Impact of Omega-3 on Endocannabinoid System Expression and Function, Enhancing Cognition and Behavior in Male Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Animal Treatment
2.3. Synaptosomal Fractionation
2.4. Western Blotting
2.5. [35S]GTPγS Binding Assays
2.6. Slice Preparation and Extracellular Field Recordings
2.7. Behavioral Tests
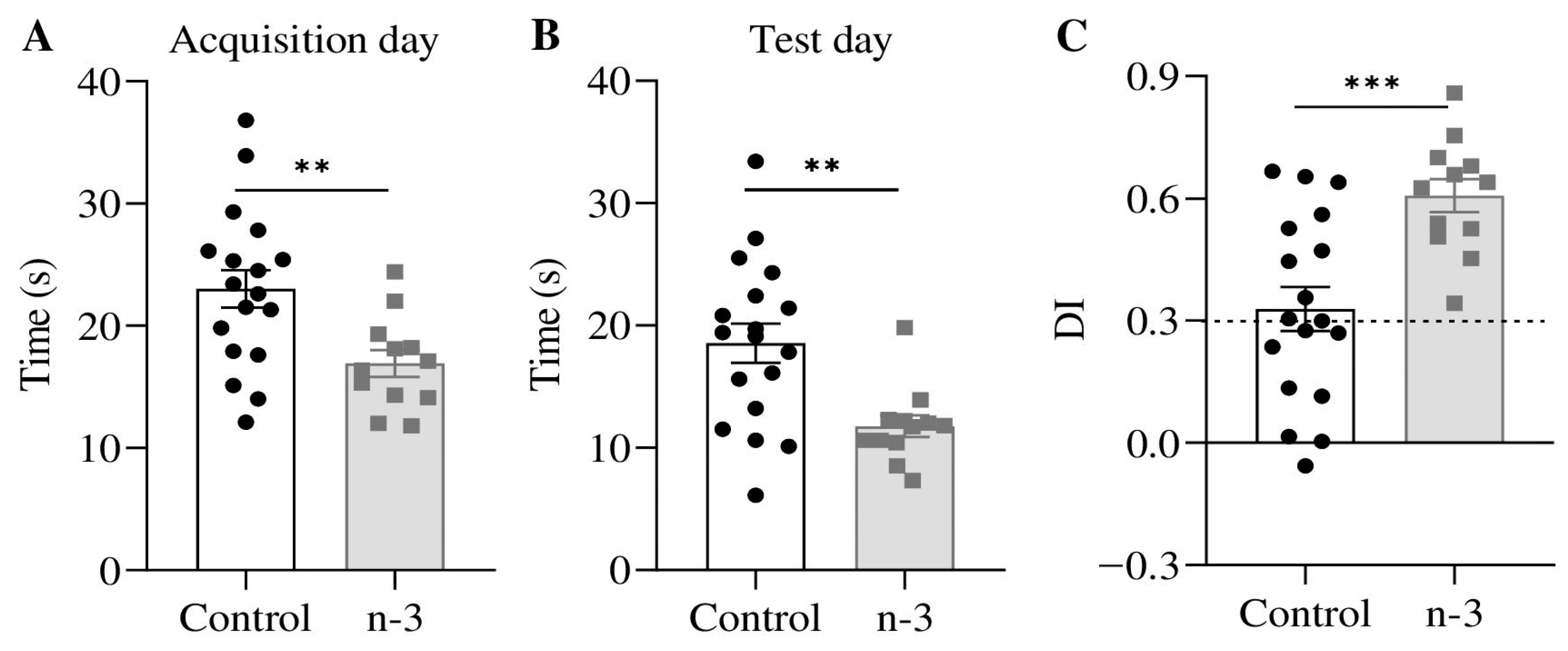
2.7.1. Novel Object Recognition Test
2.7.2. Anxiety Tests
Open Field (OF) Test
Elevated Plus Maze (EPM) Test
Light/Dark Box (LDB) Test
2.8. Statistical Analysis
3. Results
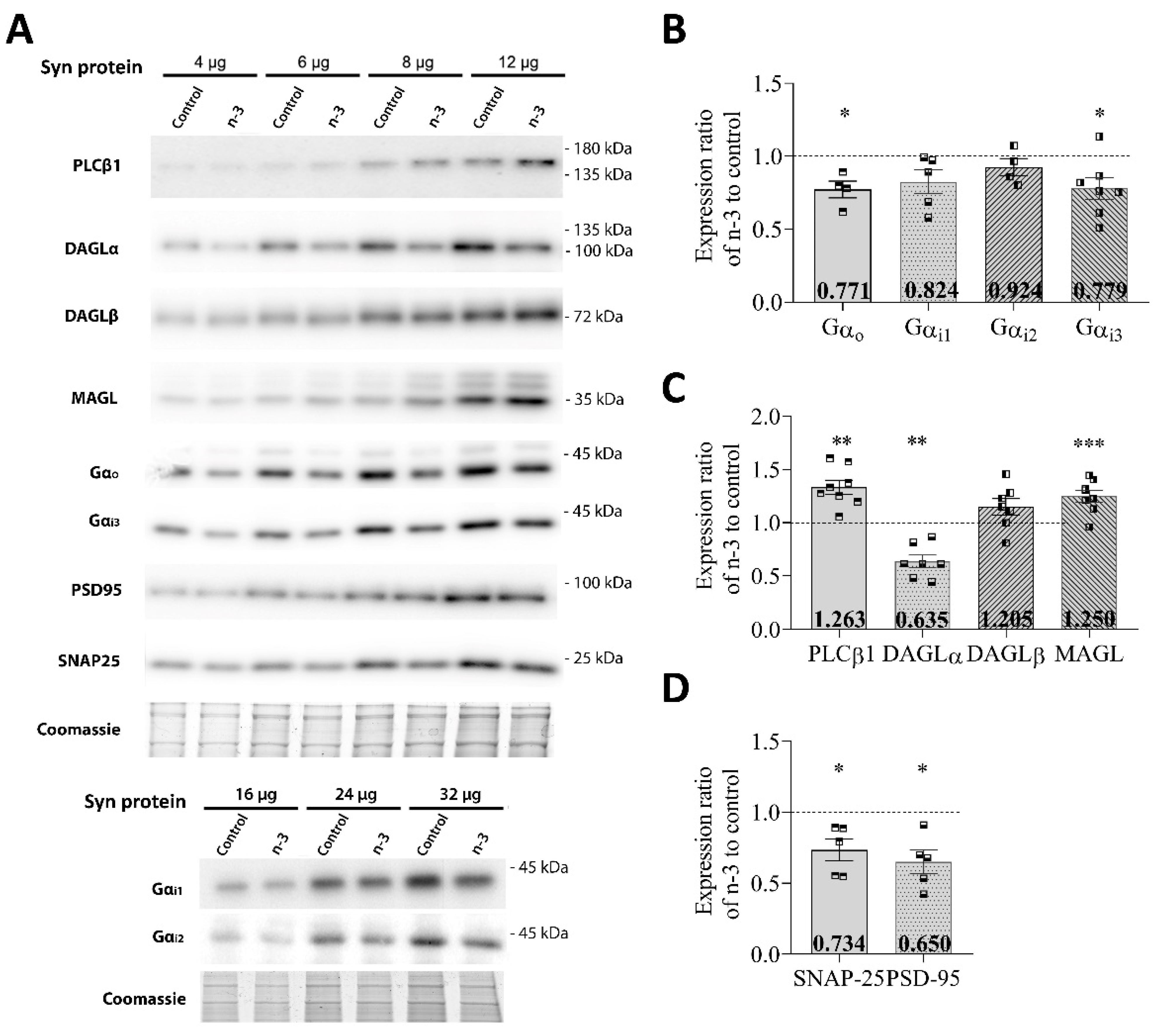
3.1. Omega-3 Enrichment Alters the Endocannabinoid System (ECS)
3.2. Effect of Omega-3 Supplementation on CB1 Receptor Coupling to Gαi/o
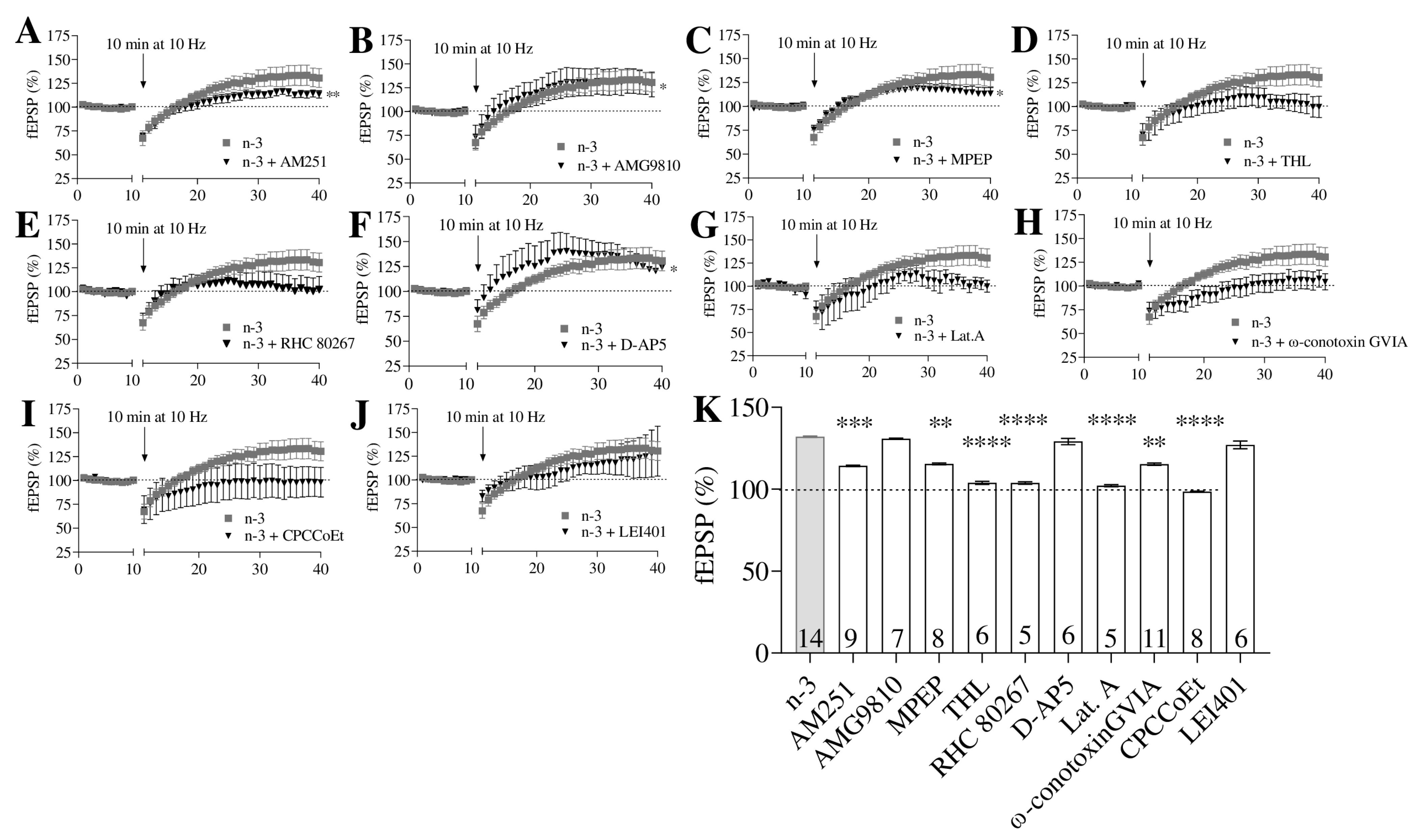
3.3. Omega-3 Enrichment Enhances Synaptic Potentiation
3.4. Omega-3 Enrichment Enhances Long-Term Recognition Memory
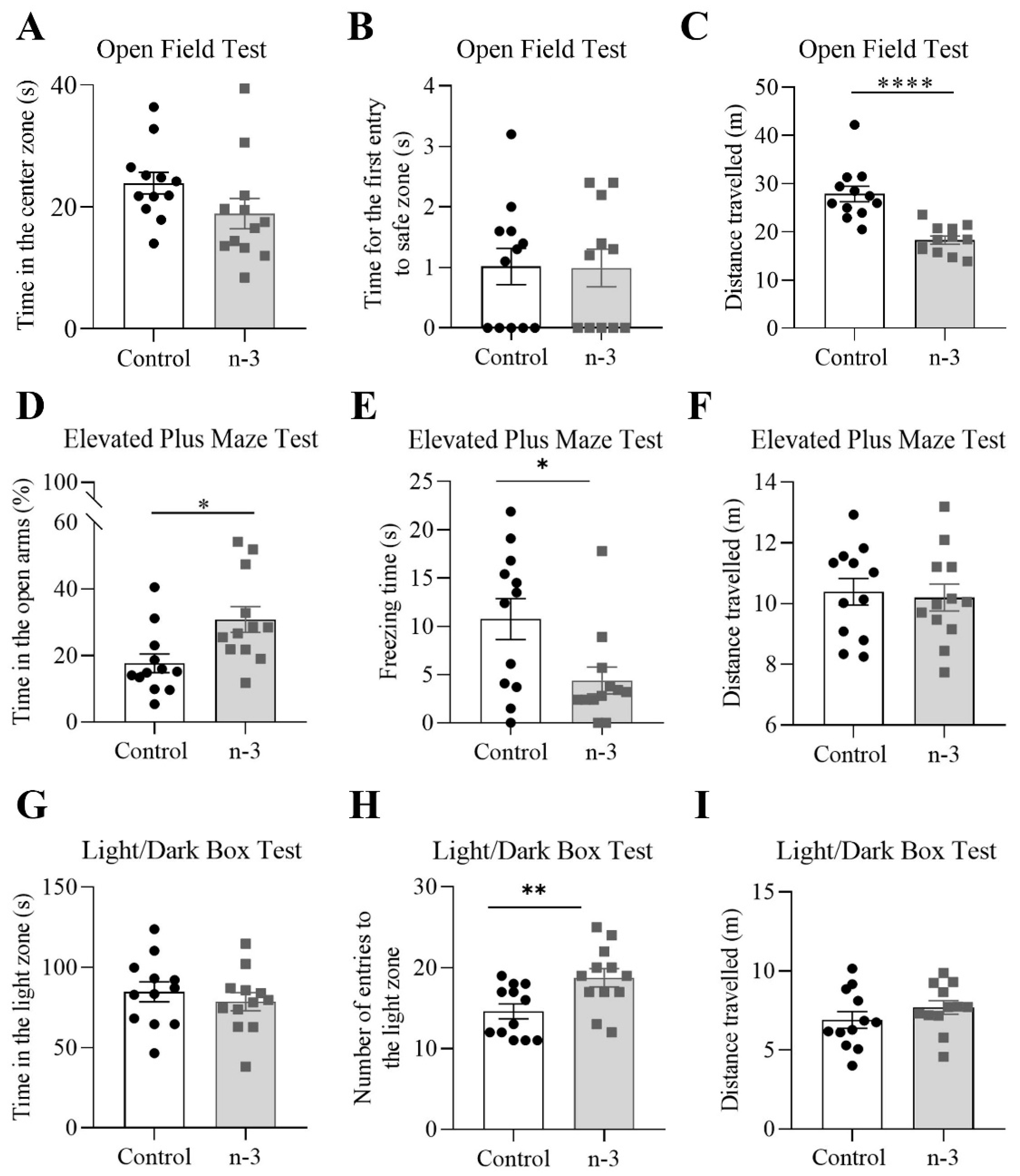
3.5. Omega-3 Supplementation Induces Anxiolytic-like Effects
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abedi, E.; Sahari, M.A. Long-chain polyunsaturated fatty acid sources and evaluation of their nutritional and functional properties. Food Sci. Nutr. 2014, 2, 443–463. [Google Scholar] [CrossRef] [PubMed]
- Aguilera García, C.; Navarro Alarcón, M.; Alonso Aperte, E.; Alvarez Mercado, A.I.; Andrés Carvajales, P.; Arredondo Olguín, M. Tratado de Nutrición. Tomo I. Bases Fisiológicas y Bioquímicas de la Nutrición; Médica Panamericana: Madrid, Spain, 2010; 963p. [Google Scholar]
- Gómez Candela, C.; Bermejo López, L.M.; Loria Kohen, V. Importance of a balanced omega 6/omega 3 ratio for the maintenance of health. Nutritional recommendations. Nutr. Hosp. 2011, 26, 323–329. [Google Scholar]
- Serrano, M.; Rico-Barrio, I.; Grandes, P. The effect of omega-3 fatty acids on alcohol-induced damage. Front. Nutr. 2023, 10, 1068343. [Google Scholar] [CrossRef]
- Venegas-Calerón, M.; Sayanova, O.; Napier, J.A. An alternative to fish oils: Metabolic engineering of oil-seed crops to produce omega-3 long chain polyunsaturated fatty acids. Prog. Lipid Res. 2010, 49, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Joffre, C.; Rey, C.; Layé, S. N-3 polyunsaturated fatty acids and the resolution of neuroinflammation. Front. Pharmacol. 2019, 10, 1022. [Google Scholar] [CrossRef]
- Calder, P.C. Docosahexaenoic acid. Ann. Nutr. Metab. 2016, 69 (Suppl. S1), 8–21. [Google Scholar] [CrossRef]
- Connor, S.; Tenorio, G.; Clandinin, M.T.; Sauvé, Y. DHA supplementation enhances high-frequency, stimulation-induced synaptic transmission in mouse hippocampus. Appl. Physiol. Nutr. Metab. 2012, 37, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Cutuli, D.; Pagani, M.; Caporali, P.; Galbusera, A.; Laricchiuta, D.; Foti, F.; Neri, C.; Spalletta, G.; Caltagirone, C.; Petrosini, L.; et al. Effects of omega-3 fatty acid supplementation on cognitive functions and neural substrates: A voxel-based morphometry study in aged mice. Front. Aging Neurosci. 2016, 8, 38. [Google Scholar] [CrossRef] [PubMed]
- Milne, G.L.; Morrow, J.D.; Picklo, M.J. Elevated oxidation of docosahexaenoic acid, 22:6 (n-3), in brain regions of rats undergoing ethanol withdrawal. Neurosci. Lett. 2006, 405, 172–174. [Google Scholar] [CrossRef]
- Pawlosky, R.J.; Bacher, J.; Salem, N. Ethanol consumption alters electroretinograms and depletes neural tissues of docosahexaenoic acid in rhesus monkeys: Nutritional consequences of a low n-3 fatty acid diet. Alcohol Clin. Exp. Res. 2001, 25, 1758–1765. [Google Scholar] [PubMed]
- Tajuddin, N.; Moon, K.H.; Marshall, S.A.; Nixon, K.; Neafsey, E.J.; Kim, H.Y.; Collins, M.A. Neuroinflammation and neurodegeneration in adult rat brain from binge ethanol exposure: Abrogation by docosahexaenoic acid. PLoS ONE 2014, 9, e101223. [Google Scholar] [CrossRef]
- Patten, A.; Sickmann, H.M.; Dyer, R.A.; Innis, S.M.; Christie, B.R. Omega-3 fatty acids can reverse the long-term deficits in hippocampal synaptic plasticity caused by prenatal ethanol exposure. Neurosci. Lett. 2013, 551, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Lafourcade, M.; Larrieu, T.; Mato, S.; Duffaud, A.; Sepers, M.; Matias, I.; Manzoni, O.J. Nutritional omega-3 deficiency abolishes endocannabinoid-mediated neuronal functions. Nat. Neurosci. 2011, 14, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Kano, M.; Ohno-Shosaku, T.; Hashimotodani, Y.; Uchigashima, M.; Watanabe, M. Endocannabinoid-mediated control of synaptic transmission. Physiol. Rev. 2009, 89, 309–380. [Google Scholar] [CrossRef]
- Booth, W.T.; Walker, N.B.; Lowther, W.T.; Howlett, A.C. Cannabinoid receptor interacting protein 1a (CRIP1a): Function and structure. Molecules 2019, 24, 3672. [Google Scholar] [CrossRef] [PubMed]
- Niehaus, J.L.; Liu, Y.; Wallis, K.T.; Egertová, M.; Bhartur, S.G.; Mukhopadhyay, S.; Shi, S.; He, H.; Selley, D.E.; Howlett, A.C.; et al. CB1 cannabinoid receptor activity is modulated by the cannabinoid receptor interacting protein CRIP1a. Mol. Pharmacol. 2007, 72, 1557–1566. [Google Scholar] [CrossRef] [PubMed]
- Oliver, E.E.; Hughes, E.K.; Puckett, M.K.; Chen, R.; Lowther, W.T.; Howlett, A.C. Cannabinoid receptor interacting protein 1a (CRIP1a) in health and disease. Biomolecules 2020, 10, 1609. [Google Scholar] [CrossRef]
- Guggenhuber, S.; Alpar, A.; Chen, R.; Schmitz, N.; Wickert, M.; Mattheus, T.; Harasta, A.E.; Purrio, M.; Kaiser, N.; Elphick, M.R.; et al. Cannabinoid receptor-interacting protein Crip1a modulates CB1 receptor signaling in mouse hippocampus. Brain Struct. Funct. 2016, 221, 2061–2074. [Google Scholar] [CrossRef]
- Blume, L.C.; Bass, C.E.; Childers, S.R.; Dalton, G.D.; Roberts, D.C.; Richardson, J.M.; Howlett, A.C. Striatal CB1 and D2 receptors regulate expression of each other, CRIP1A and delta opioid systems. J. Neurochem. 2013, 124, 808–820. [Google Scholar] [CrossRef]
- Isaac, A.R.; de Velasco, P.C.; Fraga, K.Y.D.; Tavares-do-Carmo, M.d.G.; Campos, R.M.P.; Iannotti, F.A.; Verde, R.; Martins, D.B.G.; Santos, T.A.; Ferreira, B.K.; et al. Maternal omega-3 intake differentially affects the endocannabinoid system in the progeny`s neocortex and hippocampus: Impact on synaptic markers. J. Nutr. Biochem. 2021, 96, 108782. [Google Scholar] [CrossRef] [PubMed]
- Dodd, P.R.; Hardy, J.A.; Oakley, A.E.; Edwardson, J.A.; Perry, E.K.; Delaunoy, J.P. A rapid method for preparing synaptosomes: Comparison, with alternative procedures. Brain Res. 1981, 226, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Saumell-Esnaola, M.; Barrondo, S.; García Del Caño, G.; Goicolea, M.A.; Sallés, J.; Lutz, B.; Monory, K. Subsynaptic distribution, lipid raft targeting and g protein-dependent signalling of the type 1 cannabinoid receptor in synaptosomes from the mouse hippocampus and frontal cortex. Molecules 2021, 26, 6897. [Google Scholar] [CrossRef] [PubMed]
- Montaña, M.; García del Caño, G.; López de Jesús, M.; González-Burguera, I.; Echeazarra, L.; Barrondo, S.; Sallés, J. Cellular neurochemical characterization and subcellular localization of phospholipase C β1 in rat brain. Neuroscience 2012, 222, 239–268. [Google Scholar] [CrossRef] [PubMed]
- Barrondo, S.; Sallés, J. Allosteric modulation of 5-HT1A receptors by zinc: Binding studies. Neuropharmacology 2009, 56, 455–462. [Google Scholar] [CrossRef]
- Peñasco, S.; Rico-Barrio, I.; Puente, N.; Gómez-Urquijo, S.M.; Fontaine, C.J.; Egaña-Huguet, J.; Grandes, P. Endocannabinoid long-term depression revealed at medial perforant path excitatory synapses in the dentate gyrus. Neuropharmacology 2019, 153, 32–40. [Google Scholar] [CrossRef] [PubMed]
- da Cruz, J.F.O.; Gomis-Gonzalez, M.; Maldonado, R.; Marsicano, G.; Ozaita, A.; Busquets-Garcia, A. An alternative maze to assess novel object recognition in mice. Bio-Protocol 2020, 10, e3651. [Google Scholar]
- Lezak, K.R.; Missig, G.; William, A.C., Jr. Behavioral methods to study anxiety in rodents. Dialogues Clin. Neurosci. 2017, 19, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Banni, S.; di Marzo, V. Effect of dietary fat on endocannabinoids and related mediators: Consequences on energy homeostasis, inflammation and mood. Mol. Nutr. Food Res. 2010, 54, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Doshi, M.; Hamazaki, T. n-3 Polyunsaturated fatty acid (PUFA) deficiency elevates and n-3 PUFA enrichment reduces brain 2-arachidonoylglycerol level in mice. Prostaglandins Leukot. Essent. Fat. Acids 2003, 69, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Brown, S.; Roche, J.P.; Hsieh, C.; Celver, J.P.; Kovoor, A.; Chavkin, C.; Mackie, K. Distinct domains of the CB1 cannabinoid receptor mediate desensitization and internalization. J. Neurosci. 1999, 19, 3773–3780. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.F.; Yang, L.Q.; Goschke, A.; Stumm, R.; Brandenburg, L.O.; Liang, Y.J.; Koch, T. Role of receptor internalization in the agonist-induced desensitization of cannabinoid type 1 receptors. J. Neurochem. 2008, 104, 1132–1143. [Google Scholar] [CrossRef] [PubMed]
- Mikasova, L.; Groc, L.; Choquet, D.; Manzoni, O.J. Altered surface trafficking of presynaptic cannabinoid type 1 receptor in and out synaptic terminals paralles receptor desensitization. Proc. Natl. Acad. Sci. USA 2008, 105, 18596–18601. [Google Scholar] [CrossRef] [PubMed]
- Egaña-Huguet, J.; Saumell-esnaola, M.; Achicallende, S. Lack of the transient receptor potential vanilloid 1 shifts cannabinoid-dependent excitatory synaptic plasticity in the dentate gyrus of the mouse brain hippocampus. Front. Neuroanat. 2021, 15, 701573. [Google Scholar] [CrossRef] [PubMed]
- Egaña-Huguet, J.; Bonilla-Del Río, I.; Gómez-Urquijo, S.M.; Mimenza, A.; Saumell-Esnaola, M.; Borrega-Roman, L.; del Caño, G.G.; Sallés, J.; Puente, N.; Gerrikagoitia, I.; et al. The absence of the transient receptor potential vanilloid 1 directly impacts on the expression and localization of the endocannabinoid system in the mouse hippocampus. Front. Neuroanat. 2021, 15, 645940. [Google Scholar] [CrossRef] [PubMed]
- Blume, L.C.; Eldeeb, K.; Bass, C.E.; Selley, D.E.; Howlett, A.C. Cannabinoid receptor interacting protein (CRIP1a) attenuates CB1R signaling in neuronal cells. Cell. Signal. 2015, 27, 716–726. [Google Scholar] [CrossRef]
- Mattheus, T.; Kukla, K.; Zimmermann, T.; Tenzer, S.; Lutz, B. Cell type-specific tandem affinity purification of the mouse hippocampal CB1 receptor-associated proteome. J. Proteome Res. 2016, 15, 3585–3601. [Google Scholar] [CrossRef] [PubMed]
- Peñasco, S.; Rico-Barrio, I.; Puente, N.; Fontaine, C.J.; Ramos, A.; Reguero, L.; Gerrikagoitia, I.; de Fonseca, F.R.; Suarez, J.; Barrondo, S.; et al. Intermittent ethanol exposure during adolescence impairs cannabinoid type 1 receptor- dependent long-term depression and recognition memory in adult mice. Neuropsychopharmacology 2020, 45, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Rico-Barrio, I.; Peñasco, S.; Lekunberri, L.; Serrano, M.; Egaña-Huguet, J.; Mimenza, A.; Soria-Gomez, E.; Ramos, A.; Buceta, I.; Gerrikagoitia, I.; et al. Environmental enrichment rescues endocannabinoid-dependent synaptic plasticity lost in young adult male mice after ethanol exposure during adolescence. Biomedicines 2021, 9, 825. [Google Scholar] [CrossRef]
- Wang, W.; Trieu, B.H.; Palmer, L.C.; Jia, Y.; Pham, D.T.; Jung, K.M.; Karsten, C.A.; Merrill, C.B.; Mackie, K.; Gall, C.M.; et al. A primary cortical input to hippocampus expresses a pathway-specific and endocannabinoid-dependent form of long-term potentiation. eNeuro 2016, 3, ENEURO.0160-16.2016. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Jia, Y.; Pham, D.T.; Palmer, L.C.; Jung, K.M.; Cox, C.D.; Rumbaugh, G.; Piomelli, D.; Gall, C.M.; Lynch, G. Atypical endocannabinoid signaling initiates a new form of memory-related plasticity at a cortical input to hippocampus. Cereb. Cortex 2018, 28, 2253–2266. [Google Scholar] [CrossRef]
- Xu, J.Y.; Zhang, J.; Chen, C. Long-lasting potentiation of hippocampal synaptic transmission by direct cortical input is mediated via endocannabinoids. J. Physiol. 2012, 590, 2305–2315. [Google Scholar] [CrossRef]
- Cui, Y.; Prokin, I.; Xu, H.; Delord, B.; Genet, S.; Venance, L.; Berry, H. Endocannabinoid dynamics gate spike- timing dependent depression and potentiation. Elife 2016, 5, e13185. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Perez, S.; Venance, L. Endocannabinoid-LTP mediated by CB1 and TRPV1 receptors encodes for limited occurrences of coincident activity in neocortex. Front. Cell. Neurosci. 2018, 12, 182. [Google Scholar] [CrossRef] [PubMed]
- Glass, M.; Felder, C.C. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors augments cAMP accumulation in striatal neurons: Evidence for a G(s) linkage to the CB1 receptor. J. Neurosci. 1997, 17, 5327–5333. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, B.; Paz, F.; Florán, L.; Aceves, J.; Erlij, D.; Florán, B. Cannabinoid agonists stimulate [3H]GABA release in the globus pallidus of the rat when Gi protein-receptor coupling is restricted: Role of dopamine D2 receptors. J. Pharmacol. Exp. Ther. 2009, 328, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Feng, N.; Lin, G.; Tong, Y.; Jiang, X.; Yang, Q.; Wang, S.; Chen, W.; He, Z.; Chen, Y.Q. Metabolic shift induced by ω-3 PUFAs and rapamycin lead to cancer cell death. Cell. Physiol. Biochem. 2018, 48, 2318–2336. [Google Scholar] [CrossRef]
- Bender, V.A.; Pugh, J.R.; Jahr, C.E. Presynaptically expressed long-term potentiation increases multivesicular release at parallel fiber synapses. J. Neurosci. 2009, 29, 10974–10978. [Google Scholar] [CrossRef] [PubMed]
- Chevaleyre, V.; Heifets, B.D.; Kaeser, P.S.; Sudhof, T.C.; Castillo, P.E. Endocannabinoid-mediated long-term plasticity requires cAMP/PKA signaling and RIM1α. Neuron. 2007, 54, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Fukaya, R.; Miyano, R.; Hirai, H.; Sakaba, T. Mechanistic insights into cAMP-mediated presynaptic potentiation at hippocampal mossy fiber synapses. Front. Cell. Neurosci. 2023, 17, 1237589. [Google Scholar] [CrossRef]
- Kim, H.; Spector, A. N-Docosahexaenoylethanolamine: A neurotrophic and neuroprotective metabolite of docosahexaenoic acid. Mol. Asp. Med. 2018, 64, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Huang, B.X.; Kwon, H.S.; Rashid, M.A.; Kharebava, G.; Desai, A.; Patnaik, S.; Marugan, J.; Kim, H.-Y. Orphan GPR110 (ADGRF1) targeted by N-docosahexaenoylethanolamine in development of neurons and cognitive function. Nat. Commun. 2016, 7, 13123. [Google Scholar] [CrossRef] [PubMed]
- Mottola, G.; Chatterjee, A.; Wu, B.; Chen, M.; Conte, M.S. Aspirin-triggered resolvin D1 attenuates PDGF-induced vascular smooth muscle cell migration via the cyclic adenosine monophosphate/protein kinase A (cAMP/PKA) pathway. PLoS ONE 2017, 12, e0174936. [Google Scholar] [CrossRef]
- Kim, H.; Huang, B.; Spector, A. Molecular and signaling mechanisms for docosahexaenoic acid-derived neurodevelopment and neuroprotection. Int. J. Mol. Sci. 2022, 23, 4635. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.S.; Siegelbaum, S.A. Recruitment of N-Type Ca2+ Channels during LTP enhances low release efficacy of hippocampal CA1 perforant path synapses. Neuron 2009, 63, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Hodel, A. Snap-25. Int. J. Biochem. Cell Biol. 1998, 30, 1069–1073. [Google Scholar] [CrossRef]
- Verderio, C.; Pozzi, D.; Pravettoni, E.; Inverardi, F.; Schenk, U.; Coco, S.; Proux-Gillardeaux, V.; Galli, T.; Rossetto, O.; Frassoni, C.; et al. SNAP-25 modulation of calcium dynamics underlies differences in GABAergic and glutamatergic responsiveness to depolarization. Neuron 2004, 41, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Lynch, M.A.; Voss, K.L. Membrane arachidonic acid concentration correlates with age and induction of long-term potentiation in the dentate gyrus in the rat. Eur. J. Neurosci. 1994, 6, 1008–1014. [Google Scholar] [CrossRef] [PubMed]
- Collins, D.R.; Davies, S.N. Co-administration of (1S,3R)-1 aminocyclopentane-1,3-dicarboxylic acid and arachidonic acid potentiates synaptic transmission in the hippocampus. Eur. J. Pharmacol. 1993, 240, 325–326. [Google Scholar] [CrossRef] [PubMed]
- McGahon, B.; Lynch, M.A. The synergism between metabotropic glutamate receptor activation and arachidonic acid on glutamate release is occluded by induction of long-term potentiation in the dentate gyrus. Neuroscience 1996, 72, 847–855. [Google Scholar] [CrossRef]
- Harney, S.C.; Jane, D.E.; Anwyl, R. Extrasynaptic NR2D-containing NMDARs are recruited to the synapse during LTP of NMDAR-EPSCs. J. Neurosci. 2008, 28, 11685–11694. [Google Scholar] [CrossRef]
- Hunt, D.L.; Castillo, P.E. Synaptic plasticity of NMDA receptors: Mechanisms and functional implications. Curr. Opin. Neurobiol. 2012, 22, 496–508. [Google Scholar] [CrossRef] [PubMed]
- Bliss, T.; Burns, B.D.; Uttley, A.M. Factors affecting the conductivity of pathways in the cerebral cortex. J. Physiol. 1968, 195, 339–367. [Google Scholar] [CrossRef] [PubMed]
- Volianskis, A.; Collingridge, G.L.; Jensen, M.S. The roles of STP and LTP in synaptic encoding. PeerJ 2013, 1, e3. [Google Scholar] [CrossRef]
- Bliss, T.; Collingridge, G. Persistent memories of long-term potentiation and the N -methyl-d-aspartate receptor. Brain Neurosci. Adv. 2019, 3, 2398212819848213. [Google Scholar] [CrossRef] [PubMed]
- Errington, M.L.; Lynch, M.A.; Bliss, T.V.P. Long-term potentiation in the dentate gyrus: Induction and increased glutamate release are blocked by d(−)aminophosphonovalerate. Neuroscience 1987, 20, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Sturgill, J.F.; Steiner, P.; Czervionke, B.L.; Sabatini, B.L. Distinct domains within PSD-95 mediate synaptic incorporation, stabilization, and activity-dependent trafficking. J. Neurosci. 2009, 29, 12845–12854. [Google Scholar] [CrossRef] [PubMed]
- Meyer, D.; Bonhoeffer, T.; Scheuss, V. Balance and stability of synaptic structures during synaptic plasticity. Neuron 2014, 82, 430–443. [Google Scholar] [CrossRef]
- Coley, A.A.; Gao, W.J. PSD-95 deficiency disrupts PFC-associated function and behavior during neurodevelopment. Sci. Rep. 2019, 9, 9486. [Google Scholar] [CrossRef]
- Nagura, H.; Ishikawa, Y.; Kobayashi, K.; Takao, K.; Tanaka, T.; Nishikawa, K.; Tamura, H.; Shiosaka, S.; Suzuki, H.; Miyakawa, T.; et al. Impaired synaptic clustering of postsynaptic density proteins and altered signal transmission in hippocampal neurons, and disrupted learning behavior in PDZ1 and PDZ2 ligand binding-deficient PSD-95 knockin mice. Mol. Brain 2012, 5, 43. [Google Scholar] [CrossRef]
- Pan, J.P.; Zhang, H.Q.; Wang, W.; Guo, Y.F.; Xiao, N.; Cao, X.H.; Liu, L.-J. Some subtypes of endocannabinoid/endovanilloid receptors mediate docosahexaenoic acid-induced enhanced spatial memory in rats. Brain Res. 2011, 1412, 18–27. [Google Scholar] [CrossRef]
- Satizabal, C.L.; Himali, J.J.; Beiser, A.S.; Ramachandran, V.; Melo Van Lent, D.; Himali, D.; Seshadri, S. Association of red blood cell omega-3 fatty acids with MRI markers and cognitive function in midlife: The Framingham Heart study. Neurology 2022, 99, E2572–E2582. [Google Scholar] [CrossRef]
- Pusceddu, M.M.; Kelly, P.; Ariffin, N.; Cryan, J.F.; Clarke, G.; Dinan, T.G. N-3 PUFAs have beneficial effects on anxiety and cognition in female rats: Effects of early life stress. Psychoneuroendocrinology 2015, 58, 79–90. [Google Scholar] [CrossRef]
- Yurko-Mauro, K.; McCarthy, D.; Rom, D.; Nelson, E.B.; Ryan, A.S.; Blackwell, A.; Salem, N., Jr.; Stedman, M.; MIDAS Investigators. Beneficial effects of docosahexaenoic acid on cognition in age-related cognitive decline. Alzheimer’s Dement. 2010, 6, 456–464. [Google Scholar] [CrossRef]
- Larrieu, T.; Madore, C.; Joffre, C.; Layé, S. Nutritional n-3 polyunsaturated fatty acids deficiency alters cannabinoid receptor signaling pathway in the brain and associated anxiety-like behavior in mice. J. Physiol. Biochem. 2012, 68, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Manduca, A.; Bara, A.; Larrieu, T.; Lassalle, O.; Joffre, C.; Layé, S.; Manzoni, O.J. Amplification of mGlu5-endocannabinoid signaling rescues behavioral and synaptic deficits in a mouse model of adolescent and adult dietary polyunsaturated fatty acid imbalance. J. Neurosci. 2017, 37, 6851–6868. [Google Scholar] [CrossRef]
- Thomazeau, A.; Bosch-Bouju, C.; Manzoni, O.; Layé, S. Nutritional n-3 PUFA deficiency abolishes endocannabinoid gating of hippocampal long-term potentiation. Cereb. Cortex 2017, 27, 2571–2579. [Google Scholar] [CrossRef]
- Hu, Y.W.; Ma, X.; Li, X.X.; Liu, X.H.; Xiao, J.; Mo, Z.C.; Tang, C.K. Eicosapentaenoic acid reduces ABCA1 serine phosphorylation and impairs ABCA1-dependent cholesterol efflux through cyclic AMP/protein kinase A signaling pathway in THP-1 macrophage-derived foam cells. Atherosclerosis 2009, 204, e35–e43. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, X.; Ma, L.J.; Feng, R.B.; Yan, C.; Su, H.; He, C.; Kang, J.X.; Liu, B.; Wan, J.-B. Omega-3 polyunsaturated fatty acids ameliorate ethanol-induced adipose hyperlipolysis: A mechanism for hepatoprotective effect against alcoholic liver disease. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 3190–3201. [Google Scholar] [CrossRef] [PubMed]
- Roy, J.; Lefkimmiatis, K.; Moyer, M.P.; Curci, S.; Hofer, A.M. The ω-3 fatty acid eicosapentaenoic acid elicits cAMP generation in colonic epithelial cells via a “store-operated” mechanism. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G715–G722. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.A.; Kim, H. N-Docosahexaenoylethanolamine ameliorates ethanol-induced impairment of neural stem cell neurogenic differentiation. Neuropharmacology 2016, 102, 174–185. [Google Scholar] [CrossRef]
- Wiescholleck, V.; Manahan-Vaughan, D. PDE4 inhibition enhances hippocampal synaptic plasticity in vivo and rescues MK801-induced impairment of long-term potentiation and object recognition memory in an animal model of psychosis. Transl. Psychiatry 2012, 2, e89. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Santos, A.F.; Moreira, F.A.; Guimaraes, F.S.; Aguiar, D.C. 2-arachidonoylglycerol endocannabinoid signaling coupled to metabotropic glutamate receptor type-5 modulates anxiety-like behavior in the rat ventromedial prefrontal cortex. J. Psychopharmacol. 2017, 31, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Su, K.P.; Tseng, P.T.; Lin, P.Y.; Okubo, R.; Chen, T.Y.; Chen, Y.W.; Matsuoka, Y.J. Association of use of omega-3 polyunsaturated fatty acids with changes in severity of anxiety symptoms: A systematic review and meta-analysis. JAMA Netw. Open 2018, 1, e182327. [Google Scholar] [CrossRef] [PubMed]
- Neto, J.; Jantsch, J.; De Oliveira, S.; Braga, M.F.; Castro, L.F.D.S.; Diniz, B.F.; Moreira, J.C.F.; Giovenardi, M.; Porawski, M.; Guedes, R.P. DHA/EPA supplementation decreases anxiety-like behaviour, but it does not ameliorate metabolic profile in obese male rats. Br. J. Nutr. 2022, 128, 964–974. [Google Scholar] [CrossRef] [PubMed]
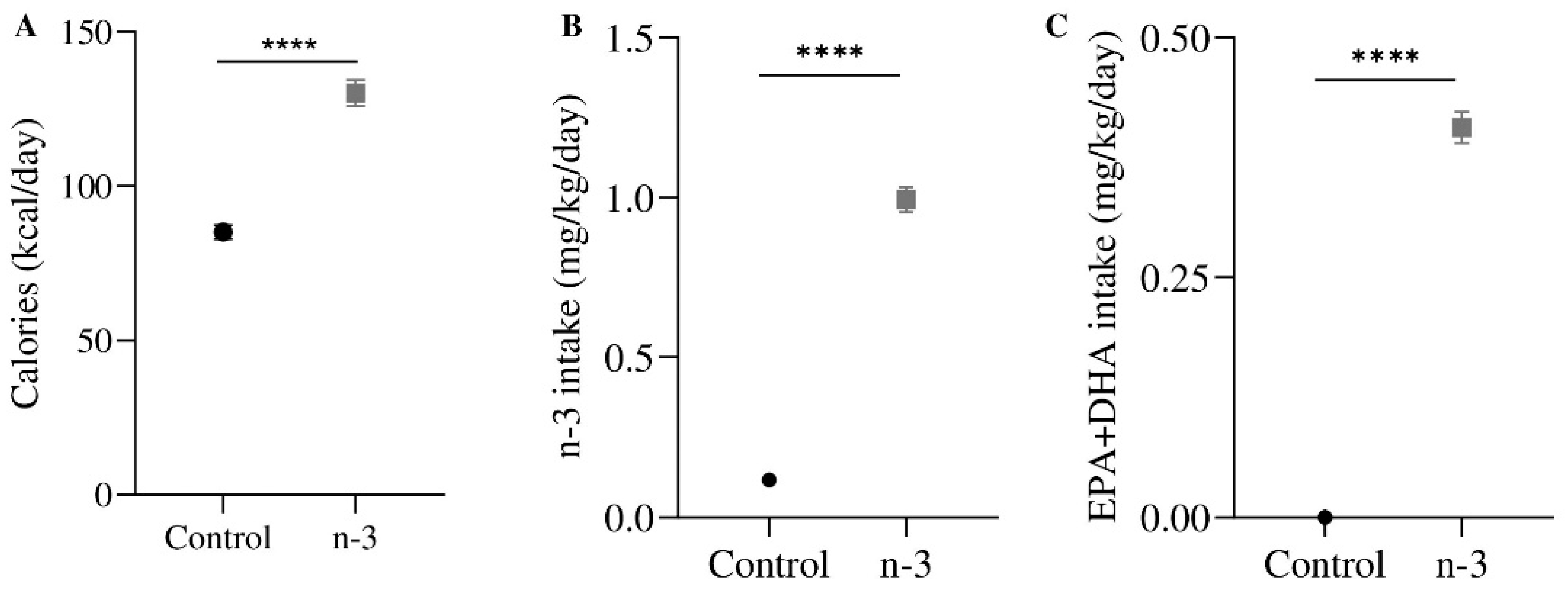



| Standard Diet | n-3-Enriched Diet | |||
|---|---|---|---|---|
| Fats (%) | 4.0 | 5.9 | ||
| mg/kg | % of Total Fats | mg/kg | % of Total Fats | |
| SFA | 6000 | 15.0 | 11,867 | 20.1 |
| PUFA | 21,000 | 52.5 | 31,129 | 52.8 |
| n-3 | 1000 | 2.5 | 5437 | 9.2 |
| n-6 | 20,000 | 50.0 | 25,679 | 43.5 |
| Ratio n-6/n-3 | - | 20.0 | - | 4.7 |
| EPA | 0 | 0 | 1325 | 2.2 |
| DHA | 0 | 0 | 899 | 1.5 |
| Antibody | Dilution | Species and Clonality | Isotype | Immunizing Antigen | Source, Cat. |
|---|---|---|---|---|---|
| CB1 | 1:1000 | Goat polyclonal | Serum | Mouse CB1, C-terminal 31 aa (NM007726) | Nittobo Medical Co. (Tokyo, Japan), CB1-Go-Af450 |
| Crip1a | 1:500 | Rabbit polyclonal | IgG | Peptide mapping within an internal region of CRIP1 of human origin | Santa Cruz Biotechnology, Inc. (Dallas, TX, USA), sc-137401 |
| Gαo | 1:200 | Mouse monoclonal | IgG1 kappa light chain | Raised against Gαo of bovine origin | Santa Cruz Biotechnology, Inc. (Dallas, TX, USA), sc-13532 |
| Gαi1 | 1:200 | Mouse monoclonal | IgG2b kappa light chain | Raised against Gαi1 of rat origin | Santa Cruz Biotechnology, Inc. (Dallas, TX, USA), sc-13533 |
| Gαi2 | 1:200 | Mouse monoclonal | IgG2b kappa light chain | Raised against Gαi2 of rat origin | Santa Cruz Biotechnology, Inc. (Dallas, TX, USA), sc-13534 |
| Gαi3 | 1:200 | Mouse monoclonal | IgG3 kappa light chain | Epitope mapping between amino acids 339–354 at the C-terminus of Gαi3 of rat origin | Santa Cruz Biotechnology, Inc. (Dallas, TX, USA), sc-365422 |
| PLCβ1 | 1:2000 | Sheep Polyclonal | IgG | E. coli-derived recombinant human PLC-β1 Lys27-Met245 | Novus Biologicals (Centennial, CO, USA), AF4466 |
| DAGLα | 1:1000 | Rabbit polyclonal | Serum | Mouse DGL-α, C-terminal 42 aa (NM198114) | Nittobo Medical Co. (Tokyo, Japan), DGLa-Rb-Af380 |
| DAGLβ | 1:2000 | Rabbit monoclonal | IgG | Synthetic peptide corresponding to residues surrounding Leu505 of human DGL-β protein | Cell Signaling Technology, Inc. (Danvers, MA, USA), 12574 |
| MAGL | 1:1000 | Rabbit polyclonal | Serum | Mouse MGL, 1–35aa (NM_011844) | Nittobo Medical Co. (Tokyo, Japan), MGL-Rb-Af200 |
| PSD-95 | 1:2500 | Goat polyclonal | IgG | Synthetic peptide derived from residues 1–100 of mouse PSD95 | Abcam (Cambridge, UK), ab12093 |
| SNAP-25 | 1:10,000 | Rabbit polyclonal | IgG | Synthetic peptide within residues 150 to the C-terminus of human SNAP25 | Abcam (Cambridge, UK), ab41455 |
| Drug | Action | Dilution (µM) |
|---|---|---|
| AM251 | CB1 antagonist | 4 |
| AMG9810 | TRPV1 antagonist | 3 |
| MPEP | mGluR5 antagonist | 10 |
| THL | DGL inhibitor | 10 |
| RHC 80267 | DGL inhibitor | 100 |
| D-AP5 | NMDA antagonist | 50 |
| Latrunculin A | Actin assembly inhibitor | 500 |
| ω-conotoxin GVIA | N-type Ca2+ channels blocker | 1 |
| CPCCoEt | mGluR1 antagonist | 50 |
| LEI401 | NAPE-PLD inhibitor | 10 |
| WIN-2 | CB1 agonist | 5 |
| Fraction | Protein | n-3 |
|---|---|---|
| wH | CB1 | 0.972 ± 0.046 (n = 3) |
| Crip1a | 1.024 ± 0.016 (n = 3) | |
| Synaptosomes | CB1 | 1.333 ± 0.065 ** (n = 8) |
| Crip1a | 1.487 ± 0.095 *** (n = 11) | |
| Gαo | 0.771 ± 0.057 * (n = 4) | |
| Gαi1 | 0.824 ± 0.081 (n = 5) | |
| Gαi2 | 0.924 ± 0.059 (n = 4) | |
| Gαi3 | 0.779 ± 0.075 * (n = 7) | |
| PLCβ1 | 1.263 ± 0.059 ** (n = 8) | |
| DAGLα | 0.635 ± 0.059 ** (n = 7) | |
| DAGLβ | 1.205 ± 0.064 (n = 7) | |
| MAGL | 1.250 ± 0.055 *** (n = 7) | |
| SNAP-25 | 0.734 ± 0.076 * (n = 5) | |
| PSD-95 | 0.650 ± 0.082 * (n = 5) |
| Control | n-3 | |
|---|---|---|
| Emax | 156.90 ± 12.80 | 159.00 ± 8.56 |
| pEC50 | 7.03 ± 0.07 | 7.13 ± 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serrano, M.; Saumell-Esnaola, M.; Ocerin, G.; García del Caño, G.; Puente, N.; Sallés, J.; Rodríguez de Fonseca, F.; Rodríguez-Arias, M.; Gerrikagoitia, I.; Grandes, P. Impact of Omega-3 on Endocannabinoid System Expression and Function, Enhancing Cognition and Behavior in Male Mice. Nutrients 2024, 16, 4344. https://doi.org/10.3390/nu16244344
Serrano M, Saumell-Esnaola M, Ocerin G, García del Caño G, Puente N, Sallés J, Rodríguez de Fonseca F, Rodríguez-Arias M, Gerrikagoitia I, Grandes P. Impact of Omega-3 on Endocannabinoid System Expression and Function, Enhancing Cognition and Behavior in Male Mice. Nutrients. 2024; 16(24):4344. https://doi.org/10.3390/nu16244344
Chicago/Turabian StyleSerrano, Maitane, Miquel Saumell-Esnaola, Garazi Ocerin, Gontzal García del Caño, Nagore Puente, Joan Sallés, Fernando Rodríguez de Fonseca, Marta Rodríguez-Arias, Inmaculada Gerrikagoitia, and Pedro Grandes. 2024. "Impact of Omega-3 on Endocannabinoid System Expression and Function, Enhancing Cognition and Behavior in Male Mice" Nutrients 16, no. 24: 4344. https://doi.org/10.3390/nu16244344
APA StyleSerrano, M., Saumell-Esnaola, M., Ocerin, G., García del Caño, G., Puente, N., Sallés, J., Rodríguez de Fonseca, F., Rodríguez-Arias, M., Gerrikagoitia, I., & Grandes, P. (2024). Impact of Omega-3 on Endocannabinoid System Expression and Function, Enhancing Cognition and Behavior in Male Mice. Nutrients, 16(24), 4344. https://doi.org/10.3390/nu16244344







