Practical Recommendations for the Diagnosis and Management of Lysosomal Acid Lipase Deficiency with a Focus on Wolman Disease
Abstract
:1. Introduction
2. Methods
3. Clinical Manifestations
3.1. Infantile-Onset LAL-D (Wolman Disease)
3.2. Later-Onset LAL-D
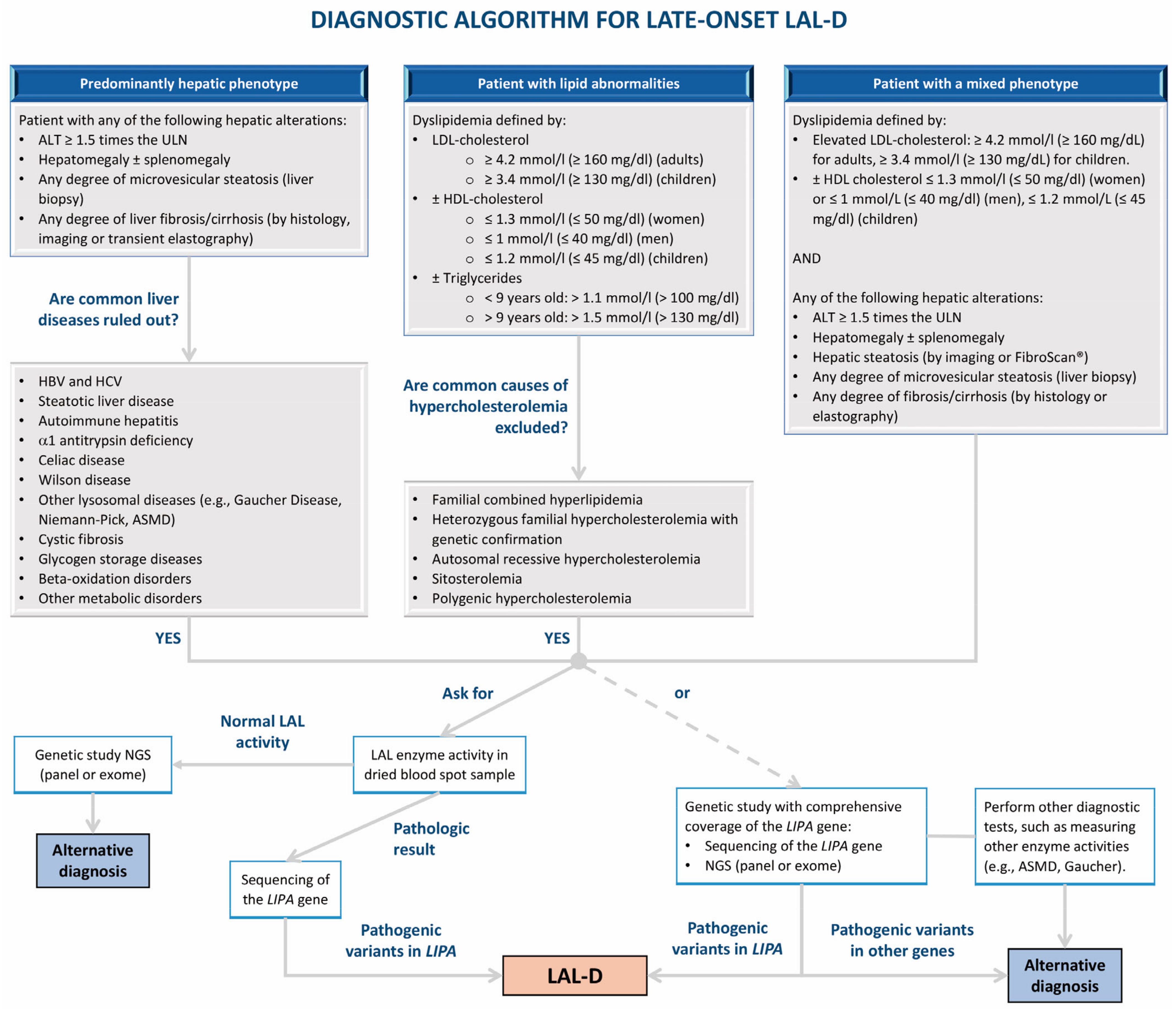
4. Diagnosis
4.1. Biochemical Suspicion
4.2. Determination of LAL Activity
4.3. Molecular Analysis of the LIPA Gene
4.4. Other Diagnostic Tests
4.4.1. Biomarkers
4.4.2. Diagnostic Tools: Imaging, Biopsy, and Histology
4.5. Differential Diagnosis
5. Treatment
5.1. Infantile-Onset LAL-D (Wolman Disease)
5.1.1. Enzyme Replacement Therapy
5.1.2. Nutrition
5.1.3. Hematopoietic Stem Cell Transplantation
5.1.4. Initial Approach and Specific Problems in Infantile-Onset LAL-D
5.2. Later-Onset LAL-D
5.2.1. Enzyme Replacement Therapy
5.2.2. Lipid-Lowering Drugs
5.2.3. Liver Transplantation
5.3. Treatments Under Investigation
6. Proposed Diagnosis and Follow-Up Algorithms
7. Transition from Pediatric to Adult Medicine
8. Strengths and Limitations
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Reiner, Z.; Guardamagna, O.; Nair, D.; Soran, H.; Hovingh, K.; Bertolini, S.; Jones, S.; Coric, M.; Calandra, S.; Hamilton, J.; et al. Lysosomal acid lipase deficiency: An under-recognized cause of dyslipidaemia and liver dysfunction. Atherosclerosis 2014, 235, 21–30. [Google Scholar] [PubMed]
- Bernstein, D.L.; Hulkova, H.; Bialer, M.G.; Desnick, R.J. Cholesteryl ester storage disease: Review of the findings in 135 reported patients with an underdiagnosed disease. J. Hepatol. 2013, 58, 1230–1243. [Google Scholar] [PubMed]
- Jones, S.A.; Valayannopoulos, V.; Schneider, E.; Eckert, S.; Banikazemi, M.; Bialer, M.; Cederbaum, S.; Chan, A.; Dhawan, A.; Di Rocco, M.; et al. Rapid progression and mortality of lysosomal acid lipase deficiency presenting in infants. Genet. Med. 2016, 18, 452–458. [Google Scholar] [CrossRef]
- Strebinger, G.; Muller, E.; Feldman, A.; Aigner, E. Lysosomal acid lipase deficiency—Early diagnosis is the key. Hepatic Med. 2019, 11, 79–88. [Google Scholar]
- Balwani, M.; Balistreri, W.; D’Antiga, L.; Evans, J.; Ros, E.; Abel, F.; Wilson, D.P. Lysosomal acid lipase deficiency manifestations in children and adults: Baseline data from an international registry. Liver Int. 2023, 43, 1537–1547. [Google Scholar]
- Burton, B.K.; Deegan, P.B.; Enns, G.M.; Guardamagna, O.; Horslen, S.; Hovingh, G.K.; Lobritto, S.J.; Malinova, V.; McLin, V.A.; Raiman, J.; et al. Clinical features of lysosomal acid lipase deficiency. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 619–625. [Google Scholar]
- Pericleous, M.; Kelly, C.; Wang, T.; Livingstone, C.; Ala, A. Wolman’s disease and cholesteryl ester storage disorder: The phenotypic spectrum of lysosomal acid lipase deficiency. Lancet Gastroenterol. Hepatol. 2017, 2, 670–679. [Google Scholar] [CrossRef]
- Scott, S.A.; Liu, B.; Nazarenko, I.; Martis, S.; Kozlitina, J.; Yang, Y.; Ramírez, C.; Kasai, Y.; Hyatt, T.; Peter, I.; et al. Frequency of the cholesteryl ester storage disease common lipa e8sjm mutation (c.894g>a) in various racial and ethnic groups. Hepatology 2013, 58, 958–965. [Google Scholar]
- Vijay, S.; Brassier, A.; Ghosh, A.; Fecarotta, S.; Abel, F.; Marulkar, S.; Jones, S.A. Long-term survival with sebelipase alfa enzyme replacement therapy in infants with rapidly progressive lysosomal acid lipase deficiency: Final results from 2 open-label studies. Orphanet J. Rare Dis. 2021, 16, 13. [Google Scholar]
- Camarena, C.; Aldamiz-Echevarria, L.J.; Polo, B.; Barba Romero, M.A.; García, I.; Cebolla, J.J.; Ros, E. Update on lysosomal acid lipase deficiency: Diagnosis, treatment and patient management. Med. Clin. 2017, 148, 429.e1–429.e10. [Google Scholar] [CrossRef]
- Aguisanda, F.; Thorne, N.; Zheng, W. Targeting wolman disease and cholesteryl ester storage disease: Disease pathogenesis and therapeutic development. Curr. Chem. Genom. Transl. Med. 2017, 11, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Alabbas, F.; Elyamany, G.; Alanzi, T.; Ali, T.B.; Albatniji, F.; Alfaraidi, H. Wolman’s disease presenting with secondary hemophagocytic lymphohistiocytosis: A case report from saudi arabia and literature review. BMC Pediatr. 2021, 21, 72. [Google Scholar]
- Rabah, F.; Al-Hashmi, N.; Beshlawi, I. Wolman’s disease with secondary hemophagocytic lymphohistiocytosis. Pediatr. Hematol. Oncol. 2014, 31, 576–578. [Google Scholar] [PubMed]
- Küçükçongar Yavas, A.; Orhaner, B.; Genc, P.; Kilic, N.; Erdogan, H.; Ozdemir, O.; Ekici, A. Secondary hemophagocytic lymphohistiocytosis in an infant with wolman disease. Turk. J. Haematol. 2017, 34, 264–265. [Google Scholar] [PubMed]
- Chabchoub, I.; Boudabbous, H.; Maaloul, I.; Ben Abdelaziz, R.; Ben Chehida, A.; Ayadi, L.; Kamoun, T.; Tebib, N.; Boudaouara, T.; Bekri, S.; et al. Hemophagocytic lymphohistiocytosis: A rare complication of an ultrarare lysosomal storage disease. J. Pediatr. Hematol. Oncol. 2020, 42, 310–312. [Google Scholar]
- Bartakke, S.; Nisal, A.; Bafna, V.; Valecha, A. Secondary hemophagocytic lymphohistiocytosis in an infant with wolman disease. Indian J. Hematol. Blood Transfus. 2021, 37, 191–192. [Google Scholar]
- Janka, G.E.; Lehmberg, K. Hemophagocytic lymphohistiocytosis: Pathogenesis and treatment. Hematol. Am. Soc. Hematol. Educ. Program 2013, 2013, 605–611. [Google Scholar]
- Santos Silva, E.; Klaudel-Dreszler, M.; Bakula, A.; Oliva, T.; Sousa, T.; Fernandes, P.C.; Tylki-Szymanska, A.; Kamenets, E.; Martins, E.; Socha, P. Early onset lysosomal acid lipase deficiency presenting as secondary hemophagocytic lymphohistiocytosis: Two infants treated with sebelipase alfa. Clin. Res. Hepatol. Gastroenterol. 2018, 42, e77–e82. [Google Scholar]
- Pisciotta, L.; Fresa, R.; Bellocchio, A.; Pino, E.; Guido, V.; Cantafora, A.; Di Rocco, M.; Calandra, S.; Bertolini, S. Cholesteryl ester storage disease (cesd) due to novel mutations in the lipa gene. Mol. Genet. Metab. 2009, 97, 143–148. [Google Scholar]
- Sheth, S.; Toth, P.P.; Baum, S.J.; Aggarwal, M. Distinguishing lysosomal acid lipase deficiency from familial hypercholesterolemia. JACC Case Rep. 2023, 24, 102023. [Google Scholar]
- Castro Narro, G.E.; Gamboa Domínguez, A.; Consuelo Sánchez, A.; Salazar Martínez, A.; Agramonte Hevia, J.; Cebolla, J.J.; Cuellar Mendoza, M.E.; Díaz Hernández, H.A. Combined hepatocellular-cholangiocarcinoma in a patient with cirrhosis due to cholesteryl ester storage disease. Hepatology 2019, 69, 1838–1841. [Google Scholar] [CrossRef] [PubMed]
- Cunha-Silva, M.; de França, E.V.C.; Veiga, C.T.; Greca, R.D.; de Moraes, P.B.S.; de Campos Mazo, D.F.; de Ataíde, E.C.; Perales, S.R.; Monici, L.T.; Sevá-Pereira, T. 15-year progression to liver cancer in the lack of treatment for lysosomal acid lipase deficiency: A case report. Medicine 2022, 101, e30315. [Google Scholar] [CrossRef]
- Wilson, D.P.; Patni, N. Lysosomal acid lipase deficiency. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2023. [Google Scholar]
- Chora, J.R.; Alves, A.C.; Medeiros, A.M.; Mariano, C.; Lobarinhas, G.; Guerra, A.; Mansilha, H.; Cortez-Pinto, H.; Bourbon, M. Lysosomal acid lipase deficiency: A hidden disease among cohorts of familial hypercholesterolemia? J. Clin. Lipidol. 2017, 11, 477–484.e2. [Google Scholar] [CrossRef]
- Sustar, U.; Groselj, U.; Trebusak Podkrajsek, K.; Mlinaric, M.; Kovac, J.; Thaler, M.; Drole Torkar, A.; Skarlovnik, A.; Battelino, T.; Hovnik, T. Early discovery of children with lysosomal acid lipase deficiency with the universal familial hypercholesterolemia screening program. Front. Genet. 2022, 13, 936121. [Google Scholar] [CrossRef]
- Cuchel, M.; Raal, F.J.; Hegele, R.A.; Al-Rasadi, K.; Arca, M.; Averna, M.; Bruckert, E.; Freiberger, T.; Gaudet, D.; Harada-Shiba, M.; et al. 2023 update on european atherosclerosis society consensus statement on homozygous familial hypercholesterolaemia: New treatments and clinical guidance. Eur. Heart J. 2023, 44, 2277–2291. [Google Scholar] [CrossRef]
- Asna Ashari, K.; Azari-Yam, A.; Shahrooei, M.; Ziaee, V. Wolman disease presenting with hemophagocytic lymphohistiocytosis syndrome and a novel lipa gene variant: A case report and review of the literature. J. Med. Case Rep. 2023, 17, 369. [Google Scholar] [CrossRef]
- Anderson, G.; Smith, V.V.; Malone, M.; Sebire, N.J. Blood film examination for vacuolated lymphocytes in the diagnosis of metabolic disorders; retrospective experience of more than 2500 cases from a single centre. J. Clin. Pathol. 2005, 58, 1305–1310. [Google Scholar] [CrossRef]
- Hamilton, J.; Jones, I.; Srivastava, R.; Galloway, P. A new method for the measurement of lysosomal acid lipase in dried blood spots using the inhibitor lalistat 2. Clin. Chim. Acta 2012, 413, 1207–1210. [Google Scholar] [CrossRef]
- Civallero, G.; De Mari, J.; Bittar, C.; Burin, M.; Giugliani, R. Extended use of a selective inhibitor of acid lipase for the diagnosis of wolman disease and cholesteryl ester storage disease. Gene 2014, 539, 154–156. [Google Scholar] [CrossRef]
- Chamoles, N.A.; Blanco, M.B.; Gaggioli, D.; Casentini, C. Hurler-like phenotype: Enzymatic diagnosis in dried blood spots on filter paper. Clin. Chem. 2001, 47, 2098–2102. [Google Scholar] [CrossRef]
- Masi, S.; Chennamaneni, N.; Turecek, F.; Scott, C.R.; Gelb, M.H. Specific substrate for the assay of lysosomal acid lipase. Clin. Chem. 2018, 64, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.A.; Rao, N.; Byrum, R.S.; Rothschild, C.B.; Bowden, D.W.; Hayworth, R.; Pettenati, M. In situ localization of the genetic locus encoding the lysosomal acid lipase/cholesteryl esterase (lipa) deficient in wolman disease to chromosome 10q23.2-q23.3. Genomics 1993, 15, 245–247. [Google Scholar] [CrossRef]
- Klima, H.; Ullrich, K.; Aslanidis, C.; Fehringer, P.; Lackner, K.J.; Schmitz, G. A splice junction mutation causes deletion of a 72-base exon from the mrna for lysosomal acid lipase in a patient with cholesteryl ester storage disease. J. Clin. Investig. 1993, 92, 2713–2718. [Google Scholar] [CrossRef]
- Ruiz-Andrés, C.; Sellés, E.; Arias, A.; Gort, L.; Spanish LAL Deficiency Working Group. Lysosomal acid lipase deficiency in 23 spanish patients: High frequency of the novel c.966+2t>g mutation in wolman disease. JIMD Rep. 2017, 37, 7–12. [Google Scholar]
- Guerreiro, G.; Deon, M.; Vargas, C.R. Evaluation of biochemical profile and oxidative damage to lipids and proteins in patients with lysosomal acid lipase deficiency. Biochem. Cell Biol. 2023, 101, 294–302. [Google Scholar] [CrossRef]
- Pajares, S.; Arias, A.; García-Villoria, J.; Macías-Vidal, J.; Ros, E.; de las Heras, J.; Girós, M.; Coll, M.J.; Ribes, A. Cholestane-3b,5a,6b-triol: High levels in niemann-pick type c, cerebrotendinous xanthomatosis, and lysosomal acid lipase deficiency. J. Lipid Res. 2015, 56, 1926–1935. [Google Scholar] [CrossRef]
- Isman, F.; Hobert, J.A.; Thompson, J.N.; Natowicz, M.R. Plasma chitotriosidase in lysosomal storage diseases. Clin. Chim. Acta 2008, 387, 165–167. [Google Scholar] [CrossRef]
- Brinkman, J.; Wijburg, F.A.; Hollak, C.E.; Groener, J.E.; Verhoek, M.; Scheij, S.; Aten, J.; Boot, R.G.; Aerts, J.M. Plasma chitotriosidase and ccl18: Early biochemical surrogate markers in type b niemann-pick disease. J. Inherit. Metab. Dis. 2005, 28, 13–20. [Google Scholar] [CrossRef]
- de Castro-Orós, I.; Irún, P.; Cebolla, J.J.; Rodríguez-Sureda, V.; Mallén, M.; Pueyo, M.J.; Mozas, P.; Domínguez, C.; Pocoví, M.; Spanish NP-C Group. Assessment of plasma chitotriosidase activity, ccl18/parc concentration and np-c suspicion index in the diagnosis of niemann-pick disease type c: A prospective observational study. J. Transl. Med. 2017, 15, 43. [Google Scholar] [CrossRef]
- Hägg, D.A.; Olson, F.J.; Kjelldahl, J.; Jernas, M.; Thelle, D.S.; Carlsson, L.M.; Fagerberg, B.; Svensson, P.A. Expression of chemokine (c-c motif) ligand 18 in human macrophages and atherosclerotic plaques. Atherosclerosis 2009, 204, e15–e20. [Google Scholar] [CrossRef]
- Boot, R.G.; Renkema, G.H.; Verhoek, M.; Strijland, A.; Bliek, J.; de Meulemeester, T.M.; Mannens, M.M.; Aerts, J.M. The human chitotriosidase gene. Nature of inherited enzyme deficiency. J. Biol. Chem. 1998, 273, 25680–25685. [Google Scholar] [CrossRef] [PubMed]
- Cebolla, J.J.; Irun, P.; Mozas, P.; Giraldo, P. Evaluation of two approaches to lysosomal acid lipase deficiency patient identification: An observational retrospective study. Atherosclerosis 2019, 285, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Boenzi, S.; Deodato, F.; Taurisano, R.; Goffredo, B.M.; Rizzo, C.; Dionisi-Vici, C. Evaluation of plasma cholestane-3b,5a,6b-triol and 7-ketocholesterol in inherited disorders related to cholesterol metabolism. J. Lipid Res. 2016, 57, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Boaz, M.; Iuliano, L.; Himmelfarb, J.; Matas, Z.; Micheletta, F.; McMonagle, E.; Friedman, V.; Natoli, S.; Gvirtz, G.; Biro, A.; et al. Baseline oxysterols and other markers of oxidative stress, inflammation and malnutrition in the vitamin e and intima media thickness progression in end-stage renal disease (viper) cohort. Nephron Clin. Pract. 2005, 100, c111–c119. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Thelwall, P.E.; Smith, F.E.; Leavitt, M.C.; Canty, D.; Hu, W.; Hollingsworth, K.G.; Thoma, C.; Trenell, M.I.; Taylor, R.; Rutkowski, J.V.; et al. Hepatic cholesteryl ester accumulation in lysosomal acid lipase deficiency: Non-invasive identification and treatment monitoring by magnetic resonance. J. Hepatol. 2013, 59, 543–549. [Google Scholar] [CrossRef]
- Hautekeete, M.L.; Degott, C.; Benhamou, J.P. Microvesicular steatosis of the liver. Acta Clin. Belg. 1990, 45, 311–326. [Google Scholar] [CrossRef]
- Hulkova, H.; Elleder, M. Distinctive histopathological features that support a diagnosis of cholesterol ester storage disease in liver biopsy specimens. Histopathology 2012, 60, 1107–1113. [Google Scholar] [CrossRef]
- Giraldo, P.; López de Frutos, L.; Cebolla, J.J. Recommendations for overcoming challenges in the diagnosis of lysosomal acid lipase deficiency. Expert Opin. Orphan Drugs 2022, 10, 11–21. [Google Scholar] [CrossRef]
- Boldrini, R.; Devito, R.; Biselli, R.; Filocamo, M.; Bosman, C. Wolman disease and cholesteryl ester storage disease diagnosed by histological and ultrastructural examination of intestinal and liver biopsy. Pathol. Res. Pract. 2004, 200, 231–240. [Google Scholar] [CrossRef]
- Lipinski, P.; Cielecka-Kuszyk, J.; Bozkiewicz-Kasperczyk, A.; Perkowska, B.; Jurkiewicz, E.; Tylki-Szymanska, A. Progressive macrophage accumulation in lysosomal acid lipase deficiency. Mol. Genet. Metab. Rep. 2020, 23, 100594. [Google Scholar] [CrossRef] [PubMed]
- vom Dahl, S.; Harzer, K.; Rolfs, A.; Albrecht, B.; Niederau, C.; Vogt, C.; van Weely, S.; Aerts, J.; Muller, G.; Haussinger, D. Hepatosplenomegalic lipidosis: What unless gaucher? Adult cholesteryl ester storage disease (cesd) with anemia, mesenteric lipodystrophy, increased plasma chitotriosidase activity and a homozygous lysosomal acid lipase -1 exon 8 splice junction mutation. J. Hepatol. 1999, 31, 741–746. [Google Scholar] [CrossRef]
- Hegele, R.A.; Boren, J.; Ginsberg, H.N.; Arca, M.; Averna, M.; Binder, C.J.; Calabresi, L.; Chapman, M.J.; Cuchel, M.; von Eckardstein, A.; et al. Rare dyslipidaemias, from phenotype to genotype to management: A european atherosclerosis society task force consensus statement. Lancet Diabetes Endocrinol. 2020, 8, 50–67. [Google Scholar] [CrossRef]
- Kohli, R.; Ratziu, V.; Fiel, M.I.; Waldmann, E.; Wilson, D.P.; Balwani, M. Initial assessment and ongoing monitoring of lysosomal acid lipase deficiency in children and adults: Consensus recommendations from an international collaborative working group. Mol. Genet. Metab. 2020, 129, 59–66. [Google Scholar] [CrossRef]
- Chatrath, H.; Keilin, S.; Attar, B.M. Cholesterol ester storage disease (cesd) diagnosed in an asymptomatic adult. Dig. Dis. Sci. 2009, 54, 168–173. [Google Scholar] [CrossRef]
- European Medicines Agency. Product Information: Kanuma® (Sebelipase Alfa). Available online: https://www.ema.europa.eu/en/documents/product-information/kanuma-epar-product-information_en.pdf (accessed on 13 September 2024).
- Jones, S.A.; Rojas-Caro, S.; Quinn, A.G.; Friedman, M.; Marulkar, S.; Ezgu, F.; Zaki, O.; Gargus, J.J.; Hughes, J.; Plantaz, D.; et al. Survival in infants treated with sebelipase a for lysosomal acid lipase deficiency: An open-label, multicenter, dose-escalation study. Orphanet J. Rare Dis. 2017, 12, 25. [Google Scholar] [CrossRef]
- Malinová, V.; Balwani, M.; Sharma, R.; Arnoux, J.B.; Kane, J.; Whitley, C.B.; Marulkar, S.; Abel, F. Sebelipase alfa for lysosomal acid lipase deficiency: 5-year treatment experience from a phase 2 open-label extension study. Liver Int. 2020, 40, 2203–2214. [Google Scholar] [CrossRef]
- Demaret, T.; Lacaille, F.; Wicker, C.; Arnoux, J.B.; Bouchereau, J.; Belloche, C.; Gitiaux, C.; Grevent, D.; Broissand, C.; Adjaoud, D.; et al. Sebelipase alfa enzyme replacement therapy in wolman disease: A nationwide cohort with up to ten years of follow-up. Orphanet J. Rare Dis. 2021, 16, 507. [Google Scholar] [CrossRef]
- Burton, B.K.; Sanchez, A.C.; Kostyleva, M.; Martins, A.M.; Marulkar, S.; Abel, F.; Baric, I. Long-term sebelipase alfa treatment in children and adults with lysosomal acid lipase deficiency. J. Pediatr. Gastroenterol. Nutr. 2022, 74, 757–764. [Google Scholar] [CrossRef]
- Potter, J.E.; Petts, G.; Ghosh, A.; White, F.J.; Kinsella, J.L.; Hughes, S.; Roberts, J.; Hodgkinson, A.; Brammeier, K.; Church, H.; et al. Enzyme replacement therapy and hematopoietic stem cell transplant: A new paradigm of treatment in wolman disease. Orphanet J. Rare Dis. 2021, 16, 235. [Google Scholar] [CrossRef]
- de Las Heras, J.; Cano, A.; Vinuesa, A.; Montes, M.; Unceta Suárez, M.; Arza, A.; Jiménez, S.; Vera, E.; del Hoyo, M.; Gendive, M.; et al. Importance of timely treatment initiation in infantile-onset pompe disease, a single-centre experience. Children 2021, 8, 1026. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Aleck, K.; Arvonen, M.; Broomfield, A.; Fecarotta, S.; Gargus, J.J.; Gokcay, G.; de las Heras, J.; Holmes, V.; Jones, R.; et al. Experience of the nutritional management of infantile onset lysosomal acid lipase deficiency (lald). Mol. Genet. Metab. 2018, 123, S51. [Google Scholar] [CrossRef]
- Dixon, M.; Skeath, R.; White, F.J. Disorders of mitochondrial fatty acid oxidation and lipid metabolism. In Clinical Paediatric Dietetics; Shaw, V., Ed.; Wile-Blackwell: Hoboken, NJ, USA, 2020; pp. 640–672. [Google Scholar]
- Krivit, W.; Freese, D.; Chan, K.W.; Kulkarni, R. Wolman’s disease: A review of treatment with bone marrow transplantation and considerations for the future. Bone Marrow Transplant. 1992, 10 (Suppl. 1), 97–101. [Google Scholar] [PubMed]
- Stein, J.; Garty, B.Z.; Dror, Y.; Fenig, E.; Zeigler, M.; Yaniv, I. Successful treatment of wolman disease by unrelated umbilical cord blood transplantation. Eur. J. Pediatr. 2007, 166, 663–666. [Google Scholar] [CrossRef]
- Lum, S.H.; Minkov, M.; Jones, S.A.; Hazelaar, S.; Sirait, T.; Potter, J.E.; Stepensky, P.; Garban, F.; Pichler, H.; Stein, J.; et al. Outcome of haematopoietic cell transplantation in children with lysosomal acid lipase deficiency: A study on behalf of the ebmt inborn errors working party. Bone Marrow Transplant. 2023, 58, 594–596. [Google Scholar] [CrossRef]
- Eskandari, S.K.; Revenich, E.G.M.; Pot, D.J.; de Boer, F.; Bierings, M.; van Spronsen, F.J.; van Hasselt, P.M.; Lindemans, C.A.; Lubout, C.M.A. High-dose ert, rituximab, and early hsct in an infant with wolman’s disease. N. Engl. J. Med. 2024, 390, 623–629. [Google Scholar] [CrossRef]
- de Castro, M.J.; Jones, S.A.; de Las Heras, J.; Sánchez-Pintos, P.; Couce, M.L.; Colón, C.; Crujeiras, P.; Unceta, M.; Church, H.; Brammeier, K.; et al. Twice weekly dosing with sebelipase alfa (kanuma®) rescues severely ill infants with wolman disease. Orphanet J. Rare Dis. 2024, 19, 244. [Google Scholar] [CrossRef]
- Burton, B.K.; Balwani, M.; Feillet, F.; Baric, I.; Burrow, T.A.; Camarena Grande, C.; Coker, M.; Consuelo-Sánchez, A.; Deegan, P.; Di Rocco, M.; et al. A phase 3 trial of sebelipase a in lysosomal acid lipase deficiency. N. Engl. J. Med. 2015, 373, 1010–1020. [Google Scholar] [CrossRef]
- Burton, B.K.; Feillet, F.; Furuya, K.N.; Marulkar, S.; Balwani, M. Sebelipase alfa in children and adults with lysosomal acid lipase deficiency: Final results of the arise study. J. Hepatol. 2022, 76, 577–587. [Google Scholar] [CrossRef]
- Wilson, D.P.; Friedman, M.; Marulkar, S.; Hamby, T.; Bruckert, E. Sebelipase a improves atherogenic biomarkers in adults and children with lysosomal acid lipase deficiency. J. Clin. Lipidol. 2018, 12, 604–614. [Google Scholar] [CrossRef]
- Korbelius, M.; Kuentzel, K.B.; Bradic, I.; Vujic, N.; Kratky, D. Recent insights into lysosomal acid lipase deficiency. Trends Mol. Med. 2023, 29, 425–438. [Google Scholar] [CrossRef]
- Block, R.C.; Razani, B. Options to consider when treating lysosomal acid lipase deficiency. J. Clin. Lipidol. 2016, 10, 1280–1281. [Google Scholar] [CrossRef]
- Levy, R.; Ostlund, R.E., Jr.; Schonfeld, G.; Wong, P.; Semenkovich, C.F. Cholesteryl ester storage disease: Complex molecular effects of chronic lovastatin therapy. J. Lipid Res. 1992, 33, 1005–1015. [Google Scholar] [CrossRef]
- Dalgic, B.; Sari, S.; Gunduz, M.; Ezgu, F.; Tumer, L.; Hasanoglu, A.; Akyol, G. Cholesteryl ester storage disease in a young child presenting as isolated hepatomegaly treated with simvastatin. Turk. J. Pediatr. 2006, 48, 148–151. [Google Scholar]
- Rassoul, F.; Richter, V.; Lohse, P.; Naumann, A.; Purschwitz, K.; Keller, E. Long-term administration of the hmg-coa reductase inhibitor lovastatin in two patients with cholesteryl ester storage disease. Int. J. Clin. Pharmacol. Ther. 2001, 39, 199–204. [Google Scholar] [CrossRef]
- Glueck, C.J.; Lichtenstein, P.; Tracy, T.; Speirs, J. Safety and efficacy of treatment of pediatric cholesteryl ester storage disease with lovastatin. Pediatr. Res. 1992, 32, 559–565. [Google Scholar] [CrossRef]
- Chuang, J.C.; López, A.M.; Posey, K.S.; Turley, S.D. Ezetimibe markedly attenuates hepatic cholesterol accumulation and improves liver function in the lysosomal acid lipase-deficient mouse, a model for cholesteryl ester storage disease. Biochem. Biophys. Res. Commun. 2014, 443, 1073–1077. [Google Scholar] [CrossRef]
- Bernstein, D.L.; Lobritto, S.; Iuga, A.; Remotti, H.; Schiano, T.; Fiel, M.I.; Balwani, M. Lysosomal acid lipase deficiency allograft recurrence and liver failure: Clinical outcomes of 18 liver transplantation patients. Mol. Genet. Metab. 2018, 124, 11–19. [Google Scholar] [CrossRef]
- Sreekantam, S.; Nicklaus-Wollenteit, I.; Orr, J.; Sharif, K.; Vijay, S.; McKiernan, P.J.; Santra, S. Successful long-term outcome of liver transplantation in late-onset lysosomal acid lipase deficiency. Pediatr. Transplant. 2016, 20, 851–854. [Google Scholar] [CrossRef]
- Herzeg, A.; Borges, B.; Lianoglou, B.R.; Gonzalez-Velez, J.; Canepa, E.; Munar, D.; Young, S.P.; Bali, D.; Gelb, M.H.; Chakraborty, P.; et al. Intrauterine enzyme replacement therapies for lysosomal storage disorders: Current developments and promising future prospects. Prenat. Diagn. 2023, 43, 1638–1649. [Google Scholar] [CrossRef]
- Massaro, G.; Geard, A.F.; Liu, W.; Coombe-Tennant, O.; Waddington, S.N.; Baruteau, J.; Gissen, P.; Rahim, A.A. Gene therapy for lysosomal storage disorders: Ongoing studies and clinical development. Biomolecules 2021, 11, 611. [Google Scholar] [CrossRef] [PubMed]
- Lam, P.; Zygmunt, D.A.; Ashbrook, A.; Yan, C.; Du, H.; Martin, P.T. Liver-directed aav gene therapy normalizes disease symptoms and provides cross-correction in a model of lysosomal acid lipase deficiency. Mol. Ther. 2024, 32, 4272–4284. [Google Scholar] [CrossRef] [PubMed]
- Genevaz, D.; Arnoux, A.; Marcel, C.; Brassier, A.; Pichard, S.; Feillet, F.; Labarthe, F.; Chabrol, B.; Berger, M.; Lapointe, A.S.; et al. Transition from child to adult health care for patients with lysosomal storage diseases in france: Current status and priorities-the tenalys study, a patient perspective survey. Orphanet J. Rare Dis. 2022, 17, 68. [Google Scholar] [CrossRef]



| Infantile-Onset LAL-D | Later-Onset LAL-D |
|---|---|
|
|
| LAL-D | Familial Hypercholesterolemia | Non-Alcoholic Fatty Liver Disease |
|---|---|---|
|
|
|
| Age: 2 Months of Life. Weight: 3.5 kg. An Enteral Low-Fat Elemental Modular Formula Is Initially Provided by Continuous Feeding at the Volume the Infant Tolerates Owing to Gut Damage, Malabsorption, and Abdominal Distension. Low-Lipid Parenteral Nutrition Combined with the Enteral Modular Formula in Most Cases. | ||||||||
|---|---|---|---|---|---|---|---|---|
| Initial Diet * | Product | Amount | Protein | Lipids | Carbohydrates | Energy (kcal) | Na | K |
| Proteins | Essential amino acid mix (Nutricia) | 17.5 g (4 g/kg protein equivalent) | 14 g | 0 | 0 | 3.16 kcal/g | 0 | 0 |
| Glucose polymer | Fantomalt (Nutricia), Vitajoule (Vitaflo) | 35 g ** (10% dextrinomaltose) | 0 | 0 | 33.25 g | 3.8 kcal/g | 0 | 0 |
| MCT oil | MCT oil (Nutricia) | 3.5 mL (1 mL/kg) | 0 | 3.3 g | 0 | 8.55 kcal/ml | 0 | 0 |
| Vitamins and mineral supplement | Paediatric Seravit (Nutricia) | 17.5 g (5 g/100 mL) | 0 | 0 | 13.12 g | 2.93 kcal/g | 40 mg/ 100 g | 30 mg/ 100 g |
| Essential Fatty acids | KeyOmega (Vitaflo) | 4 g | 0 | 0.8 g DHA 100 mg AA 200 mg | 2.8 g | 19 kcal per sachet | 0 | 0 |
| Ions *** | e.g., Bioralsuero Casen | 180 mL | 0 | 0 | 2.6 g | 5.8 kcal/ 100 mL | 146.4 mg/ 100 mL | 78.6 mg/ 100 mL |
| Fluid | Water | To 100 mL/kg | 0 | 0 | 0 | 0 | ||
| Interventions | First Year | After First Year | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline a | 1 Month | 3 Months | 6 Months | 9 Months | 12 Months | Every 6 Months | Every 12 Months | Every 24 Months | |
| Recommended | |||||||||
| Clinical evaluation b | √ | √ | √ | √ | √ | √ | √ | ||
| Abdominal ultrasound | √ | √ | √ | ||||||
| Complete blood cell count c | √ | √ Infantile-onset | √ Infantile-onset | √ Infantile-onset | √ | √ | |||
| Liver function d | √ | √ | √ | √ | √ | √ | √ | ||
| Basic lipid profile e | √ | √ | √ | √ | √ | √ | √ | ||
| Extended lipid profile f | √ | √ | √ | ||||||
| Other biomarkers g | √ | √ | √ | √ | |||||
| Iron metabolism | √ | √ Infantile-onset | √ Infantile-onset | √ Infantile-onset | √ | √ | |||
| Lipid-soluble vitamins (A, D, E, K) | √ | √ Infantile-onset | √ Infantile-onset | √ Infantile-onset | √ Infantile-onset | √ | √ | ||
| Polyunsaturated fatty acids | √ | √ Infantile-onset | √ | √ | |||||
| Diet evaluation | √ | √ | √ | √ | √ | √ | √ | ||
| Optional | |||||||||
| Liver biopsy h | √ | Repeat in 2 years (non-periodic) | |||||||
| Fibroscan | √ | √ | If abnormal | If normal | |||||
| MRI/liver ultrasound | √ | If abnormal | If normal | ||||||
| Echocardiography | √ | If abnormal | If normal | ||||||
| Carotid ultrasound i | √ | If abnormal | If normal | ||||||
| Fibrosis serological markers and scores j | √ | √ | √ | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de las Heras, J.; Almohalla, C.; Blasco-Alonso, J.; Bourbon, M.; Couce, M.-L.; de Castro López, M.J.; García Jiménez, M.C.; Gil Ortega, D.; González-Diéguez, L.; Meavilla, S.; et al. Practical Recommendations for the Diagnosis and Management of Lysosomal Acid Lipase Deficiency with a Focus on Wolman Disease. Nutrients 2024, 16, 4309. https://doi.org/10.3390/nu16244309
de las Heras J, Almohalla C, Blasco-Alonso J, Bourbon M, Couce M-L, de Castro López MJ, García Jiménez MC, Gil Ortega D, González-Diéguez L, Meavilla S, et al. Practical Recommendations for the Diagnosis and Management of Lysosomal Acid Lipase Deficiency with a Focus on Wolman Disease. Nutrients. 2024; 16(24):4309. https://doi.org/10.3390/nu16244309
Chicago/Turabian Stylede las Heras, Javier, Carolina Almohalla, Javier Blasco-Alonso, Mafalda Bourbon, Maria-Luz Couce, María José de Castro López, Mª Concepción García Jiménez, David Gil Ortega, Luisa González-Diéguez, Silvia Meavilla, and et al. 2024. "Practical Recommendations for the Diagnosis and Management of Lysosomal Acid Lipase Deficiency with a Focus on Wolman Disease" Nutrients 16, no. 24: 4309. https://doi.org/10.3390/nu16244309
APA Stylede las Heras, J., Almohalla, C., Blasco-Alonso, J., Bourbon, M., Couce, M.-L., de Castro López, M. J., García Jiménez, M. C., Gil Ortega, D., González-Diéguez, L., Meavilla, S., Moreno-Álvarez, A., Pastor-Rosado, J., Sánchez-Pintos, P., Serrano-Gonzalo, I., López, E., Valdivielso, P., Yahyaoui, R., & Quintero, J. (2024). Practical Recommendations for the Diagnosis and Management of Lysosomal Acid Lipase Deficiency with a Focus on Wolman Disease. Nutrients, 16(24), 4309. https://doi.org/10.3390/nu16244309








