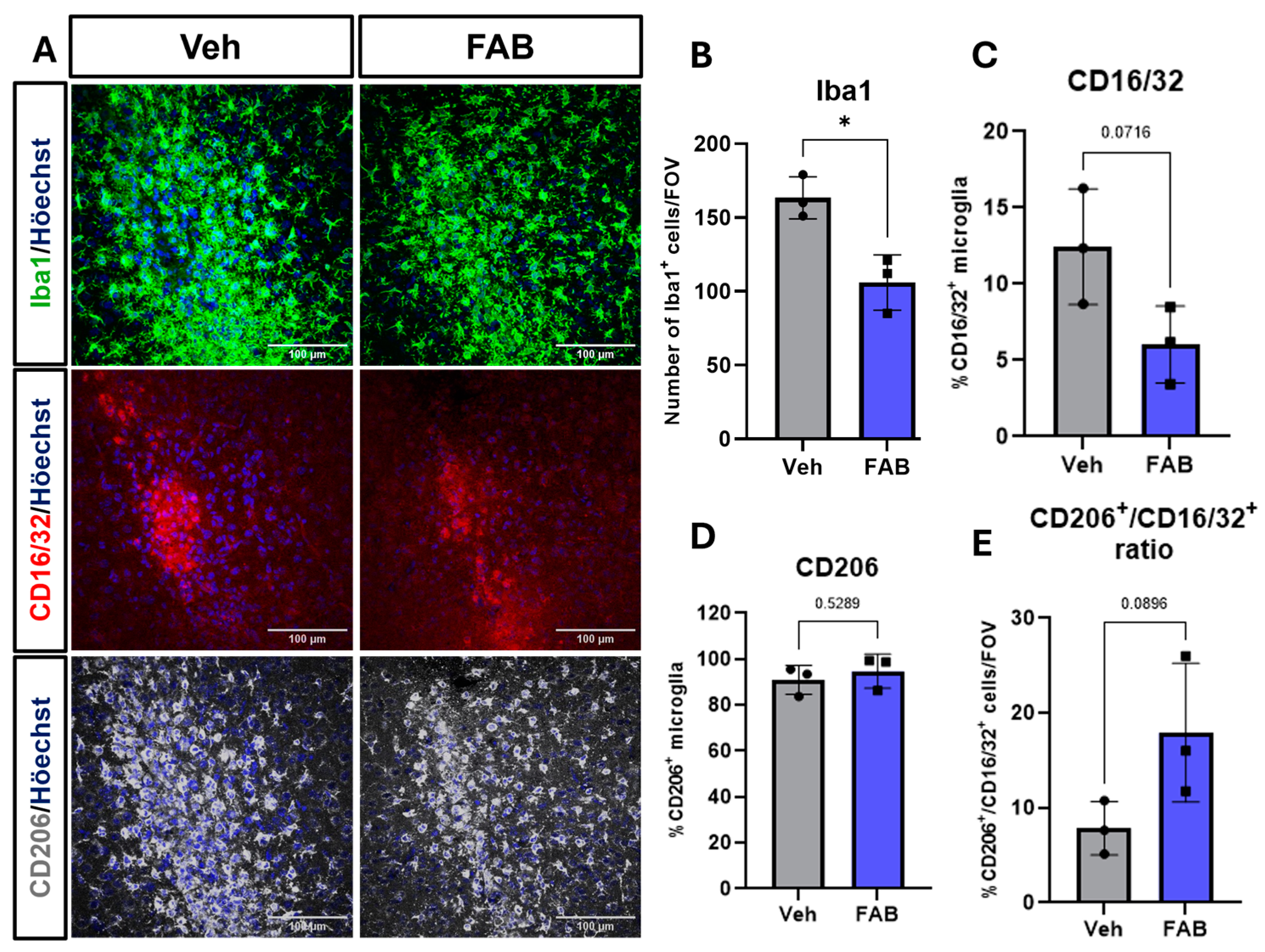
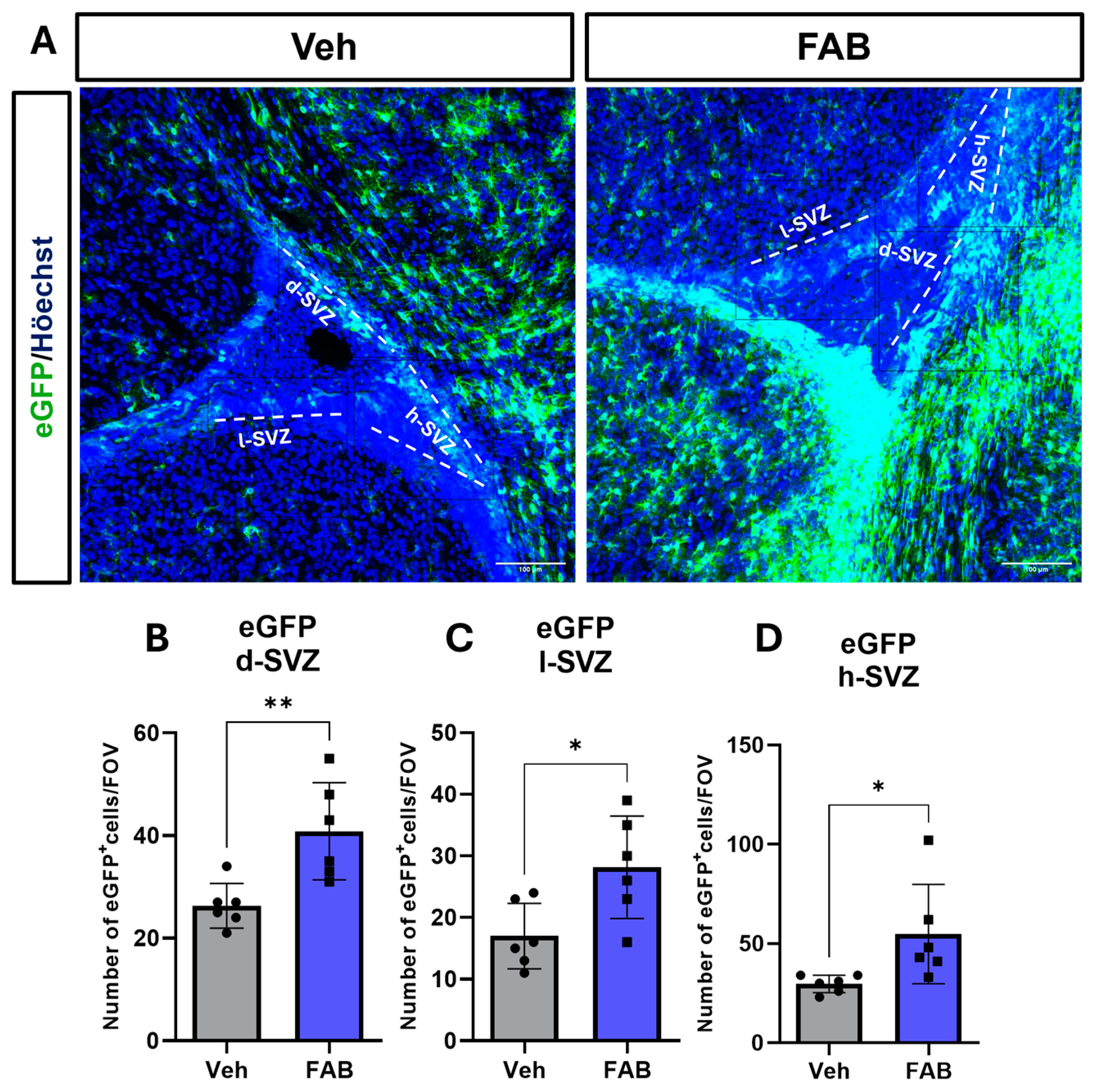
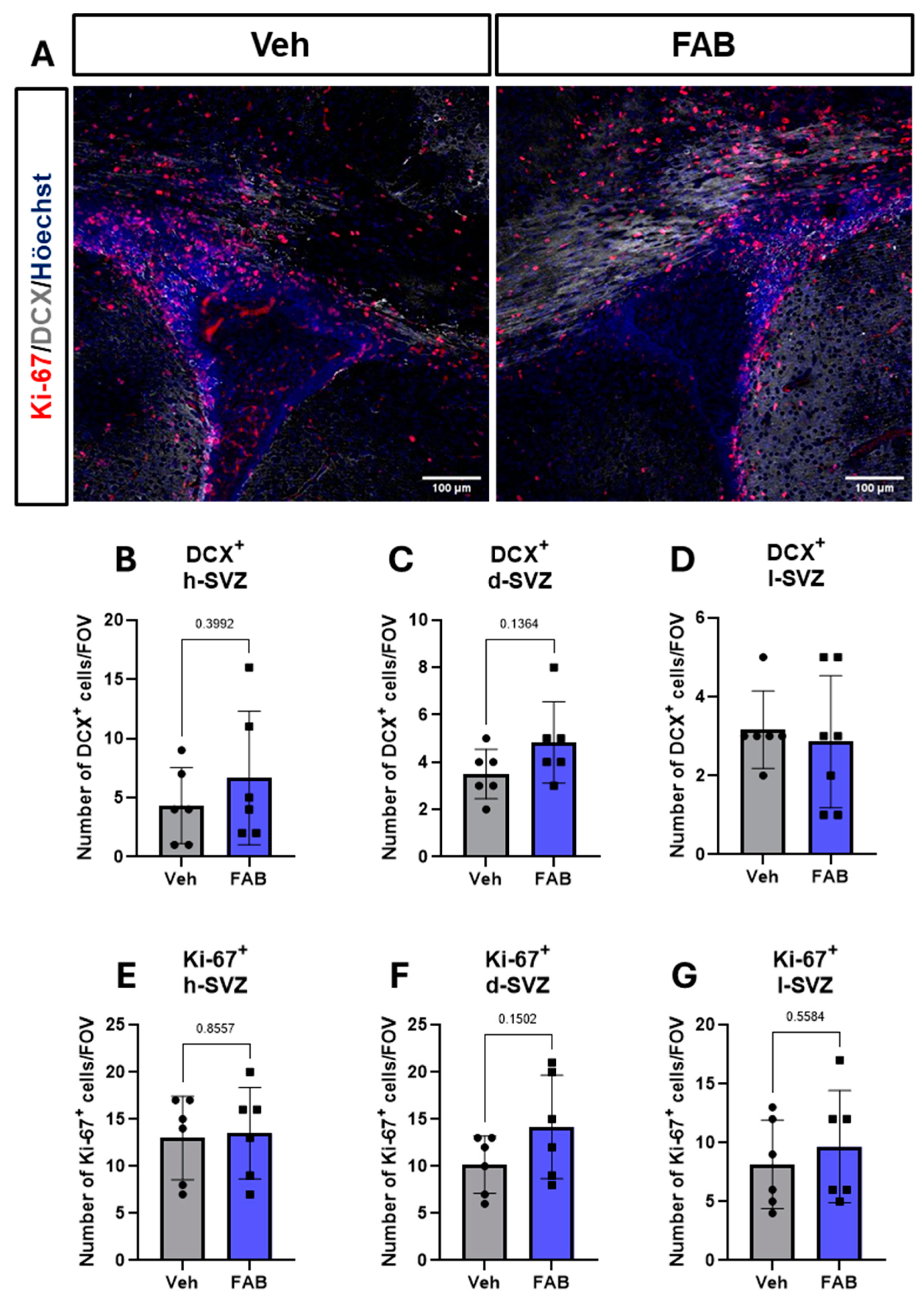
3. Results
Agathisflavone Modulates Reactive Gliosis in the Mouse Lesioned Cortex
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Postolache, T.T.; Wadhawan, A.; Can, A.; Lowry, C.A.; Woodbury, M.; Makkar, H.; Hoisington, A.J.; Scott, A.J.; Potocki, E.; Benros, M.E.; et al. Inflammation in traumatic brain injury. J. Alzheimer’s Dis. 2020, 74, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Amlerova, Z.; Chmelova, M.; Anderova, M.; Vargova, L. Reactive gliosis in traumatic brain injury: A comprehensive review. Front. Cell. Neurosci. 2024, 18, 1335849. [Google Scholar] [CrossRef]
- Pekny, M.; Wilhelmsson, U.; Pekna, M. The dual role of astrocyte activation and reactive gliosis. Neurosci. Lett. 2014, 565, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Pekny, M.; Wilhelmsson, U.; Tatlisumak, T.; Pekna, M. Astrocyte activation and reactive gliosis—A new target in stroke? Neurosci. Lett. 2019, 689, 45–55. [Google Scholar] [CrossRef]
- Hibbits, N.; Yoshino, J.; Le, T.Q.; Armstrong, R.C. Astrogliosis during acute and chronic cuprizone demyelination and implications for remyelination. ASN Neuro 2012, 4, 393–408. [Google Scholar] [CrossRef] [PubMed]
- Levine, J. The reactions and role of NG2 glia in spinal cord injury. Brain Res. 2016, 1638, 199–208. [Google Scholar] [CrossRef]
- Adams, K.L.; Gallo, V. The diversity and disparity of the glial scar. Nat. Neurosci. 2018, 21, 9–15. [Google Scholar] [CrossRef]
- Burda, J.E.; Sofroniew, M.V. Reactive Gliosis and the Multicellular Response to CNS Damage and Disease. Neuron 2014, 81, 229–248. [Google Scholar] [CrossRef]
- Bellver-Landete, V.; Bretheau, F.; Mailhot, B.; Vallières, N.; Lessard, M.; Janelle, M.-E.; Vernoux, N.; Tremblay, M.-È.; Fuehrmann, T.; Shoichet, M.S.; et al. Microglia are an essential component of the neuroprotective scar that forms after spinal cord injury. Nat. Commun. 2019, 10, 518. [Google Scholar] [CrossRef]
- Donat, C.K.; Scott, G.; Gentleman, S.M.; Sastre, M. Microglial Activation in Traumatic Brain Injury. Front. Aging Neurosci. 2017, 9, 208. [Google Scholar] [CrossRef]
- O’Shea, T.M.; Burda, J.E.; Sofroniew, M.V. Cell biology of spinal cord injury and repair. J. Clin. Invest. 2017, 127, 3259–3270. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Liu, X.; Chen, Z. Glial Scar—A Promising Target for Improving Outcomes After CNS Injury. J. Mol. Neurosci. 2019, 70, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Tani, J.; Wen, Y.T.; Hu, C.J.; Sung, J.Y. Current and Potential Pharmacologic Therapies for Traumatic Brain Injury. Pharmaceuticals 2022, 15, 838. [Google Scholar] [CrossRef]
- Clifford, T.; Finkel, Z.; Rodriguez, B.; Joseph, A.; Cai, L. Current Advancements in Spinal Cord Injury Research—Glial Scar Formation and Neural Regeneration. Cells 2023, 12, 853. [Google Scholar] [CrossRef]
- Mendes, C.C.; Bahia, M.V.; David, J.M.; David, J.P. Constituents of Caesalpinia pyramidalis. Fitoterapia 2000, 71, 205–207. [Google Scholar] [CrossRef]
- Islam, M.T.; Zihad, S.M.N.K.; Rahman, M.S.; Sifat, N.; Khan, M.R.; Uddin, S.J.; Rouf, R. Agathisflavone: Botanical sources, therapeutic promises, and molecular docking study. IUBMB Life 2019, 71, 1192–1200. [Google Scholar] [CrossRef] [PubMed]
- De Amorim, V.C.M.; Júnior, M.S.O.; da Silva, A.B.; David, J.M.; David, J.P.L.; de Fátima Dias Costa, M.; Butt, A.M.; da Silva, V.D.A.; Costa, S.L. Agathisflavone modulates astrocytic responses and increases the population of neurons in an in vitro model of traumatic brain injury. Naunyn. Schmiedebergs Arch. Pharmacol. 2020, 393, 1921–1930. [Google Scholar] [CrossRef]
- Dos Santos, B.L.; dos Santos, C.C.; Soares, J.R.P.; da Silva, K.C.; de Oliveira, J.V.R.; Pereira, G.S.; de Araújo, F.M.; de Fatima D. Costa, M.; David, J.M.; da Silva, V.D.A.; et al. The Flavonoid Agathisflavone Directs Brain Microglia/Macrophages to a Neuroprotective Anti-Inflammatory and Antioxidant State via Regulation of NLRP3 Inflammasome. Pharmaceutics 2023, 15, 1410. [Google Scholar] [CrossRef]
- De Almeida, M.M.A.; dos Santos Souza, C.; Dourado, N.S.; da Silva, A.B.; Ferreira, R.S.; David, J.M.; David, J.P.; Costa, M.d.F.D.; da Silva, V.D.A.; Butt, A.M.; et al. Phytoestrogen agathisflavone ameliorates neuroinflammation-induced by LPS and IL-1β and protects neurons in cocultures of glia/neurons. Biomolecules 2020, 10, 562. [Google Scholar] [CrossRef]
- De Almeida, M.M.A.; Pieropan, F.; de Mattos Oliveira, L.; dos Santos Junior, M.C.; David, J.M.; David, J.P.; da Silva, V.D.A.; dos Santos Souza, C.; Costa, S.L.; Butt, A.M. The flavonoid agathisflavone modulates the microglial neuroinflammatory response and enhances remyelination. Pharmacol. Res. 2020, 159, 104997. [Google Scholar] [CrossRef]
- Dos Santos Souza, C.; Grangeiro, M.S.; Lima Pereira, E.P.; dos Santos, C.C.; da Silva, A.B.; Sampaio, G.P.; Ribeiro Figueiredo, D.D.; David, J.M.; David, J.P.; da Silva, V.D.A.; et al. Agathisflavone, a flavonoid derived from Poincianella pyramidalis (Tul.), enhances neuronal population and protects against glutamate excitotoxicity. Neurotoxicology 2018, 65, 85–97. [Google Scholar] [CrossRef]
- Do Nascimento, R.P.; de Jesus, L.B.; Oliveira-Junior, M.S.; Almeida, A.M.; Moreira, E.L.T.; Paredes, B.D.; David, J.M.; Souza, B.S.F.; de Fátima, D.; Costa, M.; et al. Agathisflavone as a Single Therapy or in Association With Mesenchymal Stem Cells Improves Tissue Repair in a Spinal Cord Injury Model in Rats. Front. Pharmacol. 2022, 13, 858190. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, B.S.; Souza, C.S.; Chicaybam, L.; Bonamino, M.H.; Bahia, M.; Costa, S.L.; Borges, H.L.; Rehen, S.K. Agathisflavone enhances retinoic acid-induced neurogenesis and its receptors α and β in pluripotent stem cells. Stem Cells Dev. 2011, 20, 1711–1721. [Google Scholar] [CrossRef]
- Lalo, U.; Pankratov, Y.; Kirchhoff, F.; North, R.A.; Verkhratsky, A. NMDA receptors mediate neuron-to-glia signaling in mouse cortical astrocytes. J. Neurosci. 2006, 26, 2673–2683. [Google Scholar] [CrossRef]
- Azim, K.; Raineteau, O.; Butt, A.M. Intraventricular injection of FGF-2 promotes generation of oligodendrocyte-lineage cells in the postnatal and adult forebrain. Glia 2012, 60, 1977–1990. [Google Scholar] [CrossRef]
- Azim, K.; Rivera, A.; Raineteau, O.; Butt, A.M. GSK3β regulates oligodendrogenesis in the dorsal microdomain of the subventricular zone via Wnt-β-catenin signaling. Glia 2014, 62, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Wang, H.; Yin, Y. Microglia Polarization From M1 to M2 in Neurodegenerative Diseases. Front. Aging Neurosci. 2022, 14, 815347. [Google Scholar] [CrossRef]
- Marangon, D.; Castro e Silva, J.H.; Cerrato, V.; Boda, E.; Lecca, D. Oligodendrocyte Progenitors in Glial Scar: A Bet on Remyelination. Cells 2024, 13, 1024. [Google Scholar] [CrossRef]
- Chen, Y.; Peng, F.; Xing, Z.; Chen, J.; Peng, C.; Li, D. Beneficial effects of natural flavonoids on neuroinflammation. Front. Immunol. 2022, 13, 1006434. [Google Scholar] [CrossRef]
- Costa, S.L.; Silva, V.D.A.; Dos Santos Souza, C.; Santos, C.C.; Paris, I.; Muñoz, P.; Segura-Aguilar, J. Impact of Plant-Derived Flavonoids on Neurodegenerative Diseases. Neurotox. Res. 2016, 30, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.L.; Bi, C.W.C.; Choi, R.C.Y.; Zhu, K.Y.; Miernisha, A.; Dong, T.T.X.; Tsim, K.W.K. Flavonoids induce the synthesis and secretion of neurotrophic factors in cultured rat astrocytes: A signaling response mediated by estrogen receptor. Evidence-Based Complement. Altern. Med. 2013, 2013, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Davinelli, S.; Medoro, A.; Ali, S.; Passarella, D.; Intrieri, M.; Scapagnini, G. Dietary Flavonoids and Adult Neurogenesis: Potential Implications for Brain Aging. Curr. Neuropharmacol. 2023, 21, 651–668. [Google Scholar] [CrossRef] [PubMed]
- Cichon, N.; Saluk-Bijak, J.; Gorniak, L.; Przyslo, L.; Bijak, M. Flavonoids as a Natural Enhancer of Neuroplasticity—An Overview of the Mechanism of Neurorestorative Action. Antioxidants 2020, 9, 1035. [Google Scholar] [CrossRef]
- Escartin, C.; Galea, E.; Lakatos, A.; O’Callaghan, J.P.; Petzold, G.C.; Serrano-Pozo, A.; Steinhäuser, C.; Volterra, A.; Carmignoto, G.; Agarwal, A.; et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat. Neurosci. 2021, 24, 312–325. [Google Scholar] [CrossRef]
- Yang, T.; Dai, Y.; Chen, G.; Cui, S. Dissecting the Dual Role of the Glial Scar and Scar-Forming Astrocytes in Spinal Cord Injury. Front. Cell. Neurosci. 2020, 14, 78. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Barres, B.A. Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity 2017, 46, 957–967. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Butt, A.; Li, B.; Illes, P.; Zorec, R.; Semyanov, A.; Tang, Y.; Sofroniew, M.V. Astrocytes in human central nervous system diseases: A frontier for new therapies. Signal Transduct. Target. Ther. 2023, 8, 1–37. [Google Scholar] [CrossRef]
- Woodburn, S.C.; Bollinger, J.L.; Wohleb, E.S. The semantics of microglia activation: Neuroinflammation, homeostasis, and stress. J. Neuroinflammation 2021, 18, 258. [Google Scholar] [CrossRef]
- Schreiner, T.G.; Schreiner, O.D.; Ciobanu, R.C. Spinal Cord Injury Management Based on Microglia-Targeting Therapies. J. Clin. Med. 2024, 13, 2773. [Google Scholar] [CrossRef]
- Tang, Y.; Le, W. Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol. Neurobiol. 2016, 53, 1181–1194. [Google Scholar] [CrossRef] [PubMed]
- Kigerl, K.A.; Gensel, J.C.; Ankeny, D.P.; Alexander, J.K.; Donnelly, D.J.; Popovich, P.G. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J. Neurosci. 2009, 29, 13435–13444. [Google Scholar] [CrossRef] [PubMed]
- Jurga, A.M.; Paleczna, M.; Kuter, K.Z. Overview of General and Discriminating Markers of Differential Microglia Phenotypes. Front. Cell. Neurosci. 2020, 14, 198. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, H.G.; Toda, T.; Gage, F.H. Adult hippocampal neurogenesis: A coming-of-age story. J. Neurosci. 2018, 38, 10401–10410. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.A.; Alvarez-Buylla, A. The adult ventricular–subventricular zone (V-SVZ) and olfactory bulb (OB) neurogenesis. Cold Spring Harb. Perspect. Biol. 2016, 8, a018820. [Google Scholar] [CrossRef]
- Menn, B.; Garcia-Verdugo, J.M.; Yaschine, C.; Gonzalez-Perez, O.; Rowitch, D.; Alvarez-Buylla, A. Origin of oligodendrocytes in the subventricular zone of the adult brain. J. Neurosci. 2006, 26, 7907–7918. [Google Scholar] [CrossRef]
- Platel, J.C.; Bordey, A. The multifaceted subventricular zone astrocyte: From a metabolic and pro-neurogenic role to acting as a neural stem cell. Neuroscience 2016, 323, 20–28. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro e Silva, J.H.; Pieropan, F.; Rivera, A.D.; Butt, A.M.; Costa, S.L. Agathisflavone Modulates Reactive Gliosis After Trauma and Increases the Neuroblast Population at the Subventricular Zone. Nutrients 2024, 16, 4053. https://doi.org/10.3390/nu16234053
Castro e Silva JH, Pieropan F, Rivera AD, Butt AM, Costa SL. Agathisflavone Modulates Reactive Gliosis After Trauma and Increases the Neuroblast Population at the Subventricular Zone. Nutrients. 2024; 16(23):4053. https://doi.org/10.3390/nu16234053
Chicago/Turabian StyleCastro e Silva, Juliana Helena, Francesca Pieropan, Andrea Domenico Rivera, Arthur Morgan Butt, and Silvia Lima Costa. 2024. "Agathisflavone Modulates Reactive Gliosis After Trauma and Increases the Neuroblast Population at the Subventricular Zone" Nutrients 16, no. 23: 4053. https://doi.org/10.3390/nu16234053
APA StyleCastro e Silva, J. H., Pieropan, F., Rivera, A. D., Butt, A. M., & Costa, S. L. (2024). Agathisflavone Modulates Reactive Gliosis After Trauma and Increases the Neuroblast Population at the Subventricular Zone. Nutrients, 16(23), 4053. https://doi.org/10.3390/nu16234053






