Preoperative Body Composition Correlates with Postoperative Muscle Volume and Degeneration after Total Hip Arthroplasty
Abstract
:1. Introduction
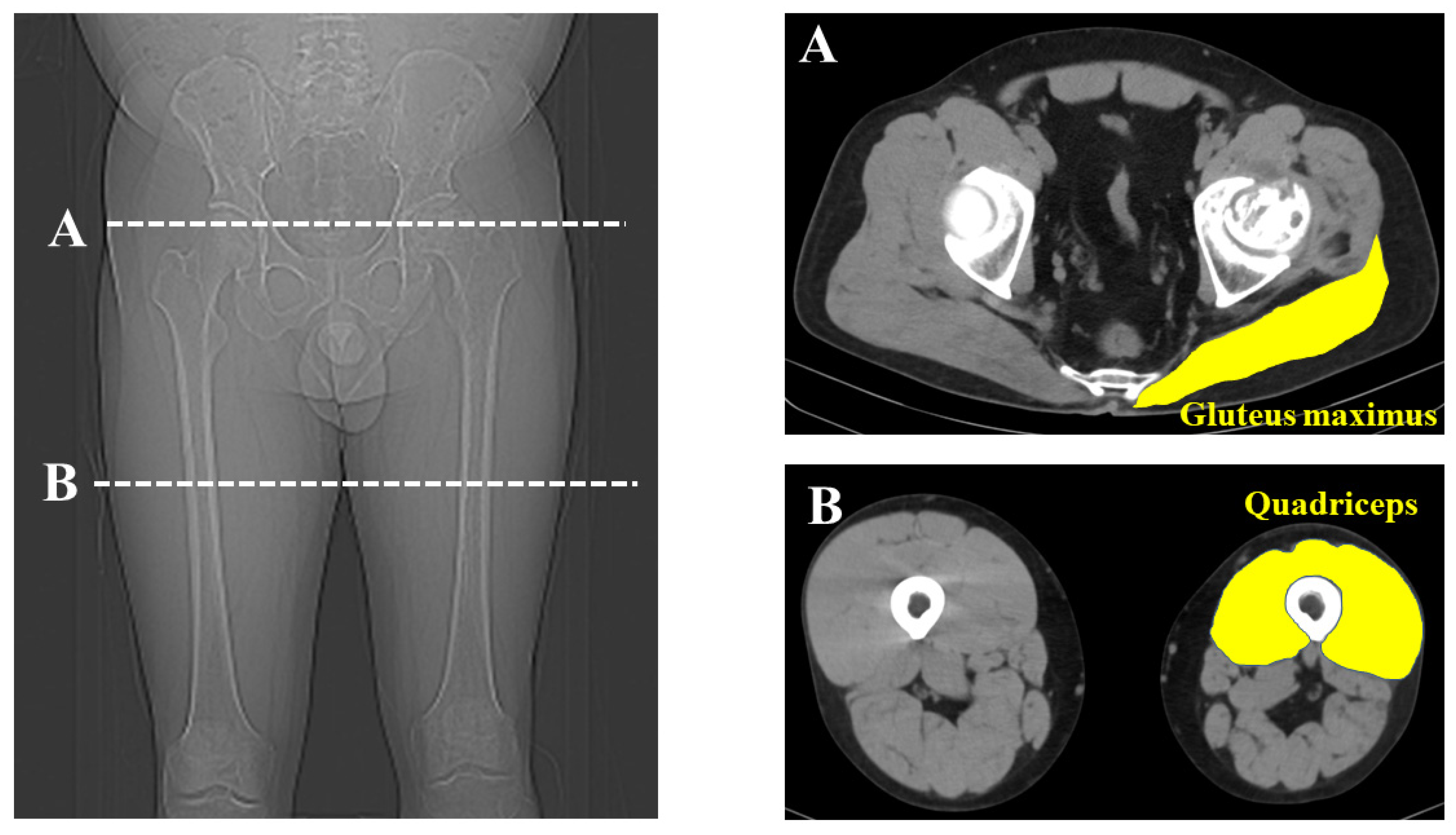
2. Materials and Methods
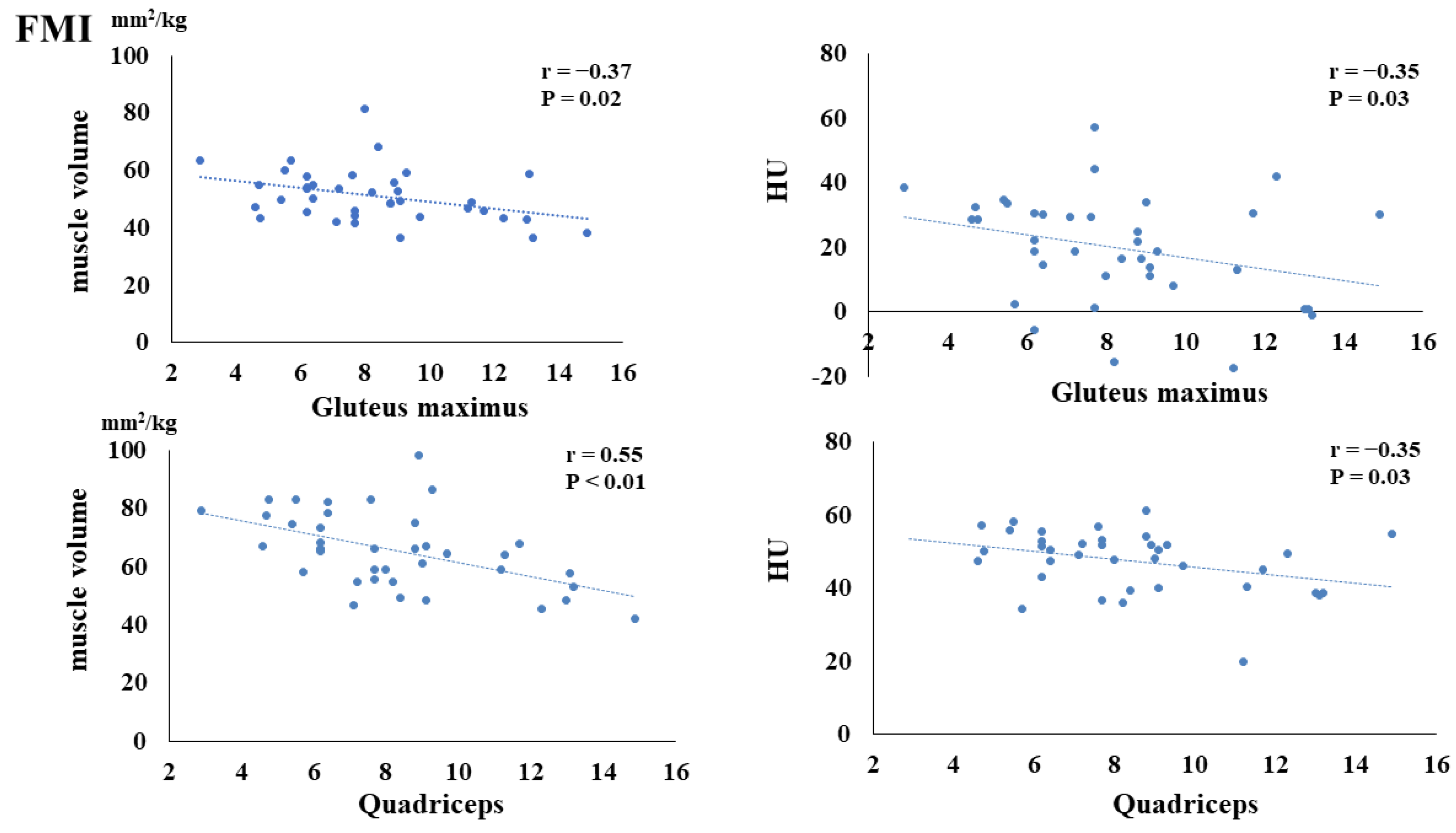
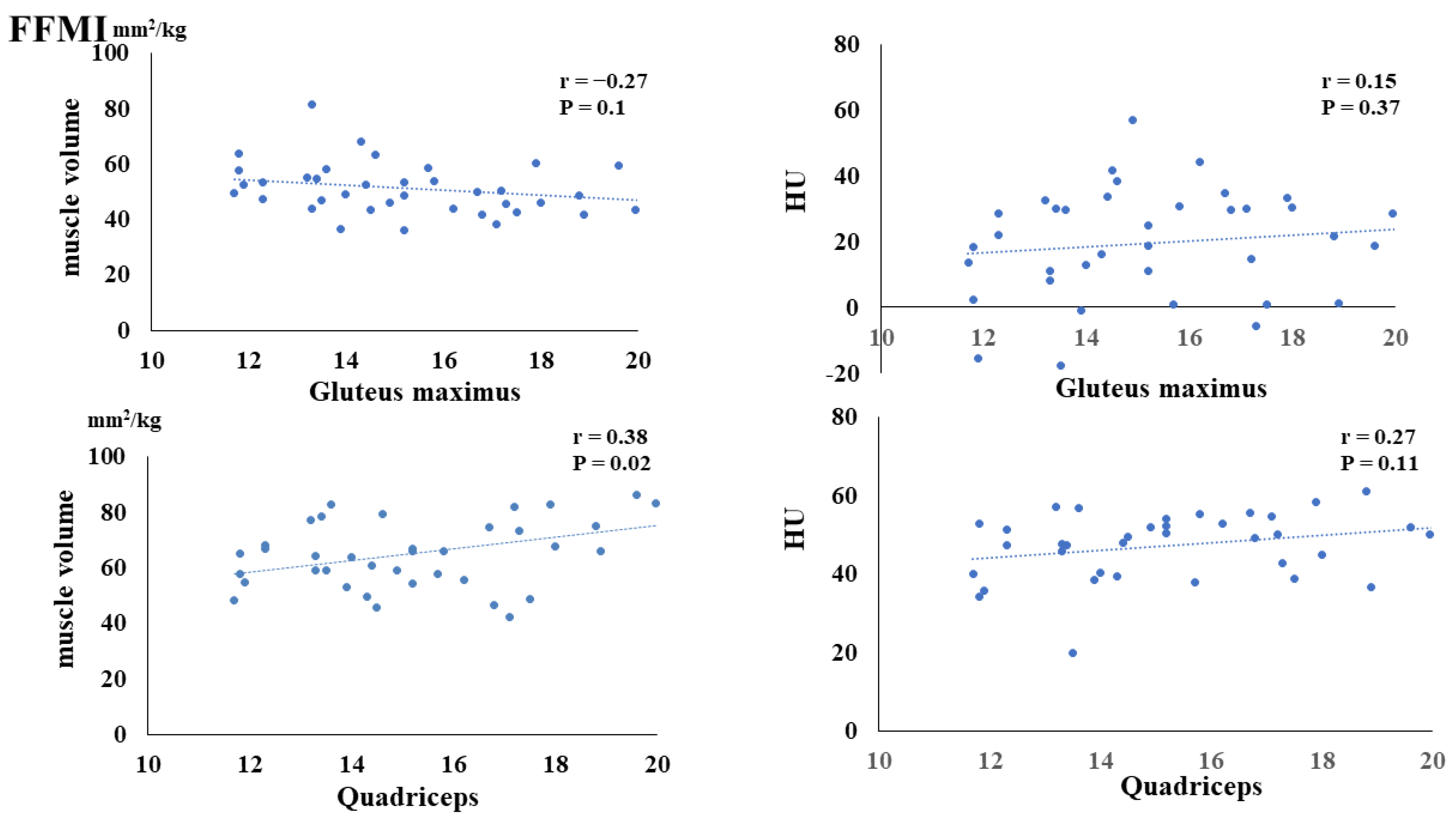
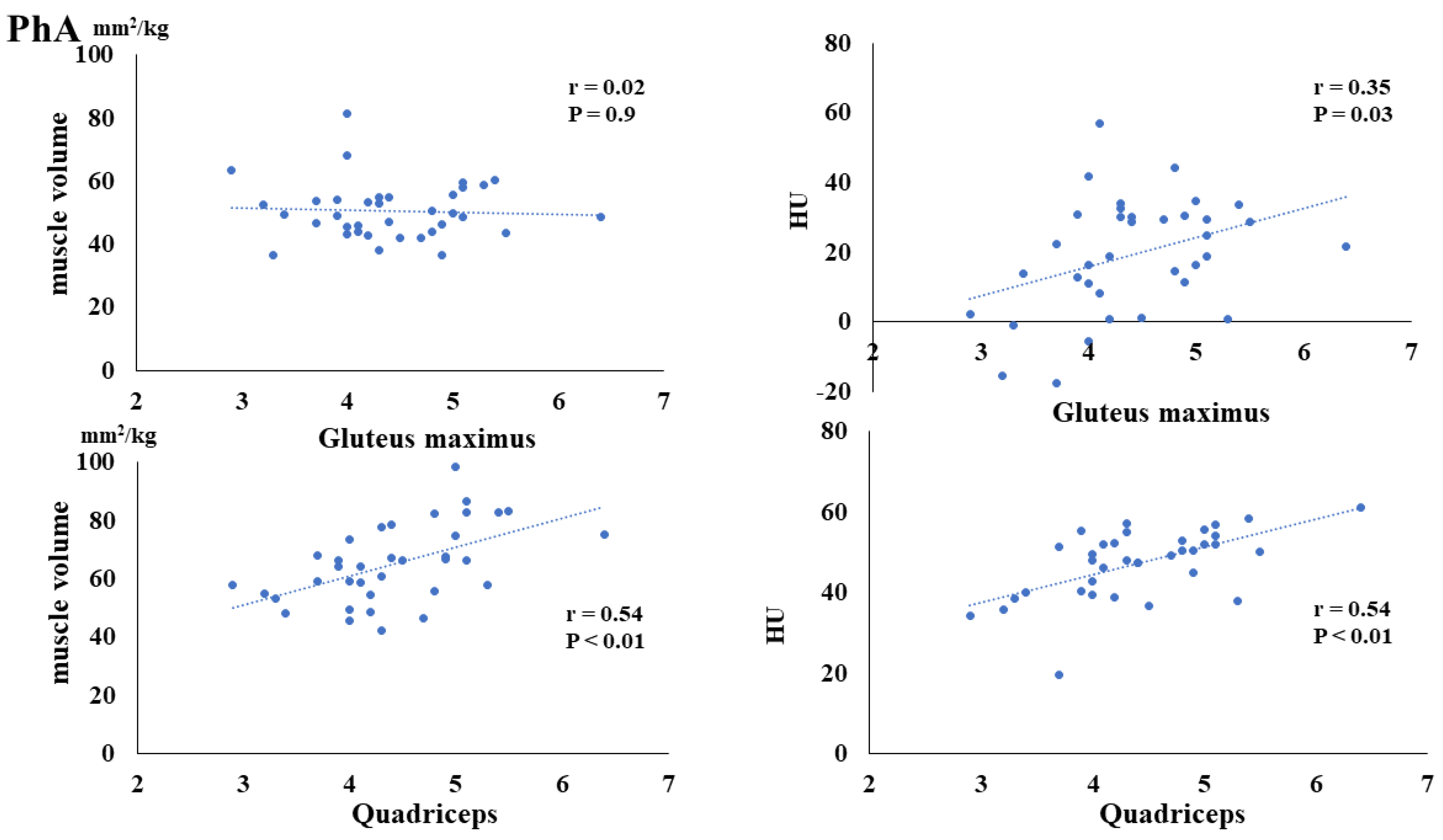
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kurtz, S.; Ong, K.; Lau, E.; Mowat, F.; Halpern, M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J. Bone Jt. Surg. Am. 2007, 89, 780–785. [Google Scholar] [CrossRef]
- Majewski, M.; Bischoff-Ferrari, H.A.; Grüneberg, C.; Dick, W.; Allum, J.H. Improvements in balance after total hip replacement. J. Bone Jt. Surg. Br. 2005, 87, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Lugade, V.; Klausmeier, V.; Jewett, B.; Collis, D.; Chou, L.S. Short-term recovery of balance control after total hip arthroplasty. Clin. Orthop. Relat. Res. 2008, 466, 3051–3058. [Google Scholar] [CrossRef] [PubMed]
- Rasch, A.; Dalén, N.; Berg, H.E. Muscle strength, gait, and balance in 20 patients with hip osteoarthritis followed for 2 years after THA. Acta Orthop. 2010, 81, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Judd, D.L.; Dennis, D.A.; Thomas, A.C.; Wolfe, P.; Dayton, M.R.; Stevens-Lapsley, J.E. Muscle strength and functional recovery during the first year after THA. Clin. Orthop. Relat. Res. 2014, 472, 654–664. [Google Scholar] [CrossRef]
- Denison, H.J.; Cooper, C.; Sayer, A.A.; Robinson, S.M. Prevention and optimal management of sarcopenia: A review of combined exercise and nutrition interventions to improve muscle outcomes in older people. Clin. Interv. Aging 2015, 10, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, T.; Miyatani, M.; Tachi, M.; Kouzaki, M.; Kawakami, Y.; Kanehira, H. Muscle volume is a major determinant of joint torque in humans. Acta Physiol. Scand. 2001, 172, 249–255. [Google Scholar] [CrossRef]
- Baxter, J.R.; Piazza, S.J. Plantar flexor moment arm and muscle volume predict torque-generating capacity in young men. J. Appl. Physiol. 2014, 116, 538–544. [Google Scholar] [CrossRef]
- Westcott, W.L. Resistance training is medicine: Effects of strength training on health. Curr. Sports Med. Rep. 2012, 11, 209–216. [Google Scholar] [CrossRef]
- Mcleod, J.C.; Stokes, T.; Phillips, S.M. Resistance exercise training as a primary countermeasure to age-related chronic disease. Front. Physiol. 2019, 10, 645. [Google Scholar] [CrossRef]
- Tyrovolas, S.; Panagiotakos, D.; Georgouspoulou, E.; Chrysohoou, C.; Tousoulis, D.; Haro, J.M.; Pitsavos, C. Skeletal muscle mass in relation to 10 year cardiovascular disease incidence among middle aged and older adults: The ATTICA study. J. Epidemiol. Commun. Health 2020, 74, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, E.; Skalli, W.; Lafage, V.; Maillot, C.; Carlier, R.; Feydy, A.; Felter, A.; Khalifé, M.; Guigui, P. Relationships between radiographic parameters and spinopelvic muscles in adult spinal deformity patients. Eur. Spine J. 2020, 29, 1328–1339. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Moal, B.; Vira, S.; Bronsard, N.; Amabile, C.; Errico, T.; Schwab, F.; Skalli, W.; Dubousset, J.; Lafage, V. Spino-femoral muscles affect sagittal alignment and compensatory recruitment: A new look into soft tissues in adult spinal deformity. Eur. Spine 2020, 29, 2998–3005. [Google Scholar] [CrossRef] [PubMed]
- Manabe, T.; Ogawa, C.; Takuma, M.; Nakahara, M.; Oura, K.; Tadokoro, T.; Fujita, K.; Tani, J.; Shibatoge, M.; Morishita, A.; et al. Usefulness of the measurement of psoas muscle volume for sarcopenia diagnosis in patients with liver disease. Diagnostics 2023, 13, 1245. [Google Scholar] [CrossRef] [PubMed]
- Nankaku, M.; Tsuboyama, T.; Akiyama, H.; Kakinoki, R.; Fujita, Y.; Nishimura, J.; Yoshioka, Y.; Kawai, H.; Matsuda, S. Preoperative prediction of ambulatory status at 6 months after total hip arthroplasty. Phys. Ther. 2013, 93, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Holstege, M.S.; Lindeboom, R.; Lucas, C. Preoperative quadriceps strength as a predictor for short-term functional outcome after total hip replacement. Arch. Phys. Med. Rehabil. 2011, 92, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Uemura, K.; Takao, M.; Sakai, T.; Nishii, T.; Sugano, N. Volume increases of the gluteus maximus, gluteus medius, and thigh muscles after hip arthroplasty. J. Arthroplast. 2016, 31, 906–912. [Google Scholar] [CrossRef] [PubMed]
- Ukai, T.; Ebihara, G.; Omura, H.; Watanabe, M. Evaluation of muscle volume and degeneration after total hip arthroplasty: A comparison of the posterolateral approach and the anterolateral supine approach. J. Orthop. Surg. Res. 2021, 16, 145. [Google Scholar] [CrossRef]
- Homma, D.; Minato, I.; Imai, N.; Miyasaka, D.; Sakai, Y.; Horigome, Y.; Suzuki, H.; Dohmae, Y.; Endo, N. Relationship between the hip abductor muscles and abduction strength in patients with hip osteoarthritis. Acta Med. Okayama 2023, 77, 461–469. [Google Scholar] [CrossRef]
- Rasch, A.; Byström, A.H.; Dalen, N.; Berg, H.E. Reduced muscle radiological density, cross-sectional area, and strength of major hip and knee muscles in 22 patients with hip osteoarthritis. Acta Orthop. 2007, 78, 505–510. [Google Scholar] [CrossRef]
- Liu, R.; Wen, X.; Tong, Z.; Wang, K.; Wang, C. Changes of gluteus medius muscle in the adult patients with unilateral developmental dysplasia of the hip. BMC Musculoskelet. Disord. 2012, 13, 101. [Google Scholar] [CrossRef] [PubMed]
- Rasch, A.; Byström, A.H.; Dalén, N.; Martinez-Carranza, N.; Berg, H.E. Persisting muscle atrophy two years after replacement of the hip. J. Bone Jt. Surg. Br. 2009, 91, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, A.; Richardson, C.; Stanton, W.; Durbridge, G.; Donnelly, W.; Hides, J. The association between degenerative hip joint pathology and size of the gluteus medius, gluteus minimus and piriformis muscles. Man. Ther. 2009, 14, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Takao, M.; Otake, Y.; Yokota, F.; Hamada, H.; Sakai, T.; Sato, Y.; Sugano, N. Validation study of the CT-based cross-sectional evaluation of muscular atrophy and fatty degeneration around the pelvis and the femur. J. Orthop. Sci. 2020, 25, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Milanović, Z.; Pantelić, S.; Trajković, N.; Sporiš, G.; Kostić, R.; James, N. Age-related decrease in physical activity and functional fitness among elderly men and women. Clin. Interv. Aging 2013, 8, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, A.J.; Guépet-Sordet, H.; Manckoundia, P. Changes in olfaction during ageing and in certain neurodegenerative diseases: Up-to-date. Rev. Med. Interne 2015, 36, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Panel, N.O. On the identification, evaluation, and treatment of overweight and obesity in adults. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults—The evidence report. Obes. Res. 1998, 6, 51S–209S. [Google Scholar]
- Skrzypek, M.; Szponar, B.; Drop, B.; Panasiuk, L.; Malm, M. Anthropometric, body composition and behavioural predictors of bioelectrical impedance phase angle in Polish young adults—Preliminary results. Ann. Agric. Environ. Med. 2020, 27, 91–98. [Google Scholar] [CrossRef]
- Matthews, L.; Bates, A.; Wootton, S.A.; Levett, D. The use of bioelectrical impedance analysis to predict post-operative complications in adult patients having surgery for cancer: A systematic review. Clin. Nutr. 2021, 40, 2914–2922. [Google Scholar] [CrossRef]
- Pfeifer, M.; Begerow, B.; Minne, H.W.; Schlotthauer, T.; Pospeschill, M.; Scholz, M.; Lazarescu, A.; Pollähne, W. Vitamin D status, trunk muscle strength, body sway, falls, and fractures among 237 postmenopausal women with osteoporosis. Exp. Clin. Endocrinol. Diabetes 2001, 109, 87–92. [Google Scholar] [CrossRef]
- Sinaki, M. Exercise for patients with osteoporosis: Management of vertebral compression fractures and trunk strengthening for fall prevention. PM R 2012, 4, 882–888. [Google Scholar] [CrossRef]
- Lukaski, H.C.; Kyle, U.G.; Kondrup, J. Assessment of adult malnutrition and prognosis with bioelectrical impedance analysis: Phase angle and impedance ratio. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Matias, C.N.; Monteiro, C.P.; Santos, D.A.; Martins, F.; Silva, A.M.; Laires, M.J.; Sardinha, L.B. Magnesium and Phase Angle: A prognostic tool for monitoring cellular integrity in judo athletes. Magnes. Res. 2015, 28, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Norman, K.; Stobäus, N.; Pirlich, M.; Bosy-Westphal, A. Bioelectrical phase angle and impedance vector analysis-clinical relevance and applicability of impedance parameters. Clin. Nutr. 2012, 31, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Kilic, M.K.; Kizilarslanoglu, M.C.; Arik, G.; Bolayir, B.; Kara, O.; Varan, H.D.; Sumer, F.; Kuyumcu, M.E.; Halil, M.; Ulger, Z. Association of bioelectrical impedance analysis-derived phase angle and sarcopenia in older adults. Nutr. Clin. Pract. 2017, 32, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Bosy-Westphal, A.; Danielzik, S.; Dörhöfer, R.P.; Later, W.; Wiese, S.; Müller, M.J. Phase angle from bioelectrical impedance analysis: Population reference values by age, sex, and body mass index. JPEN J. Parenter. Enter. Nutr. 2006, 30, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Nagano, M.; Suita, S.; Yamanouchi, T. The validity of bioelectrical impedance phase angle for nutritional assessment in children. J. Pediatr. Surg. 2000, 35, 1035–1039. [Google Scholar] [CrossRef] [PubMed]
- Pupim, L.B.; Kent, P.; Ikizler, T.A. Bioelectrical impedance analysis in dialysis patients. Min. Electrolyte Metab. 1999, 25, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Marini, E.; Campa, F.; Buffa, R.; Stagi, S.; Matias, C.N.; Toselli, S.; Sardinha, L.B.; Silva, A.M. Phase angle and bioelectrical impedance vector analysis in the evaluation of body composition in athletes. Clin. Nutr. 2020, 39, 447–454. [Google Scholar] [CrossRef]
- Selberg, O.; Selberg, D. Norms and correlates of bioimpedance phase angle in healthy human subjects, hospitalized patients, and patients with liver cirrhosis. Eur. J. Appl. Physiol. 2002, 86, 509–516. [Google Scholar] [CrossRef]
- Kyle, U.G.; Soundar, E.P.; Genton, L.; Pichard, C. Can phase angle determined by bioelectrical impedance analysis assess nutritional risk? A comparison between healthy and hospitalized subjects. Clin. Nutr. 2012, 31, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lu, K.; Chen, H.; Hu, N.; Chen, J.; Liang, X.; Qin, J.; Huang, W. Trunk skeletal muscle mass and phase angle measured by bioelectrical impedance analysis are associated with the chance of femoral neck fracture in very elderly people. Clin. Interv. Aging 2020, 15, 889–895. [Google Scholar] [CrossRef]
- Day, K.; Kwok, A.; Evans, A.; Mata, F.; Verdejo-Garcia, A.; Hart, K.; Ward, L.C.; Truby, H. Comparison of a bioelectrical impedance device against the reference method dual energy X-ray absorptiometry and anthropometry for the evaluation of body composition in adults. Nutrients 2018, 10, 1469. [Google Scholar] [CrossRef] [PubMed]
- Merchant, R.A.; Seetharaman, S.; Au, L.; Wong, M.W.K.; Wong, B.L.L.; Tan, L.F.; Chen, M.Z.; Ng, S.E.; Soong, J.T.Y.; Hui, R.J.Y.; et al. Relationship of fat mass index and fat free mass index with body mass index and association with function, cognition and sarcopenia in pre-frail older adults. Front. Endocrinol. 2021, 12, 765415. [Google Scholar] [CrossRef] [PubMed]
- Kyle, U.G.; Genon, L.; Karsegard, V.L.; Raguso, C.A.; Dupertuis, Y.M.; Pichard, C. Percentile (10, 25, 75 and 90th) for phase angle (PhA), determined by bioelectrical impedance analysis (BIA) in 2740 healthy adults aged 20–75 yr. Clin. Nutr. 2004, 23, 758. [Google Scholar]
- Tomeleri, C.M.; Cavaglieri, C.R.; de Souza, M.F.; Cavalcante, E.F.; Antunes, M.; Nabbuco, H.C.G.; Venturini, D.; Barbosa, D.S.; Silva, A.M.; Cyrino, E.S. Phase angle is related with inflammatory and oxidative stress biomarkers in older women. Exp. Gerontol. 2018, 102, 12–18. [Google Scholar] [CrossRef]
- Nunes, J.P.; Ribeiro, A.S.; Silva, A.M.; Schoenfeld, B.J.; dos Santos, L.; Cunha, P.M.; Nascimento, M.A.; Tomeleri, C.M.; Nabuco, H.C.G.; Antunes, M.; et al. Improvements in phase angle are related with muscle quality index after resistance training in older women. J. Aging Phys. Act. 2019, 27, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.F.; Tomeleri, C.M.; Ribeiro, A.S.; Schoenfeld, B.J.; Silva, A.M.; Sardinha, L.B.; Cyrino, E.S. Effect of resistance training on phase angle in older women: A randomized controlled trial. Scand. J. Med. Sci. Sports 2017, 27, 1308–1316. [Google Scholar] [CrossRef]
- Ten Haaf, D.S.M.; Nuijten, M.A.H.; Maessen, M.F.H.; Horstman, A.M.H.; Eijsvogels, T.M.H.; Hopman, M.T.E. Effects of protein supplementation on lean body mass, muscle strength, and physical performance in nonfrail community-dwelling older adults: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2018, 108, 1043–1059. [Google Scholar] [CrossRef]
- Wada, O.; Kurita, N.; Yamada, M.; Mizuno, K. Structural severity, phase angle, and quadriceps strength among patients with knee osteoarthritis: The SPSS-OK study. Clin. Rheumatol. 2020, 39, 3049–3056. [Google Scholar] [CrossRef]
- Gunn, S.M.; Halbert, J.A.; Giles, L.C.; Stepien, J.M.; Miller, M.D.; Crotty, M. Bioelectrical phase angle values in a clinical sample of ambulatory rehabilitation patients. Dyn. Med. 2008, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Ando, K.; Kobayashi, K.; Hida, T.; Seki, T.; Hamada, T.; Ito, K.; Tsushima, M.; Morozumi, M.; Machino, M.; et al. The decrease in phase angle measured by bioelectrical impedance analysis reflects the increased locomotive syndrome risk in community-dwelling people: The Yakumo study. Mod. Rheumatol. 2019, 29, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Norman, K.; Stobäus, N.; Zocher, D.; Bosy-Westphal, A.; Szramek, A.; Scheufele, R.; Smoliner, C.; Pirlich, M. Cutoff percentiles of bioelectrical phase angle predict functionality, quality of life, and mortality in patients with cancer. Am. J. Clin. Nutr. 2010, 92, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Kimura, Y.; Ishiyama, D.; Nishio, N.; Otobe, Y.; Tanaka, T.; Ohji, S.; Koyama, S.; Sato, A.; Suzuki, M.; et al. Phase angle is a useful indicator for muscle function in older adults. J. Nutr. Health Aging 2019, 23, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Matias, C.N.; Nunes, C.L.; Francisco, S.; Tomeleri, C.; Cyrino, E.; Sardinha, L.; Silva, A. Phase angle predicts physical function in older adults. Arch. Gerontol. Geriatr. 2020, 90, 104151. [Google Scholar] [CrossRef] [PubMed]
- Streb, A.R.; Hansen, F.; Gabiatti, M.P.; Tozetto, W.R.; Del Duca, G.F. Phase angle associated with different indicators of health-related physical fitness in adults with obesity. Physiol. Behav. 2020, 225, 113104. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Muscogiuri, G.; Laudisio, D.; Di Somma, C.; Salzano, C.; Pugliese, G.; De Alteriis, G.; Colao, A.; Savastano, S. Phase Angle: A possible biomarker to quantify inflammation in subjects with obesity and 25(OH)D deficiency. Nutrients 2019, 11, 1747. [Google Scholar] [CrossRef]
- Ukai, T.; Watanabe, M. Do metal implants for total hip arthroplasty affect bioelectrical impedance analysis? A retrospective study. BMC Musculoskelet. Disord. 2023, 24, 763. [Google Scholar] [CrossRef]

| Characteristic | Patients (n = 38) |
|---|---|
| Age (years) | 61.6 ± 16.0 |
| Sex (male:female) | 15:23 |
| BMI (kg/m2) | 23.8 ± 5.8 |
| Diagnosis (OA:ION:RA:RDC) | 18:17:2:1 |
| Preoperative leg discrepancy (mm) | −9.5 ± 8.2 |
| Postoperative leg discrepancy (mm) | −3.2 ± 8.8 |
| Preoperative offset (mm) | 41.0 ± 6.4 |
| Postoperative offset (mm) | 40.0 ± 6.4 |
| Preoperative | Postoperative | p Value | |
|---|---|---|---|
| Muscle strength of hip flexion (N/kg) | 1.1 ± 0.7 | 2.2 ± 0.8 | <0.01 * |
| Muscle strength of hip extension (N/kg) | 1.7 ± 0.6 | 2.2 ± 0.4 | <0.01 * |
| Muscle strength of hip abduction (N/kg) | 1.3 ± 0.5 | 1.8 ± 0.4 | <0.01 * |
| FMI (kg/m2) | 8.3 ± 2.8 | 8.3 ± 2.5 | 0.50 |
| FFMI (kg/m2) | 15.3 ± 2.4 | 15.5 ± 2.6 | <0.01 * |
| PhA (degrees) | 4.4 ± 0.7 | 4.4 ± 0.7 | 0.33 |
| Muscle volume of gluteus maximus (mm2/kg) | 45.9 ± 10.4 | 51.1 ± 9.2 | <0.01 * |
| HU of gluteus maximus | 21.0 ± 13.5 | 19.7 ± 16.4 | 0.16 |
| Muscle volume of quadriceps (mm2/kg) | 65.5 ± 13.1 | 70.6 ± 13.1 | <0.01 * |
| HU of quadriceps | 47.1 ± 8.3 | 47.4 ± 8.4 | 0.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ukai, T.; Yokoyama, K.; Watanabe, M. Preoperative Body Composition Correlates with Postoperative Muscle Volume and Degeneration after Total Hip Arthroplasty. Nutrients 2024, 16, 386. https://doi.org/10.3390/nu16030386
Ukai T, Yokoyama K, Watanabe M. Preoperative Body Composition Correlates with Postoperative Muscle Volume and Degeneration after Total Hip Arthroplasty. Nutrients. 2024; 16(3):386. https://doi.org/10.3390/nu16030386
Chicago/Turabian StyleUkai, Taku, Katsuya Yokoyama, and Masahiko Watanabe. 2024. "Preoperative Body Composition Correlates with Postoperative Muscle Volume and Degeneration after Total Hip Arthroplasty" Nutrients 16, no. 3: 386. https://doi.org/10.3390/nu16030386






