The Interactions Between Diet and Gut Microbiota in Preventing Gestational Diabetes Mellitus: A Narrative Review
Abstract
1. Introduction
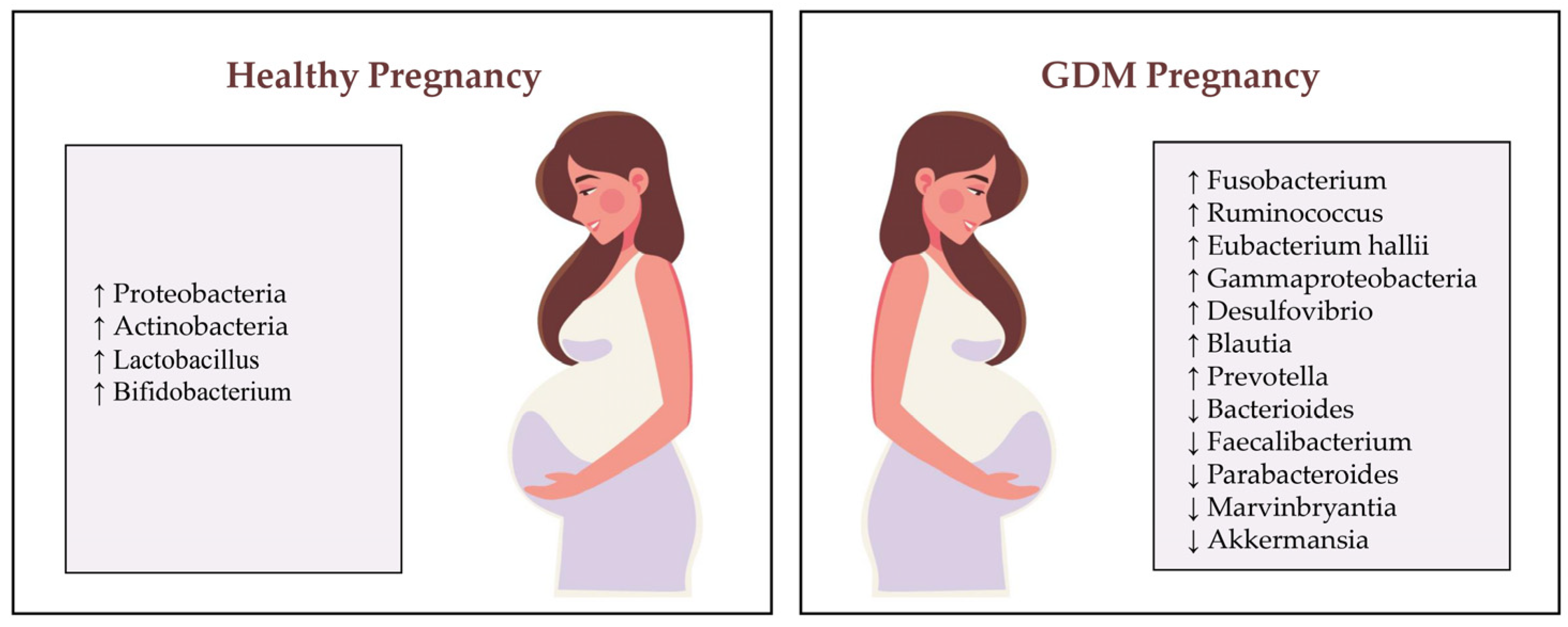
2. Pathophysiology of GDM and the Role of Gut Microbiota
3. Dietary Modulation of Gut Microbiota and Its Impact on Glucose Metabolism
3.1. Dietary Patterns and Gut Microbiota
3.2. Mechanisms of Dietary Modulation
3.3. Nutritional Interventions in Pregnancy
4. Interactions Between Diet, Gut Microbiota and Gestational Diabetes Mellitus Prevention
5. Clinical Evidence on Diet, Gut Microbiota and GDM
6. Future Directions and Personalized Approaches
6.1. Potential for Personalized Nutrition
6.2. Emerging Microbiota-Based Therapies
6.3. Gaps in Research
Author Contributions
Funding
Conflicts of Interest
References
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2020. Diabetes Care 2020, 43 (Suppl. S1), S14–S31. [Google Scholar] [CrossRef]
- Vounzoulaki, E.; Khunti, K.; Abner, S.C.; Tan, B.K.; Davies, M.J.; Gillies, C.L. Progression to type 2 diabetes in women with a known history of gestational diabetes: Systematic review and meta-analysis. BMJ 2020, 369, m1361. [Google Scholar] [CrossRef] [PubMed]
- Scholtens, D.M.; Kuang, A.; Lowe, L.P.; Hamilton, J.; Lawrence, J.M.; Lebenthal, Y.; Brickman, W.J.; Clayton, P.; Ma, R.C.; McCance, D.; et al. HAPO Follow-Up Study Cooperative Research Group. Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study (HAPO FUS): Maternal Glycemia and Childhood Glucose Metabolism. Diabetes Care 2019, 42, 381–392. [Google Scholar] [CrossRef]
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021; Available online: https://diabetesatlas.org/atlas/tenth-edition/ (accessed on 1 October 2024).
- Vladu, I.M.; Clenciu, D.; Mitrea, A.; Amzolini, A.; Micu, S.E.; Crisan, A.E.; Efrem, I.C.; Fortofoiu, M.; Fortofoiu, M.C.; Mita, A.; et al. Maternal and Fetal Metabolites in Gestational Diabetes Mellitus: A Narrative Review. Metabolites 2022, 12, 383. [Google Scholar] [CrossRef] [PubMed]
- Hartling, L.; Dryden, D.M.; Guthrie, A.; Muise, M.; Vandermeer, B.; Aktary, W.M.; Pasichnyk, D.; Seida, J.C.; Donovan, L. Screening and diagnosing gestational diabetes mellitus. Evid. Rep. Technol. Assess. Full Rep. 2012, 210, 1–327. [Google Scholar]
- Mor, G.; Cardenas, I. The immune system in pregnancy: A unique complexity. Am. J. Reprod. Immunol. 2010, 63, 425–433. [Google Scholar] [CrossRef]
- Plows, J.F.; Stanley, J.L.; Baker, P.N.; Reynolds, C.M.; Vickers, M.H. The Pathophysiology of Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2018, 19, 3342. [Google Scholar] [CrossRef]
- Kunz, C.; Kuntz, S.; Rudloff, S. Intestinal flora. Adv. Exp. Med. Biol. 2009, 639, 67–79. [Google Scholar] [CrossRef]
- Clarke, G.; Stilling, R.M.; Kennedy, P.J.; Stanton, C.; Cryan, J.F.; Dinan, T.G. Minireview: Gut microbiota: The neglected endocrine organ. Mol. Endocrinol. 2014, 28, 1221–1238. [Google Scholar] [CrossRef]
- Dualib, P.M.; Ogassavara, J.; Mattar, R.; da Silva, E.M.; Dib, S.A.; de Almeida Pititto, B. Gut microbiota and gestational diabetes mellitus: A systematic review. Diabetes Res. Clin. Pract. 2021, 180, 109078. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, T.A.; Xiang, A.H. Gestational diabetes mellitus. J. Clin. Investig. 2005, 115, 485–491. [Google Scholar] [CrossRef]
- Sebastián Domingo, J.J.; Sánchez Sánchez, C. From the intestinal flora to the microbiome. Rev. Esp. Enferm. Dig. 2018, 110, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Catalano, P.M.; Ehrenberg, H.M. The short-and long-term implications of maternal obesity on the mother and her offspring. BJOG 2006, 113, 1126–1133. [Google Scholar] [CrossRef]
- Butte, N.F. Carbohydrate and lipid metabolism in pregnancy: Normal compared with gestational diabetes mellitus. Am. J. Clin. Nutr. 2000, 71, 1256S–1261S. [Google Scholar] [CrossRef]
- Barbour, L.A.; McCurdy, C.E.; Hernandez, T.L.; Kirwan, J.P.; Catalano, P.M.; Friedman, J.E. Cellular mechanisms for insulin resistance in normal pregnancy and gestational diabetes. Diabetes Care 2007, 30 (Suppl. S2), S112–S119. [Google Scholar] [CrossRef]
- Catalano, P.M.; Hauguel-De Mouzon, S. Is it time to revisit the Pedersen hypothesis in the face of the obesity epidemic? Am. J. Obs. Gynecol. 2011, 204, 479–487. [Google Scholar] [CrossRef]
- Ryan, E.A.; Enns, L. Role of gestational hormones in the induction of insulin resistance. J. Clin. Endocrinol. Metab. 1988, 67, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Preda, A.; Preda, S.D.; Mota, M.; Iliescu, D.G.; Zorila, L.G.; Comanescu, A.C.; Mitrea, A.; Clenciu, D.; Mota, E.; Vladu, I.M. Dyslipidemia in Pregnancy: A Systematic Review of Molecular Alterations and Clinical Implications. Biomedicines 2024, 12, 2252. [Google Scholar] [CrossRef] [PubMed]
- Radaelli, T.; Varastehpour, A.; Catalano, P.; Hauguel-De Mouzon, S. Gestational diabetes induces placental genes for chronic stress and inflammatory pathways. Diabetes 2003, 52, 2951–2958. [Google Scholar] [CrossRef] [PubMed]
- Kirwan, J.P.; Hauguel-De Mouzon, S.; Lepercq, J.; Challier, J.C.; Huston-Presley, L.; Friedman, J.E.; Kalhan, S.C.; Catalano, P.M. TNF-α is a predictor of insulin resistance in human pregnancy. Diabetes 2002, 51, 2207–2213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Ju, Z.; Zuo, T. Time for food: The impact of diet on gut microbiota and human health. Nutrition 2018, 51–52, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Gill, P.A.; van Zelm, M.C.; Muir, J.G.; Gibson, P.R. Review article: Short chain fatty acids as potential therapeutic agents in human gastrointestinal and inflammatory disorders. Aliment. Pharmacol. Ther. 2018, 48, 15–34. [Google Scholar] [CrossRef]
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Delzenne, N.M. The role of the gut microbiota in energy metabolism and metabolic disease. Curr. Pharm. Des. 2009, 15, 1546–1558. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Price, J.; Abu-Ali, G.; Huttenhower, C. The healthy human microbiome. Genome Med. 2016, 8, 51. [Google Scholar] [CrossRef]
- Cotillard, A.; Kennedy, S.P.; Kong, L.C.; Prifti, E.; Pons, N.; Le Chatelier, E.; Almeida, M.; Quinquis, B.; Levenez, F.; Galleron, N.; et al. Dietary intervention impact on gut microbial gene richness. Nature 2013, 500, 585–588. [Google Scholar] [CrossRef] [PubMed]
- Koren, O.; Goodrich, J.K.; Cullender, T.C.; Spor, A.; Laitinen, K.; Bäckhed, H.K.; Gonzalez, A.; Werner, J.J.; Angenent, L.T.; Knight, R.; et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 2012, 150, 470–480. [Google Scholar] [CrossRef]
- Crudele, L.; Gadaleta, R.M.; Cariello, M.; Moschetta, A. Gut microbiota in the pathogenesis and therapeutic approaches of diabetes. eBioMedicine 2023, 97, 104821. [Google Scholar] [CrossRef]
- Kunasegaran, T.; Balasubramaniam, V.R.M.T.; Arasoo, V.J.T.; Palanisamy, U.D.; Ramadas, A. The Modulation of Gut Microbiota Composition in the Pathophysiology of Gestational Diabetes Mellitus: A Systematic Review. Biology 2021, 10, 1027. [Google Scholar] [CrossRef]
- Roberfroid, M.B. Prebiotics and synbiotics: Concepts and nutritional properties. Br. J. Nutr. 1998, 80, S197–S202. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, J.; Shi, W.; Du, N.; Xu, X.; Zhang, Y.; Ji, P.; Zhang, F.; Jia, Z.; Wang, Y.; et al. Dysbiosis of maternal and neonatal microbiota associated with gestational diabetes mellitus. Gut 2018, 67, 1614–1625. [Google Scholar] [CrossRef] [PubMed]
- Crusell, M.K.W.; Hansen, T.H.; Nielsen, T.; Allin, K.H.; Rühlemann, M.C.; Damm, P.; Vestergaard, H.; Rørbye, C.; Jørgensen, N.R.; Christiansen, O.B.; et al. Gestational diabetes is associated with change in the gut microbiota composition in third trimester of pregnancy and postpartum. Microbiome 2018, 6, 89. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Zhang, L.; Wang, M.; Chen, Y.; Gu, S.; Wang, K.; Leng, J.; Gu, Y.; Xie, X. The Gut Microbiota in Women Suffering from Gestational Diabetes Mellitus with the Failure of Glycemic Control by Lifestyle Modification. J. Diabetes Res. 2019, 2019, 6081248. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, M.; Zhang, J.; Sun, Z.; Ran, L.; Ban, Y.; Wang, B.; Hou, X.; Zhai, S.; Ren, L.; et al. Differential intestinal and oral microbiota features associated with gestational diabetes and maternal inflammation. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E247–E253. [Google Scholar] [CrossRef]
- Cortez, R.V.; Taddei, C.R.; Sparvoli, L.G.; Ângelo, A.G.S.; Padilha, M.; Mattar, R.; Daher, S. Microbiome and its relation to gestational diabetes. Endocrine 2019, 64, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.T.; Davis-Richardson, A.G.; Giongo, A.; Gano, K.A.; Crabb, D.B.; Mukherjee, N.; Casella, G.; Drew, J.C.; Ilonen, J.; Knip, M.; et al. Gut microbiome metagenomics analysis suggests a functional model for the development of autoimmunity for type 1 diabetes. PLoS ONE 2011, 6, e25792. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, H.K.; Gudmundsdottir, V.; Nielsen, H.B.; Hyotylainen, T.; Nielsen, T.; Jensen, B.A.; Forslund, K.; Hildebrand, F.; Prifti, E.; Falony, G.; et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 2016, 535, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; Molinaro, A.; Ståhlman, M.; Khan, M.T.; Schmidt, C.; Mannerås-Holm, L.; Wu, H.; Carreras, A.; Jeong, H.; Olofsson, L.E.; et al. Microbially Produced Imidazole Propionate Impairs Insulin Signaling through mTORC1. Cell 2018, 175, 947–961.e17. [Google Scholar] [CrossRef] [PubMed]
- Rivière, A.; Selak, M.; Lantin, D.; Leroy, F.; De Vuyst, L. Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front. Microbiol. 2016, 7, 979. [Google Scholar] [CrossRef]
- Donohoe, D.R.; Garge, N.; Zhang, X.; Sun, W.; O’Connell, T.M.; Bunger, M.K.; Bultman, S.J. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011, 13, 517–526. [Google Scholar] [CrossRef]
- Chriett, S.; Zerzaihi, O.; Vidal, H.; Pirola, L. The histone deacetylase inhibitor sodium butyrate improves insulin signalling in palmitate-induced insulin resistance in L6 rat muscle cells through epigenetically-mediated up-regulation of Irs1. Mol. Cell. Endocrinol. 2017, 439, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Jena, G. The role of butyrate, a histone deacetylase inhibitor in diabetes mellitus: Experimental evidence for therapeutic intervention. Epigenomics 2015, 7, 669–680. [Google Scholar] [CrossRef]
- Engels, C.; Ruscheweyh, H.J.; Beerenwinkel, N.; Lacroix, C.; Schwab, C. The Common Gut Microbe Eubacterium hallii also Contributes to Intestinal Propionate Formation. Front. Microbiol. 2016, 7, 713. [Google Scholar] [CrossRef]
- Zhang, J.; Sturla, S.; Lacroix, C.; Schwab, C. Gut Microbial Glycerol Metabolism as an Endogenous Acrolein Source. mBio 2018, 9, e01947-17. [Google Scholar] [CrossRef]
- Lappas, M.; Hiden, U.; Desoye, G.; Froehlich, J.; Hauguel-de Mouzon, S.; Jawerbaum, A. The role of oxidative stress in the pathophysiology of gestational diabetes mellitus. Antioxid. Redox Signal. 2011, 15, 3061–3100. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, P.; Bobe, A.; Dolan, K.; Leone, V.; Martinez, K. Nutritional modulation of gut microbiota—The impact on metabolic disease pathophysiology. J. Nutr. Biochem. 2016, 28, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Kant, A.K. Dietary patterns and health outcomes. J. Am. Diet. Assoc. 2004, 104, 615–635. [Google Scholar] [CrossRef]
- Choi, Y.; Hoops, S.L.; Thoma, C.J.; Johnson, A.J. A guide to dietary pattern–microbiome data integration. J. Nutr. 2022, 152, 1187–1199. [Google Scholar] [CrossRef] [PubMed]
- Neuhouser, M.L. The importance of healthy dietary patterns in chronic disease prevention. Nutr. Res. 2019, 70, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Allam-Ndoul, B.; Castonguay-Paradis, S.; Veilleux, A. Gut microbiota and intestinal trans-epithelial permeability. Int. J. Mol. Sci. 2020, 21, 6402. [Google Scholar] [CrossRef] [PubMed]
- Sender, R.; Fuchs, S.; Milo, R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef]
- Penders, J.; Thijs, C.; Vink, C.; Stelma, F.F.; Snijders, B.; Kummeling, I.; van den Brandt, P.A.; Stobberingh, E.E. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics 2006, 118, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; Carnielli, V.P.; Ksiazyk, J.; Luna, M.S.; Migacheva, N.; Mosselmans, J.M.; Picaud, J.C.; Possner, M.; Singhal, A.; Wabitsch, M. Factors affecting early-life intestinal microbiota development. Nutrition 2020, 78, 110812. [Google Scholar] [CrossRef]
- Roswall, J.; Olsson, L.M.; Kovatcheva-Datchary, P.; Nilsson, S.; Tremaroli, V.; Simon, M.C.; Kiilerich, P.; Akrami, R.; Krämer, M.; Uhlén, M.; et al. Developmental trajectory of the healthy human gut microbiota during the first 5 years of life. Cell Host Microbe 2021, 29, 765–776. [Google Scholar] [CrossRef]
- Perler, B.K.; Friedman, E.S.; Wu, G.D. The role of the gut microbiota in the relationship between diet and human health. Annu. Rev. Physiol. 2023, 85, 449–468. [Google Scholar] [CrossRef] [PubMed]
- Neuman, H.; Debelius, J.W.; Knight, R.; Koren, O. Microbial endocrinology: The interplay between the microbiota and the endocrine system. FEMS Microbiol. Rev. 2015, 39, 509–521. [Google Scholar] [CrossRef]
- Haiser, H.J.; Gootenberg, D.B.; Chatman, K.; Sirasani, G.; Balskus, E.P.; Turnbaugh, P.J. Predicting and manipulating cardiac drug inactivation by the human gut bacterium Eggerthella lenta. Science 2013, 341, 295–298. [Google Scholar] [CrossRef]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell 2016, 167, 1339–1353. [Google Scholar] [CrossRef] [PubMed]
- Malesza, I.J.; Malesza, M.; Walkowiak, J.; Mussin, N.; Walkowiak, D.; Aringazina, R.; Bartkowiak-Wieczorek, J.; Mądry, E. High-fat, western-style diet, systemic inflammation, and gut microbiota: A narrative review. Cells 2021, 10, 3164. [Google Scholar] [CrossRef] [PubMed]
- Patterson, E.; Ryan, P.M.; Cryan, J.F.; Dinan, T.G.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Gut microbiota, obesity and diabetes. Postgrad. Med. J. 2016, 92, 286–300. [Google Scholar] [CrossRef] [PubMed]
- Carrera-Bastos, P.; Fontes-Villalba, M.; O’Keefe, J.H.; Lindeberg, S.; Cordain, L. The western diet and lifestyle and diseases of civilization. Res. Rep. Clin. Cardiol. 2011, 2011, 15–35. [Google Scholar] [CrossRef]
- Thomson, C.; Garcia, A.L.; Edwards, C.A. Interactions between dietary fibre and the gut microbiota. Proc. Nutr. Soc. 2021, 80, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Satokari, R. High Intake of Sugar and the Balance between Pro- and Anti-Inflammatory Gut Bacteria. Nutrients 2020, 12, 1348. [Google Scholar] [CrossRef] [PubMed]
- Birkeland, E.; Gharagozlian, S.; Valeur, J.; Aas, A.M. Short-chain fatty acids as a link between diet and cardiometabolic risk: A narrative review. Lipids Health Dis. 2023, 22, 40. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef]
- Pinto, Y.; Frishman, S.; Turjeman, S.; Eshel, A.; Nuriel-Ohayon, M.; Shrossel, O.; Ziv, O.; Walters, W.; Parsonnet, J.; Ley, C.; et al. Gestational diabetes is driven by microbiota-induced inflammation months before diagnosis. Gut 2023, 72, 918–928. [Google Scholar] [CrossRef] [PubMed]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.; Weickert, M.O. The effects of the Mediterranean diet on health and gut microbiota. Nutrients 2023, 15, 2150. [Google Scholar] [CrossRef] [PubMed]
- Beam, A.; Clinger, E.; Hao, L. Effect of diet and dietary components on the composition of the gut microbiota. Nutrients 2021, 13, 2795. [Google Scholar] [CrossRef]
- Florkowski, M.; Abiona, E.; Frank, K.M.; Brichacek, A.L. Obesity-associated inflammation countered by a Mediterranean diet: The role of gut-derived metabolites. Front. Nutr. 2024, 11, 1392666. [Google Scholar] [CrossRef]
- Farràs, M.; Martinez-Gili, L.; Portune, K.; Arranz, S.; Frost, G.; Tondo, M.; Blanco-Vaca, F. Modulation of the Gut Microbiota by Olive Oil Phenolic Compounds: Implications for Lipid Metabolism, Immune System, and Obesity. Nutrients 2020, 12, 2200. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Tohumcu, E.; Raoul, P.; Fiorani, M.; Cintoni, M.; Mele, M.C.; Cammarota, G.; Gasbarrini, A.; Ianiro, G. The role of diet in shaping human gut microbiota. Best Pract. Clin. Gastroenterol. 2023, 62, 101828. [Google Scholar] [CrossRef]
- Moreira de Gouveia, M.I.; Bernalier-Donadille, A.; Jubelin, G. Enterobacteriaceae in the Human Gut: Dynamics and Ecological Roles in Health and Disease. Biology 2024, 13, 142. [Google Scholar] [CrossRef]
- Mudgil, D.; Barak, S. Composition, properties and health benefits of indigestible carbohydrate polymers as dietary fiber: A review. Int. J. Biol. Macromol. 2013, 61, 1–6. [Google Scholar] [CrossRef]
- Blaut, M. Gut microbiota and energy balance: Role in obesity. Proc. Nutr. Soc. 2015, 74, 227–234. [Google Scholar] [CrossRef]
- Hernández, M.A.G.; Canfora, E.E.; Jocken, J.W.E.; Blaak, E.E. The Short-Chain Fatty Acid Acetate in Body Weight Control and Insulin Sensitivity. Nutrients 2019, 11, 1943. [Google Scholar] [CrossRef]
- Hamamah, S.; Amin, A.; Al-Kassir, A.L.; Chuang, J.; Covasa, M. Dietary Fat Modulation of Gut Microbiota and Impact on Regulatory Pathways Controlling Food Intake. Nutrients 2023, 15, 3365. [Google Scholar] [CrossRef]
- Iacob, S.; Iacob, D.G.; Luminos, L.M. Intestinal Microbiota as a Host Defense Mechanism to Infectious Threats. Front. Microbiol. 2019, 9, 3328. [Google Scholar] [CrossRef]
- Scott, K.P.; Gratz, S.W.; Sheridan, P.O.; Flint, H.J.; Duncan, S.H. The influence of diet on the gut microbiota. Pharmacol. Res. 2013, 69, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Portune, K.J.; Beaumont, M.; Davila, A.-M.; Tomé, D.; Blachier, F.; Sanz, Y. Gut microbiota role in dietary protein metabolism and health-related outcomes: The two sides of the coin. Trends Food Sci. Technol. 2016, 57, 213–232. [Google Scholar] [CrossRef]
- Li, H.; Page, A.J. Altered vagal signaling and its pathophysiological roles in functional dyspepsia. Front. Neurosci. 2022, 16, 858612. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, X.; Liu, H.; Brown, M.A.; Qiao, S. Dietary Protein and Gut Microbiota Composition and Function. Curr. Protein Pept. 2019, 20, 145–154. [Google Scholar] [CrossRef]
- Zoetendal, E.G.; Raes, J.; van den Bogert, B.; Arumugam, M.; Booijink, C.C.; Troost, F.J.; Bork, P.; Wels, M.; de Vos, W.M.; Kleerebezem, M. The human small intestinal microbiota is driven by rapid uptake and conversion of simple carbohydrates. ISME J. 2012, 6, 1415–1426. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P. Influence of Foods and Nutrition on the Gut Microbiome and Implications for Intestinal Health. Int. J. Mol. Sci. 2022, 23, 9588. [Google Scholar] [CrossRef]
- Sajdel-Sulkowska, E.M. The Impact of Maternal Gut Microbiota during Pregnancy on Fetal Gut-Brain Axis Development and Life-Long Health Outcomes. Microorganisms 2023, 11, 2199. [Google Scholar] [CrossRef]
- Nighot, M.; Rawat, M.; Al-Sadi, R.; Castillo, E.F.; Nighot, P.; Ma, T.Y. Lipopolysaccharide-induced increase in intestinal permeability is mediated by TAK-1 activation of IKK and MLCK/MYLK gene. Am. J. Pathol. 2019, 189, 797–812. [Google Scholar] [CrossRef]
- Machate, D.J.; Figueiredo, P.S.; Marcelino, G.; Guimarães, R.C.A.; Hiane, P.A.; Bogo, D.; Pinheiro, V.A.Z.; Oliveira, L.C.S.; Pott, A. Fatty Acid Diets: Regulation of Gut Microbiota Composition and Obesity and Its Related Metabolic Dysbiosis. Int. J. Mol. Sci. 2020, 21, 4093. [Google Scholar] [CrossRef] [PubMed]
- Ferenc, K.; Sokal-Dembowska, A.; Helma, K.; Motyka, E.; Jarmakiewicz-Czaja, S.; Filip, R. Modulation of the Gut Microbiota by Nutrition and Its Relationship to Epigenetics. Int. J. Mol. Sci. 2024, 25, 1228. [Google Scholar] [CrossRef]
- Kraiczy, J.; Zilbauer, M. Intestinal epithelial organoids as tools to study epigenetics in gut health and disease. Stem Cells Int. 2019, 2019, 7242415. [Google Scholar] [CrossRef]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef]
- Barker, D.J.; Osmond, C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet 1986, 327, 1077–1081. [Google Scholar] [CrossRef] [PubMed]
- Girard, A.W.; Olude, O. Nutrition education and counselling provided during pregnancy: Effects on maternal, neonatal and child health outcomes. Paediatr. Périnat. Epidemiol. 2012, 26, 191–204. [Google Scholar] [CrossRef]
- Killeen, S.L.; Geraghty, A.A.; O’Brien, E.C.; O’Reilly, S.L.; Yelverton, C.A.; McAuliffe, F.M. Addressing the gaps in nutritional care before and during pregnancy. Proc. Nutr. Soc. 2022, 81, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Preda, A.; Pădureanu, V.; Moța, M.; Ștefan, A.G.; Comănescu, A.C.; Radu, L.; Mazilu, E.R.; Vladu, I.M. Analysis of Maternal and Neonatal Complications in a Group of Patients with Gestational Diabetes Mellitus. Medicina 2021, 57, 1170. [Google Scholar] [CrossRef] [PubMed]
- Cirulli, F.; De Simone, R.; Musillo, C.; Ajmone-Cat, M.A.; Berry, A. Inflammatory signatures of maternal obesity as risk factors for neurodevelopmental disorders: Role of maternal microbiota and nutritional intervention strategies. Nutrients 2022, 14, 3150. [Google Scholar] [CrossRef]
- Cheng, L.; Hu, T.; Shi, H.; Chen, X.; Wang, H.; Zheng, K.; Huang, X.F.; Yu, Y. DHA reduces hypothalamic inflammation and improves central leptin signaling in mice. Life Sci. 2020, 257, 118036. [Google Scholar] [CrossRef]
- Dahl, W.J.; Mendoza, D.R.; Lambert, J.M. Diet, nutrients and the microbiome. Prog. Mol. Biol. Transl. Sci. 2020, 171, 237–263. [Google Scholar] [CrossRef] [PubMed]
- Newman, T.M.; Shively, C.A.; Register, T.C.; Appt, S.E.; Yadav, H.; Colwell, R.R.; Fanelli, B.; Dadlani, M.; Graubics, K.; Nguyen, U.T.; et al. Diet, obesity, and the gut microbiome as determinants modulating metabolic outcomes in a non-human primate model. Microbiome 2021, 9, 100. [Google Scholar] [CrossRef]
- Meslier, V.; Laiola, M.; Roager, H.M.; De Filippis, F.; Roume, H.; Quinquis, B.; Giacco, R.; Mennella, I.; Ferracane, R.; Pons, N.; et al. Mediterranean diet intervention in overweight and obese subjects lowers plasma cholesterol and causes changes in the gut microbiome and metabolome independently of energy intake. Gut 2020, 69, 1258–1268. [Google Scholar] [CrossRef] [PubMed]
- Vasile, F.C.; Preda, A.; Ștefan, A.G.; Vladu, M.I.; Forțofoiu, M.C.; Clenciu, D.; Gheorghe, I.O.; Forțofoiu, M.; Moța, M. An Update of Medical Nutrition Therapy in Gestational Diabetes Mellitus. J. Diabetes Res. 2021, 2021, 5266919. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee. 15. Management of Diabetes in Pregnancy: Standards of Care in Diabetes—2024. Diabetes Care 2024, 47 (Suppl. S1), S282–S294. [Google Scholar] [CrossRef]
- Hernandez, T.L. Carbohydrate Content in the GDM Diet: Two Views: View 1: Nutrition Therapy in Gestational Diabetes: The Case for Complex Carbohydrates. Diabetes Spectr. 2016, 29, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.; Poulsen, C.W.; Kampmann, U.; Smedegaard, S.B.; Ovesen, P.G.; Fuglsang, J. Diet and Healthy Lifestyle in the Management of Gestational Diabetes Mellitus. Nutrients 2020, 12, 3050. [Google Scholar] [CrossRef]
- Canfora, E.E.; Jocken, J.W.; Blaak, E.E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 2015, 11, 577–591. [Google Scholar] [CrossRef]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef]
- Li, T.; Chiang, J.Y. Bile acid signaling in metabolic disease and drug therapy. Pharmacol. Rev. 2014, 66, 948–983. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J. Fiber and prebiotics: Mechanisms and health benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef]
- Lindsay, K.L.; Walsh, C.A.; Brennan, L.; McAuliffe, F.M. Probiotics in pregnancy and maternal outcomes: A systematic review. J. Matern. Fetal Neonatal Med. 2013, 26, 772–778. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.S.; Chambers, E.S.; Morrison, D.J.; Frost, G. The role of short chain fatty acids in appetite regulation and energy homeostasis. Int. J. Obes. 2015, 39, 1331–1338. [Google Scholar] [CrossRef]
- Li, H.; Zhang, L.; Li, J.; Wu, Q.; Qian, L.; He, J.; Ni, Y.; Kovatcheva-Datchary, P.; Yuan, R.; Liu, S.; et al. Resistant starch intake facilitates weight loss in humans by reshaping the gut microbiota. Nat. Metab. 2024, 6, 578–597. [Google Scholar] [CrossRef]
- Golden, J.M.; Escobar, O.H.; Nguyen, M.V.L.; Mallicote, M.U.; Kavarian, P.; Frey, M.R.; Gayer, C.P. Ursodeoxycholic acid protects against intestinal barrier breakdown by promoting enterocyte migration via EGFR- and COX-2-dependent mechanisms. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G259–G271. [Google Scholar] [CrossRef]
- Quintero, P.; Pizarro, M.; Solís, N.; Arab, J.P.; Padilla, O.; Riquelme, A.; Arrese, M. Bile acid supplementation improves established liver steatosis in obese mice independently of glucagon-like peptide-1 secretion. J. Physiol. Biochem. 2014, 70, 667–674. [Google Scholar] [CrossRef]
- Nie, B.; Park, H.M.; Kazantzis, M.; Lin, M.; Henkin, A.; Ng, S.; Song, S.; Chen, Y.; Tran, H.; Lai, R.; et al. Specific bile acids inhibit hepatic fatty acid uptake in mice. Hepatology 2012, 56, 1300–1310. [Google Scholar] [CrossRef] [PubMed]
- Assaf-Balut, C.; García de la Torre, N.; Durán, A.; Fuentes, M.; Bordiú, E.; Del Valle, L.; Familiar, C.; Ortolá, A.; Jiménez, I.; Herraiz, M.A.; et al. A Mediterranean diet with additional extra virgin olive oil and pistachios reduces the incidence of gestational diabetes mellitus (GDM): A randomized controlled trial: The St. Carlos GDM prevention study. PLoS ONE 2017, 12, e0185873. [Google Scholar] [CrossRef]
- Martínez-González, M.A. Benefits of the Mediterranean diet beyond the Mediterranean Sea and beyond food patterns. BMC Med. 2016, 14, 157. [Google Scholar] [CrossRef] [PubMed]
- Moses, R.G.; Barker, M.; Winter, M.; Petocz, P.; Brand-Miller, J.C. Can a low-glycemic index diet reduce the need for insulin in gestational diabetes mellitus? A randomized trial. Diabetes Care 2009, 32, 996–1000. [Google Scholar] [CrossRef]
- Miklankova, D.; Markova, I.; Hüttl, M.; Stankova, B.; Malinska, H. The Different Insulin-Sensitising and Anti-Inflammatory Effects of Palmitoleic Acid and Oleic Acid in a Prediabetes Model. J. Diabetes Res. 2022, 2022, 4587907. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.H.; Pryor, M.; Noguchi, A.; Sampson, M.; Johnson, B.; Pryor, M.; Donkor, K.; Amar, M.; Remaley, A.T. Dietary Palmitoleic Acid Attenuates Atherosclerosis Progression and Hyperlipidemia in Low-Density Lipoprotein Receptor-Deficient Mice. Mol. Nutr. Food Res. 2019, 63, e1900120. [Google Scholar] [CrossRef]
- Ravaut, G.; Légiot, A.; Bergeron, K.F.; Mounier, C. Monounsaturated Fatty Acids in Obesity-Related Inflammation. Int. J. Mol. Sci. 2020, 22, 330. [Google Scholar] [CrossRef] [PubMed]
- Brand-Miller, J.; Dickinson, S.; Barclay, A.; Celermajer, D. The glycemic index and cardiovascular disease risk. Curr. Atheroscler. Rep. 2007, 9, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Satija, A.; Hu, F.B. Plant-based diets and cardiovascular health. Trends Cardiovasc. Med. 2018, 28, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Schiattarella, A.; Lombardo, M.; Morlando, M.; Rizzo, G. The Impact of a Plant-Based Diet on Gestational Diabetes: A Review. Antioxidants 2021, 10, 557. [Google Scholar] [CrossRef] [PubMed]
- Salinas-Roca, B.; Rubió-Piqué, L.; Montull-López, A. Polyphenol Intake in Pregnant Women on Gestational Diabetes Risk and Neurodevelopmental Disorders in Offspring: A Systematic Review. Nutrients 2022, 14, 3753. [Google Scholar] [CrossRef]
- Wan, M.L.Y.; Co, V.A.; El-Nezami, H. Dietary polyphenol impact on gut health and microbiota. Crit. Rev. Food Sci. Nutr. 2021, 61, 690–711. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.K.; Mukherjee, S. Evolving Interplay Between Dietary Polyphenols and Gut Microbiota—An Emerging Importance in Healthcare. Front. Nutr. 2021, 8, 634944. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Selma, M.V.; Espín, J.C. Interactions of gut microbiota with dietary polyphenols and consequences to human health. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 471–476. [Google Scholar] [CrossRef]
- Lippolis, T.; Cofano, M.; Caponio, G.R.; De Nunzio, V.; Notarnicola, M. Bioaccessibility and Bioavailability of Diet Polyphenols and Their Modulation of Gut Microbiota. Int. J. Mol. Sci. 2023, 24, 3813. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef]
- Dehghan, P.; Gargari, B.P.; Jafar-Abadi, M.A.; Aliasgharzadeh, A. Inulin controls inflammation and metabolic endotoxemia in women with type 2 diabetes mellitus: A randomized-controlled clinical trial. Int. J. Food Sci. Nutr. 2014, 65, 117–123. [Google Scholar] [CrossRef]
- Hasain, Z.; Mokhtar, N.M.; Kamaruddin, N.A.; Mohamed Ismail, N.A.; Razalli, N.H.; Gnanou, J.V.; Raja Ali, R.A. Gut Microbiota and Gestational Diabetes Mellitus: A Review of Host-Gut Microbiota Interactions and Their Therapeutic Potential. Front. Cell. Infect. Microbiol. 2020, 10, 188. [Google Scholar] [CrossRef]
- Sweeting, A.; Wong, J.; Murphy, H.R.; Ross, G.P. A Clinical Update on Gestational Diabetes Mellitus. Endocr. Rev. 2022, 43, 763–793. [Google Scholar] [CrossRef] [PubMed]
- Johns, E.C.; Denison, F.C.; Norman, J.E.; Reynolds, R.M. Gestational Diabetes Mellitus: Mechanisms, Treatment, and Complications. Trends Endocrinol. Metab. 2018, 29, 743–754. [Google Scholar] [CrossRef]
- Kamińska, K.; Stenclik, D.; Błażejewska, W.; Bogdański, P.; Moszak, M. Probiotics in the Prevention and Treatment of Gestational Diabetes Mellitus (GDM): A Review. Nutrients 2022, 14, 4303. [Google Scholar] [CrossRef]
- Luoto, R.; Laitinen, K.; Nermes, M.; Isolauri, E. Impact of maternal probiotic-supplemented dietary counselling on pregnancy outcome and prenatal and postnatal growth: A double-blind, placebo-controlled study. Br. J. Nutr. 2010, 103, 1792–1799. [Google Scholar] [CrossRef]
- Asemi, Z.; Samimi, M.; Tabassi, Z.; Naghibi Rad, M.; Rahimi Foroushani, A.; Khorammian, H.; Esmaillzadeh, A. Effect of daily consumption of probiotic yoghurt on insulin resistance in pregnant women: A randomized controlled trial. Eur. J. Clin. Nutr. 2013, 67, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, K.L.; Brennan, L.; Kennelly, M.A.; Maguire, O.C.; Smith, T.; Curran, S.; Coffey, M.; Foley, M.E.; Hatunic, M.; Shanahan, F.; et al. Impact of probiotics in women with gestational diabetes mellitus on metabolic health: A randomized controlled trial. Am. J. Obs. Gynecol. 2015, 212, 496-e1. [Google Scholar] [CrossRef]
- Jafarnejad, S.; Saremi, S.; Jafarnejad, F.; Arab, A. Effects of a multispecies probiotic mixture on glycemic control and inflammatory status in women with gestational diabetes: A randomized controlled clinical trial. J. Nutr. Metab. 2016, 2016, 5190846. [Google Scholar] [CrossRef] [PubMed]
- Kijmanawat, A.; Panburana, P.; Reutrakul, S.; Tangshewinsirikul, C. Effects of probiotic supplements on insulin resistance in gestational diabetes mellitus: A double-blind randomized controlled trial. J. Diabetes Investig. 2019, 10, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Taghizadeh, M.; Asemi, Z. Effects of synbiotic food consumption on glycemic status and serum hs-CRP in pregnant women: A randomized controlled clinical trial. Hormones 2014, 13, 398–406. [Google Scholar] [CrossRef]
- Ahmadi, S.; Jamilian, M.; Tajabadi-Ebrahimi, M.; Jafari, P.; Asemi, Z. The effects of synbiotic supplementation on markers of insulin metabolism and lipid profiles in gestational diabetes: A randomised, double-blind, placebo-controlled trial. Br. J. Nutr. 2016, 116, 1394–1401. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.B.; Benny, P.; Riel, J.; Boushey, C.; Perez, R.; Khadka, V.; Qin, Y.; Maunakea, A.K.; Lee, M.J. Adherence to Mediterranean diet impacts gastrointestinal microbial diversity throughout pregnancy. BMC Pregnancy Childbirth 2021, 21, 558. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Wang, H.K.; Gan, X.P.; Chen, L.; Cao, Y.N.; Cheng, D.C.; Zhang, D.Y.; Liu, W.Y.; Li, F.F.; Xu, X.M. Alterations of gut microbiota in gestational diabetes patients during the second trimester of pregnancy in the Shanghai Han population. J. Transl. Med. 2021, 19, 366. [Google Scholar] [CrossRef] [PubMed]
- Zeevi, D.; Korem, T.; Zmora, N.; Israeli, D.; Rothschild, D.; Weinberger, A.; Ben-Yacov, O.; Lador, D.; Avnit-Sagi, T.; Lotan-Pompan, M.; et al. Prediction of Glycemic Responses. Cell 2015, 163, 1079–1094. [Google Scholar] [CrossRef]
- Torres, N.; Tovar, A.R. The Present and Future of Personalized Nutrition. Rev. Investig. Clin. 2021, 73, 321–325. [Google Scholar] [CrossRef]
- Collins, F.S.; Varmus, H. A new initiative on precision medicine. N. Engl. J. Med. 2015, 372, 793–795. [Google Scholar] [CrossRef]
- Kolodziejczyk, A.A.; Zheng, D.; Elinav, E. Diet-microbiota interactions and personalized nutrition. Nature reviews. Nat. Rev. Microbiol. 2019, 17, 742–753. [Google Scholar] [CrossRef]
- Matusheski, N.V.; Caffrey, A.; Christensen, L.; Mezgec, S.; Surendran, S.; Hjorth, M.F.; McNulty, H.; Pentieva, K.; Roager, H.M.; Seljak, B.K.; et al. Diets, nutrients, genes and the microbiome: Recent advances in personalised nutrition. Br. J. Nutr. 2021, 126, 1489–1497. [Google Scholar] [CrossRef]
- Mathers, J.C. Paving the way to better population health through personalised nutrition. EFSA J. 2019, 17 (Suppl. S1), e170713. [Google Scholar] [CrossRef] [PubMed]
- Korpela, K.; Flint, H.J.; Johnstone, A.M.; Lappi, J.; Poutanen, K.; Dewulf, E.; Delzenne, N.; de Vos, W.M.; Salonen, A. Gut microbiota signatures predict host and microbiota responses to dietary interventions in obese individuals. PLoS ONE 2014, 9, e90702. [Google Scholar] [CrossRef]
- Sánchez-García, J.C.; Saraceno López-Palop, I.; Piqueras-Sola, B.; Cortés-Martín, J.; Mellado-García, E.; Muñóz Sánchez, I.; Rodríguez-Blanque, R. Advancements in Nutritional Strategies for Gestational Diabetes Management: A Systematic Review of Recent Evidence. J. Clin. Med. 2023, 13, 37. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 5. Facilitating Positive Health Behaviors and Well-being to Improve Health Outcomes: Standards of Care in Diabetes—2024. Diabetes Care 2024, 47 (Suppl. S1), S77–S110. [Google Scholar] [CrossRef]
- Sheykhsaran, E.; Abbasi, A.; Ebrahimzadeh Leylabadlo, H.; Sadeghi, J.; Mehri, S.; Naeimi Mazraeh, F.; Feizi, H.; Bannazadeh Baghi, H. Gut microbiota and obesity: An overview of microbiota to microbial-based therapies. Postgrad. Med. J. 2023, 99, 384–402. [Google Scholar] [CrossRef]
- Everard, A.; Cani, P.D. Diabetes, obesity and gut microbiota. Best Pract. Res. Clin. Gastroenterol. 2013, 27, 73–83. [Google Scholar] [CrossRef]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Delzenne, N.M. The gut microbiome as therapeutic target. Pharmacol. Ther. 2011, 130, 202–212. [Google Scholar] [CrossRef]
- Chen, Z.; Guo, L.; Zhang, Y.; Walzem, R.L.; Pendergast, J.S.; Printz, R.L.; Morris, L.C.; Matafonova, E.; Stien, X.; Kang, L.; et al. Incorporation of therapeutically modified bacteria into gut microbiota inhibits obesity. J. Clin. Investig. 2014, 124, 3391–3406. [Google Scholar] [CrossRef]
- Kang, Y.; Cai, Y. Gut microbiota and obesity: Implications for fecal microbiota transplantation therapy. Hormones 2017, 16, 223–234. [Google Scholar] [CrossRef] [PubMed]
- van Nood, E.; Vrieze, A.; Nieuwdorp, M.; Fuentes, S.; Zoetendal, E.G.; de Vos, W.M.; Visser, C.E.; Kuijper, E.J.; Bartelsman, J.F.; Tijssen, J.G.; et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N. Engl. J. Med. 2013, 368, 407–415. [Google Scholar] [CrossRef]
- Davidson, S.J.; Barrett, H.L.; Price, S.A.; Callaway, L.K.; Nitert, M.D. Probiotics for preventing gestational diabetes. Cochrane Database Syst. Rev. 2021, 4, CD009951. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- De Palma, G.; Blennerhassett, P.; Lu, J.; Deng, Y.; Park, A.J.; Green, W.; Denou, E.; Silva, M.A.; Santacruz, A.; Sanz, Y.; et al. Microbiota and host determinants of behavioural phenotype in maternally separated mice. Nat. Commun. 2015, 6, 7735. [Google Scholar] [CrossRef]
- Vandeputte, D. Personalized nutrition through the gut microbiota: Current insights and future perspectives. Nutr. Rev. 2020, 78 (Suppl. S2), 66–74. [Google Scholar] [CrossRef]
- Ren, Y.; Zeng, Y.; Wu, Y.; Yu, J.; Zhang, Q.; Xiao, X. The Role of Gut Microbiota in Gestational Diabetes Mellitus Affecting Intergenerational Glucose Metabolism: Possible Mechanisms and Interventions. Nutrients 2023, 15, 4551. [Google Scholar] [CrossRef]
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.; Duncan, A.E.; Kau, A.L.; Griffin, N.W.; Lombard, V.; Henrissat, B.; Bain, J.R.; et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 2013, 341, 1241214. [Google Scholar] [CrossRef]
- Ordovas, J.M.; Ferguson, L.R.; Tai, E.S.; Mathers, J.C. Personalised nutrition and health. BMJ 2018, 361, bmj.k2173. [Google Scholar] [CrossRef]

| Assessed Dietary Intervention | Ref. | Type of Study | Methods | No. of Participants | Results |
|---|---|---|---|---|---|
| Probiotic supplementation | Luoto et al. (2010) [136] | Double-blind placebo-controlled randomized trial | Probiotic supplement containing Lactobacillus rhamnosus GG and Bifidobacterium lactis Bb12 | 256 | This study demonstrated lower incidence of GDM compared to the control group without perinatal deaths or serious adverse incidences in mothers/newborns. |
| Asemi et al. (2013) [137] | Randomized controlled clinical trial | Probiotic yoghurt prepared with cultures of Streptococcus thermophilus and Lactobacillus bulgaricus and enriched with two strains of lactobacilli (Lactobacillus acidophilus LA5) and bifidobacteria (Bifidobacterium animalis BB12) | 70 | The outcome of the study was that daily consumption of probiotic yogurt might help pregnant women prevent developing insulin resistance by maintaining insulin levels. | |
| Lindsay et al. (2015) [138] | Double-blind placebo-controlled randomized trial | Probiotic supplement containing Lactobacillus salivarius | 149 | Supplementation with probiotic capsules among women with abnormal glucose tolerance had no impact on glycemic control. | |
| Jafarneiad et al. (2016) [139] | Randomized clinical trial | Probiotic supplement containing 112.5 × 109 CFU/capsule of eight strains of lactic acid bacteria (Streptococcus thermophilus, Bifidobacterium breve, Bifidobacterium longum, Bifidobacterium infantis, Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus paracasei and Lactobacillus delbrueckii subsp. Bulgaricus) | 89 | Probiotic supplementation may have a slightly favorable effect on glycemic status as the product did not significantly affect FPG and HbA1c but prevented the rise in serum insulin concentration and increase in insulin resistance. Therefore, it improved intestinal permeability function and regulated concentration of proinflammatory mediators. | |
| Kijmanawat et al. (2019) [140] | Double-blind randomized controlled trial. | Probiotic supplements containing Bifidobacterium and Lactobacillus | 57 | Probiotic supplements in women with diet-controlled gestational diabetes in the late second and early third trimester had positive effects on fasting glucose levels and increased insulin sensitivity; therefore, they may be considered as an adjunct treatment for glycemic control in these patients. | |
| Synbiotic supplementation | Taghizadeh et al. (2014) [141] | Randomized placebo-controlled | Synbiotic food consisting of a probiotic Lactobacillus sporogenes, inulin isomalt, sorbitol and stevia | 52 | This study illustrated that consumption of synbiotic food in pregnant women improved the insulin response compared to the control food; however, it had no effect on fasting plasma glucose and serum hs-CRP concentrations. |
| Ahmadi et al. (2016) [142] | Randomized, double-blind, placebo-controlled trial. | Probiotic supplementation with Lactobacillus acidophilus, Lactobacillus casei and Bifidobacterium bifidum (2 × 109 colony-forming units/g each) plus 800 mg inulin | 70 | Synbiotic supplementation in GDM women was associated with a significant reduction in serum TAG and VLDL-cholesterol concentrations, but did not influence lipid profiles or PFG. | |
| Studies on gut microbiota in pregnancy | Koren et al. (2012) [30] | Cohort study | Stool samples (from T1 and T3 of pregnancy as well as woman’s infants at 1 month of age, 6 months of age and 4 years of age), diet information and clinical data | 91 | During pregnancy, gut microbiota reshapes, particularly in the third trimester, resembling a disease-associated state (dysbiosis) that differs among women, having an increased number of Proteobacteria and Actinobacteria species. These microbial shifts were linked to increased insulin resistance and higher inflammatory response. |
| Miller et al. (2021) [143] | Longitudinal cohort study | Adherence to Mediterranean diet pattern was scored by the Alternate Mediterranean Diet Quality Score | 41 | Mediterranean diet pattern is associated with greater diversity of the microbiota community, promoting the production of SCFAs. | |
| Su et al. (2021) [144] | Cohort study | Fecal microbiota profiles from women with GDM normoglycemic women were assessed by 16S rRNA gene sequencing; fasting metabolic hormone concentrations were measured using multiplex ELISA. | 53 | Dysbiosis of the gut microbiome exists in patients with GDM in the second trimester of pregnancy; specifically, the phylum Bacteroidetes increased in GDM, as did Bacteroides, Incertae sedis, Citrobacter, Parabacteroides, and Fusicatenibacter genus. There are connections between gut microbiome and glucose plasma levels; thus, it might be possible that dysbiosis can be involved in the pathogenesis of GDM revealing the potential of these biomarkers in prevention and intervention strategies in GDM. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beldie, L.-A.; Dica, C.-C.; Moța, M.; Pirvu, B.-F.; Burticală, M.-A.; Mitrea, A.; Clenciu, D.; Efrem, I.C.; Vladu, B.E.; Timofticiuc, D.C.P.; et al. The Interactions Between Diet and Gut Microbiota in Preventing Gestational Diabetes Mellitus: A Narrative Review. Nutrients 2024, 16, 4131. https://doi.org/10.3390/nu16234131
Beldie L-A, Dica C-C, Moța M, Pirvu B-F, Burticală M-A, Mitrea A, Clenciu D, Efrem IC, Vladu BE, Timofticiuc DCP, et al. The Interactions Between Diet and Gut Microbiota in Preventing Gestational Diabetes Mellitus: A Narrative Review. Nutrients. 2024; 16(23):4131. https://doi.org/10.3390/nu16234131
Chicago/Turabian StyleBeldie, Luiza-Andreea, Cristina-Camelia Dica, Maria Moța, Bianca-Florentina Pirvu, Marilena-Alexandra Burticală, Adina Mitrea, Diana Clenciu, Ion Cristian Efrem, Beatrice Elena Vladu, Diana Cristina Protasiewicz Timofticiuc, and et al. 2024. "The Interactions Between Diet and Gut Microbiota in Preventing Gestational Diabetes Mellitus: A Narrative Review" Nutrients 16, no. 23: 4131. https://doi.org/10.3390/nu16234131
APA StyleBeldie, L.-A., Dica, C.-C., Moța, M., Pirvu, B.-F., Burticală, M.-A., Mitrea, A., Clenciu, D., Efrem, I. C., Vladu, B. E., Timofticiuc, D. C. P., Roșu, M. M., Gheonea, T. C., Amzolini, A. M., Moța, E., & Vladu, I. M. (2024). The Interactions Between Diet and Gut Microbiota in Preventing Gestational Diabetes Mellitus: A Narrative Review. Nutrients, 16(23), 4131. https://doi.org/10.3390/nu16234131






