Carbohydrate Oral Rinsing, Cycling Performance and Individual Complex Carbohydrate Taste Sensitivity
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
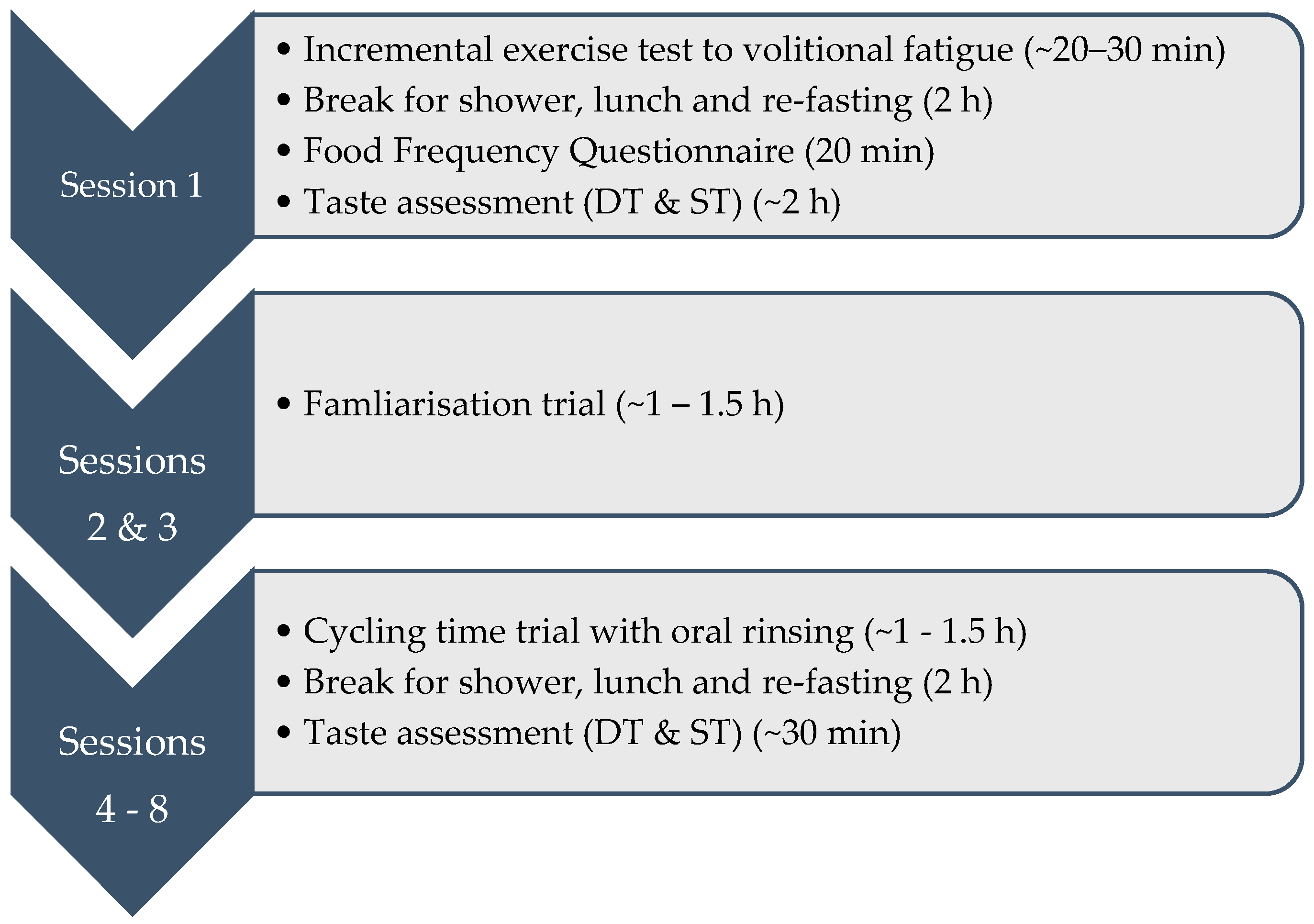
2.2. Experimental Design
2.3. Session 1
2.3.1. Sensory Stimuli
2.3.2. Sensory Methods
2.3.3. Food Frequency Questionnaire
2.4. Sessions 2–8
2.4.1. Oral Rinse Protocol
2.4.2. Dietary Procedures
2.4.3. Sensory Methods
2.5. Statistical Analysis
3. Results
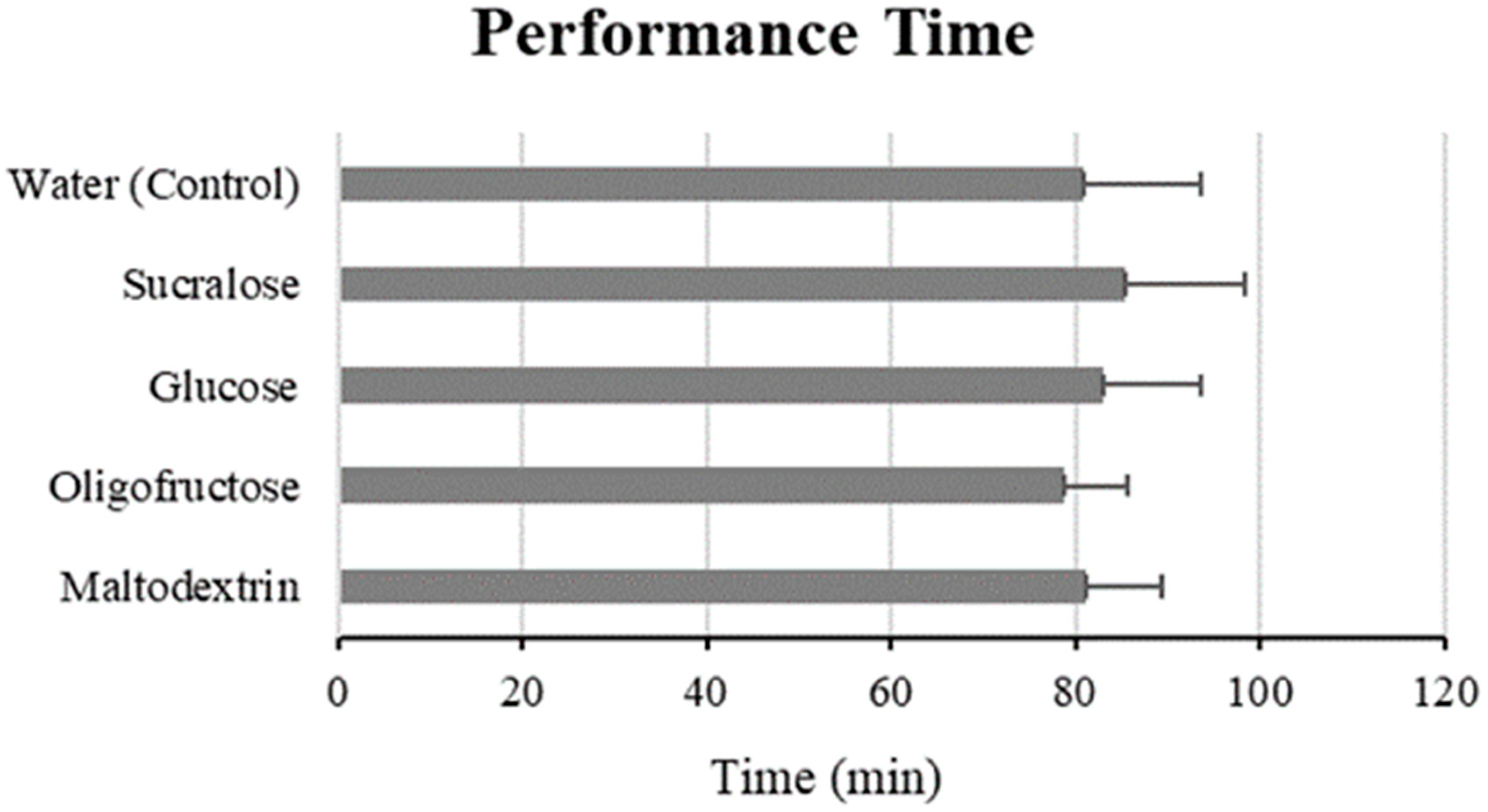
3.1. Performance Time and Power Output
3.2. Heart Rate, Rating of Perceived Exertion and Body Mass
3.3. Oral Detection Thresholds
3.4. Oral Suprathreshold Intensities
3.5. Taste Sensitivity and Time Trial Performance
3.6. Dietary Data
3.7. Correlations
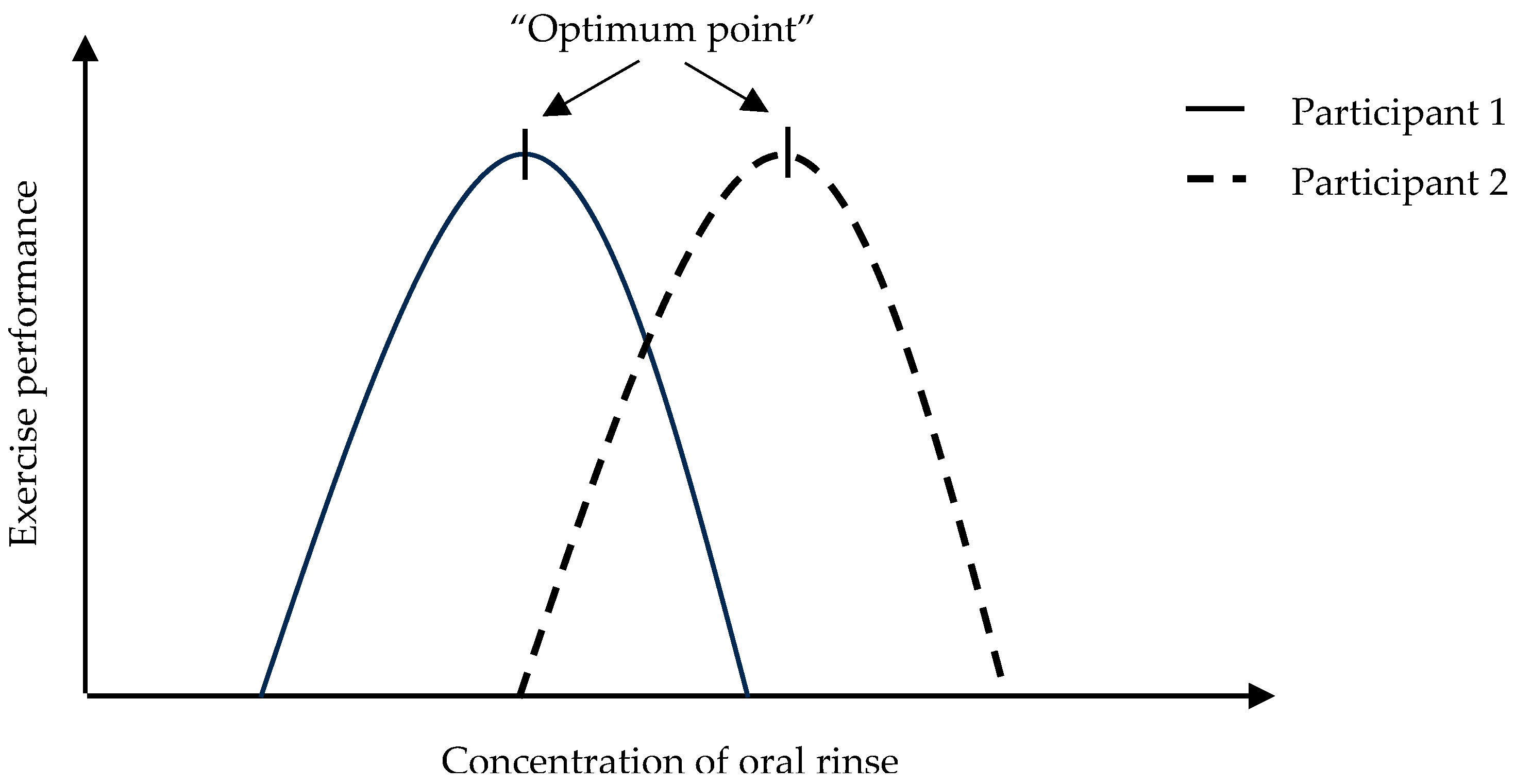
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krogh, A.; Lindhard, J. The Relative Value of Fat and Carbohydrate as Sources of Muscular Energy: With Appendices on the Correlation between Standard Metabolism and the Respiratory Quotient during Rest and Work. Biochem. J. 1920, 14, 290–363. [Google Scholar] [CrossRef]
- Burke, L.; Deakin, V. Clinical Sports Nutrition, 4th ed.; Sports Medicine Series; McGraw-Hill Education: North Ryde, Australia, 2010. [Google Scholar]
- Jeukendrup, A. A step towards personalized sports nutrition: Carbohydrate intake during exercise. Sports Med. 2014, 44 (Suppl. S1), S25–S33. [Google Scholar] [CrossRef]
- Rodriguez, N.R.; Di Marco, N.M.; Langley, S. American College of Sports Medicine position stand. Nutrition and athletic performance. Med. Sci. Sports Exerc. 2009, 41, 709–731. [Google Scholar]
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. Position of the Academy of Nutrition and Dietetics, Dietitians of Canada, and the American College of Sports Medicine: Nutrition and Athletic Performance. J. Acad. Nutr. Diet. 2016, 116, 501–528. [Google Scholar] [CrossRef]
- Burke, L.M.; Hawley, J.A.; Wong, S.H.S.; Jeukendrup, A.E. Carbohydrates for training and competition. J. Sports Sci. 2011, 29 (Suppl. S1), S17–S27. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. Nutrition and Athletic Performance. Med. Sci. Sports Exerc. 2016, 48, 543–568. [Google Scholar] [PubMed]
- De Oliveira, E.P.; Burini, R.C. Carbohydrate-dependent, exercise-induced gastrointestinal distress. Nutrients 2014, 6, 4191–4199. [Google Scholar] [CrossRef]
- Jeukendrup, A.E. Carbohydrate feeding during exercise. Eur. J. Sport. Sci. 2008, 8, 77–86. [Google Scholar] [CrossRef]
- Peters, H.P.; van Schelven, F.W.; Verstappen, P.A.; de Boer, R.W.; Bol, E.; Erich, W.B.; van der Togt, C.R.; de Vries, W.R. Gastrointestinal problems as a function of carbohydrate supplements and mode of exercise. Med. Sci. Sports Exerc. 1993, 25, 1211–1224. [Google Scholar] [CrossRef]
- Peters, H.P.; Wiersma, J.W.; Koerselman, J.; Akkermans, L.M.; Bol, E.; Mosterd, W.L.; de Vries, W.R. The effect of a sports drink on gastroesophageal reflux during a run-bike-run test. Int. J. Sports Med. 2000, 21, 65–70. [Google Scholar] [CrossRef]
- Peters, H.P.; Bos, M.; Seebregts, L.; Akkermans, L.M.; van Berge Henegouwen, G.P.; Bol, E.; Mosterd, W.L.; de Vries, W.R. Gastrointestinal symptoms in long-distance runners, cyclists, and triathletes: Prevalence, medication, and etiology. Am. J. Gastroenterol. 1999, 94, 1570–1581. [Google Scholar] [CrossRef]
- Peters, H.P.F.; Akkermans, L.M.A.; Bol, E.; Mosterd, W.L. Gastrointestinal Symptoms During Exercise. Sports Med. 1995, 20, 65–76. [Google Scholar] [CrossRef]
- Rehrer, N.J.; Janssen, G.M.; Brouns, F.; Saris, W.H. Fluid intake and gastrointestinal problems in runners competing in a 25-km race and a marathon. Int. J. Sports Med. 1989, 10 (Suppl. S1), S22–S25. [Google Scholar] [CrossRef]
- Carter, J.M.; Jeukendrup, A.E.; Jones, D.A. The effect of carbohydrate mouth rinse on 1-h cycle time trial performance. Med. Sci. Sports Exerc. 2004, 36, 2107–2111. [Google Scholar] [CrossRef]
- Chambers, E.S.; Bridge, M.W.; Jones, D. Carbohydrate sensing in the human mouth: Effects on exercise performance and brain activity. J. Physiol. 2009, 587 Pt 8, 1779–1794. [Google Scholar] [CrossRef]
- Murray, K.O.; Paris, H.L.; Fly, A.D.; Chapman, R.F.; Mickleborough, T.D. Carbohydrate Mouth Rinse Improves Cycling Time-Trial Performance without Altering Plasma Insulin Concentration. J. Sports Sci. Med. 2018, 17, 145–152. [Google Scholar] [PubMed]
- Rollo, I.; Williams, C.; Gant, N.; Nute, M. The influence of carbohydrate mouth rinse on self-selected speeds during a 30-min treadmill run. Int. J. Sport. Nutr. Exerc. Metab. 2008, 18, 585–600. [Google Scholar] [CrossRef] [PubMed]
- Rollo, I.; Cole, M.; Miller, R.; Williams, C. Influence of mouth rinsing a carbohydrate solution on 1-h running performance. Med. Sci. Sports Exerc. 2010, 42, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Decimoni, L.; Curty, V.; Almeida, L.; Koch, A.; Willardson, J.; Machado, M. Carbohydrate mouth rinsing improves resistance training session performance. Int. J. Sports Sci. Coach. 2018, 13, 804–809. [Google Scholar] [CrossRef]
- Bastos-Silva, V.J.; Prestes, J.; Geraldes, A.A. Effect of Carboyhdrate Mouth Rinse on Training Load Volume in Resistance Exercise. J. Strength. Cond. Res. 2019, 33, 1653–1657. [Google Scholar] [CrossRef]
- Hartley, C.; Carr, A.; Bowe, S.J.; Bredie, W.L.P.; Keast, R.S.J. Maltodextrin-Based Carbohydrate Oral Rinsing and Exercise Performance: Systematic Review and Meta-Analysis. Sports Med. 2022, 52, 1833–1862. [Google Scholar] [CrossRef] [PubMed]
- Lapis, T.J.; Penner, M.H.; Lim, J. Humans Can Taste Glucose Oligomers Independent of the hT1R2/hT1R3 Sweet Taste Receptor. Chem. Senses 2016, 41, 755–762. [Google Scholar] [CrossRef]
- Feigin, M.B.; Sclafani, A.; Sunday, S.R. Species differences in polysaccharide and sugar taste preferences. Neurosci. Biobehav. Rev. 1987, 11, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Hettinger, T.P.; Frank, M.E.; Myers, W.E. Are the tastes of polycose and monosodium glutamate unique? Chem. Senses 1996, 21, 341–347. [Google Scholar] [CrossRef]
- Bachmanov, A.A.; Beauchamp, G.K. Taste receptor genes. Annu. Rev. Nutr. 2007, 27, 389–414. [Google Scholar] [CrossRef] [PubMed]
- Simon, S.A.; de Araujo, I.E.; Gutierrez, R.; Nicolelis, M.A. The neural mechanisms of gustation: A distributed processing code. Nat. Rev. Neurosci. 2006, 7, 890–901. [Google Scholar] [CrossRef]
- Hartley, I.E.; Liem, D.G.; Keast, R. Umami as an ‘Alimentary’ Taste. A New Perspective on Taste Classification. Nutrients 2019, 11, 182. [Google Scholar] [CrossRef]
- Mandel, A.L.; Peyrot des Gachons, C.; Plank, K.L.; Alarcon, S.; Breslin, P.A.S. Individual Differences in AMY1 Gene Copy Number, Salivary α-Amylase Levels, and the Perception of Oral Starch. PLoS ONE 2010, 5, e13352. [Google Scholar] [CrossRef]
- Holesh, J.E.; Aslam, S.; Martin, A. Physiology, Carbohydrates; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2024. [Google Scholar]
- Low, J.Y.; Lacy, K.E.; McBride, R.L.; Keast, R.S.J. The associations between sweet taste function, oral complex carbohydrate sensitivity, liking and consumption of ad libitum sweet and non-sweet carbohydrate milkshakes among female adults. Br. J. Nutr. 2019, 122, 829–840. [Google Scholar] [CrossRef]
- Low, J.Y.Q.; Lacy, K.E.; McBride, R.L.; Keast, R.S.J. Evidence supporting oral sensitivity to complex carbohydrates independent of sweet taste sensitivity in humans. PLoS ONE 2017, 12, e0188784. [Google Scholar] [CrossRef]
- Low, J.Y.; Lacy, K.E.; McBride, R.L.; Keast, R.S.J. Carbohydrate Taste Sensitivity Is Associated with Starch Intake and Waist Circumference in Adults. J. Nutr. 2017, 147, 2235–2242. [Google Scholar] [CrossRef]
- Andersson, H.; Knight, A.; Buscombe, R.; Sinclair, J.; Edmonds, C.J.; Bottoms, L. The effect of carbohydrate mouth rinse on a 30-minute arm cranking performance. Comp. Exerc. Physiol. 2016, 12, 41–47. [Google Scholar] [CrossRef]
- Ataide-Silva, T.; Ghiarone, T.; Bertuzzi, R.; Stathis, C.G.; Leandro, C.G.; Lima-Silva, A.E. CHO Mouth Rinse Ameliorates Neuromuscular Response with Lower Endogenous CHO Stores. Med. Sci. Sports Exerc. 2016, 48, 1810–1820. [Google Scholar] [CrossRef]
- Bailey, S.P.; Hibbard, J.; La Forge, D.; Mitchell, M.; Roelands, B.; Harris, G.K.; Folger, S. Impact of a Carbohydrate Mouth Rinse on Quadriceps Muscle Function and Corticomotor Excitability. Int. J. Sports Physiol. Perform. 2019, 14, 927–933. [Google Scholar] [CrossRef]
- Bavaresco Gambassi, B.; Gomes de Santana Barros Leal, Y.; Pinheiro Dos Anjos, E.R.; Antonelli, B.A.; Gomes Goncalves, E.S.D.C.; Hermes Pires de Melo Montenegro, I.; di Cassia de Oliveira Angelo, R.; Suruagy Correia Moura, I.; Schwingel, P.A. Carbohydrate mouth rinse improves cycling performance carried out until the volitional exhaustion. J. Sports Med. Phys. Fitness 2019, 59, 1–5. [Google Scholar] [CrossRef]
- Bazzucchi, I.; Patrizio, F.; Felici, F.; Nicolò, A.; Sacchetti, M. Carbohydrate Mouth Rinsing: Improved Neuromuscular Performance During Isokinetic Fatiguing Exercise. Int. J. Sports Physiol. Perform. 2017, 12, 1031–1038. [Google Scholar] [CrossRef]
- Beelen, M.; Berghuis, J.; Bonaparte, B.; Ballak, S.B.; Jeukendrup, A.E.; van Loon, L.J. Carbohydrate mouth rinsing in the fed state: Lack of enhancement of time-trial performance. Int. J. Sport. Nutr. Exerc. Metab. 2009, 19, 400–409. [Google Scholar] [CrossRef]
- Chong, E.; Guelfi, K.J.; Fournier, P.A. Effect of a carbohydrate mouth rinse on maximal sprint performance in competitive male cyclists. J. Sci. Med. Sport. 2011, 14, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Deighton, K.; Duckworth, L.; Matu, J.; Suter, M.; Fletcher, C.; Stead, S.; Ali, S.; Gunby, N.; Korsness, K. Mouth rinsing with a sweet solution increases energy expenditure and decreases appetite during 60 min of self-regulated walking exercise. Appl. Physiol. Nutr. Metab. 2016, 41, 1255–1261. [Google Scholar] [CrossRef] [PubMed]
- Dorling, J.L.; Earnest, C.P. Effect of carbohydrate mouth rinsing on multiple sprint performance. J. Int. Soc. Sports Nutr. 2013, 10, 41. [Google Scholar] [CrossRef] [PubMed]
- Durkin, M.; Akeroyd, H.; Holliday, A. Carbohydrate mouth rinse improves resistance exercise capacity in the glycogen-lowered state. Appl. Physiol. Nutr. Metab. 2021, 46, 126–132. [Google Scholar] [CrossRef]
- Fares, E.J.; Kayser, B. Carbohydrate mouth rinse effects on exercise capacity in pre- and postprandial States. J. Nutr. Metab. 2011, 2011, 385962. [Google Scholar] [CrossRef]
- Gam, S.; Guelfi, K.J.; Fournier, P.A. Opposition of Carbohydrate in a Mouth-Rinse Solution to the Detrimental Effect of Mouth Rinsing During Cycling Time Trials. Int. J. Sport. Nutr. Exerc. Metab. 2013, 23, 48. [Google Scholar] [CrossRef]
- Green, M.S.; Kimmel, C.S.; Martin, T.D.; Mouser, J.G.; Brune, M.P. Effect of Carbohydrate Mouth Rinse on Resistance Exercise Performance. J. Strength Cond. Res. 2020, 36, 1916–1921. [Google Scholar] [CrossRef]
- Jeffers, R.; Shave, R.; Ross, E.; Stevenson, E.J.; Goodall, S. The effect of a carbohydrate mouth-rinse on neuromuscular fatigue following cycling exercise. Appl. Physiol. Nutr. Metab. 2015, 40, 557–564. [Google Scholar] [CrossRef]
- Sinclair, J.; Bottoms, L.; Flynn, C.; Bradley, E.; Alexander, G.; McCullagh, S.; Finn, T.; Hurst, H.T. The effect of different durations of carbohydrate mouth rinse on cycling performance. Eur. J. Sport Sci. 2014, 14, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Pires, F.O.; Brietzke, C.; Pinheiro, F.A.; Veras, K.; de Mattos, E.C.T.; Rodacki, A.L.F.; Ugrinowitsch, C. Carbohydrate Mouth Rinse Fails to Improve Four-Kilometer Cycling Time Trial Performance. Nutrients 2018, 10, 342. [Google Scholar] [CrossRef]
- Trommelen, J.; Beelen, M.; Mullers, M.; Gibala, M.J.; van Loon, L.J.; Cermak, N.M. A Sucrose Mouth Rinse Does Not Improve 1-hr Cycle Time Trial Performance When Performed in the Fasted or Fed State. Int. J. Sport. Nutr. Exerc. Metab. 2015, 25, 576–583. [Google Scholar] [CrossRef]
- Hawkins, K.R.; Krishnan, S.; Ringos, L.; Garcia, V.; Cooper, J.A. Running Performance With Nutritive and Nonnutritive Sweetened Mouth Rinses. Int. J. Sports Physiol. Perform. 2017, 12, 1105–1110. [Google Scholar] [CrossRef][Green Version]
- De Pauw, K.; Roelands, B.; Cheung, S.S.; de Geus, B.; Rietjens, G.; Meeusen, R. Guidelines to classify subject groups in sport-science research. Int. J. Sports Physiol. Perform. 2013, 8, 111–122. [Google Scholar] [CrossRef]
- McLennan, W.; Podger, A.S. National Nutrition Survey: Foods Eaten. Australia 1995. 1999: Australian Bureau of Statistics. Available online: https://www.abs.gov.au/AUSSTATS/abs@.nsf/0/9A125034802F94CECA2568A9001393CE%3FOpenDocument (accessed on 9 December 2019).
- ISO 3972:2011; International Standards Organisation, Sensory Analysis Methodology: Method of Investigating Sensitivity of Taste. ISO: Geneva, Switzerland, 1991.
- Green, B.G.; Shaffer, G.S.; Gilmore, M.M. Derivation and evaluation of a semantic scale of oral sensation magnitude with apparent ratio properties. Chem. Senses 1993, 18, 683–702. [Google Scholar] [CrossRef]
- Hartley, I.; Orellana, L.; Liem, D.G.; Keast, R. Assessment of the triangle test methodology for determining umami discrimination status. Chem. Senses 2022, 47, bjac003. [Google Scholar] [CrossRef]
- Low, J.Y. The associations between Sweet Raste Function, Oral Complex Carbohydrate Sensitivity, Dietary Intake Patterns and Body Composition. Ph.D. Thesis, Deakin University, Victoria, Australia, 2017. [Google Scholar]
- Low, J.Y.; Lacy, K.E.; McBride, R.L.; Keast, R.S.J. The Associations Between Oral Complex Carbohydrate Sensitivity, BMI, Liking, and Consumption of Complex Carbohydrate Based Foods. J. Food Sci. 2018, 83, 2227–2236. [Google Scholar] [CrossRef]
- Kourouniotis, S.; Keast, R.S.J.; Riddell, L.J.; Lacy, K.; Thorpe, M.G.; Cicerale, S. The importance of taste on dietary choice, behaviour and intake in a group of young adults. Appetite 2016, 103, 1–7. [Google Scholar] [CrossRef]
- Jeukendrup, A.; Saris, W.H.; Brouns, F.; Kester, A.D. A new validated endurance performance test. Med. Sci. Sports Exerc. 1996, 28, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Borg, G. Borg’s Perceived Exertion and Pan Scales; Human Kinetics: Champaign, IL, USA, 1998. [Google Scholar]
- Thomas, K.; Stone, M.R.; Thompson, K.G.; St Clair Gibson, A.; Ansley, L. Reproducibility of pacing strategy during simulated 20-km cycling time trials in well-trained cyclists. Eur. J. Appl. Physiol. 2012, 112, 223–229. [Google Scholar] [CrossRef]
- Hibbert, A.W.; Billaut, F.; Varley, M.C.; Polman, R.C.J. Familiarization Protocol Influences Reproducibility of 20-km Cycling Time-Trial Performance in Novice Participants. Front. Physiol. 2017, 8, 488. [Google Scholar] [CrossRef]
- Burke, L.M.; Peeling, P. Methodologies for Investigating Performance Changes With Supplement Use. Int. J. Sport. Nutr. Exerc. Metab. 2018, 28, 159–169. [Google Scholar] [CrossRef]
- Ide, B.N.; Leme, T.C.; Lopes, C.R.; Moreira, A.; Dechechi, C.J.; Sarraipa, M.F.; Da Mota, G.R.; Brenzikofer, R.; Macedo, D.V. Time course of strength and power recovery after resistance training with different movement velocities. J. Strength. Cond. Res. 2011, 25, 2025–2033. [Google Scholar] [CrossRef]
- Close, G.L.; Kasper, A.M.; Morton, J.P. From Paper to Podium: Quantifying the Translational Potential of Performance Nutrition Research. Sports Med. 2019, 49 (Suppl. S1), 25–37. [Google Scholar] [CrossRef]
- Hamlin, M.; Manimmanakorn, A.; Creasy, R.H.; Manimmanakorn, N. Live High-Train Low Altitude Training: Responders and Non- Responders. J. Athl. Enhanc. 2015, 4, 1–8. [Google Scholar]
- Sparks, L.M. Exercise training response heterogeneity: Physiological and molecular insights. Diabetologia 2017, 60, 2329–2336. [Google Scholar] [CrossRef]
- Cramer, M.N.; Thompson, M.W.; Périard, J.D. Thermal and Cardiovascular Strain Mitigate the Potential Benefit of Carbohydrate Mouth Rinse During Self-Paced Exercise in the Heat. Front. Physiol. 2015, 6, 354. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.M.; Findlay, S.; Kavaliauskas, M.; Grant, M.C. The influence of serial carbohydrate mouth rinsing on power output during a cycle sprint. J. Sports Sci. Med. 2014, 13, 252–258. [Google Scholar] [PubMed]
- Lane, S.C.; Bird, S.R.; Burke, L.M.; Hawley, J.A. Effect of a carbohydrate mouth rinse on simulated cycling time-trial performance commenced in a fed or fasted state. Appl. Physiol. Nutr. Metab. 2013, 38, 134–139. [Google Scholar] [CrossRef]
- Chatterton, R.T., Jr.; Vogelsong, K.M.; Lu, Y.C.; Ellman, A.B.; Hudgens, G.A. Salivary alpha-amylase as a measure of endogenous adrenergic activity. Clin. Physiol. 1996, 16, 433–448. [Google Scholar] [CrossRef]
- Dawes, C. The effects of exercise on protein and electrolyte secretion in parotid saliva. J. Physiol. 1981, 320, 139–148. [Google Scholar] [CrossRef]
- Walsh, N.P.; Blannin, A.K.; Clark, A.M.; Cook, L.; Robson, P.J.; Gleeson, M. The effects of high-intensity intermittent exercise on saliva IgA, total protein and alpha-amylase. J. Sports Sci. 1999, 17, 129–134. [Google Scholar] [CrossRef]
- Squires, B.T. Human salivary amylase secretion in relation to diet. J. Physiol. 1953, 119, 153–156. [Google Scholar] [CrossRef]
- Granger, D.A.; Kivlighan, K.T.; El-Sheikh, M.; Gordis, E.B.; Stroud, L.R. Salivary α -amylase in biobehavioral research: Recent developments and applications. Ann. N. Y. Acad. Sci. 2007, 1098, 122–144. [Google Scholar] [CrossRef]
- Kringelbach, M.L. Food for thought: Hedonic experience beyond homeostasis in the human brain. Neuroscience 2004, 126, 807–819. [Google Scholar] [CrossRef]
- Rolls, E.T. Sensory processing in the brain related to the control of food intake. Proc. Nutr. Soc. 2007, 66, 96–112. [Google Scholar] [CrossRef]
- Frank, G.K.; Oberndorfer, T.A.; Simmons, A.N.; Paulus, M.P.; Fudge, J.L.; Yang, T.T.; Kaye, W.H. Sucrose activates human taste pathways differently from artificial sweetener. Neuroimage 2008, 39, 1559–1569. [Google Scholar] [CrossRef] [PubMed]
- Haase, L.; Cerf-Ducastel, B.; Murphy, C. Cortical activation in response to pure taste stimuli during the physiological states of hunger and satiety. Neuroimage 2009, 44, 1008–1021. [Google Scholar] [CrossRef] [PubMed]
- Lambert, E.V.; St Clair Gibson, A.; Noakes, T.D. Complex systems model of fatigue: Integrative homoeostatic control of peripheral physiological systems during exercise in humans. Br. J. Sports Med. 2005, 39, 52–62. [Google Scholar] [CrossRef] [PubMed]
- St Clair Gibson, A.; Lambert, M.L.; Noakes, T.D. Neural control of force output during maximal and submaximal exercise. Sports Med. 2001, 31, 637–650. [Google Scholar] [CrossRef] [PubMed]
- BeMiller, J.N. 19–Carbohydrate and Noncarbohydrate Sweeteners. In Carbohydrate Chemistry for Food Scientists, 3rd ed.; BeMiller, J.N., Ed.; AACC International Press: Cambridge, MA, USA, 2019; pp. 371–399. [Google Scholar]
- Birch, G.G.; Azudin, M.N.; Grigor, J.M. Solution Properties and Composition of Dextrins; ACS Publications: Washington, DC, USA, 1991. [Google Scholar]
- Marchal, L.M.; Beeftink, H.H.; Tramper, J. Towards a rational design of commercial maltodextrins. Trends Food Sci. Technol. 1999, 10, 345–355. [Google Scholar] [CrossRef]
| Taste Quality | Stimulus | Concentration (% w/v) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | ||
| Sweet | Glucose | 0.05 | 0.09 | 0.1 | 0.2 | 0.4 | 0.6 | 1.1 | 1.8 | 2.9 |
| Sour | Citric Acid | 0.013 | 0.016 | 0.020 | 0.025 | 0.031 | 0.038 | 0.048 | 0.06 | 0.10 |
| Carbohydrate | Maltodextrin | 0.1 | 0.2 | 0.3 | 0.6 | 1.1 | 1.9 | 3.6 | 6.3 | 11.2 |
| Taste Quality | Stimulus | Concentration (% w/v) | ||
|---|---|---|---|---|
| Weak | Medium | Strong | ||
| Sweet | Glucose | 5.3 | 10.6 | 21.2 |
| Sour | Citric Acid | 0.02 | 0.06 | 0.13 |
| Carbohydrate | Maltodextrin | 3.6 | 6.3 | 11.2 |
| Rinse | Concentration (% w/v) |
|---|---|
| Maltodextrin | 6.4 |
| Oligofructose | 6.4 |
| Glucose | 6.4 |
| Sucralose | 0.0057 |
| Oral Rinse | Mean Time (min) | Mean Difference Compared to Control | Confidence Interval (CI) |
|---|---|---|---|
| Water (Control) | 81.00 ± 12.48 | - | - |
| Sucralose | 85.29 ± 13.22 | +3.61 | −2.26–9.48 |
| Glucose | 82.90 ± 10.61 | +0.98 | −4.85–6.81 |
| Oligofructose | 78.68 ± 6.86 | −3.99 | −10.14–2.16 |
| Maltodextrin | 81.25 ± 8.09 | +0.58 | −5.27–6.42 |
| Taste Quality | Timepoint | (log) Mean ± SD | (log) Range |
|---|---|---|---|
| Maltodextrin | Baseline | −0.6 ± 1.6 | −2.3–2.0 |
| Session 8 | −1.0 ± 1.4 | −2.3–1.2 | |
| Intervention | −0.5 ± 1.2 | −2.3–1.3 | |
| Sweet | Baseline | −1.9 ± 1.1 | −3.0–0.2 |
| Session 8 | −1.7 ± 1.6 | −3.0–1.1 | |
| Intervention | −1.4 ± 1.3 | −3.0–0.3 | |
| Sour | Baseline | −4.3 ± 0.0 | - |
| Session 8 | −4.3 ± 0.0 | - | |
| Intervention | −4.3 ± 0.01 | −4.34–−4.30 |
| Taste Quality | Timepoint | Mean ± SD | Range |
|---|---|---|---|
| Maltodextrin | Baseline | 6.9 ± 3.9 | 0.7–13.2 |
| Session 8 | 12.6 ± 11.5 | 0.7–31.3 | |
| Intervention | 11.9 ± 10.6 | 2.3–35.6 | |
| Sweet | Baseline | 39.7 ± 15.4 | 20.2–69.8 |
| Session 8 | 29.5 ± 12.7 | 7.7–53.2 | |
| Intervention | 35.6 ± 9.5 | 22.9–56.9 | |
| Sour | Baseline | 42.8 ± 18.1 | 15.1–77.0 |
| Session 8 | 43.6 ± 12.8 | 26.8–61.9 | |
| Intervention | 41.5 ± 13.6 | 24.8–64.5 |
| (log) Mean ± SD | (log) Median ± SD | (log) Range | ||
|---|---|---|---|---|
| Complex Carbohydrate Responders | Baseline | −0.3 ± 1.4 | −0.4 | −2.3–1.7 |
| Session 8 | −0.5 ± 1.4 | −0.1 | −2.1–1.3 | |
| Intervention | −1.3 ± 1.4 | −1.9 | −2.3–1.2 | |
| Overall | −0.1 ± 1.1 | 0.2 | −1.3–1.1 | |
| Complex Carbohydrate Non-Responders | Baseline | −0.9 ± 1.8 | −1.4 | −2.3–2.0 |
| Session 8 | −0.6 ± 1.1 | −0.5 | −2.3–0.7 | |
| Intervention | −0.8 ± 1.5 | −0.4 | −2.3–1.0 | |
| Overall | −0.6 ± 1.2 | −0.6 | −2.3–1.1 |
| Mean ± SD | Median | Range | ||
|---|---|---|---|---|
| Complex Carbohydrate Responders | Baseline | 4.8 ± 2.9 | 6.7 * | 0.7–6.8 |
| Session 8 | 7.0 ± 8.9 | 4.1 | 0.7–22.3 | |
| Intervention | 6.9 ± 5.4 | 6.0 | 2.3–16.1 | |
| Overall | 6.6 ± 4.5 | 6.2 # | 2.0–13.9 | |
| Complex Carbohydrate Non-Responders | Baseline | 9.0 ± 3.8 | 8.585 * | 3.8–13.2 |
| Session 8 | 18.1 ± 11.9 | 18.4 | 4.3–31.3 | |
| Intervention | 16.8 ± 12.7 | 9.7 | 6.6–35.6 | |
| Overall | 15.5 ± 10.3 | 9.3 # | 7.7–30.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hartley, C.; Carr, A.; Roberts, S.S.H.; Bredie, W.L.P.; Keast, R.S.J. Carbohydrate Oral Rinsing, Cycling Performance and Individual Complex Carbohydrate Taste Sensitivity. Nutrients 2024, 16, 459. https://doi.org/10.3390/nu16030459
Hartley C, Carr A, Roberts SSH, Bredie WLP, Keast RSJ. Carbohydrate Oral Rinsing, Cycling Performance and Individual Complex Carbohydrate Taste Sensitivity. Nutrients. 2024; 16(3):459. https://doi.org/10.3390/nu16030459
Chicago/Turabian StyleHartley, Claudia, Amelia Carr, Spencer S. H. Roberts, Wender L. P. Bredie, and Russell S. J. Keast. 2024. "Carbohydrate Oral Rinsing, Cycling Performance and Individual Complex Carbohydrate Taste Sensitivity" Nutrients 16, no. 3: 459. https://doi.org/10.3390/nu16030459
APA StyleHartley, C., Carr, A., Roberts, S. S. H., Bredie, W. L. P., & Keast, R. S. J. (2024). Carbohydrate Oral Rinsing, Cycling Performance and Individual Complex Carbohydrate Taste Sensitivity. Nutrients, 16(3), 459. https://doi.org/10.3390/nu16030459






