Influence of CReatine Supplementation on mUScle Mass and Strength After Stroke (ICaRUS Stroke Trial): A Randomized Controlled Trial
Abstract
1. Introduction
2. Methods
2.1. Trial Design
2.2. Participants and Eligibility Criteria
2.3. Interventions
2.4. Outcomes
2.5. Follow-Up
2.6. Sample Size
2.7. Randomization
2.8. Blinding
2.9. Statistical Analysis
3. Results
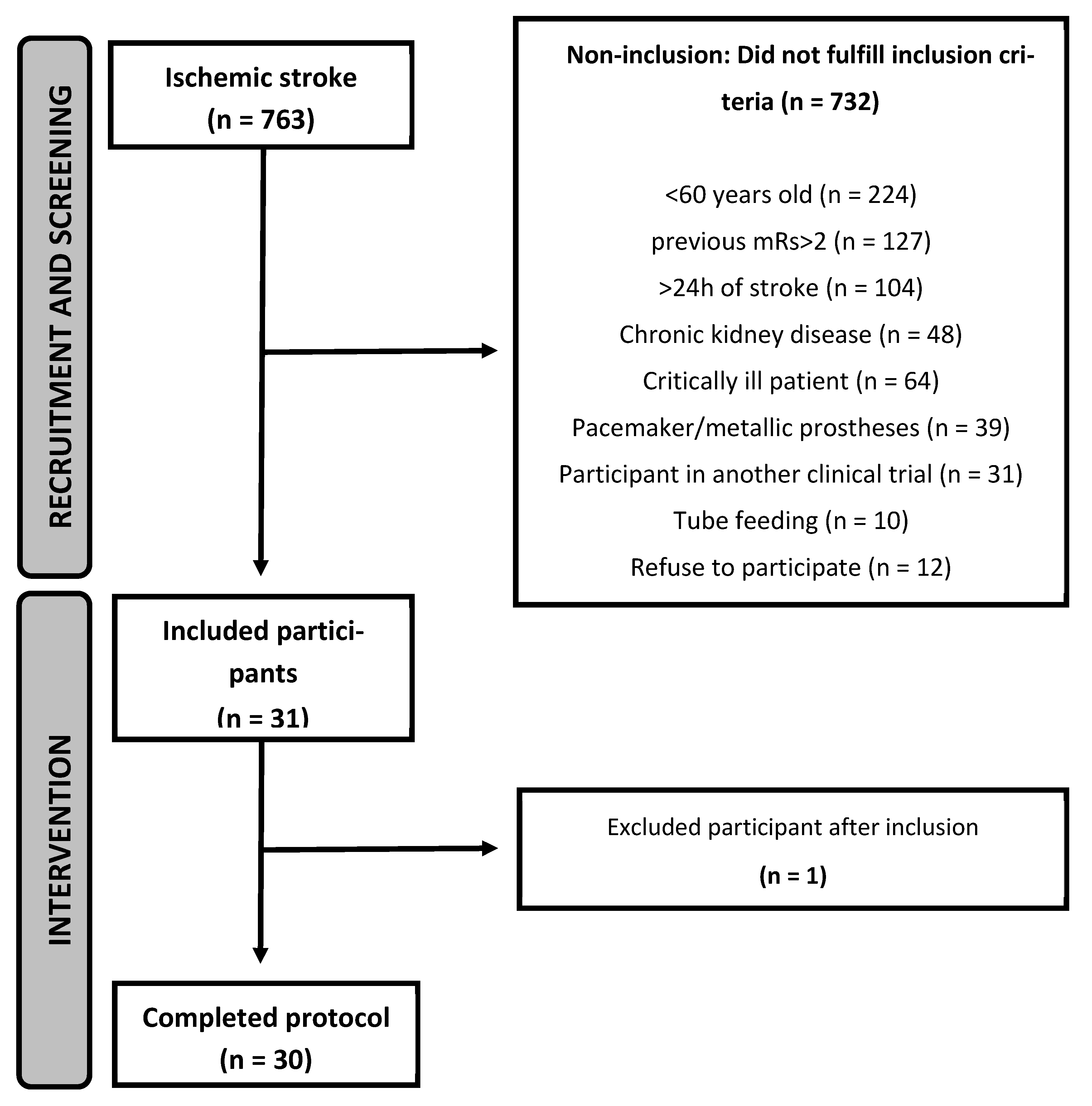
3.1. Recruitment and Characteristics of the Participants
3.2. Primary Outcomes
3.3. Secondary Outcomes
3.4. Follow-Up Visit
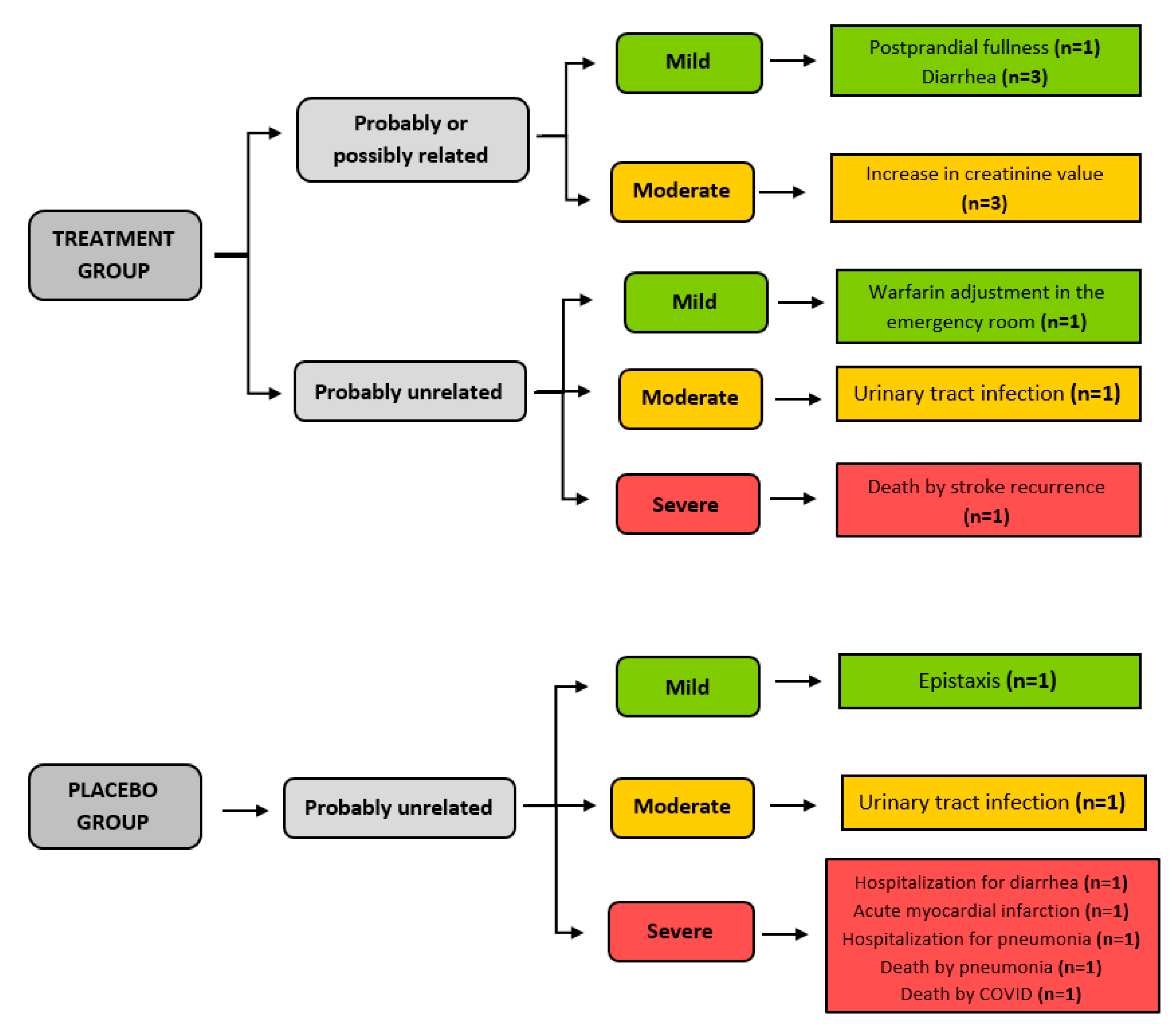
3.5. Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1459–1544, Erratum in Lancet 2017, 389, e1. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Katan, M.; Luft, A. Global Burden of Stroke. Semin. Neurol. 2018, 38, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Garcia, J.M. Sarcopenia, cachexia and aging: Diagnosis, mechanisms and therapeutic options—A mini-review. Gerontology 2014, 60, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Carin-Levy, G.; Greig, C.; Young, A.; Lewis, S.; Hannan, J.; Mead, G. Longitudinal changes in muscle strength and mass after acute stroke. Cerebrovasc. Dis. 2006, 21, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, L.; Jacobsen, B.K. Changes in muscle mass, fat mass, and bone mineral content in the legs after stroke: A 1 year prospective study. Bone 2001, 28, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.S.; Buscemi, A.; Forrester, L.; Hafer-Macko, C.E.; Ivey, F.M. Atrophy and intramuscular fat in specific muscles of the thigh: Associated weakness and hyperinsulinemia in stroke survivors. Neurorehabilit. Neural Repair. 2011, 25, 865–872. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ryan, A.S.; Dobrovolny, C.L.; Smith, G.V.; Silver, K.H.; Macko, R.F. Hemiparetic muscle atrophy and increased intramuscular fat in stroke patients. Arch. Phys. Med. Rehabil. 2002, 83, 1703–1707. [Google Scholar] [CrossRef] [PubMed]
- Scherbakov, N.; von Haehling, S.; Anker, S.D.; Dirnagl, U.; Doehner, W. Stroke induced Sarcopenia: Muscle wasting and disability after stroke. Int. J. Cardiol. 2013, 170, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Scherbakov, N.; Sandek, A.; Doehner, W. Stroke-related sarcopenia: Specific characteristics. J. Am. Med. Dir. Assoc. 2015, 16, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Scherbakov, N.; Pietrock, C.; Sandek, A.; Ebner, N.; Valentova, M.; Springer, J.; Schefold, J.C.; von Haehling, S.; Anker, S.D.; Norman, K.; et al. Body weight changes and incidence of cachexia after stroke. J. Cachexia Sarcopenia Muscle 2019, 10, 611–620. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Perasso, L.; Spallarossa, P.; Gandolfo, C.; Ruggeri, P.; Balestrino, M. Therapeutic use of creatine in brain or heart ischemia: Available data and future perspectives. Med. Res. Rev. 2013, 33, 336–363. [Google Scholar] [CrossRef] [PubMed]
- Gualano, B.; Rawson, E.S.; Candow, D.G.; Chilibeck, P.D. Creatine supplementation in the aging population: Effects on skeletal muscle, bone and brain. Amino Acids 2016, 48, 1793–1805. [Google Scholar] [CrossRef] [PubMed]
- Dolan, E.; Artioli, G.G.; Pereira, R.M.R.; Gualano, B. Muscular Atrophy and Sarcopenia in the Elderly: Is There a Role for Creatine Supplementation? Biomolecules 2019, 9, 642. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Candow, D.G.; Forbes, S.C.; Kirk, B.; Duque, G. Current Evidence and Possible Future Applications of Creatine Supplementation for Older Adults. Nutrients 2021, 13, 745. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Izquierdo, M.; Ibañez, J.; González-Badillo, J.J.; Gorostiaga, E.M. Effects of creatine supplementation on muscle power, endurance, and sprint performance. Med. Sci. Sports Exerc. 2002, 34, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Cordingley, D.M.; Cornish, S.M.; Candow, D.G. Anti-Inflammatory and Anti-Catabolic Effects of Creatine Supplementation: A Brief Review. Nutrients 2022, 14, 544. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kurzepa, J.; Kurzepa, J.; Golab, P.; Czerska, S.; Bielewicz, J. The significance of matrix metalloproteinase (MMP)-2 and MMP-9 in the ischemic stroke. Int. J. Neurosci. 2014, 124, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Lam, R.; Shaywitz, D.; Hendrickson, R.C.; Opiteck, G.J.; Wishengrad, D.; Liaw, A.; Song, Q.; Stewart, A.J.; Cummings, C.E.; et al. Evaluation of early biomarkers of muscle anabolic response to testosterone. J. Cachexia Sarcopenia Muscle 2011, 2, 45–56. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cardoso, A.L.; Fernandes, A.; Aguilar-Pimentel, J.A.; de Angelis, M.H.; Guedes, J.R.; Brito, M.A.; Ortolano, S.; Pani, G.; Athanasopoulou, S.; Gonos, E.S.; et al. Towards frailty biomarkers: Candidates from genes and pathways regulated in aging and age-related diseases. Ageing Res. Rev. 2018, 47, 214–277. [Google Scholar] [CrossRef] [PubMed]
- Crossland, H.; Smith, K.; Atherton, P.J.; Wilkinson, D.J. A novel stable isotope tracer method to simultaneously quantify skeletal muscle protein synthesis and breakdown. Metabol. Open 2020, 5, 100022. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de Souza, J.T.; Minicucci, M.F.; Ferreira, N.C.; Polegato, B.F.; Okoshi, M.P.; Modolo, G.P.; Phillips, B.E.; Atherton, P.J.; Smith, K.; Wilkinson, D.; et al. Influence of CReatine supplementation on mUScle mass and strength after stroke (ICaRUS Stroke Trial): Study protocol for a randomized controlled trial. Trials 2023, 24, 214. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bernhardt, J.; Churilov, L.; Ellery, F.; Collier, J.; Chamberlain, J.; Langhorne, P.; Lindley, R.I.; Moodie, M.; Dewey, H.; Thrift, A.G.; et al. AVERT Collaboration Group. Prespecified dose-response analysis for A Very Early Rehabilitation Trial (AVERT). Neurology 2016, 86, 2138–2145, Erratum in Neurology 2017, 89, 107. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Winstein, C.J.; Stein, J.; Arena, R.; Bates, B.; Cherney, L.R.; Cramer, S.C.; Deruyter, F.; Eng, J.J.; Fisher, B.; Harvey, R.L.; et al. Guidelines for Adult Stroke Rehabilitation and Recovery: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2016, 47, e98–e169, Erratum in Stroke 2017, 48, e78; Erratum in Stroke 2017, 48, e369. [Google Scholar] [CrossRef] [PubMed]
- Brasil. Ministério da Saúde. Secretaria de Atenção à Saúde. Departamento de Atenção Básica. Guia alimentar para a população brasileira/Ministério da Saúde, Secretaria de Atenção à Saúde, Departamento de Atenção Básica, 2nd ed., 1 reimpr, Brasília: Ministério da Saúde. 2014. Available online: https://www.gov.br/saude/pt-br/assuntos/saude-brasil/publicacoes-para-promocao-a-saude/guia_alimentar_populacao_brasileira_2ed.pdf/view (accessed on 6 March 2023).
- Cincura, C.; Pontes-Neto, O.M.; Neville, I.S.; Mendes, H.F.; Menezes, D.F.; Mariano, D.C.; Pereira, I.F.; Teixeira, L.A.; Jesus, P.A.; de Queiroz, D.C.; et al. Validation of the National Institutes of Health Stroke Scale, modified Rankin Scale and Barthel Index in Brazil: The role of cultural adaptation and structured interviewing. Cerebrovasc. Dis. 2009, 27, 119–122. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31, Erratum in Age Ageing 2019, 48, 601. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Smith-Ryan, A.E.; Fultz, S.N.; Melvin, M.N.; Wingfield, H.L.; Woessner, M.N. Reproducibility and validity of A-mode ultrasound for body composition measurement and classification in overweight and obese men and women. PLoS ONE 2014, 9, e91750. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eickemberg, M.; Oliveira, C.C.; RORIZ, A.K.C.; Sampaio, L.R. Bioelectric impedance analysis and its use for nutritional assessments. Rev. Nutr. 2011, 24, 883–893. [Google Scholar] [CrossRef]
- Barry, E.; Galvin, R.; Keogh, C.; Horgan, F.; Fahey, T. Is the Timed Up and Go test a useful predictor of risk of falls in community dwelling older adults: A systematic review and meta-analysis. BMC Geriatr. 2014, 14, 14. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jones, C.J.; Rikli, R.E.; Beam, W.C. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res. Q. Exerc. Sport 1999, 70, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Pinto, E.B.; Maso, I.; Vilela, R.N.; Santos, L.C.; Oliveira-Filho, J. Validation of the EuroQol quality of life questionnaire on stroke victims. Arq. Neuropsiquiatr. 2011, 69, 320–333. [Google Scholar] [CrossRef] [PubMed]
- Gotshalk, L.A.; Volek, J.S.; Staron, R.S.; Denegar, C.R.; Hagerman, F.C.; Kraemer, W.J. Creatine supplementation improves muscular performance in older men. Med. Sci. Sports Exerc. 2002, 34, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Lanhers, C.; Pereira, B.; Naughton, G.; Trousselard, M.; Lesage, F.X.; Dutheil, F. Creatine Supplementation and Lower Limb Strength Performance: A Systematic Review and Meta-Analyses. Sports Med. 2015, 45, 1285–1294. [Google Scholar] [CrossRef] [PubMed]
- Balch, M.H.H.; Nimjee, S.M.; Rink, C.; Hannawi, Y. Beyond the Brain: The Systemic Pathophysiological Response to Acute Ischemic Stroke. J. Stroke 2020, 22, 159–172. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Finnerty, C.C.; Mabvuure, N.T.; Ali, A.; Kozar, R.A.; Herndon, D.N. The surgically induced stress response. JPEN J. Parenter. Enteral. Nutr. 2013, 37 (Suppl. 5), 21S–29S. [Google Scholar] [CrossRef] [PubMed]
- Chitramuthu, B.P.; Bennett, H.P.J.; Bateman, A. Progranulin: A new avenue towards the understanding and treatment of neurodegenerative disease. Brain 2017, 140, 3081–3104. [Google Scholar] [CrossRef] [PubMed]
- Gualano, B.; Macedo, A.R.; Alves, C.R.; Roschel, H.; Benatti, F.B.; Takayama, L.; de Sá Pinto, A.L.; Lima, F.R.; Pereira, R.M. Creatine supplementation and resistance training in vulnerable older women: A randomized double-blind placebo-controlled clinical trial. Exp. Gerontol. 2014, 53, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Forbes, S.C.; Candow, D.G.; Ostojic, S.M.; Roberts, M.D.; Chilibeck, P.D. Meta-Analysis Examining the Importance of Creatine Ingestion Strategies on Lean Tissue Mass and Strength in Older Adults. Nutrients 2021, 13, 1912. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, C.C.; Fang, C.C.; Lee, Y.H.; Yang, M.T.; Chan, K.H. Effects of 4-Week Creatine Supplementation Combined with Complex Training on Muscle Damage and Sport Performance. Nutrients 2018, 10, 1640. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lambertsen, K.L.; Finsen, B.; Clausen, B.H. Post-stroke inflammation-target or tool for therapy? Acta Neuropathol. 2019, 137, 693–714. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kato, E.T.; Morrow, D.A.; Guo, J.; Berg, D.D.; Blazing, M.A.; Bohula, E.A.; Bonaca, M.P.; Cannon, C.P.; de Lemos, J.A.; Giugliano, R.P.; et al. Growth differentiation factor 15 and cardiovascular risk: Individual patient meta-analysis. Eur. Heart J. 2023, 44, 293–300. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Swift, I.J.; Rademakers, R.; Finch, N.; Baker, M.; Ghidoni, R.; Benussi, L.; Binetti, G.; Rossi, G.; Synofzik, M.; Wilke, C.; et al. A systematic review of progranulin concentrations in biofluids in over 7000 people-assessing the pathogenicity of GRN mutations and other influencing factors. Alzheimer’s Res. Ther. 2024, 16, 66. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, C.J.; Bosch, X. Progranulin: A growth factor, a novel TNFR ligand and a drug target. Pharmacol. Ther. 2012, 133, 124–132. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Horinokita, I.; Hayashi, H.; Oteki, R.; Mizumura, R.; Yamaguchi, T.; Usui, A.; Yuan, B.; Takagi, N. Involvement of Progranulin and Granulin Expression in Inflammatory Responses after Cerebral Ischemia. Int. J. Mol. Sci. 2019, 20, 5210. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lasek-Bal, A.; Jedrzejowska-Szypulka, H.; Student, S.; Warsz-Wianecka, A.; Zareba, K.; Puz, P.; Bal, W.; Pawletko, K.; Lewin-Kowalik, J. The importance of selected markers of inflammation and blood-brain barrier damage for short-term ischemic stroke prognosis. J. Physiol. Pharmacol. 2019, 70, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Lu, L.; Liu, L.; Bi, G.; Zheng, L. Progranulin and short-term outcome in patients with acute ischaemic stroke. Eur. J. Neurol. 2016, 23, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, R.S.; Østergaard, A.; Kjær, P.; Skerris, A.; Skou, C.; Christoffersen, J.; Seest, L.S.; Poulsen, M.B.; Rønholt, F.; Overgaard, K. Stroke rehabilitation at home before and after discharge reduced disability and improved quality of life: A randomised controlled trial. Clin. Rehabil. 2016, 30, 225–236. [Google Scholar] [CrossRef] [PubMed]



| Treatment (n = 15) | Placebo (n = 15) | p | |
|---|---|---|---|
| Male sex, N (%) | 10.0 (66.7) | 10.0 (66.7) | 0.70 |
| Age (years) | 69.0 (64.0–79.0) | 66.0 (63.0–73.0) | 0.88 |
| NRS 2002 ≥ 3, N (%) | 8.0 (53.3) | 9.0 (60.0) | 1.00 |
| Diabetes mellitus, N (%) | 4.0 (26.7) | 6.0 (40.0) | 0.70 |
| Arterial hypertension, N (%) | 10.0 (66.7) | 12.0 (80.0) | 0.68 |
| Dyslipidemia, N (%) | 5.0 (33.3) | 6.0 (40.0) | 1.00 |
| Previous MI, N (%) | 4.0 (26.7) | 2.0 (13.3) | 0.65 |
| Previous stroke, N (%) | 1.0 (6.7) | 2.0 (13.3) | 1.00 |
| Thrombolysis, N (%) | 6.0 (40.0) | 6.0 (40.0) | 0.71 |
| Insulin use, N (%) | 5.0 (33.3) | 3.0 (20.0) | 0.68 |
| Statin use, N (%) | 5.0 (33.3) | 2.0 (13.3) | 0.39 |
| Dysphagia, N (%) | 3.0 (20.0) | 6.0 (40.0) | 0.43 |
| FOIS, N (%) | 6.0 (40.0) | 6.0 (40.0) | 0.15 |
| ASPECTS score | 10.0 (8.0–10.0) | 8.0 (8.0–9.0) | 0.13 |
| NIHSS at admission | 7.0 (4.0–13.0) | 8.0 (6.0–12.0) | 0.55 |
| Bamford classification | 0.85 | ||
| LACS, N (%) | 7.0 (23.3) | 6.0 (20.0) | |
| PACS, N (%) | 4.0 (13.3) | 6.0 (20.0) | |
| TACS, N (%) | 2.0 (6.7) | 1.0 (3.3) | |
| POCS, N (%) | 2.0 (6.7) | 2.0 (6.7) | |
| Toast classification | 0.41 | ||
| Cardioembolism, N (%) | 3.0 (10.0) | 4.0 (13.3) | |
| Large-vessel, N (%) atherothrombosis, N (%) | 4.0 (13.3) | 2.0 (6.7) | |
| Undetermined causes, N (%) | 8.0 (26.7) | 7.0 (23.3) | |
| Small-vessel disease, N (%) | 0.0 (0.0) | 2.0 (6.7) |
| Outcomes | 95% Confidence Interval | ||||
|---|---|---|---|---|---|
| Variables | Estimate | Lower | Upper | p | |
| Modified Rankin Scale | Moment | −0.30 | −0.55 | −0.05 | 0.03 |
| Treatment | 0.03 | −1.26 | 1.33 | 0.96 | |
| Interaction of time × treatment | −0.20 | 0.71 | 0.31 | 0.44 | |
| Handgrip unaffected side (kgf) | Moment | 1.45 | −0.40 | 3.29 | 0.14 |
| Treatment | −3.29 | −12.41 | 5.84 | 0.49 | |
| Interaction of time × treatment | 3.16 | −0.53 | 6.85 | 0.10 | |
| Handgrip affected side (kgf) | Moment | 0.59 | −1.90 | 3.08 | 0.64 |
| Treatment | −0.34 | −10.36 | 9.67 | 0.95 | |
| Interaction of time × treatment | −0.02 | −5.00 | 4.97 | 0.99 | |
| FFMI (kg/m2) | Moment | −0.71 | −1.97 | 0.55 | 0.28 |
| Treatment | 0.66 | −1.59 | 2.92 | 0.57 | |
| Interaction of time × treatment | −0.49 | −3.01 | 2.03 | 0.70 | |
| AMMI (kg/m2) | Moment | −0.45 | −1.51 | 0.60 | 0.41 |
| Treatment | 0.15 | −2.69 | 2.99 | 0.92 | |
| Interaction of time × treatment | 0.46 | −1.65 | 2.57 | 0.67 | |
| BBM thickness—affected side (mm) | Moment | −2.29 | −4.34 | −0.25 | 0.03 |
| Treatment | 2.00 | −3.28 | 7.28 | 0.46 | |
| Interaction of time × treatment | 1.47 | −2.62 | 5.56 | 0.48 | |
| BBM thickness—unaffected side (mm) | Moment | −0.17 | −1.05 | 0.71 | 0.70 |
| Treatment | 2.90 | −2.32 | 8.12 | 0.28 | |
| Interaction of time × treatment | 0.92 | −0.83 | 2.68 | 0.30 | |
| RFM thickness—affected side (mm) | Moment | −1.45 | −2.76 | −0.14 | 0.03 |
| Treatment | −0.42 | −4.80 | 3.95 | 0.85 | |
| Interaction of time × treatment | −0.74 | −3.36 | 1.88 | 0.58 | |
| RFM thickness—unaffected side (mm) | Moment | −0.71 | −1.61 | 0.19 | 0.12 |
| Treatment | 0.53 | −3.81 | 4.87 | 0.81 | |
| Interaction of time × treatment | 0.06 | −1.74 | 1.85 | 0.95 | |
| 3MH—labeled isotope (%/h) | Moment | −0.00 | −0.01 | 0.01 | 0.67 |
| Treatment | −0.00 | −0.03 | 0.03 | 0.95 | |
| Interaction of time × treatment | −0.01 | −0.03 | 0.00 | 0.13 | |
| 3MH—ELISA (nmol/mL) | Moment | −1.71 | −11.9 | 8.47 | 0.74 |
| Treatment | −7.15 | −36.2 | 21.88 | 0.63 | |
| Interaction of time × treatment | −11.79 | −32.0 | 8.44 | 0.25 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza, J.T.; Minicucci, M.F.; Ferreira, N.C.; Polegato, B.F.; Okoshi, M.P.; Modolo, G.P.; Leal-Pereira, F.W.; Phillips, B.E.; Atherton, P.J.; Smith, K.; et al. Influence of CReatine Supplementation on mUScle Mass and Strength After Stroke (ICaRUS Stroke Trial): A Randomized Controlled Trial. Nutrients 2024, 16, 4148. https://doi.org/10.3390/nu16234148
Souza JT, Minicucci MF, Ferreira NC, Polegato BF, Okoshi MP, Modolo GP, Leal-Pereira FW, Phillips BE, Atherton PJ, Smith K, et al. Influence of CReatine Supplementation on mUScle Mass and Strength After Stroke (ICaRUS Stroke Trial): A Randomized Controlled Trial. Nutrients. 2024; 16(23):4148. https://doi.org/10.3390/nu16234148
Chicago/Turabian StyleSouza, Juli T., Marcos F. Minicucci, Natália C. Ferreira, Bertha F. Polegato, Marina P. Okoshi, Gabriel P. Modolo, Filipe W. Leal-Pereira, Bethan E. Phillips, Philip J. Atherton, Kenneth Smith, and et al. 2024. "Influence of CReatine Supplementation on mUScle Mass and Strength After Stroke (ICaRUS Stroke Trial): A Randomized Controlled Trial" Nutrients 16, no. 23: 4148. https://doi.org/10.3390/nu16234148
APA StyleSouza, J. T., Minicucci, M. F., Ferreira, N. C., Polegato, B. F., Okoshi, M. P., Modolo, G. P., Leal-Pereira, F. W., Phillips, B. E., Atherton, P. J., Smith, K., Wilkinson, D. J., Gordon, A. L., Tanni, S. E., Costa, V. E., Fernandes, M. F., Bazan, S. G., Zornoff, L. M., Paiva, S. R., Bazan, R., & Azevedo, P. S. (2024). Influence of CReatine Supplementation on mUScle Mass and Strength After Stroke (ICaRUS Stroke Trial): A Randomized Controlled Trial. Nutrients, 16(23), 4148. https://doi.org/10.3390/nu16234148












