The Protective Role of Vitamin K in Aging and Age-Related Diseases
Abstract
:1. Introduction
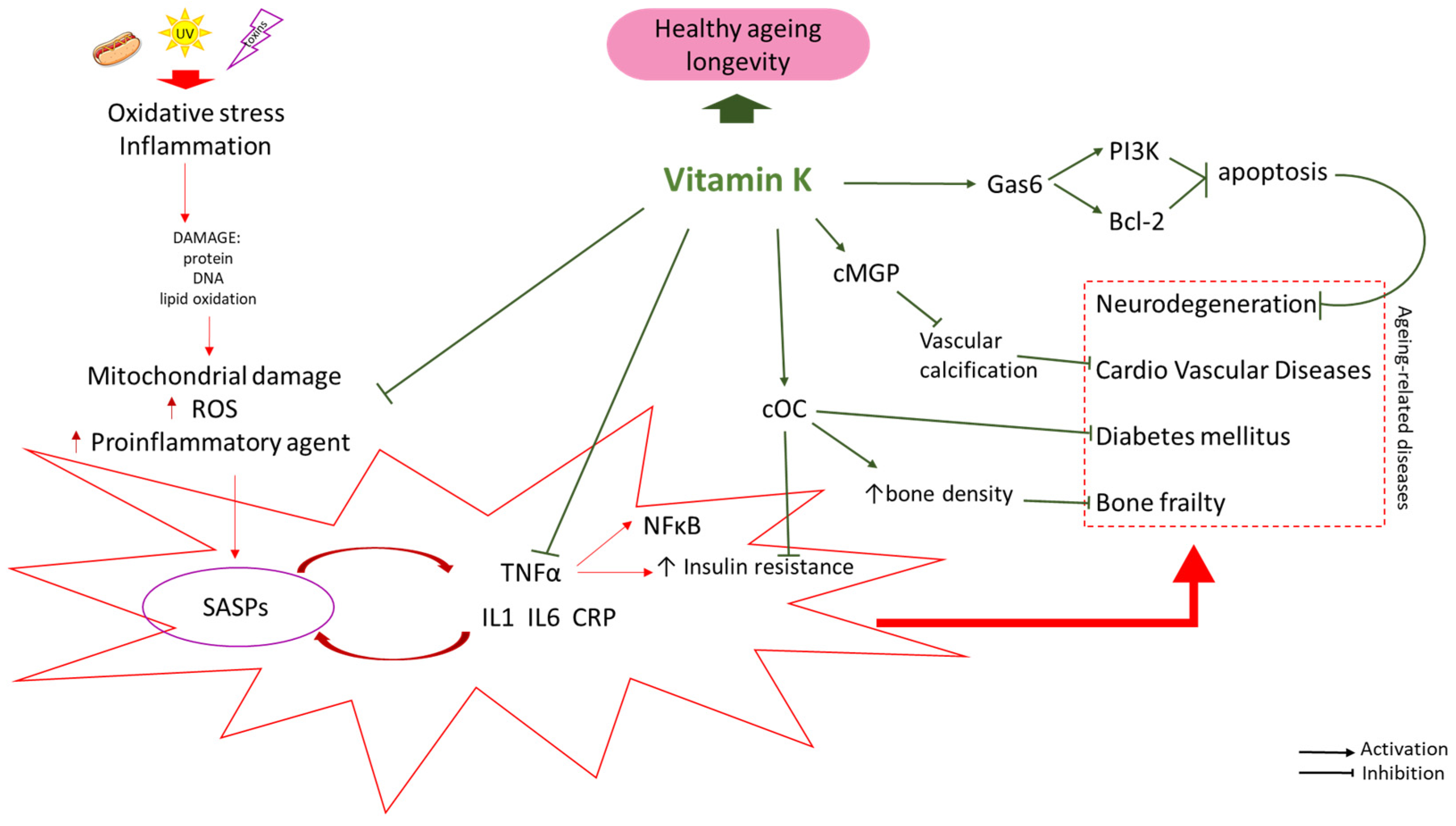
2. Vitamin K—Promoting Healthy Aging
2.1. Vitamin K—Reducing the Risk of Age-Related Diseases
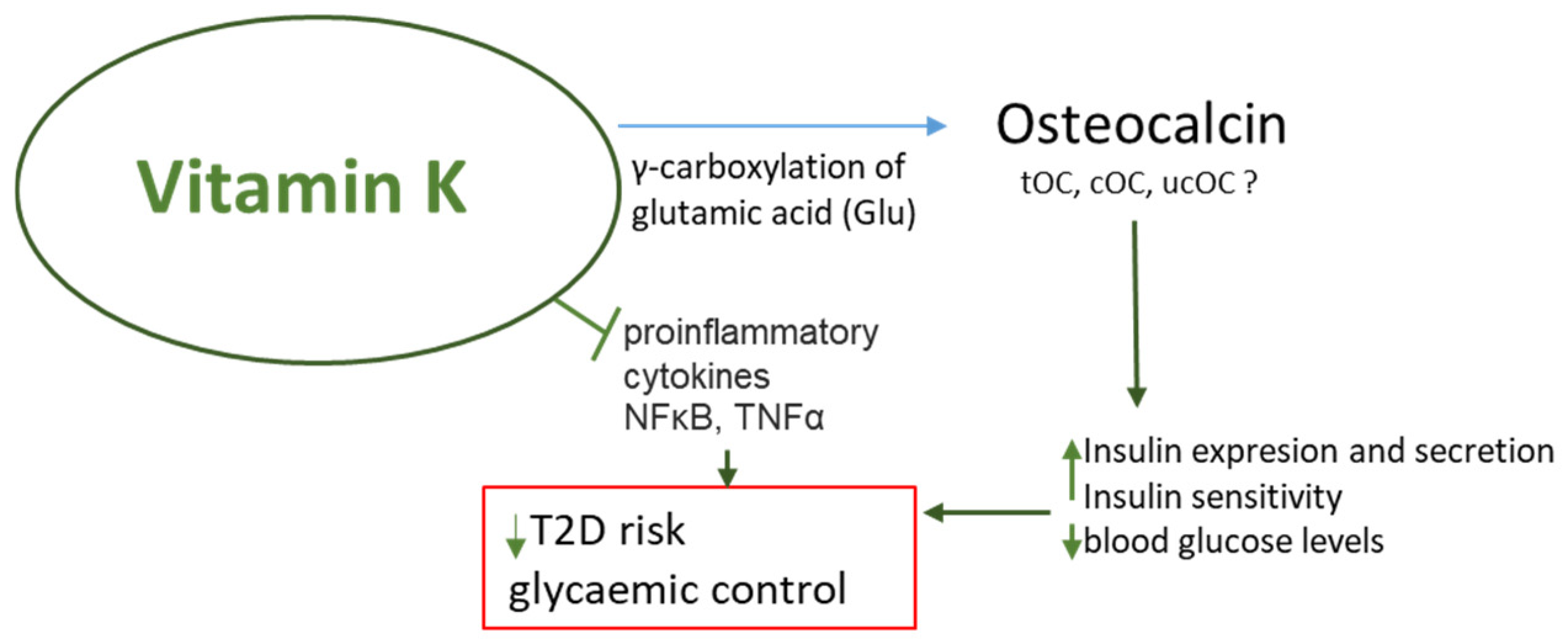
2.2. VK Improves the Sensitivity of Cells to Insulin
2.3. Vitamin K Is a Factor That Reduces the Risk of Osteoporosis
2.4. Vitamin K and the Risk of Cardiovascular Diseases
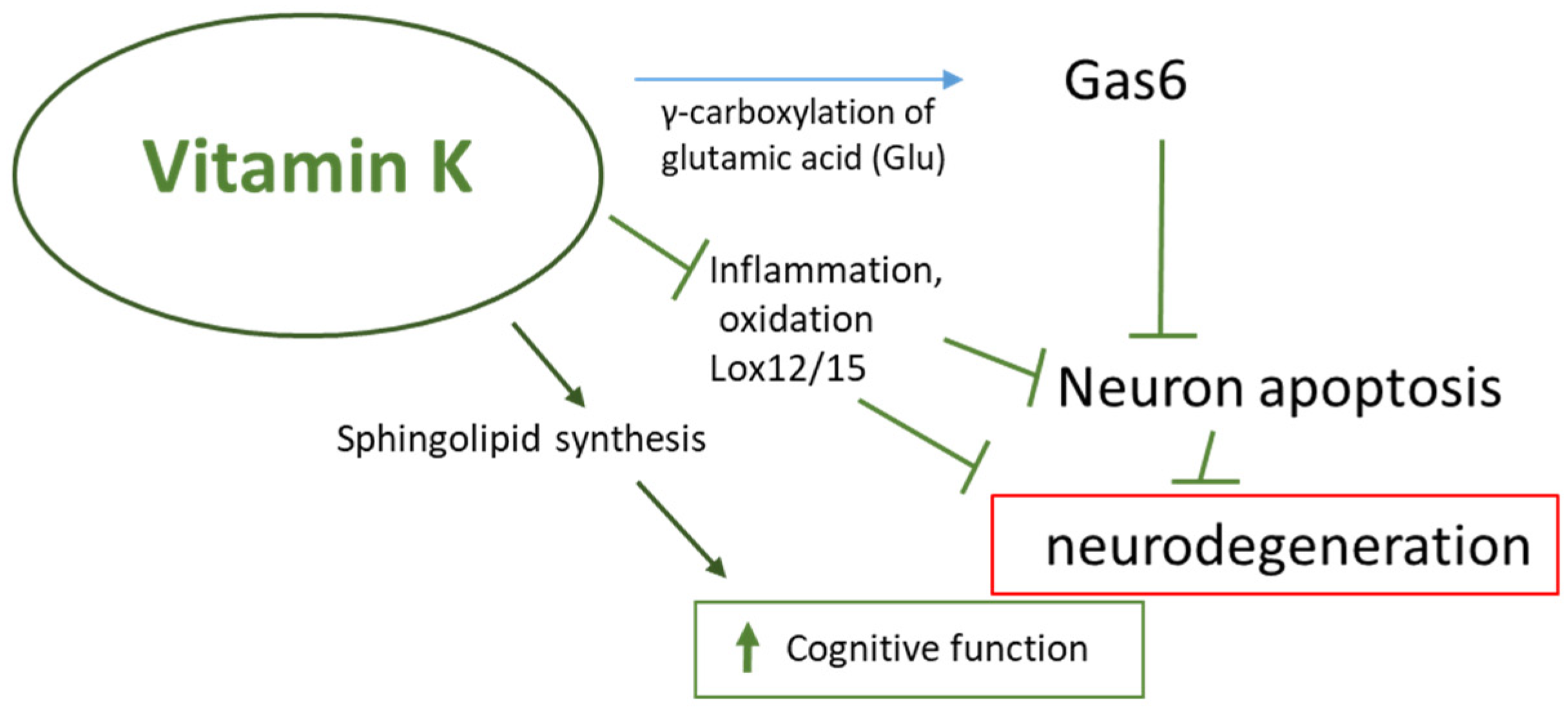
2.5. Vitamin K Supports Brain Health and Reduces the Risk of Cognitive Impairment
3. Vitamin K Status and Biomarkers
4. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Da Costa, J.P.; Vitorino, R.; Silva, G.M.; Vogel, C.; Duarte, A.C.; Rocha-Santos, T. A synopsis on aging-Theories, mechanisms and future prospects. Ageing Res. Rev. 2016, 29, 90–112. [Google Scholar] [CrossRef] [PubMed]
- Gems, D.; Virk, R.S.; de Magalhães, J.P. Epigenetic clocks and programmatic aging. Ageing Res. Rev. 2024, 101, 102546. [Google Scholar] [CrossRef]
- Marsman, D.; Belsky, D.W.; Gregori, D.; Johnson, M.A.; Low Dog, T.; Meydani, S.; Pigat, S.; Sadana, R.; Shao, A.; Griffiths, J.C. Healthy ageing: The natural consequences of good nutrition—A conference report. Eur. J. Nutr. 2018, 57 (Suppl. S2), 15–34. [Google Scholar] [CrossRef] [PubMed]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Mc Auley, M.T.; Guimera, A.M.; Hodgson, D.; Mcdonald, N.; Mooney, K.M.; Morgan, A.E.; Proctor, C.J. Modelling the molecular mechanisms of aging. Biosci. Rep. 2017, 37, BSR20160177. [Google Scholar] [CrossRef]
- Gorgoulis, V.; Adams, P.D.; Alimonti, A.; Bennett, D.C.; Bischof, O.; Bishop, C.; Campisi, J.; Collado, M.; Evangelou, K.; Ferbeyre, G.; et al. Cellular Senescence: Defining a Path Forward. Cell 2019, 179, 813–827. [Google Scholar] [CrossRef] [PubMed]
- Lecot, P.; Alimirah, F.; Desprez, P.Y.; Campisi, J.; Wiley, C. Context-dependent effects of cellular senescence in cancer development. Br. J. Cancer 2016, 114, 1180–1184. [Google Scholar] [CrossRef]
- Antonangeli, F.; Soriani, A.; Ricci, B.; Ponzetta, A.; Benigni, G.; Morrone, S.; Bernardini, G.; Santoni, A. Natural killer cell recognition of in vivo drug-induced senescent multiple myeloma cells. Oncoimmunology 2016, 5, e1218105. [Google Scholar] [CrossRef]
- Hanley, S.; Chen, Y.Y.; Hazeldine, J.; Lord, J.M. Senescent cell-derived extracellular vesicles as potential mediators of innate immunosenescence and inflammaging. Exp. Gerontol. 2024, 187, 112365. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cong, R.; Lv, T.; Liu, K.; Chang, X.; Li, Y.; Han, X.; Zhu, Y. Islet-resident macrophage-derived miR-155 promotes β cell decompensation via targeting PDX1. iScience 2024, 27, 109540. [Google Scholar] [CrossRef]
- Ge, W.; Zhang, X.; Wang, Q.; Mao, J.; Jia, P.; Cai, J. Dicoumarol attenuates NLRP3 inflammasome activation to inhibit inflammation and fibrosis in knee osteoarthritis. Mol. Med. Rep. 2024, 29, 100. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wen, Z. The mediating role of inflammaging between mitochondrial dysfunction and sarcopenia in aging: A review. Am. J. Clin. Exp. Immunol. 2023, 12, 109–126. [Google Scholar]
- Alberro, A.; Iribarren-Lopez, A.; Sáenz-Cuesta, M. Inflammaging markers characteristic of advanced age show similar levels with frailty and dependency. Sci. Rep. 2021, 11, 4358. [Google Scholar] [CrossRef] [PubMed]
- Bartke, A. Insulin and aging. Cell Cycle 2008, 7, 3338–3343. [Google Scholar] [CrossRef]
- Paolisso, G.; Barbieri, M.; Rizzo, M.R.; Carella, C.; Rotondi, M.; Bonafe, M. Low insulin resistance and preserved beta-cell function contribute to human longevity but are not associated with TH-INS genes. Exp. Gerontol. 2001, 37, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Schuetz, P.; Bally, M.; Stanga, Z.; Keller, U. Loss of appetite in acutely ill medical inpatients: Physiological response or therapeutic target? An area of current uncertainty. Swiss. Med. Wkly. 2014, 144, w13957. [Google Scholar]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 5280483, Vitamin K. 2024. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Vitamin-K (accessed on 23 July 2024).
- Mizuiri, S.; Nishizawa, Y.; Yamashita, K.; Ono, K.; Naito, T.; Tanji, C. Relationship of matrix Gla protein and vitamin K with vascular calcification in hemodialysis patients. Ren. Fail. 2019, 41, 770–777. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Mills, A.D.; Ibrahim, A. The extracellular matrix protein TGFBI induces microtubule stabilization and sensitizes ovarian cancers to paclitaxel. Cancer Cell 2007, 12, 514–527. [Google Scholar] [CrossRef]
- Shearer, M.J.; Newman, P. Metabolism and cell biology of vitamin K. Thromb. Haemost. 2008, 100, 530–547. [Google Scholar] [PubMed]
- Suematsu, N.; Tsutsui, H.; Wen, J.; Kang, D.; Ikeuchi, M.; Ide, T.; Hayashidani, S.; Shiomi, T.; Kubota, T.; Hamasaki, N.; et al. Oxidative stress mediates tumor necrosis factor-alpha-induced mitochondrial DNA damage and dysfunction in cardiac myocytes. Circulation 2003, 107, 1418–1423. [Google Scholar] [CrossRef]
- Remels, A.H.; Gosker, H.R.; Bakker, J.; Guttridge, D.C.; Schols, A.M.; Langen, R.C. Regulation of skeletal muscle oxidative phenotype by classical NF-κB signalling. Biochim. Biophys. Acta 2013, 1832, 1313–1325. [Google Scholar] [CrossRef]
- Vos, M.; Esposito, G.; Edirisinghe, J.N.; Vilain, S.; Haddad, D.M.; Slabbaert, J.R.; Van Meensel, S.; Schaap, O.; De Strooper, B.; Meganathan, R.; et al. Vitamin K2 is a mitochondrial electron carrier that rescues pink1 deficiency. Science 2012, 336, 1306–1310. [Google Scholar] [CrossRef] [PubMed]
- Proudfoot, D.; Skepper, J.N.; Hegyi, L.; Bennett, M.R.; Shanahan, C.M.; Weissberg, P.L. Apoptosis regulates human vascular calcification in vitro: Evidence for initiation of vascular calcification by apoptotic bodies. Circ. Res. 2000, 87, 1055–1062. [Google Scholar] [CrossRef]
- Qiu, C.; Zheng, H.; Tao, H.; Yu, W.; Jiang, X.; Li, A.; Jin, H.; Lv, A.; Li, H. Vitamin K2 inhibits rat vascular smooth muscle cell calcification by restoring the Gas6/Axl/Akt anti-apoptotic pathway. Mol. Cell Biochem. 2017, 433, 149–159. [Google Scholar] [CrossRef]
- Mukai, K.; Itoh, S.; Morimoto, H. Stopped-flow kinetic study of vitamin E regeneration reaction with biological hydroquinones (reduced forms of ubiquinone, vitamin K, and tocopherolquinone) in solution. J. Biol. Chem. 1992, 267, 22277–22281. [Google Scholar] [CrossRef]
- Vervoort, L.M.; Ronden, J.E.; Thijssen, H.H. The potent antioxidant activity of the vitamin K cycle in microsomal lipid peroxidation. Biochem. Pharmacol. 1997, 54, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zou, H. Lipoxygenase Metabolism: Critical Pathways in Microglia-mediated Neuroinflammation and Neurodevelopmental Disorders. Neurochem. Res. 2022, 47, 3213–3220. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lin, J.C.; Wang, H.; Peterson, J.W.; Furie, B.C.; Furie, B.; Booth, S.L.; Volpe, J.J.; Rosenberg, P.A. Novel role of vitamin K in preventing oxidative injury to developing oligodendrocytes and neurons. J. Neurosci. 2003, 23, 5816–5826. [Google Scholar] [CrossRef]
- Li, J.; Wang, H.; Rosenberg, P.A. Vitamin K prevents oxidative cell death by inhibiting activation of 12-lipoxygenase in developing oligodendrocytes. J. Neurosci. Res. 2009, 87, 1997–2005. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhuo, J.-M.; Chu, J.; Chinnici, C.; Praticò, D. Amelioration of the Alzheimer’s Disease Phenotype by Absence of 12/15-Lipoxygenase. Biol. Psychiatry 2010, 68, 922–929. [Google Scholar] [CrossRef]
- Kulkarni, A.; Nadler, J.L.; Mirmira, R.G.; Casimiro, I. Regulation of Tissue Inflammation by 12-Lipoxygenases. Biomolecules 2021, 11, 717. [Google Scholar] [CrossRef] [PubMed]
- Weaver, J.R.; Holman, T.R.; Imai, Y.; Jadhav, A.; Kenyon, V.; Maloney, D.J.; Nadler, J.L.; Rai, G.; Simeonov, A.; Taylor-Fishwick, D.A. Integration of pro-inflammatory cytokines, 12-lipoxygenase and NOX-1 in pancreatic islet beta cell dysfunction. Mol. Cell Endocrinol. 2012, 358, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Wang, H.; Chen, K.; Li, Y. Roles of hydroxyeicosatetraenoic acids in diabetes (HETEs and diabetes). Biomed. Pharmacother. 2022, 156, 113981. [Google Scholar] [CrossRef] [PubMed]
- Kołakowski, A.; Kurzyna, P.F.; Bzdęga, W.; Żywno, H.; Harasim-Symbor, E.; Chabowski, A.; Konstantynowicz-Nowicka, K. Influence of vitamin K2 on lipid precursors of inflammation and fatty acids pathway activities in HepG2 cells. Eur. J. Cell Biol. 2021, 100, 151188. [Google Scholar] [CrossRef] [PubMed]
- Dihingia, A.; Ozah, D.; Ghosh, S.; Sarkar, A.; Baruah, P.K.; Kalita, J.; Sil, P.C.; Manna, P. Vitamin K1 inversely correlates with glycemia and insulin resistance in patients with type 2 diabetes (T2D) and positively regulates SIRT1/AMPK pathway of glucose metabolism in liver of T2D mice and hepatocytes cultured in high glucose. J. Nutr. Biochem. 2018, 52, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Yeung, F.; Hoberg, J.E.; Ramsey, C.S.; Keller, M.D.; Jones, D.R.; Frye, R.A.; Mayo, M.W. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004, 23, 2369–2380. [Google Scholar] [CrossRef]
- Ohsaki, Y.; Shirakawa, H.; Miura, A.; Giriwono, P.E.; Sato, S.; Ohashi, A. Vitamin K suppresses the lipopolysaccharide-induced expression of inflammatory cytokines in cultured macrophage-like cells via the inhibition of the activation of nuclear factor κB through the repression of IKKα/β phosphorylation. J. Nutr. Biochem. 2010, 21, 1120–1126. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Yu, J.; Choi, H.; An, J.H.; Kim, S.W.; Park, K.S. Vitamin K2 Supplementation Improves Insulin Sensitivity via Osteocalcin Metabolism: A Placebo-Controlled Trial. Diabetes Care 2011, 34, e147. [Google Scholar] [CrossRef] [PubMed]
- Aguayo-Ruiz, J.I.; García-Cobián, T.A.; Pascoe-González, S.; Sánchez-Enríquez, S.; Llamas-Covarrubias, I.M.; García-Iglesias, T.; López-Quintero, A.; Llamas-Covarrubias, M.A.; Trujillo-Quiroz, J.; Rivera-Leon, E.A. Effect of supplementation with vitamins D3 and K2 on undercarboxylated osteocalcin and insulin serum levels in patients with type 2 diabetes mellitus: A randomized, double-blind, clinical trial. Diabetol. Metab. Syndr. 2020, 12, 73. [Google Scholar] [CrossRef]
- Baez-Duarte, B.G.; Sánchez-Guillén Mdel, C.; Perez-Fuentes, R.; Zamora-Ginez, I.; Leon-Chavez, B.A.; Revilla-Monsalve, C.; Islas-Andrade, S. Beta-cell function is associated with metabolic syndrome in Mexican subjects. Diabetes Metab. Syndr. Obes. Targets Ther. 2010, 3, 30. [Google Scholar]
- Lee, N.K.; Sowa, H.; Hinoi, E.; Ferron, M.; Ahn, J.D.; Confavreux, C. Endocrine regulation of energy metabolism by the skeleton. Cell 2007, 130, 456–469. [Google Scholar] [CrossRef] [PubMed]
- Cooper, I.D.; Brookler, K.H.; Crofts, C.A.P. Rethinking Fragility Fractures in Type 2 Diabetes: The Link between Hyperinsulinaemia and Osteofragilitas. Biomedicines 2021, 9, 1165. [Google Scholar] [CrossRef] [PubMed]
- Mera, P.; Ferron, M.; Mosialou, I. Regulation of energy metabolism by bone-derived hormones. Cold Spring Harb. Perspect. Med. 2018, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Lacombe, J.; Al Rifai, O.; Loter, L.; Moran, T.; Turcotte, A.F.; Grenier-Larouche, T.; Tchernof, A.; Biertho, L.; Carpentier, A.C.; Prud’homme, D.; et al. Measurement of bioactive osteocalcin in humans using a novel immunoassay reveals association with glucose metabolism and β-cell function. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E381–E391. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.M.; Guo, X.Z.; Tong, H.J.; Tao, B.; Sun, L.H.; Zhao, H.Y.; Ning, G.; Liu, J.M. Association between osteocalcin and glucose metabolism: A meta-analysis. Osteoporos. Int. 2015, 26, 2823–2833. [Google Scholar] [CrossRef]
- Losada-Grande, E.; Hawley, S.; Soldevila, B.; Martinez-Laguna, D.; Nogues, X.; Diez-Perez, A. Insulin use and Excess Fracture Risk in Patients with Type 2 Diabetes: A Propensity-Matched cohort analysis. Sci. Rep. 2017, 7, 3781. [Google Scholar] [CrossRef]
- Napoli, N.; Strotmeyer, E.S.; Ensrud, K.E.; Sellmeyer, D.E.; Bauer, D.C.; Hoffman, A.R. Fracture risk in diabetic elderly men: The MrOS study. Diabetology 2014, 57, 2057–2065. [Google Scholar] [CrossRef]
- Jin, C.; Tan, K.; Yao, Z.; Lin, B.H.; Zhang, D.P.; Chen, W.K. A Novel Anti-Osteoporosis Mechanism of VK2: Interfering with Ferroptosis via AMPK/SIRT1 Pathway in Type 2 Diabetic Osteoporosis. J. Agric. Food Chem. 2023, 71, 2745–2761. [Google Scholar] [CrossRef]
- Compston, J.E.; McClung, M.R.; Leslie, W.D. Osteoporosis. Lancet 2019, 393, 364–376. [Google Scholar] [CrossRef]
- Xu, Y.; Shen, L.; Liu, L.; Zhang, Z.; Hu, W. Undercarboxylated osteocalcin and its associations with bone mineral density, bone turnover markers, and prevalence of osteopenia and osteoporosis in Chinese population: A cross-sectional study. Front. Endocrinol. 2022, 13, 843912. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, T.; Kazama, H.; Araki, A.; Inoue, J.; Hosoi, T.; Onouchi, T. Impaired gamma carboxylation of osteocalcin in elderly women with type II diabetes mellitus: Relationship between increase in undercarboxylated osteocalcin levels and low bone mineral density. J. Bone Miner. Metab. 2004, 22, 236–240. [Google Scholar] [CrossRef]
- Emaus, N.; Nguyen, N.D.; Almaas, B.; Berntsen, G.K.; Center, J.R.; Christensen, M. Serum level of under-carboxylated osteocalcin and bone mineral density in early menopausal Norwegian women. Eur. J. Nutr. 2013, 52, 49–55. [Google Scholar] [CrossRef]
- Ma, M.L.; Ma, Z.J.; He, Y.L.; Sun, H.; Yang, B.; Ruan, B.J. Efficacy of vitamin K2 in the prevention and treatment of postmenopausal osteoporosis: A systematic review and meta-analysis of randomized controlled trials. Front. Public Health 2022, 10, 979649. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Arima, K.; Nishimura, T. Vitamin K deficiency, evaluated with higher serum ucOC, was correlated with poor bone status in women. J. Physiol. Anthropol. 2020, 39, 9. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Olleros Rodríguez, C.; Díaz Curiel, M. Vitamin K and Bone Health: A Review on the Effects of Vitamin K Deficiency and Supplementation and the Effect of Non-Vitamin K Antagonist Oral Anticoagulants on Different Bone Parameters. J. Osteoporos. 2019, 2019, 2069176. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Han, S.; Zhang, W. Efficacy and safety of vitamin K2 for postmenopausal women with osteoporosis at a long-term follow-up: Meta-analysis and systematic review. J. Bone Miner. Metab. 2022, 40, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Inaba, N.; Sato, T.; Yamashita, T. Low-Dose Daily Intake of Vitamin K2 (Menaquinone-7) Improves Osteocalcin γ-Carboxylation: A Double-Blind, Randomized Controlled Trials. J. Nutr. Sci. Vitaminol. 2015, 61, 471–480. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Weitzmann, M.N. Vitamin K2 stimulates osteoblastogenesis and suppresses osteoclastogenesis by suppressing NF-κB activation. Int. J. Mol. Med. 2011, 27, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Lees, J.S.; Chapman, F.A.; Witham, M.D.; Jardine, A.G.; Mark, P.B. Vitamin K status, supplementation and vascular disease: A systematic review and meta-analysis. Heart 2019, 105, 938–945. [Google Scholar] [CrossRef]
- Schurgers, L.J.; Teunissen, K.J.; Knapen, M.H.; Kwaijtaal, M.; van Diest, R.; Appels, A.; Reutelingsperger, C.P.; Cleutjens, J.P.; Vermeer, C. Novel conformation-specific antibodies against matrix gamma-carboxyglutamic acid (Gla) protein: Undercarboxylated matrix Gla protein as marker for vascular calcification. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1629–1633. [Google Scholar] [CrossRef]
- Juanola-Falgarona, M.; Salas-Salvadó, J.; Martínez-González, M.Á.; Corella, D.; Estruch, R.; Ros, E. Dietary intake of vitamin K is inversely associated with mortality risk. J. Nutr. 2014, 144, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Dupuy, M.; Radavelli-Bagatini, S.; Zhong, L.; Dalla Via, J.; Zhu, K.; Blekkenhorst, L.C. Vitamin K1 intake is associated with lower risk for all-cause and cardiovascular disease mortality in community-dwelling older Australian women. Nutr. Metab. Cardiovasc. Dis. 2024, 34, 1189–1197. [Google Scholar] [CrossRef]
- Beulens, J.W.; Bots, M.L.; Atsma, F.; Bartelink, M.L.; Prokop, M.; Geleijnse, J.M. High dietary menaquinone intake is associated with reduced coronary calcification. Atherosclerosis 2009, 203, 489–493. [Google Scholar] [CrossRef]
- Gast, G.C.; de Roos, N.M.; Sluijs, I.; Bots, M.L.; Beulens, J.W.; Geleijnse, J.M. A high menaquinone intake reduces the incidence of coronary heart disease. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Zwakenberg, S.R.; den Braver, N.R.; Engelen, A.I.P.; Feskens, E.J.M.; Vermeer, C.; Boer, J.M.A.; Verschuren, W.M.M. Vitamin K intake and all-cause and cause specific mortality. Clin. Nutr. 2017, 36, 1294–1300. [Google Scholar] [CrossRef]
- Shea, M.K.; Berkner, K.L.; Ferland, G.; Fu, X.; Holden, R.M.; Booth, S.L. Perspective: Evidence before Enthusiasm—A Critical Review of the Potential Cardiovascular Benefits of Vitamin K. Adv. Nutr. 2021, 12, 632–646. [Google Scholar] [CrossRef]
- Vlasschaert, C.; Goss, C.J.; Pilkey, N.G.; McKeown, S.; Holden, R.M. Vitamin K Supplementation for the Prevention of Cardiovascular Disease: Where Is the Evidence? A Systematic Review of Controlled Trials. Nutrients 2020, 12, 2909. [Google Scholar] [CrossRef] [PubMed]
- Bellinge, J.W.; Francis, R.J.; Lee, S.C.; Bondonno, N.P.; Sim, M.; Lewis, J.R.; Watts, G.F.; Schultz, C.J. The effect of vitamin K1 on arterial calcification activity in subjects with diabetes mellitus: A post hoc analysis of a double-blind, randomized, placebo-controlled trial. Am. J. Clin. Nutr. 2022, 115, 45–52. [Google Scholar] [CrossRef]
- Gillis, C.; Mirzaei, F.; Potashman, M.; Ikram, M.A.; Maserejian, N. The incidence of mild cognitive impairment: A systematic review and data synthesis. Alzheimer’s Dement 2019, 11, 248–256. [Google Scholar] [CrossRef]
- Kazibwe, R.; Schaich, C.L.; Muhammad, A.I.; Epiu, I.; Namutebi, J.H.; Chevli, P.A.; Kazibwe, J.; Hughes, T.; Rikhi, R.R.; Shapiro, M.D.; et al. Effect of vigorous-intensity physical activity on incident cognitive impairment in high-risk hypertension. Alzheimers Dement 2024, 20, 4602–4612. [Google Scholar] [CrossRef]
- Sal-Sarria, S.; López-Taboada, I.; González-Pardo, H.; Conejo, N.M. A shift to a standard diet after exposure to a high-fat, high-sucrose diet from gestation to weaning restores brain metabolism and behavioral flexibility in adult rats. Behav. Brain Res. 2024, 467, 115020. [Google Scholar] [CrossRef]
- Shapiro, A.L.B.; Tjaden, A.H.; Edelstein, S.L.; Kahn, S.E.; Srikanthan, P.; Knowler, W.C.; Venditti, E.M.; Golden, S.H.; Carmichael, O.; Luchsinger, J.A.; et al. The association of insulin responses and insulin sensitivity with cognition in adults with pre-diabetes: The Diabetes Prevention Program Outcomes Study. J. Diabetes Complicat. 2024, 38, 108764. [Google Scholar] [CrossRef] [PubMed]
- Shang, G.; Shao, Q.; Lv, K.; Xu, W.; Ji, J.; Fan, S.; Kang, X.; Cheng, F.; Wang, X.; Wang, Q. Hypercholesterolemia and the Increased Risk of Vascular Dementia: A Cholesterol Perspective. Curr. Atheroscler. Rep. 2024, 26, 435–449. [Google Scholar] [CrossRef]
- Moheet, A.; Mangia, S.; Seaquist, E.R. Impact of diabetes on cognitive function and brain structure. Ann. N. Y. Acad. Sci. 2015, 1353, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Profenno, L.A.; Porsteinsson, A.P.; Faraone, S.V. Meta-Analysis of Alzheimer’s Disease Risk with Obesity, Diabetes, and Related Disorders. Biol. Psychiatry 2010, 67, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Scarmeas, N.; Anastasiou, C.A.; Yannakoulia, M. Nutrition and prevention of cognitive impairment. Lancet Neurol. 2018, 17, 1006–1015. [Google Scholar] [CrossRef] [PubMed]
- Posse de Chaves, E.; Sipione, S. Sphingolipids and gangliosides of the nervous system in membrane function and dysfunction. FEBS Lett. 2010, 584, 1748–1759. [Google Scholar] [CrossRef]
- Yagami, T.; Ueda, K.; Asakura, K.; Sakaeda, T.; Nakazato, H.; Kuroda, T.; Hata, S.; Sakaguchi, G.; Itoh, N.; Nakano, T.; et al. Gas6 rescues cortical neurons from amyloid beta protein-induced apoptosis. Neuropharmacology 2002, 43, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.H.; Fang, S.T.; Chen, Y.C. Molecular Mechanism of Vitamin K2 Protection against Amyloid-β-Induced Cytotoxicity. Biomolecules 2021, 11, 423. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, M.; Ye, J.; Zhao, G.; Hong, G.; Hu, X.; Cao, K.; Wu, Y.; Lu, Z. Gas6 attenuates lipopolysaccharide-induced TNF-α expression and apoptosis in H9C2 cells through NF-κB and MAPK inhibition via the Axl/PI3K/Akt pathway. Int. J. Mol. Med. 2019, 44, 982–994. [Google Scholar] [CrossRef]
- Hadipour, E.; Tayarani-Najaran, Z.; Fereidoni, M. Vitamin K2 protects PC12 cells against Aβ (1-42) and H2O2-induced apoptosis via p38 MAP kinase pathway. Nutr. Neurosci. 2020, 23, 343–352. [Google Scholar] [CrossRef]
- Presse, N.; Shatenstein, B.; Kergoat, M.J.; Ferland, G. Low vitamin K intakes in community-dwelling elders at an early stage of Alzheimer’s disease. J. Am. Diet. Assoc. 2008, 108, 2095–2099. [Google Scholar] [CrossRef]
- Kohlmeier, M.; Salomon, A.; Saupe, J.; Shearer, M.J. Transport of vitamin K to bone in humans. J. Nutr. 1996, 126, 1192S–1196S. [Google Scholar] [CrossRef] [PubMed]
- Brangier, A.; Ferland, G.; Rolland, Y.; Gautier, J.; Féart, C.; Annweiler, C. Vitamin K Antagonists and Cognitive Decline in Older Adults: A 24-Month Follow-Up. Nutrients 2018, 10, 666. [Google Scholar] [CrossRef] [PubMed]
- Tamadon-Nejad, S.; Ouliass, B.; Rochford, J.; Ferland, G. Vitamin K deficiency induced by warfarin is associated with cognitive and behavioral perturbations, and alterations in brain sphingolipids in rats. Front. Aging Neurosci. 2018, 10, 213. [Google Scholar] [CrossRef] [PubMed]
- Booth, S.L.; Shea, M.K.; Barger, K.; Leurgans, S.E.; James, B.D.; Holland, T.M.; Agarwal, P.; Fu, X.; Wang, J.; Matuszek, G.; et al. Association of vitamin K with cognitive decline and neuropathology in community-dwelling older persons. Alzheimers Dement 2022, 8, e12255. [Google Scholar] [CrossRef]
- Allison, A.C. The possible role of vitamin K deficiency in the pathogenesis of Alzheimer’s disease and in augmenting brain damage associated with cardiovascular disease. Med. Hypotheses 2001, 57, 151–155. [Google Scholar] [CrossRef] [PubMed]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies); Turck, D.; Bresson, J.-L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.J. Scientific Opinion on the die-tary reference values for vitamin K. EFSA J. 2017, 15, 4780. [Google Scholar] [CrossRef]
- Shearer, M.J. Vitamin K. Lancet 1995, 345, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Cranenburg, E.C.; Schurgers, L.J.; Vermeer, C. Vitamin K: The coagulation vitamin that became omnipotent. Thromb. Haemost. 2007, 98, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Ronden, J.E.; Drittij-Reijnders, M.J.; Vermeer, C.; Thijssen, H.H. Intestinal flora is not an intermediate in thephylloquinone-menaquinone-4 conversion in the rat. Biochim. Biophys. Acta 1998, 1379, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Schurgers, L.J.; Vermeer, C. Differential lipoprotein transport pathways of K-vitamins in healthy subjects. Biochim. Biophys. Acta 2002, 1570, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.L.; Fu, X.; Karl, J.P.; Hernandez, C.J.; Mason, J.B.; DeBose-Boyd, R.A.; Booth, S.L. Multiple Dietary Vitamin K Forms Are Converted to Tissue Menaquinone-4 in Mice. J. Nutr. 2022, 152, 981–993. [Google Scholar] [CrossRef]
- Shearer, M.J.; Newman, P. Recent trends in the metabolism and cell biology of vitamin K with special reference to vitamin K cycling and MK4 biosynthesis. J. Lipid Res. 2014, 55, 345–362. [Google Scholar] [CrossRef] [PubMed]
- Binkley, N.; Harke, J.; Krueger, D.; Engelke, J.; Vallarta-Ast, N.; Gemar, D.; Checovich, M.; Chappell, R.; Suttie, J. Vitamin K treatment reduces undercarboxylated osteocalcin but does not alter bone turnover, density, or geometry in healthy postmenopausal North American women. J. Bone Miner. Res. 2009, 24, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Schurgers, L.J.; Vermeer, C. Determination of Phylloquinone and Menaquinones in Food. Pathophysiol. Haemost. Thromb. 2000, 30, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Schurgers, L.J.; Uenishi, K. Comparison of menaquinone-4 and menaquinone-7 bioavailability in healthy women. Nutr. J. 2012, 11, 93. [Google Scholar] [CrossRef] [PubMed]
- Schurgers, L.J.; Teunissen, K.J.; Hamulyak, K.; Knapen, M.H.; Vik, H.; Vermeer, C. Vitamin K-containing dietary supplements: Comparison of synthetic vitamin K1 and natto-derived menaquinone-7. Blood 2007, 109, 3279–3283. [Google Scholar] [CrossRef] [PubMed]
- Buitenhuis, H.; Soute, B.; Vermeer, C. Comparison of the vitamins K1, K2 and K3 as cofactors for the hepatic vitamin K-dependent carboxylase. Biochim. Biophys. Acta 1990, 1034, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Hirota, Y.; Sawada, N.; Yuge, N.; Watanabe, M.; Uchino, Y.; Okuda, N.; Shimomura, Y.; Suhara, Y.; Okano, T. Identification of UBIAD1 as a novel human menaquinone-4 biosynthetic enzyme. Nature 2010, 468, 117–121. [Google Scholar] [CrossRef]
- Gentili, A.; Cafolla, A.; Gasperi, T.; Bellante, S.; Caretti, F.; Curini, R.; Fernández, V.P. Rapid, high performance method for the determination of vitamin K1, menaquinone-4 and vitamin K1 2,3-epoxide in human serum and plasma using liquid chromatography-hybrid quadrupole linear ion trap mass spectrometry. J. Chromatogr. A 2014, 1338, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Tsugawa, N.; Shiraki, M.; Suhara, Y.; Kamao, M.; Tanaka, K.; Okano, T. Vitamin K status of healthy Japanese women: Age-related vitamin K requirement for gamma-carboxylation of osteocalcin. Am. J. Clin. Nutr. 2006, 83, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Suhara, Y.; Kamao, M.; Tsugawa, N.; Okano, T. Method for the determination of vitamin K homologues in human plasma using high-performance liquid chromatography-tandem mass spectrometry. Anal Chem. 2005, 77, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Kaneki, M.; Hodges, S.J.; Hosoi, T.; Fujiwara, S.; Lyons, A.; Crean, S.J.; Ishida, N.; Nakagawa, M.; Takechi, M.; Sano, Y.; et al. Japanese fermented soybean food as the major determinant of the large geographic difference in circulating levels of vitamin K2: Possible implications for hip-fracture risk. Nutrition 2001, 17, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Card, D.J.; Gorska, R.; Harrington, D.J. Laboratory assessment of vitamin K status. J. Clin. Path 2020, 73, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Card, D.J.; Shearer, M.J.; Schurgers, L.J.; Harrington, D.J. The external quality assurance of phylloquinone (vitamin K1) analysis in human serum. Biomed. Chromatogr. 2009, 23, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- Card, D.; Freke, E.; Harrington, D. Characterization and traceability of two generations of standard reference material for the measurement of vitamin K1 (phylloquinone) at endogenous concentrations in human plasma and serum. Biomed. Chromatogr. 2022, 36, e5378. [Google Scholar] [CrossRef]
- Ryu, M.R.; Kang, E.S.; Park, H.D. Performance evaluation of serum PIVKA-II measurement using HISCL-5000 and a method comparison of HISCL-5000, LUMIPULSE G1200, and ARCHITECT i2000. J. Clin. Lab. Anal. 2019, 33, e22921. [Google Scholar] [CrossRef]
- Holden, R.M.; Morton, A.R.; Garland, J.S.; Pavlov, A.; Day, A.G.; Booth, S.L. Vitamins K and D status in stages 3–5 chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2010, 5, 590–597. [Google Scholar] [CrossRef]
- Westenfeld, R.; Krueger, T.; Schlieper, G.; Cranenburg, E.C.; Magdeleyns, E.J.; Heidenreich, S.; Holzmann, S.; Vermeer, C.; Jahnen-Dechent, W.; Ketteler, M.; et al. Effect of vitamin K2 supplementation on functional vitamin K deficiency in hemodialysis patients: A randomized trial. Am. J. Kidney Dis. 2012, 59, 186–195. [Google Scholar] [CrossRef]
- Elliott, M.J.; Booth, S.L.; Hopman, W.M.; Holden, R.M. Assessment of potential biomarkers of subclinical vitamin K deficiency in patients with end-stage kidney disease. Can. J. Kidney Health Dis. 2014, 1, 13. [Google Scholar] [CrossRef]
- Yasui, T.; Miyatani, Y.; Tomita, J.; Yamada, M.; Uemura, H.; Miura, M.; Irahara, M. Effect of vitamin K2 treatment on carboxylation of osteocalcin in early postmenopausal women. Gynecol. Endocrinol. 2006, 22, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Kremer, D.; Groothof, D.; Keyzer, C.A.; Eelderink, C.; Knobbe, T.J.; Post, A.; van Londen, M.; Eisenga, M.F.; TransplantLines Investigators; Schurgers, L.J.; et al. Kidney Function-Dependence of Vitamin K-Status Parameters: Results from the TransplantLines Biobank and Cohort Studies. Nutrients 2021, 13, 3069. [Google Scholar] [CrossRef] [PubMed]
- Jespersen, T.; Møllehave, L.T.; Thuesen, B.H.; Skaaby, T.; Rossing, P.; Toft, U.; Jørgensen, N.R.; Corfixen, B.L.; Jakobsen, J.; Frimodt-Møller, M.; et al. Uncarboxylated matrix Gla-protein: A biomarker of vitamin K status and cardiovascular risk. Clin. Biochem. 2020, 83, 49–56. [Google Scholar] [CrossRef]
- Schlieper, G.; Westenfeld, R.; Krüger, T.; Cranenburg, E.C.; Magdeleyns, E.J.; Brandenburg, V.M.; Djuric, Z.; Damjanovic, T.; Ketteler, M.; Vermeer, C.; et al. Circulating nonphosphorylated carboxylated matrix gla protein predicts survival in ESRD. J. Am. Soc. Nephrol. 2011, 22, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Knapen, M.H.; Braam, L.A.; Drummen, N.E.; Bekers, O.; Hoeks, A.P.; Vermeer, C. Menaquinone-7 supplementation improves arterial stiffness in healthy postmenopausal women. A double-blind randomised clinical trial. Thromb. Haemost. 2015, 113, 1135–1144. [Google Scholar] [CrossRef]
- Ames, B.N. Low micronutrient intake may accelerate the degenerative diseases of aging through allocation of scarce micronutrients by triage. Proc. Natl. Acad. Sci. USA 2006, 103, 17589–17594. [Google Scholar] [CrossRef] [PubMed]
- Sadler, R.A.; Shoveller, A.K.; Shandilya, U.K.; Charchoglyan, A.; Wagter-Lesperance, L.; Bridle, B.W.; Mallard, B.A.; Karrow, N.A. Beyond the Coagulation Cascade: Vitamin K and Its Multifaceted Impact on Human and Domesticated Animal Health. Curr. Issues Mol. Biol. 2024, 46, 7001–7031. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.P.; Duan, L.; Li, S. Effect of vitamin K2 on type 2 diabetes mellitus: A review. Diabetes Res. Clin. Pract. 2018, 136, 39–51. [Google Scholar] [CrossRef]
- Lacombe, J.; Guo, K.; Bonneau, J.; Faubert, D.; Gioanni, F.; Vivoli, A.; Muir, S.M.; Hezzaz, S.; Poitout, V.; Ferron, M. Vitamin K-dependent carboxylation regulates Ca2+ flux and adaptation to metabolic stress in β cells. Cell Rep. 2023, 42, 112500. [Google Scholar] [CrossRef] [PubMed]

| The Scope of Vitamins K’s Actions | Pro-Health Effect | |
|---|---|---|
| Brain | ↓ ROS ↓ Lox12/15 | Neuroprotection Lower risk of neurodegeneration (AD, PD) Improved cognitive function [77,79,82,87] |
| ↑ Gas6 ↓ neurons apoptosis | ||
| ↓ β amyloid ↑ synthesis of sphingolipids | ||
| Blood vessels | ↑ cMGP ↑ cOC ↓ calcification of vessels | Lower risk of calciphylaxis and new vascular and renal calcification * [68,69,119] |
| Bone structure | ↑ cOC ↑ Ca sequestration in the bone matrix ↑ promoting bone mineralisation | Lower risk of osteoporosis Reduced incidence of fractures [54,57] |
| ↓ bone resorption | ||
| Metabolism of glucose | ↓ inflammation ↓ blood glucose level ↓ level of lipids in blood ↑ insulin sensitivity ↑ cOC | Improved insulin sensitivity Reduced insulin resistance Reduced risk of T2D [40,120,121] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaźmierczak-Barańska, J.; Karwowski, B.T. The Protective Role of Vitamin K in Aging and Age-Related Diseases. Nutrients 2024, 16, 4341. https://doi.org/10.3390/nu16244341
Kaźmierczak-Barańska J, Karwowski BT. The Protective Role of Vitamin K in Aging and Age-Related Diseases. Nutrients. 2024; 16(24):4341. https://doi.org/10.3390/nu16244341
Chicago/Turabian StyleKaźmierczak-Barańska, Julia, and Bolesław T. Karwowski. 2024. "The Protective Role of Vitamin K in Aging and Age-Related Diseases" Nutrients 16, no. 24: 4341. https://doi.org/10.3390/nu16244341
APA StyleKaźmierczak-Barańska, J., & Karwowski, B. T. (2024). The Protective Role of Vitamin K in Aging and Age-Related Diseases. Nutrients, 16(24), 4341. https://doi.org/10.3390/nu16244341







