Maternal Diet Associates with Offspring Bone Mineralization, Fracture Risk and Enamel Defects in Childhood and Influences the Prenatal Effect of High-Dose Vitamin D Supplementation
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Measurements
2.2.1. FFQ
2.2.2. DXA Scans at Age 6 Years
2.2.3. Bone Fractures
2.2.4. Dental Examination at Age 6 Years
2.3. Maternal Blood Metabolomic Profile
2.4. Statistical Analysis
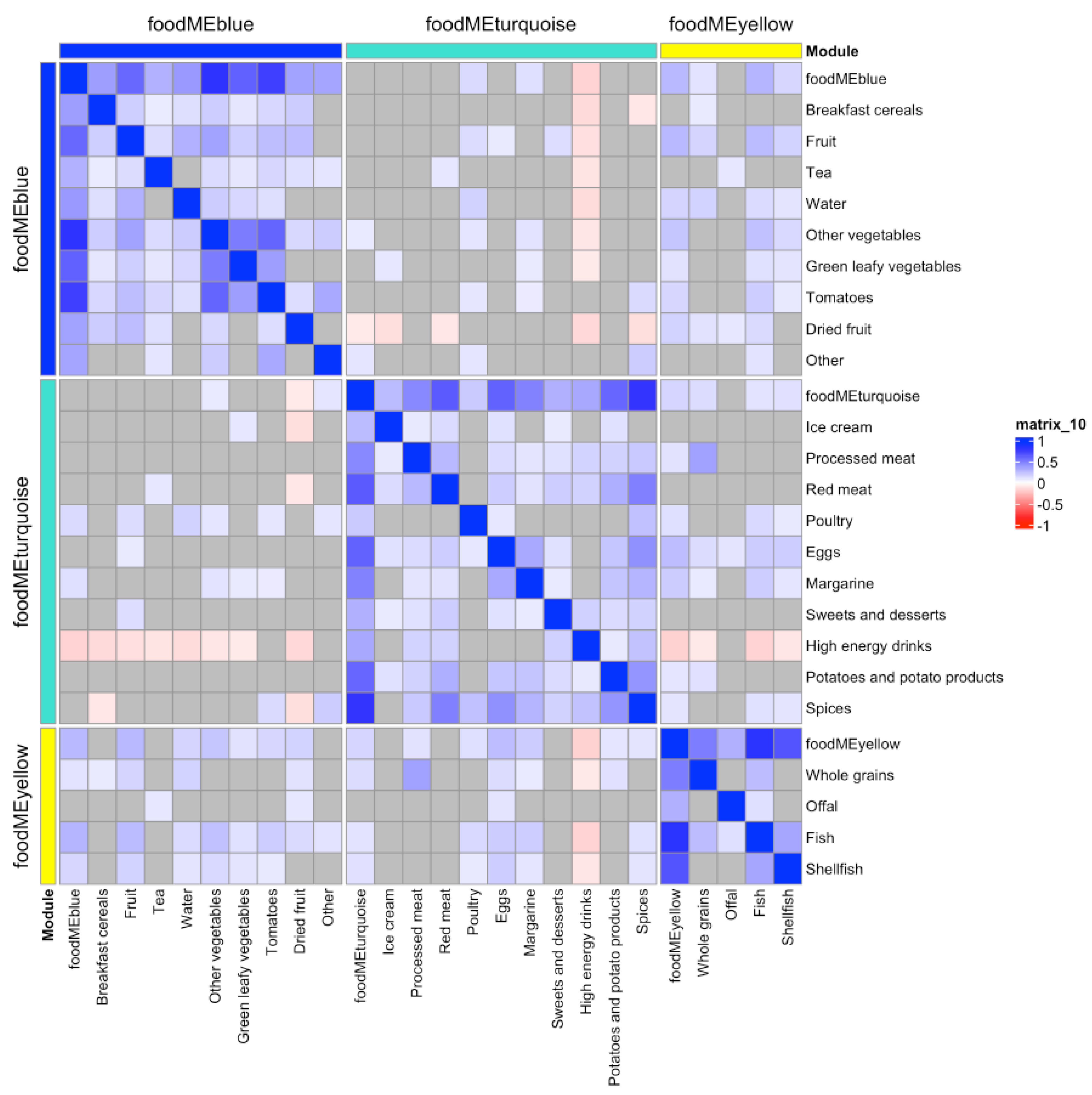
2.4.1. Dietary Patterns Based on WGCNA on FFQ
2.4.2. Food Modules and Bone and Dental Outcomes
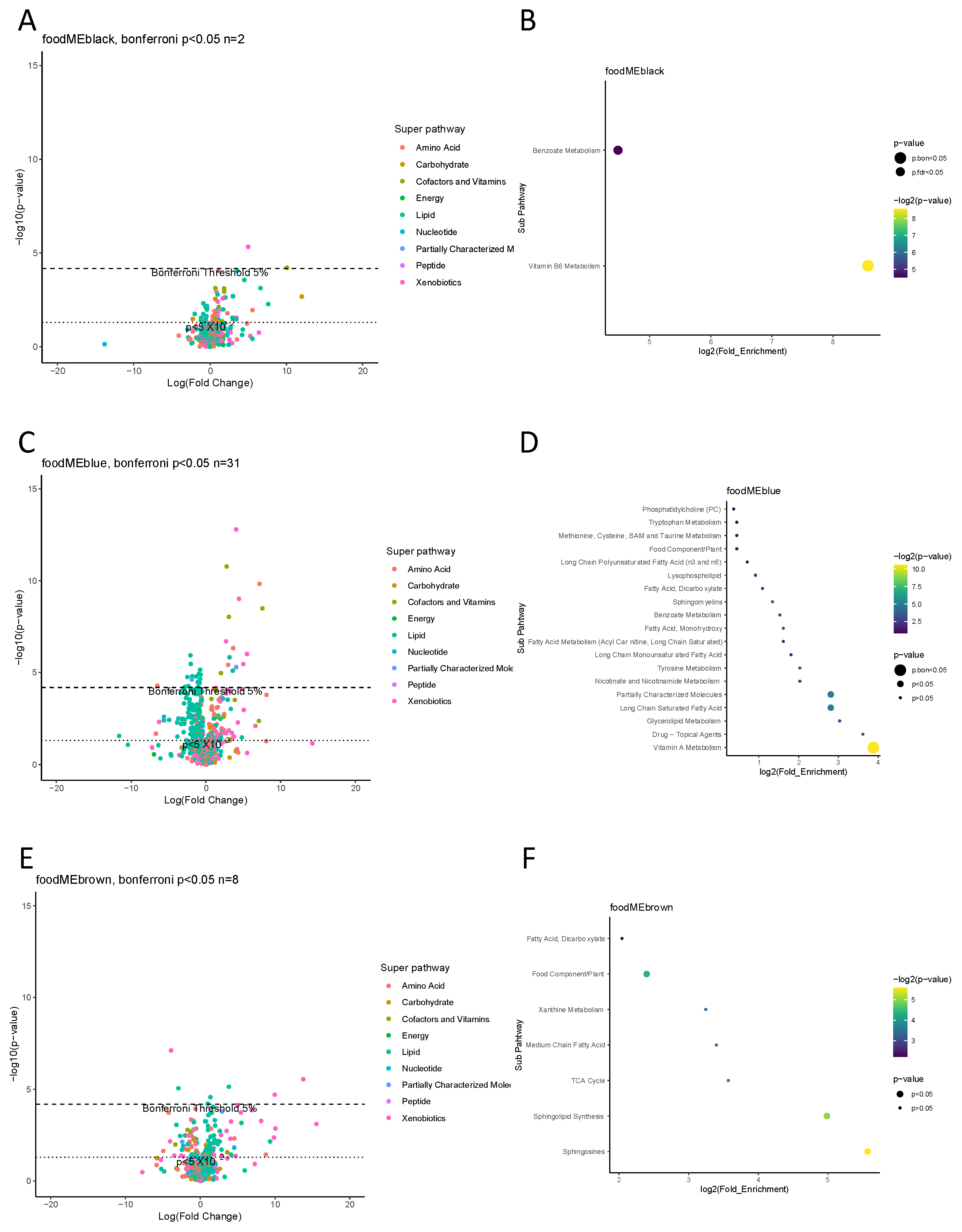
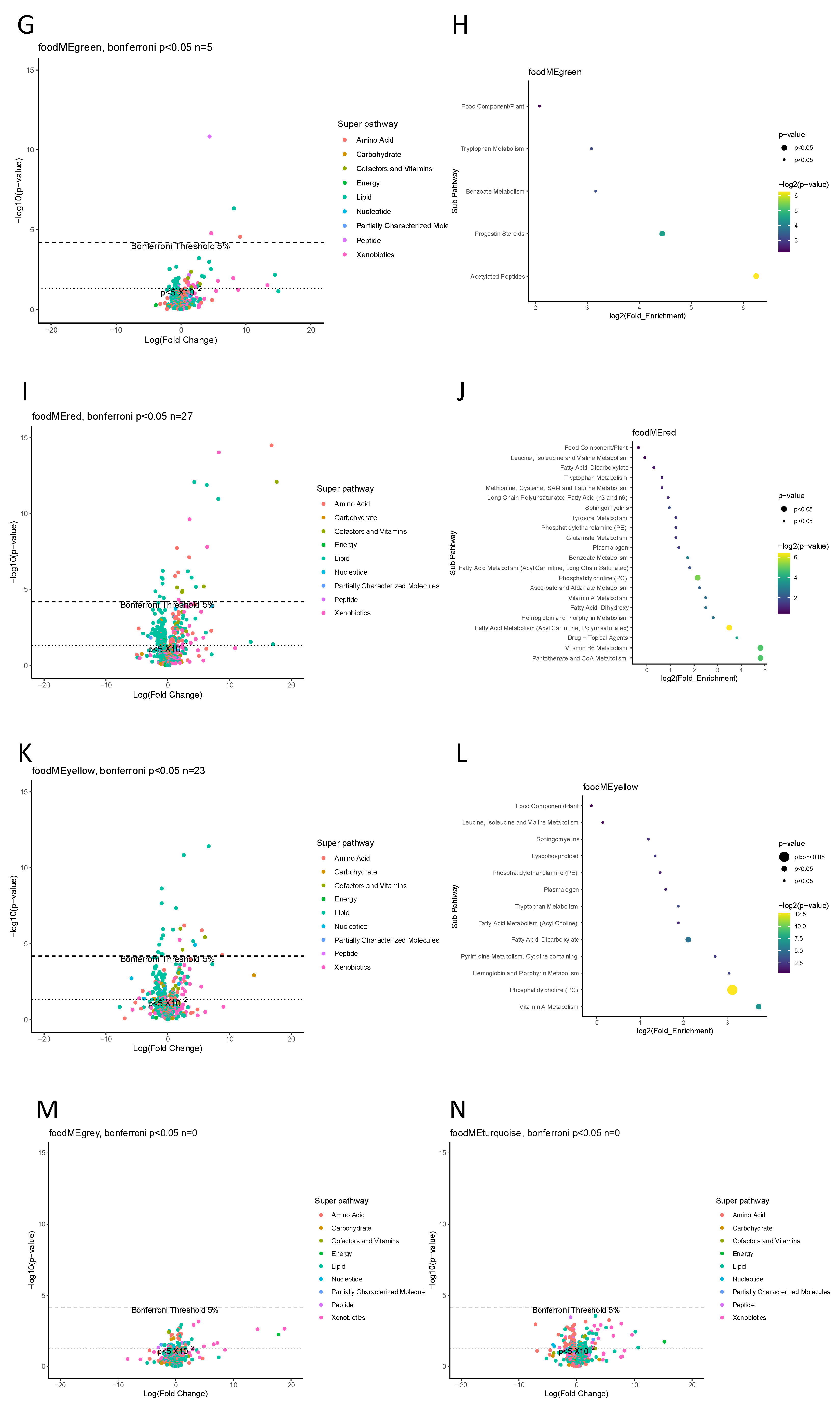
2.4.3. Food Modules and Maternal Blood Metabolomic Profiles
3. Results
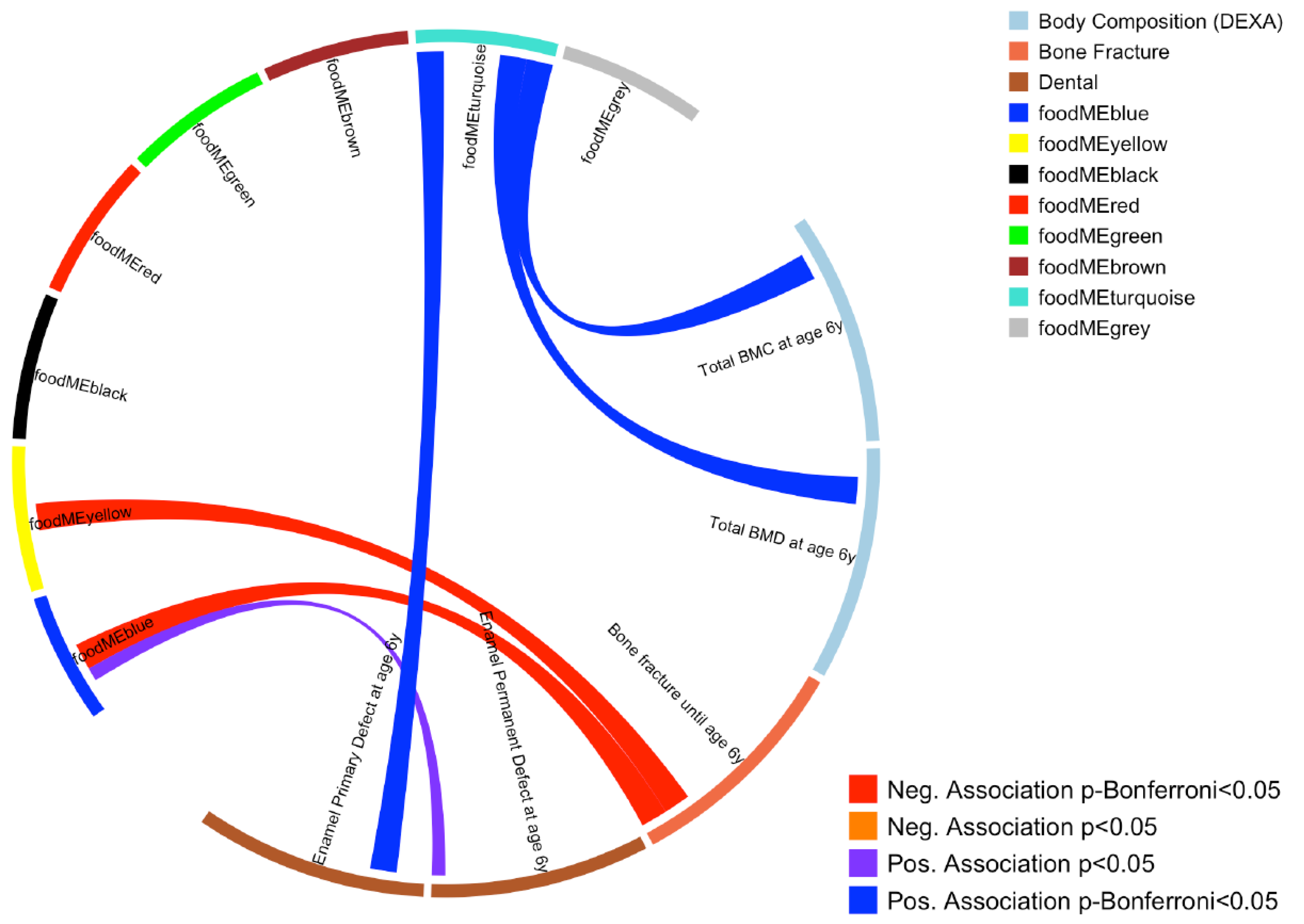
3.1. Dietary Patterns during Pregnancy and Offspring Bone and Dental Outcomes
3.2. Dietary Patterns and the Maternal Blood Metabolomic Profile
3.3. Effect of High-Dose Vitamin D Supplementation in Relation to Pregnancy Dietary Patterns on Offspring Bone and Dental Outcomes
4. Discussion
4.1. Interpretation
4.2. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DeLuca, H.F. Overview of general physiologic features and functions of vitamin D. Am. J. Clin. Nutr. 2004, 80, 1689S–1696S. [Google Scholar] [CrossRef] [PubMed]
- Koussoulakou, D.S.; Margaritis, L.H.; Koussoulakos, S.L. A curriculum vitae of teeth: Evolution, generation, regeneration. Int. J. Biol. Sci. 2009, 5, 226–243. [Google Scholar] [CrossRef] [PubMed]
- Schroth, R.J.; Christensen, J.; Morris, M.; Gregory, P.; Mittermuller, B.A.; Rockman-Greenberg, C. The Influence of Prenatal Vitamin D Supplementation on Dental Caries in Infants. J. Can. Dent. Assoc. 2020, 86, k13. [Google Scholar] [PubMed]
- Schroth, R.J.; Lavelle, C.; Tate, R.; Bruce, S.; Billings, R.J.; Moffatt, M.E. Prenatal vitamin D and dental caries in infants. Pediatrics 2014, 133, e1277–e1284. [Google Scholar] [CrossRef]
- van der Tas, J.T.; Elfrink, M.E.C.; Heijboer, A.C.; Rivadeneira, F.; Jaddoe, V.W.V.; Tiemeier, H.; Schoufour, J.D.; Moll, H.A.; Ongkosuwito, E.M.; Wolvius, E.B.; et al. Foetal, neonatal and child vitamin D status and enamel hypomineralization. Community Dent. Oral. Epidemiol. 2018, 46, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Reed, S.G.; Voronca, D.; Wingate, J.S.; Murali, M.; Lawson, A.B.; Hulsey, T.C.; Ebeling, M.D.; Hollis, B.W.; Wagner, C.L. Prenatal vitamin D and enamel hypoplasia in human primary maxillary central incisors: A pilot study. Pediatr. Dent. J. 2017, 27, 21–28. [Google Scholar] [CrossRef]
- Lee, J.Y.; So, T.Y.; Thackray, J. A review on vitamin d deficiency treatment in pediatric patients. J. Pediatr. Pharmacol. Ther. 2013, 18, 277–291. [Google Scholar] [CrossRef] [PubMed]
- Weinert, L.S.; Silveiro, S.P. Maternal-fetal impact of vitamin D deficiency: A critical review. Matern. Child Health J. 2015, 19, 94–101. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Moon, R.J.; Green, H.D.; D’Angelo, S.; Godfrey, K.M.; Davies, J.H.; Curtis, E.M.; Cooper, C.; Harvey, N.C. The effect of pregnancy vitamin D supplementation on offspring bone mineral density in childhood: A systematic review and meta-analysis. Osteoporos. Int. 2023, 34, 1269–1279. [Google Scholar] [CrossRef]
- Luo, T.; Lin, Y.; Lu, J.; Lian, X.; Guo, Y.; Han, L.; Guo, Y. Effects of vitamin D supplementation during pregnancy on bone health and offspring growth: A systematic review and meta-analysis of randomized controlled trials. PLoS ONE 2022, 17, e0276016. [Google Scholar] [CrossRef] [PubMed]
- Harvey, N.C.; Holroyd, C.; Ntani, G.; Javaid, K.; Cooper, P.; Moon, R.; Cole, Z.; Tinati, T.; Godfrey, K.; Dennison, E.; et al. Vitamin D supplementation in pregnancy: A systematic review. Health Technol. Assess. 2014, 18, 1–190. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Hitsumoto, S.; Miyake, Y.; Okubo, H.; Sasaki, S.; Miyatake, N.; Arakawa, M. Higher vitamin D intake during pregnancy is associated with reduced risk of dental caries in young Japanese children. Ann. Epidemiol. 2015, 25, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Norrisgaard, P.E.; Haubek, D.; Kuhnisch, J.; Chawes, B.L.; Stokholm, J.; Bonnelykke, K.; Bisgaard, H. Association of High-Dose Vitamin D Supplementation During Pregnancy With the Risk of Enamel Defects in Offspring: A 6-Year Follow-up of a Randomized Clinical Trial. JAMA Pediatr. 2019, 173, 924–930. [Google Scholar] [CrossRef]
- Brustad, N.; Garland, J.; Thorsen, J.; Sevelsted, A.; Krakauer, M.; Vinding, R.K.; Stokholm, J.; Bonnelykke, K.; Bisgaard, H.; Chawes, B.L. Effect of High-Dose vs Standard-Dose Vitamin D Supplementation in Pregnancy on Bone Mineralization in Offspring Until Age 6 Years: A Prespecified Secondary Analysis of a Double-Blinded, Randomized Clinical Trial. JAMA Pediatr. 2020, 174, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Sobiech, P.; Olczak-Kowalczyk, D.; Hosey, M.T.; Gozdowski, D.; Turska-Szybka, A. Vitamin D Supplementation, Characteristics of Mastication, and Parent-Supervised Toothbrushing as Crucial Factors in the Prevention of Caries in 12- to 36-Month-Old Children. Nutrients 2022, 14, 4358. [Google Scholar] [CrossRef] [PubMed]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]
- Chawes, B.L.; Bonnelykke, K.; Stokholm, J.; Vissing, N.H.; Bjarnadottir, E.; Schoos, A.M.; Wolsk, H.M.; Pedersen, T.M.; Vinding, R.K.; Thorsteinsdottir, S.; et al. Effect of Vitamin D3 Supplementation during Pregnancy on Risk of Persistent Wheeze in the Offspring: A Randomized Clinical Trial. JAMA 2016, 315, 353–361. [Google Scholar] [CrossRef]
- Brustad, N.; Olarini, A.; Kim, M.; Chen, L.; Ali, M.; Wang, T.; Cohen, A.S.; Ernst, M.; Hougaard, D.; Schoos, A.M.; et al. Diet-associated vertically transferred metabolites and risk of asthma, allergy, eczema, and infections in early childhood. Pediatr. Allergy Immunol. 2023, 34, e13917. [Google Scholar] [CrossRef]
- Brustad, N.; Chawes, B.L.; Thorsen, J.; Krakauer, M.; Lasky-Su, J.; Weiss, S.T.; Stokholm, J.; Bonnelykke, K.; Bisgaard, H. High-dose vitamin D supplementation in pregnancy and 25(OH)D sufficiency in childhood reduce the risk of fractures and improve bone mineralization in childhood: Follow-up of a randomized clinical trial. EClinicalMedicine 2022, 43, 101254. [Google Scholar] [CrossRef]
- Lygidakis, N.A.; Wong, F.; Jalevik, B.; Vierrou, A.M.; Alaluusua, S.; Espelid, I. Best Clinical Practice Guidance for clinicians dealing with children presenting with Molar-Incisor-Hypomineralisation (MIH): An EAPD Policy Document. Eur. Arch. Paediatr. Dent. 2010, 11, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Elfrink, M.E.; Ghanim, A.; Manton, D.J.; Weerheijm, K.L. Standardised studies on Molar Incisor Hypomineralisation (MIH) and Hypomineralised Second Primary Molars (HSPM): A need. Eur. Arch. Paediatr. Dent. 2015, 16, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Brustad, N.; Ali, M.; Gurdeniz, G.; Arendt, M.; Litonjua, A.A.; Wheelock, C.E.; Kelly, R.S.; Chen, Y.; Prince, N.; et al. Maternal vitamin D-related metabolome and offspring risk of asthma outcomes. J. Allergy Clin. Immunol. 2023, 152, 1646–1657. [Google Scholar] [CrossRef] [PubMed]
- Kalkwarf, H.J.; Gilsanz, V.; Lappe, J.M.; Oberfield, S.; Shepherd, J.A.; Hangartner, T.N.; Huang, X.; Frederick, M.M.; Winer, K.K.; Zemel, B.S. Tracking of bone mass and density during childhood and adolescence. J. Clin. Endocrinol. Metab. 2010, 95, 1690–1698. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.C.; Swinburn, B.A. What are the key food groups to target for preventing obesity and improving nutrition in schools? Eur. J. Clin. Nutr. 2004, 58, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Echeverria, M.S.; Schuch, H.S.; Cenci, M.S.; Motta, J.V.S.; Bertoldi, A.D.; Hallal, P.C.; Demarco, F.F. Trajectories of Sugar Consumption and Dental Caries in Early Childhood. J. Dent. Res. 2022, 101, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Bolland, M.J.; Grey, A.; Avenell, A. Effects of vitamin D supplementation on musculoskeletal health: A systematic review, meta-analysis, and trial sequential analysis. Lancet Diabetes Endocrinol. 2018, 6, 847–858. [Google Scholar] [CrossRef] [PubMed]
- LeBoff, M.S.; Chou, S.H.; Murata, E.M.; Donlon, C.M.; Cook, N.R.; Mora, S.; Lee, I.M.; Kotler, G.; Bubes, V.; Buring, J.E.; et al. Effects of Supplemental Vitamin D on Bone Health Outcomes in Women and Men in the VITamin D and OmegA-3 TriaL (VITAL). J. Bone Miner. Res. 2020, 35, 883–893. [Google Scholar] [CrossRef]
- Gharibeh, N.; Razaghi, M.; Vanstone, C.A.; Sotunde, O.F.; Glenn, L.; Mullahoo, K.; Farahnak, Z.; Khamessan, A.; Wei, S.Q.; McNally, D.; et al. Effect of Vitamin D Supplementation on Bone Mass in Infants with 25-Hydroxyvitamin D Concentrations Less Than 50 nmol/L: A Prespecified Secondary Analysis of a Randomized Clinical Trial. JAMA Pediatr. 2023, 177, 353–362. [Google Scholar] [CrossRef]
- Shim, J.S.; Oh, K.; Kim, H.C. Dietary assessment methods in epidemiologic studies. Epidemiol. Health 2014, 36, e2014009. [Google Scholar] [CrossRef]
- Zouine, N.; Lhilali, I.; Menouni, A.; Godderis, L.; El Midaoui, A.; El Jaafari, S.; Zegzouti Filali, Y. Development and Validation of Vitamin D- Food Frequency Questionnaire for Moroccan Women of Reproductive Age: Use of the Sun Exposure Score and the Method of Triad’s Model. Nutrients 2023, 15, 796. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Xia, Y.; Wu, Q.; Chang, Q.; Niu, K.; Zhao, Y. A meta-analysis of the reproducibility of food frequency questionnaires in nutritional epidemiological studies. Int. J. Behav. Nutr. Phys. Act. 2021, 18, 12. [Google Scholar] [CrossRef] [PubMed]
- Ganji, V.; Abu-Dbaa, R.; Othman, H.; Zewein, M.; Al-Abdi, T.; Shi, Z. Validation of Vitamin D-Specific Food Frequency Questionnaire against Food Records for Qatari Women. Foods 2020, 9, 195. [Google Scholar] [CrossRef] [PubMed]
- Watkins, S.; Freeborn, E.; Mushtaq, S. A validated FFQ to determine dietary intake of vitamin D. Public Health Nutr. 2021, 24, 4001–4006. [Google Scholar] [CrossRef] [PubMed]
- Benetou, V.; Orfanos, P.; Feskanich, D.; Michaelsson, K.; Pettersson-Kymmer, U.; Eriksson, S.; Grodstein, F.; Wolk, A.; Bellavia, A.; Ahmed, L.A.; et al. Fruit and Vegetable Intake and Hip Fracture Incidence in Older Men and Women: The CHANCES Project. J. Bone Miner. Res. 2016, 31, 1743–1752. [Google Scholar] [CrossRef] [PubMed]
- Khodabakhshi, A.; Davoodi, S.H.; Vahid, F. Vitamin D status, including serum levels and sun exposure are associated or correlated with bone mass measurements diagnosis, and bone density of the spine. BMC Nutr. 2023, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Dwyer, T.; Riley, M.; Cochrane, J.; Jones, G. The association between maternal diet during pregnancy and bone mass of the children at age 16. Eur. J. Clin. Nutr. 2010, 64, 131–137. [Google Scholar] [CrossRef]
- Palacios, C.; Rivas-Tumanyan, S.; Morou-Bermudez, E.; Colon, A.M.; Torres, R.Y.; Elias-Boneta, A.R. Association between Type, Amount, and Pattern of Carbohydrate Consumption with Dental Caries in 12-Year-Olds in Puerto Rico. Caries Res. 2016, 50, 560–570. [Google Scholar] [CrossRef]
- Ohlund, I.; Holgerson, P.L.; Backman, B.; Lind, T.; Hernell, O.; Johansson, I. Diet intake and caries prevalence in four-year-old children living in a low-prevalence country. Caries Res. 2007, 41, 26–33. [Google Scholar] [CrossRef]
- Javed, K.; Nasir, M.Z.; Jalees, M.; Manzoor, M.A. Role of diet and dietary habits in causing dental caries among adults reporting to a tertiary care hospital in Pakistan; a case-control study. Heliyon 2023, 9, e23117. [Google Scholar] [CrossRef]
- Mazurkiewicz, D.; Pustulka, M.; Ambrozik-Haba, J.; Bienkiewicz, M. Dietary Habits and Oral Hygiene as Determinants of the Incidence and Intensity of Dental Caries—A Pilot Study. Nutrients 2023, 15, 4833. [Google Scholar] [CrossRef]

| All (n = 623) | Standard-Dose (n = 250) | High-Dose (n = 240) | p Value | ||
|---|---|---|---|---|---|
| # | 25(OH)D level at gestation week 24 (nmol/l) | 75.35 (24.78), n = 621 | 75.36 (25.61), n = 250 | 74.81 (23.76), n = 238 | 0.80 |
| § | Fish oil intervention, No/Yes | 307/295, n = 602 | 133/117, n = 250 | 127/113, n = 240 | 0.95 |
| § | Offspring Sex, male/female | 291/313, n = 602 | 123/127, n = 250 | 113/127, n = 240 | 0.64 |
| § | Birth season, Spring/Summer/Fall/Winter | 145/181/141/135/21, n = 602 | 73/51/57/69, n = 250 | 69/52/55/64, n = 240 | 0.99 |
| # | Daycare start age, years | 0.89 (0.23), n = 595 | 0.89 (0.27), n = 245 | 0.88 (0.20), n = 238 | 0.65 |
| # | Gestational age at birth, weeks | 278.93 (12.00), n = 621 | 278.91 (10.67), n = 250 | 280.17 (9.69), n = 239 | 0.17 |
| # | Number of cigarettes mother smoked during 3rd trimester per day | 2.14 (13.85), n = 606 | 3.25 (16.02), n = 250 | 1.80 (14.46), n = 240 | 0.30 |
| # | Length of exclusive breastfeeding, days | 104.06 (59.46), n = 599 | 108.57 (60.03), n = 247 | 102.27 (57.09), n = 239 | 0.0001 * |
| # | Offspring weight at age 6 y, kg | 21.75 (2.96), n = 549 | 21.76 (2.87), n = 228 | 21.49 (3.01), n = 218 | 0.33 |
| # | Offspring height at age 6 y, cm | 118.44 (4.99), n = 549 | 118.32 (5.11), n = 228 | 118.03 (4.64), n = 218 | 0.53 |
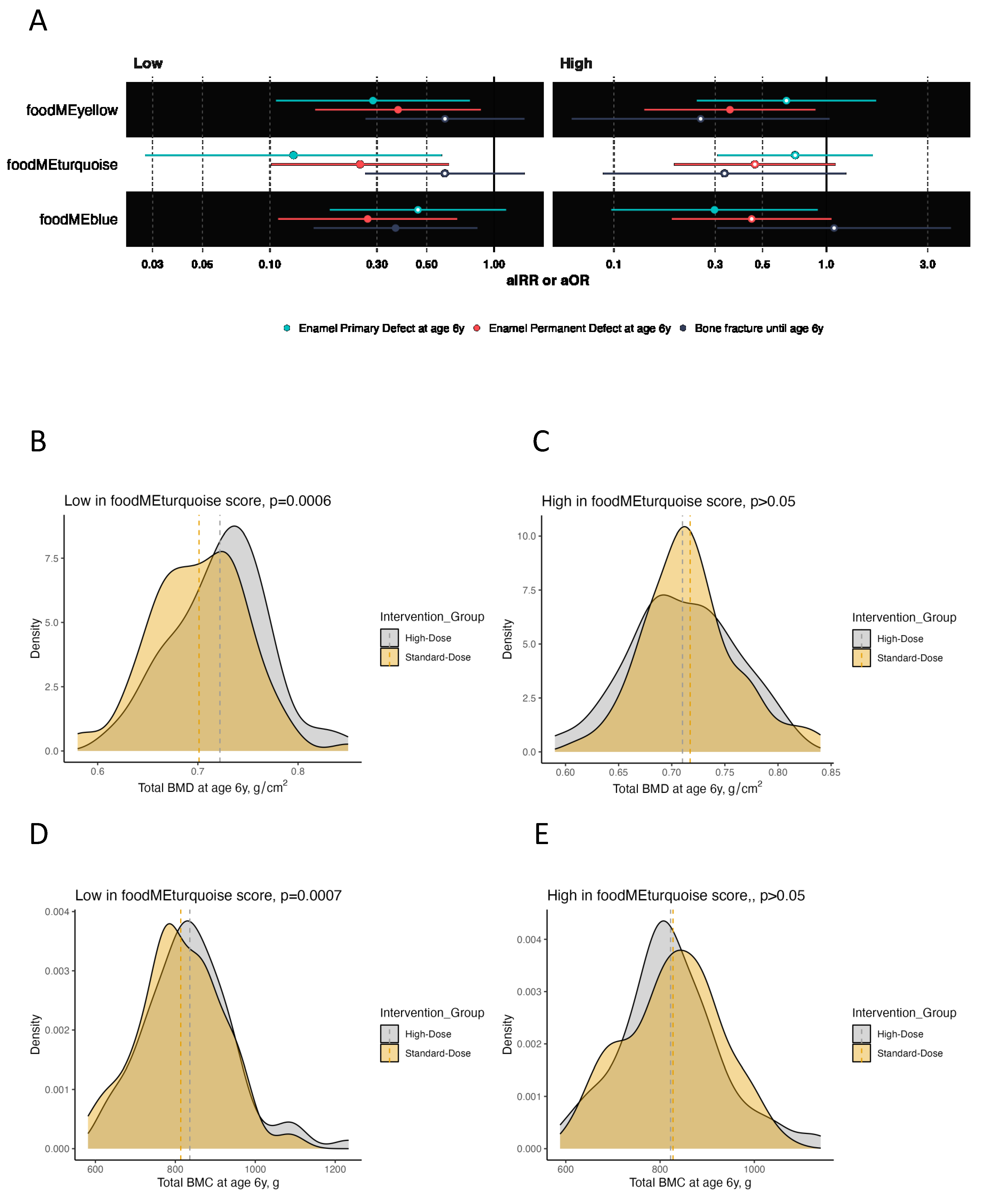
| Clinical Outcomes | Food Module Low | Food Module High | |||
|---|---|---|---|---|---|
| Effect Measure [95 CI%] | p Value | Effect Measure [95 CI%] | p Value | ||
| foodMEyellow | Total BMC at age 6 y, g | 22 [2–42] | 0.04 * | 10 [−7–27] | 0.26 |
| Total BMD at age 6 y, g/cm2 | 0.01 [−0.01–0.02] | 0.31 | 0.01 [−0.00–0.02] | 0.08 | |
| Bone fractures | 0.60 [0.27–1.37] | 0.23 | 0.26 [0.06–1.04] | 0.06 | |
| Enamel Permanent Defect at age 6 y | 0.37 [0.16–0.87] | 0.02 * | 0.35 [0.14–0.89] | 0.03 * | |
| Enamel Primary Defect at age 6 y | 0.29 [0.11–0.78] | 0.01 * | 0.65 [0.25–1.72] | 0.38 | |
| foodMEturquoise | Total BMC at age 6 y, g | 33 [14–52] | 0.0007 # | −6 [−23–12] | 0.53 |
| Total BMD at age 6 y, g/cm2 | 0.02 [0.01–0.04] | 0.0006 # | −0.01 [−0.02–0.01] | 0.3 | |
| Bone fractures | 0.60 [0.27–1.36] | 0.28 | 0.33 [0.09–1.24] | 0.1 | |
| Enamel Permanent Defect at age 6 y | 0.25 [0.10–0.63] | 0.003 # | 0.46 [0.19–1.10] | 0.08 | |
| Enamel Primary Defect at age 6 y | 0.13 [0.03–0.58] | 0.008 # | 0.71 [0.31–1.65] | 0.43 | |
| foodMEblue | Total BMC at age 6 y, g | 10 [−8–28] | 0.28 | 16 [−3–35] | 0.10 |
| Total BMD at age 6 y, g/cm2 | 0.01 [−0.01–0.02] | 0.43 | 0.01 [−0.00–0.03] | 0.06 | |
| Bone fractures | 0.36 [0.16–0.84] | 0.02 * | 1.09 [0.31–3.86] | 0.90 | |
| Enamel Permanent Defect at age 6 y | 0.27 [0.11–0.68] | 0.006 # | 0.45 [0.19–1.06] | 0.07 | |
| Enamel Primary Defect at age 6 y | 0.46 [0.18–1.13] | 0.09 | 0.30 [0.10–0.91] | 0.03 * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Nørrisgaard, P.E.; Vahman, N.; Cexus, O.N.F.; Townsend, P.A.; Stokholm, J.; Bønnelykke, K.; Chawes, B.; Brustad, N. Maternal Diet Associates with Offspring Bone Mineralization, Fracture Risk and Enamel Defects in Childhood and Influences the Prenatal Effect of High-Dose Vitamin D Supplementation. Nutrients 2024, 16, 405. https://doi.org/10.3390/nu16030405
Kim M, Nørrisgaard PE, Vahman N, Cexus ONF, Townsend PA, Stokholm J, Bønnelykke K, Chawes B, Brustad N. Maternal Diet Associates with Offspring Bone Mineralization, Fracture Risk and Enamel Defects in Childhood and Influences the Prenatal Effect of High-Dose Vitamin D Supplementation. Nutrients. 2024; 16(3):405. https://doi.org/10.3390/nu16030405
Chicago/Turabian StyleKim, Min, Pia E. Nørrisgaard, Nilo Vahman, Olivier N. F. Cexus, Paul A. Townsend, Jakob Stokholm, Klaus Bønnelykke, Bo Chawes, and Nicklas Brustad. 2024. "Maternal Diet Associates with Offspring Bone Mineralization, Fracture Risk and Enamel Defects in Childhood and Influences the Prenatal Effect of High-Dose Vitamin D Supplementation" Nutrients 16, no. 3: 405. https://doi.org/10.3390/nu16030405
APA StyleKim, M., Nørrisgaard, P. E., Vahman, N., Cexus, O. N. F., Townsend, P. A., Stokholm, J., Bønnelykke, K., Chawes, B., & Brustad, N. (2024). Maternal Diet Associates with Offspring Bone Mineralization, Fracture Risk and Enamel Defects in Childhood and Influences the Prenatal Effect of High-Dose Vitamin D Supplementation. Nutrients, 16(3), 405. https://doi.org/10.3390/nu16030405







