Impact of Dietary Niacin on Metabolic Dysfunction-Associated Steatotic Liver Disease in Mediterranean Subjects: A Population-Based Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Settings
2.2. Participants
2.3. Variables
2.4. Dietary Niacin Consumption
2.5. Dietary Macronutrient Consumption
2.6. Statistical Analysis
3. Results
3.1. Dietary Intake
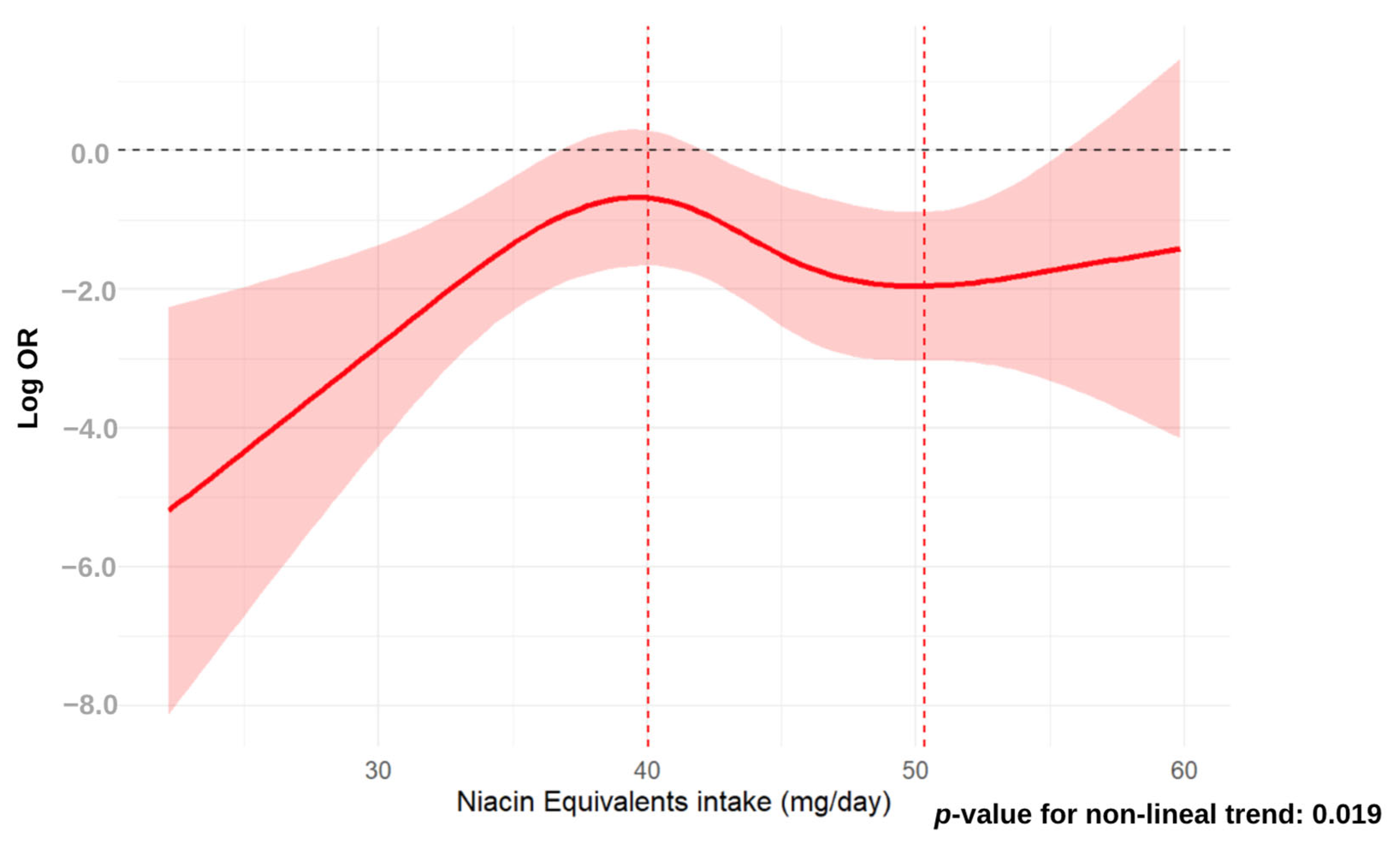
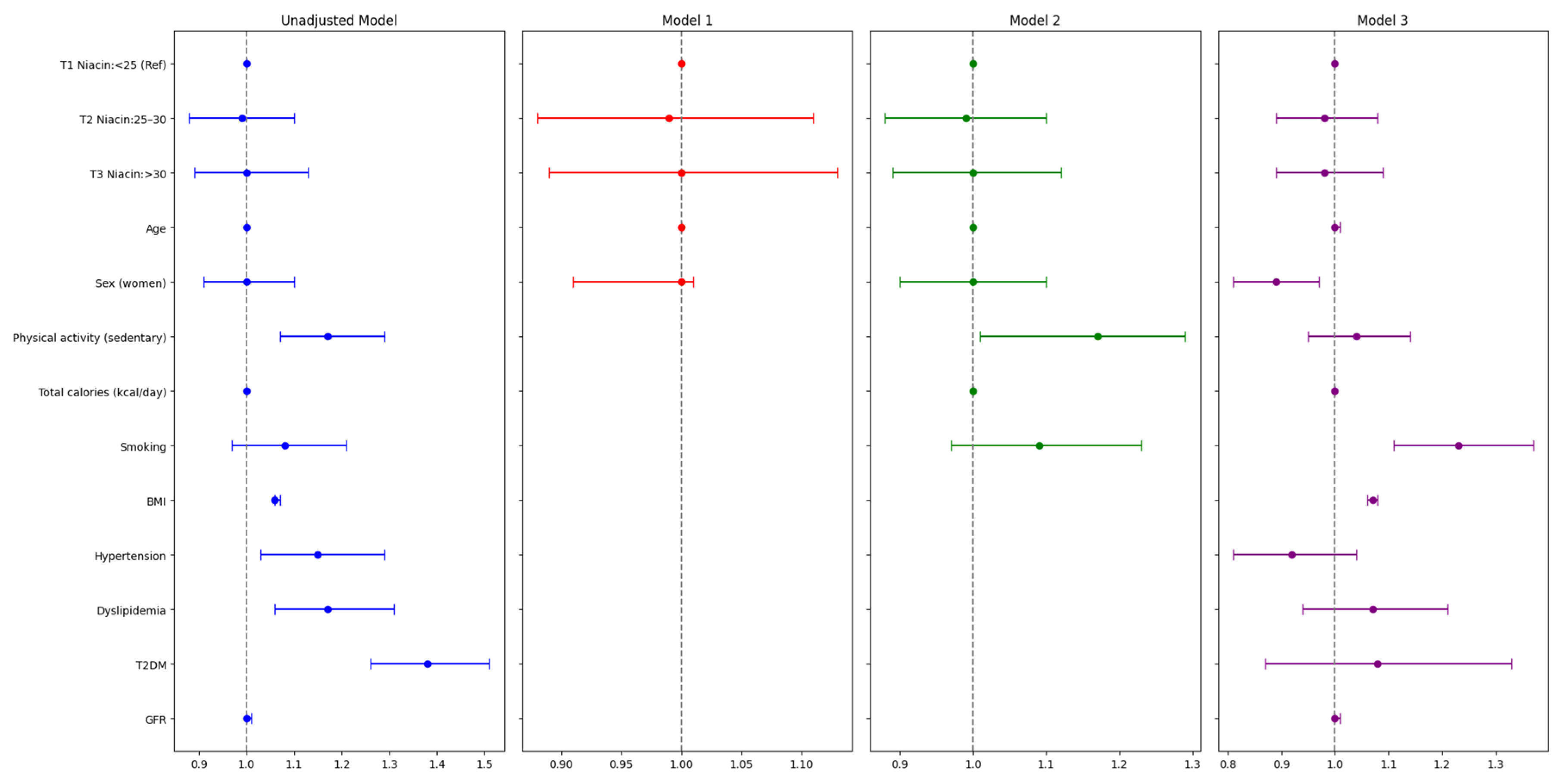
3.2. Association Analysis Between Dietary Niacin and MASLD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines on the Management of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Obes. Facts 2024, 17, 374–444. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, M.L.; Ganji, S.; Nakra, N.K.; Kamanna, V.S. Niacin for treatment of nonalcoholic fatty liver disease (NAFLD): Novel use for an old drug? J. Clin. Lipidol. 2019, 13, 873–879. [Google Scholar] [CrossRef]
- Boccatonda, A.; Andreetto, L.; D’Ardes, D.; Cocco, G.; Rossi, I.; Vicari, S.; Schiavone, C.; Cipollone, F.; Guagnano, M.T. From NAFLD to MAFLD: Definition, Pathophysiological Basis and Cardiovascular Implications. Biomedicines 2023, 11, 883. [Google Scholar] [CrossRef]
- Marchesini, G.; Petta, S.; Dalle Grave, R. Diet, weight loss, and liver health in nonalcoholic fatty liver disease: Pathophysiology, evidence, and practice. Hepatology 2016, 63, 2032–2043. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): A systematic review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef]
- Paternostro, R.; Trauner, M. Current treatment of non-alcoholic fatty liver disease. J. Intern. Med. 2022, 292, 190–204. [Google Scholar] [CrossRef] [PubMed]
- Fraser, A.; Abel, R.; Lawlor, D.A.; Fraser, D.; Elhayany, A. A modified Mediterranean diet is associated with the greatest reduction in alanine aminotransferase levels in obese type 2 diabetes patients: Results of a quasi-randomised controlled trial. Diabetologia 2008, 51, 1616–1622. [Google Scholar] [CrossRef] [PubMed]
- Hydes, T.; Alam, U.; Cuthbertson, D.J. The Impact of Macronutrient Intake on Non-alcoholic Fatty Liver Disease (NAFLD): Too Much Fat, Too Much Carbohydrate, or Just Too Many Calories? Front. Nutr. 2021, 8, 640557. [Google Scholar] [CrossRef]
- Orliacq, J.; Pérez-Cornago, A.; Parry, S.A.; Kelly, R.K.; Koutoukidis, D.A.; Carter, J.L. Associations between types and sources of dietary carbohydrates and liver fat: A UK Biobank study. BMC Med. 2023, 21, 444. [Google Scholar] [CrossRef]
- Yki-Järvinen, H.; Luukkonen, P.K.; Hodson, L.; Moore, J.B. Dietary carbohydrates and fats in nonalcoholic fatty liver disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 770–786. [Google Scholar] [CrossRef]
- Hashemi Kani, A.; Alavian, S.M.; Haghighatdoost, F.; Azadbakht, L. Diet macronutrients composition in nonalcoholic Fatty liver disease: A review on the related documents. Hepat. Mon. 2014, 14, e10939. [Google Scholar] [CrossRef] [PubMed]
- Freese, R.; Lysne, V. Niacin—A scoping review for Nordic Nutrition Recommendations 2023. Food Nutr. Res. 2023, 67, 10299. [Google Scholar] [CrossRef] [PubMed]
- Dietary Reference Values for Niacin|EFSA. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/3759 (accessed on 19 February 2024).
- Mousa, T.Y.; Mousa, O.Y. Nicotinic Acid Deficiency (Archived). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Colak, Y.; Yesil, A.; Mutlu, H.H.; Caklili, O.T.; Ulasoglu, C.; Senates, E.; Takir, M.; Kostek, O.; Yilmaz, Y.; Enc, F.Y.; et al. A potential treatment of non-alcoholic fatty liver disease with SIRT1 activators. J. Gastrointest. Liver Dis. 2014, 23, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Ponugoti, B.; Kim, D.-H.; Xiao, Z.; Smith, Z.; Miao, J.; Zang, M.; Wu, S.-Y.; Chiang, C.-M.; Veenstra, T.D.; Kemper, J.K. SIRT1 deacetylates and inhibits SREBP-1C activity in regulation of hepatic lipid metabolism. J. Biol. Chem. 2010, 285, 33959–33970. [Google Scholar] [CrossRef] [PubMed]
- Ganji, S.H.; Kukes, G.D.; Lambrecht, N.; Kashyap, M.L.; Kamanna, V.S. Therapeutic role of niacin in the prevention and regression of hepatic steatosis in rat model of nonalcoholic fatty liver disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 306, G320–G327. [Google Scholar] [CrossRef]
- Zhou, C.-C.; Yang, X.; Hua, X.; Liu, J.; Fan, M.-B.; Li, G.-Q.; Song, J.; Xu, T.-Y.; Li, Z.-Y.; Guan, Y.-F.; et al. Hepatic NAD(+) deficiency as a therapeutic target for non-alcoholic fatty liver disease in ageing. Br. J. Pharmacol. 2016, 173, 2352–2368. [Google Scholar] [CrossRef]
- Hu, G.; Ling, C.; Chi, L.; Thind, M.K.; Furse, S.; Koulman, A.; Swann, J.R.; Lee, D.; Calon, M.M.; Bourdon, C.; et al. The role of the tryptophan-NAD + pathway in a mouse model of severe malnutrition induced liver dysfunction. Nat. Commun. 2022, 13, 7576. [Google Scholar] [CrossRef]
- Dall, M.; Hassing, A.S.; Treebak, J.T. NAD+ and NAFLD—Caution, causality and careful optimism. J. Physiol. 2022, 600, 1135–1154. [Google Scholar] [CrossRef]
- Fukuwatari, T.; Shibata, K. Nutritional Aspect of Tryptophan Metabolism. Int. J. Tryptophan Res. 2013, 6, 3–8. [Google Scholar] [CrossRef]
- Romani, M.; Hofer, D.C.; Katsyuba, E.; Auwerx, J. Niacin: An old lipid drug in a new NAD+ dress. J. Lipid Res. 2019, 60, 741–746. [Google Scholar] [CrossRef]
- Bogan, K.L.; Brenner, C. Nicotinic acid, nicotinamide, and nicotinamide riboside: A molecular evaluation of NAD+ precursor vitamins in human nutrition. Annu. Rev. Nutr. 2008, 28, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Lara, K.A.; Rodríguez-Millán, E.; Sebastián, D.; Blanco-Soto, R.; Camacho, M.; Nan, M.N.; Diarte-Añazco, E.M.G.; Mato, E.; Lope-Piedrafita, S.; Roglans, N.; et al. Nicotinamide Protects Against Diet-Induced Body Weight Gain, Increases Energy Expenditure, and Induces White Adipose Tissue Beiging. Mol. Nutr. Food Res. 2021, 65, e2100111. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-J.; Sun, S.-J.; Fu, J.-T.; Ouyang, S.-X.; Zhao, Q.-J.; Su, L.; Ji, Q.-X.; Sun, D.-Y.; Zhu, J.-H.; Zhang, G.-Y.; et al. NAD+-boosting therapy alleviates nonalcoholic fatty liver disease via stimulating a novel exerkine Fndc5/irisin. Theranostics 2021, 11, 4381–4402. [Google Scholar] [CrossRef]
- Pham, T.X.; Bae, M.; Kim, M.-B.; Lee, Y.; Hu, S.; Kang, H.; Park, Y.-K.; Lee, J.-Y. Nicotinamide riboside, an NAD+ precursor, attenuates the development of liver fibrosis in a diet-induced mouse model of liver fibrosis. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 2451–2463. [Google Scholar] [CrossRef]
- Sambeat, A.; Ratajczak, J.; Joffraud, M.; Sanchez-Garcia, J.L.; Giner, M.P.; Valsesia, A.; Giroud-Gerbetant, J.; Valera-Alberni, M.; Cercillieux, A.; Boutant, M.; et al. Endogenous nicotinamide riboside metabolism protects against diet-induced liver damage. Nat. Commun. 2019, 10, 4291. [Google Scholar] [CrossRef]
- Ganji, S.H.; Kashyap, M.L.; Kamanna, V.S. Niacin inhibits fat accumulation, oxidative stress, and inflammatory cytokine IL-8 in cultured hepatocytes: Impact on non-alcoholic fatty liver disease. Metabolism 2015, 64, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Cao, Z.; Lai, X.; Shi, Y.; Zhou, N. Niacin Ameliorates Hepatic Steatosis by Inhibiting De Novo Lipogenesis via a GPR109A-Mediated PKC-ERK1/2-AMPK Signaling Pathway in C57BL/6 Mice Fed a High-Fat Diet. J. Nutr. 2020, 150, 672–684. [Google Scholar] [CrossRef]
- El-Kady, R.R.; Ali, A.K.; El Wakeel, L.M.; Sabri, N.A.; Shawki, M.A. Nicotinamide supplementation in diabetic nonalcoholic fatty liver disease patients: Randomized controlled trial. Ther. Adv. Chronic Dis. 2022, 13, 20406223221077958. [Google Scholar] [CrossRef]
- Dellinger, R.W.; Holmes, H.E.; Hu-Seliger, T.; Butt, R.W.; Harrison, S.A.; Mozaffarian, D.; Chen, O.; Guarente, L. Nicotinamide riboside and pterostilbene reduces markers of hepatic inflammation in NAFLD: A double-blind, placebo-controlled clinical trial. Hepatology 2023, 78, 863–877. [Google Scholar] [CrossRef]
- Pan, J.; Hu, Y.; Pang, N.; Yang, L. Association between Dietary Niacin Intake and Nonalcoholic Fatty Liver Disease: NHANES 2003–2018. Nutrients 2023, 15, 4128. [Google Scholar] [CrossRef]
- Linder, K.; Willmann, C.; Kantartzis, K.; Machann, J.; Schick, F.; Graf, M.; Kümmerle, S.; Häring, H.-U.; Fritsche, A.; Stefan, N.; et al. Dietary Niacin Intake Predicts the Decrease of Liver Fat Content During a Lifestyle Intervention. Sci. Rep. 2019, 9, 1303. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Han, J. Association of niacin intake and metabolic dysfunction-associated steatotic liver disease: Findings from National Health and Nutrition Examination Survey. BMC Public Health 2024, 24, 2742. [Google Scholar] [CrossRef]
- Falguera, M.; Vilanova, M.B.; Alcubierre, N.; Granado-Casas, M.; Marsal, J.R.; Miró, N.; Cebrian, C.; Molló, À.; Franch-Nadal, J.; Mata-Cases, M.; et al. Prevalence of pre-diabetes and undiagnosed diabetes in the Mollerussa prospective observational cohort study in a semi-rural area of Catalonia. BMJ Open 2020, 10, e033332. [Google Scholar] [CrossRef] [PubMed]
- Vilanova, M.B.; Falguera, M.; Marsal, J.R.; Rubinat, E.; Alcubierre, N.; Castelblanco, E.; Granado-Casas, M.; Miró, N.; Molló, À.; Mata-Cases, M.; et al. Prevalence, clinical features and risk assessment of pre-diabetes in Spain: The prospective Mollerussa cohort study. BMJ Open 2017, 7, e015158. [Google Scholar] [CrossRef]
- Alcubierre, N.; Rubinat, E.; Traveset, A.; Martinez-Alonso, M.; Hernandez, M.; Jurjo, C.; Mauricio, D. A prospective cross-sectional study on quality of life and treatment satisfaction in type 2 diabetic patients with retinopathy without other major late diabetic complications. Health Qual. Life Outcomes 2014, 12, 131. [Google Scholar] [CrossRef] [PubMed]
- Alcubierre, N.; Granado-Casas, M.; Real, J.; Perpiñán, H.; Rubinat, E.; Falguera, M.; Castelblanco, E.; Franch-Nadal, J.; Mauricio, D. Spanish People with Type 2 Diabetes Show an Improved Adherence to the Mediterranean Diet. Nutrients 2020, 12, 560. [Google Scholar] [CrossRef]
- Byrne, C.D.; Targher, G. NAFLD: A multisystem disease. J. Hepatol. 2015, 62, S47–S64. [Google Scholar] [CrossRef]
- Han, A.L. Validation of fatty liver index as a marker for metabolic dysfunction-associated fatty liver disease. Diabetol. Metab. Syndr. 2022, 14, 44. [Google Scholar] [CrossRef]
- Han, A.L.; Lee, H.K. Comparison of the Diagnostic Performance of Steatosis Indices for Discrimination of CT-Diagnosed Metabolic Dysfunction-Associated Fatty Liver Disease. Metabolites 2022, 12, 664. [Google Scholar] [CrossRef]
- Castellana, M.; Donghia, R.; Guerra, V.; Procino, F.; Lampignano, L.; Castellana, F.; Zupo, R.; Sardone, R.; De Pergola, G.; Romanelli, F.; et al. Performance of Fatty Liver Index in Identifying Non-Alcoholic Fatty Liver Disease in Population Studies. A Meta-Analysis. J. Clin. Med. 2021, 10, 1877. [Google Scholar] [CrossRef]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. NAFLD Nomenclature consensus group A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023, 78, 1966–1986. [Google Scholar] [CrossRef] [PubMed]
- Ruescas-Nicolau, M.-A.; Sánchez-Sánchez, M.L.; Cortés-Amador, S.; Pérez-Alenda, S.; Arnal-Gómez, A.; Climent-Toledo, A.; Carrasco, J.J. Validity of the International Physical Activity Questionnaire Long form for Assessing Physical Activity and Sedentary Behavior in Subjects with Chronic Stroke. Int. J. Environ. Res. Public Health 2021, 18, 4729. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, M.S.; Morabia, A.; Sloutskis, D. Definition and prevalence of sedentarism in an urban population. Am. J. Public Health 1999, 89, 862–867. [Google Scholar] [CrossRef]
- Physical Activity. Available online: https://www.who.int/news-room/fact-sheets/detail/physical-activity (accessed on 13 June 2024).
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes. Diabetes Care 2017, 40, S11–S24. [Google Scholar] [CrossRef] [PubMed]
- Olson, D.E.; Rhee, M.K.; Herrick, K.; Ziemer, D.C.; Twombly, J.G.; Phillips, L.S. Screening for diabetes and pre-diabetes with proposed A1C-based diagnostic criteria. Diabetes Care 2010, 33, 2184–2189. [Google Scholar] [CrossRef]
- Vioque, J.; Navarrete-Muñoz, E.-M.; Gimenez-Monzó, D.; García-de-la-Hera, M.; Granado, F.; Young, I.S.; Ramón, R.; Ballester, F.; Murcia, M.; Rebagliato, M.; et al. INMA-Valencia Cohort Study Reproducibility and validity of a food frequency questionnaire among pregnant women in a Mediterranean area. Nutr. J. 2013, 12, 26. [Google Scholar] [CrossRef]
- CFA 101 EMBARAZADAS|Unidad de Epidemiología de la Nutrición. Available online: https://epinut.umh.es/cfa-101-inma-embarazadas/ (accessed on 7 March 2024).
- ARS Home: USDA ARS. Available online: https://www.ars.usda.gov/ (accessed on 3 May 2024).
- Tablas de Composición de Alimentos Del CESNID. Available online: https://www.sennutricion.org/es/2013/05/13/tablas-de-composicin-de-alimentos-del-cesnid (accessed on 7 March 2024).
- Composition of Foods Integrated Dataset (CoFID). Available online: https://www.gov.uk/government/publications/composition-of-foods-integrated-dataset-cofid (accessed on 3 May 2024).
- EFSA Sets Population Reference Intakes for Protein|EFSA. Available online: https://www.efsa.europa.eu/en/press/news/120209 (accessed on 28 March 2024).
- Harrell, F.E. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis; Springer: Nashville, TN, USA, 2024. [Google Scholar] [CrossRef]
- R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 7 March 2024).
- Chaturvedi, S.; Tripathi, D.; Vikram, N.K.; Madhusudan, K.S.; Pandey, R.M.; Bhatia, N. Association of nutrient intake with non-alcoholic fatty liver disease and liver steatosis in adult Indian population—A case control study. Hum. Nutr. Metab. 2023, 32, 200188. [Google Scholar] [CrossRef]
- Salehi-Sahlabadi, A.; Teymoori, F.; Ahmadirad, H.; Mokhtari, E.; Azadi, M.; Seraj, S.S.; Hekmatdoost, A. Nutrient patterns and non-alcoholic fatty liver disease in Iranian Adul: A case-control study. Front. Nutr. 2022, 9, 977403. [Google Scholar] [CrossRef]
- Pan, J.; Zhou, Y.; Pang, N.; Yang, L. Dietary Niacin Intake and Mortality Among Individuals with Nonalcoholic Fatty Liver Disease. JAMA Netw. Open 2024, 7, e2354277. [Google Scholar] [CrossRef]
- Li, L.; Sun, J.; Wang, H.; Ouyang, Y.; Zhang, J.; Li, T.; Wei, Y.; Gong, W.; Zhou, X.; Zhang, B. Spatial Distribution and Temporal Trends of Dietary Niacin Intake in Chinese Residents ≥ 5 Years of Age between 1991 and 2018. Nutrients 2023, 15, 638. [Google Scholar] [CrossRef]
- Rezayat, A.A.; Moghadam, M.D.; Nour, M.G.; Shirazinia, M.; Ghodsi, H.; Zahmatkesh, M.R.R.; Noghabi, M.T.; Hoseini, B.; Rezayat, K.A. Association between smoking and non-alcoholic fatty liver disease: A systematic review and meta-analysis. SAGE Open Med. 2018, 6, 2050312117745223. [Google Scholar] [CrossRef] [PubMed]
- Rivera, J.C.; Espinoza-Derout, J.; Hasan, K.M.; Molina-Mancio, J.; Martínez, J.; Lao, C.J.; Lee, M.L.; Lee, D.L.; Wilson, J.; Sinha-Hikim, A.P.; et al. Hepatic steatosis induced by nicotine plus Coca-ColaTM is prevented by nicotinamide riboside (NR). Front. Endocrinol. 2024, 15, 1282231. [Google Scholar] [CrossRef] [PubMed]
- El-Zayadi, A.-R. Heavy smoking and liver. World J. Gastroenterol. 2006, 12, 6098–6101. [Google Scholar] [CrossRef] [PubMed]
- Ballestri, S.; Nascimbeni, F.; Baldelli, E.; Marrazzo, A.; Romagnoli, D.; Lonardo, A. NAFLD as a Sexual Dimorphic Disease: Role of Gender and Reproductive Status in the Development and Progression of Nonalcoholic Fatty Liver Disease and Inherent Cardiovascular Risk. Adv. Ther. 2017, 34, 1291–1326. [Google Scholar] [CrossRef]
- Kojima, S.-I.; Watanabe, N.; Numata, M.; Ogawa, T.; Matsuzaki, S. Increase in the prevalence of fatty liver in Japan over the past 12 years: Analysis of clinical background. J. Gastroenterol. 2003, 38, 954–961. [Google Scholar] [CrossRef]
- Zhou, M.; Yang, N.; Xing, X.; Chang, D.; Li, J.; Deng, J.; Chen, Y.; Hu, C.; Zhang, R.; Lu, X.; et al. Obesity interacts with hyperuricemia on the severity of non-alcoholic fatty liver disease. BMC Gastroenterol. 2021, 21, 43. [Google Scholar] [CrossRef]
- Zimmermann, E.; Gamborg, M.; Holst, C.; Baker, J.L.; Sørensen, T.I.A.; Berentzen, T.L. Body mass index in school-aged children and the risk of routinely diagnosed non-alcoholic fatty liver disease in adulthood: A prospective study based on the Copenhagen School Health Records Register. BMJ Open 2015, 5, e006998. [Google Scholar] [CrossRef]


| Characteristics | Non-MASLD (n = 222) | MASLD (n = 222) | p-Value |
|---|---|---|---|
| Age, years | 55.1 (11.9) | 55.2 (11.9) | 0.975 |
| Women, n (%) | 113 (50.9) | 113 (50.9) | 1.000 |
| Caucasian, n (%) | 218 (98.2) | 211 (95.0) | 0.096 |
| Secondary high cycle, n (%) | 123 (55.4) | 95 (42.8) | 0.004 |
| Weight, kg | 70.2 (10.8) | 89.2 (13.3) | <0.001 |
| Height, cm | 166 (9.7) | 165 (9.3) | 0.080 |
| WC, cm | 92.1 (9.7) | 110 (10.0) | <0.001 |
| BMI, kg/m2 | 25.4 (3.1) | 33.0 (5.0) | <0.001 |
| Glucose, mg/dL | 102 (30.6) | 130 (54.1) | <0.001 |
| HbA1c, % | 5.9 (0.9) | 6.8 (1.7) | <0.001 |
| sBP, mm/Hg | 126 (18.9) | 136 (18.4) | <0.001 |
| dBP, mm/Hg | 77.0 (9.9) | 79.8 (10.9) | 0.005 |
| Total cholesterol, mg/dL | 203 (34.4) | 200 (38.9) | 0.360 |
| HDL, mg/dL | 59.6 (15.1) | 50.5 (12.8) | <0.001 |
| LDL, mg/dL | 125 (29.1) | 121 (33.1) | 0.139 |
| Triglycerides, mg/dL | 94.5 (39.9) | 163 (134) | <0.001 |
| GGT, U/L | 22.6 (30.3) | 45.9 (107) | 0.002 |
| ALT, U/L | 21.3 (25.8) | 27.3 (16.6) | 0.004 |
| FLI | 31.5 (17.1) | 81.4 (12.4) | <0.001 |
| GFR | 91.0 (13.3) | 93.6 (16.2) | 0.150 |
| Physically sedentary, n, (%) | 76 (34.2) | 109 (49.5) | 0.002 |
| Smoking, n, (%) | 42 (18.9) | 54 (24.3) | 0.384 |
| Alcohol, g/day | 5.25 (6.3) | 5.02 (7.1) | 0.724 |
| T2DM, n, (%) | 50 (22.5) | 117 (52.7) | <0.001 |
| Prediabetes, n, (%) | 66 (29.7) | 48 (21.6) | 0.065 |
| Dyslipidemia, n, (%) | 43 (19.4) | 70 (31.5) | 0.005 |
| Hypertension, n, (%) | 90 (15.2) | 74 (25.4) | <0.001 |
| Dietary Intake | Non-MASLD (n = 222) | MASLD (n = 222) | p-Value |
|---|---|---|---|
| Energy intake (kcal/day) | 2197.0 (533.0) | 2168.0 (543.0) | 0.569 |
| Carbohydrates (g/day) | 219.0 (35.6) | 217.0 (36.8) | 0.712 |
| Proteins (g/day) | 98.4 (14.6) | 99.6 (15.8) | 0.400 |
| Protein intake (g/kg/day) | 1.4 (0.3) | 1.1 (0.2) | <0.001 |
| Total fat (g/day) | 89.1 (14.4) | 89.2 (15.1) | 0.923 |
| Saturated fat (g/day) | 25.0 (5.7) | 24.8 (4.9) | 0.805 |
| Monounsaturated fat (g/day) | 42.2 (10.1) | 42.8 (10.7) | 0.533 |
| Polyunsaturated fat (g/day) | 15.1 (4.2) | 14.9 (4.4) | 0.482 |
| Total fiber (g/day) | 24.7 (5.5) | 24.4 (5.5) | 0.517 |
| Fiber soluble (g/day) | 3.8 (1.1) | 3.9 (1.2) | 0.468 |
| Insoluble fiber (g/day) | 14.2 (4.6) | 14.2 (4.5) | 0.915 |
| Niacin (mg/day) | 27.2 (5.13) | 27.2 (5.16) | 0.931 |
| Niacin Equivalents (mg/day) | 43.1 (6.9) | 43.3 (6.9) | 0.800 |
| Niacin Intake (mg/day) | ||||
|---|---|---|---|---|
| Tertile 1 (<25) (n = 151) | Tertile 2 (25–30) (n = 157) | Tertile 3 (>30) (n = 136) | p-Value | |
| Non-MASLD | 75 (49.7) | 80 (51.0) | 67 (49.3) | 0.954 |
| MASLD | 76 (50.3) | 77 (49.0) | 69 (50.7) | |
| Niacin Equivalents Intake (NE) (mg/day) | ||||
|---|---|---|---|---|
| Tertile 1 (<40) (n = 141) | Tertile 2 (40–46) (n = 154) | Tertile 3 (>46) (n = 149) | p-Value | |
| Non-MASLD | 73 (51.8) | 76 (49.4) | 73 (49.0) | 0.876 |
| MASLD | 68 (48.2) | 78 (50.6) | 76 (51.0) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antentas, M.; Rojo-López, M.I.; Vendrell, P.; Granado-Casas, M.; Genua, I.; Fernandez-Camins, B.; Rossell, J.; Niño-Narvión, J.; Moreira, E.; Castelblanco, E.; et al. Impact of Dietary Niacin on Metabolic Dysfunction-Associated Steatotic Liver Disease in Mediterranean Subjects: A Population-Based Study. Nutrients 2024, 16, 4178. https://doi.org/10.3390/nu16234178
Antentas M, Rojo-López MI, Vendrell P, Granado-Casas M, Genua I, Fernandez-Camins B, Rossell J, Niño-Narvión J, Moreira E, Castelblanco E, et al. Impact of Dietary Niacin on Metabolic Dysfunction-Associated Steatotic Liver Disease in Mediterranean Subjects: A Population-Based Study. Nutrients. 2024; 16(23):4178. https://doi.org/10.3390/nu16234178
Chicago/Turabian StyleAntentas, Maria, Marina Idalia Rojo-López, Pau Vendrell, Minerva Granado-Casas, Idoia Genua, Berta Fernandez-Camins, Joana Rossell, Julia Niño-Narvión, Estefanía Moreira, Esmeralda Castelblanco, and et al. 2024. "Impact of Dietary Niacin on Metabolic Dysfunction-Associated Steatotic Liver Disease in Mediterranean Subjects: A Population-Based Study" Nutrients 16, no. 23: 4178. https://doi.org/10.3390/nu16234178
APA StyleAntentas, M., Rojo-López, M. I., Vendrell, P., Granado-Casas, M., Genua, I., Fernandez-Camins, B., Rossell, J., Niño-Narvión, J., Moreira, E., Castelblanco, E., Ortega, E., Vlacho, B., Alonso, N., Mauricio, D., & Julve, J. (2024). Impact of Dietary Niacin on Metabolic Dysfunction-Associated Steatotic Liver Disease in Mediterranean Subjects: A Population-Based Study. Nutrients, 16(23), 4178. https://doi.org/10.3390/nu16234178









