Beneficial Effects of Dietary Flaxseed on Non-Alcoholic Fatty Liver Disease
Abstract
1. Introduction
2. Experimental Design, Materials and Methods
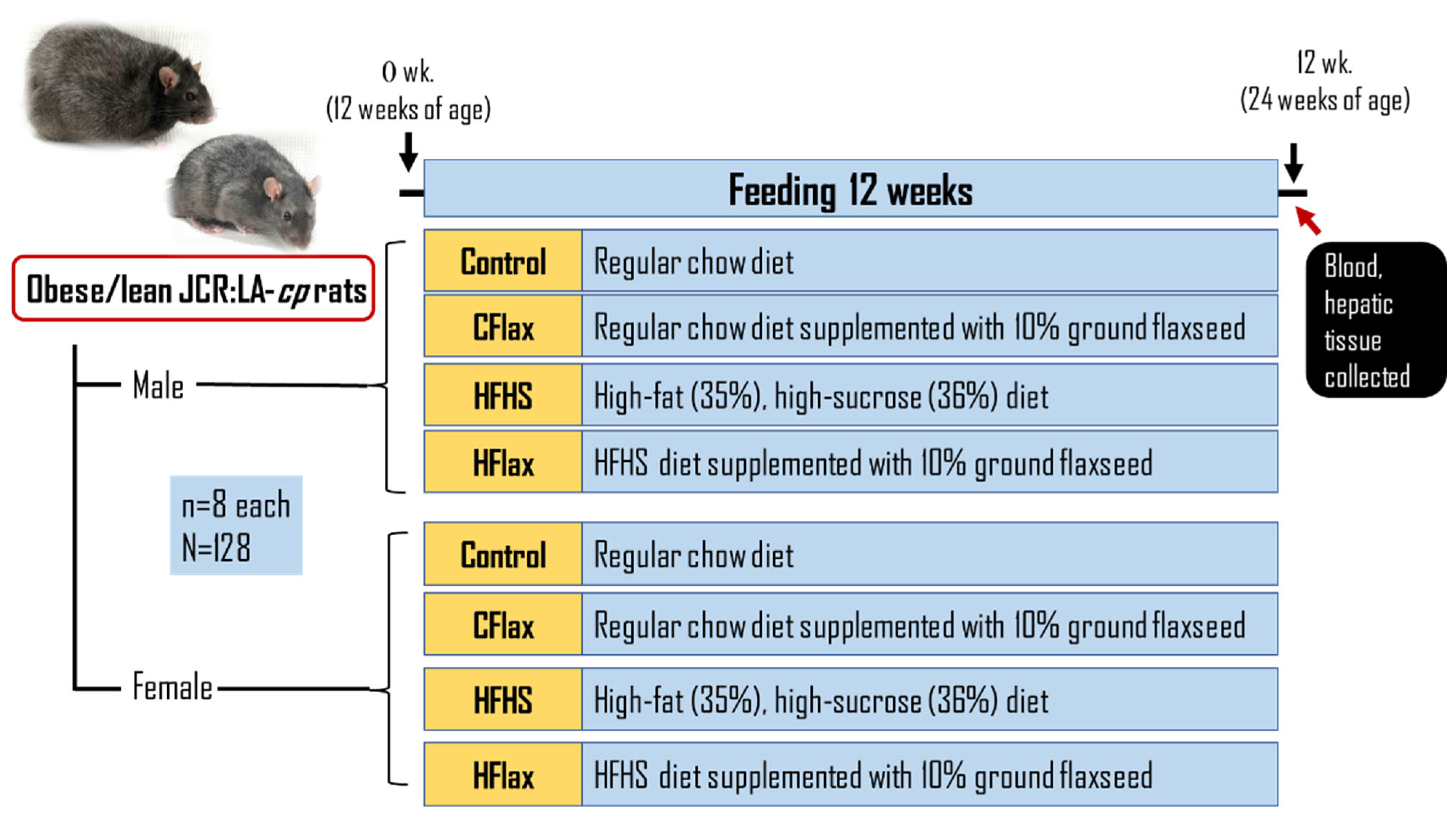
2.1. Experimental Design
2.2. Biological Sample Collection and Analysis
2.3. Hepatic Protein Expression
2.4. Statistical Analysis
3. Results
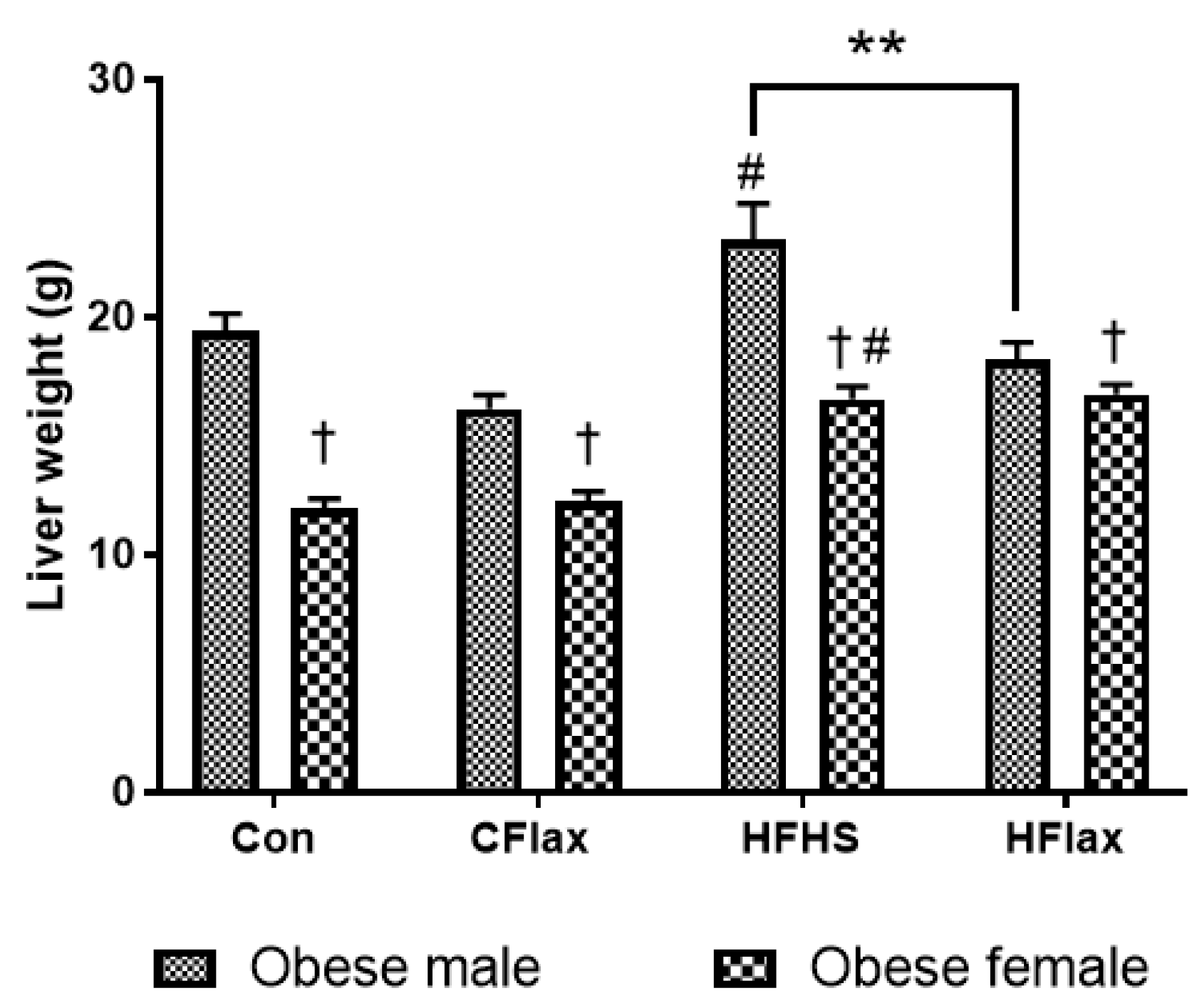
3.1. Liver Weight
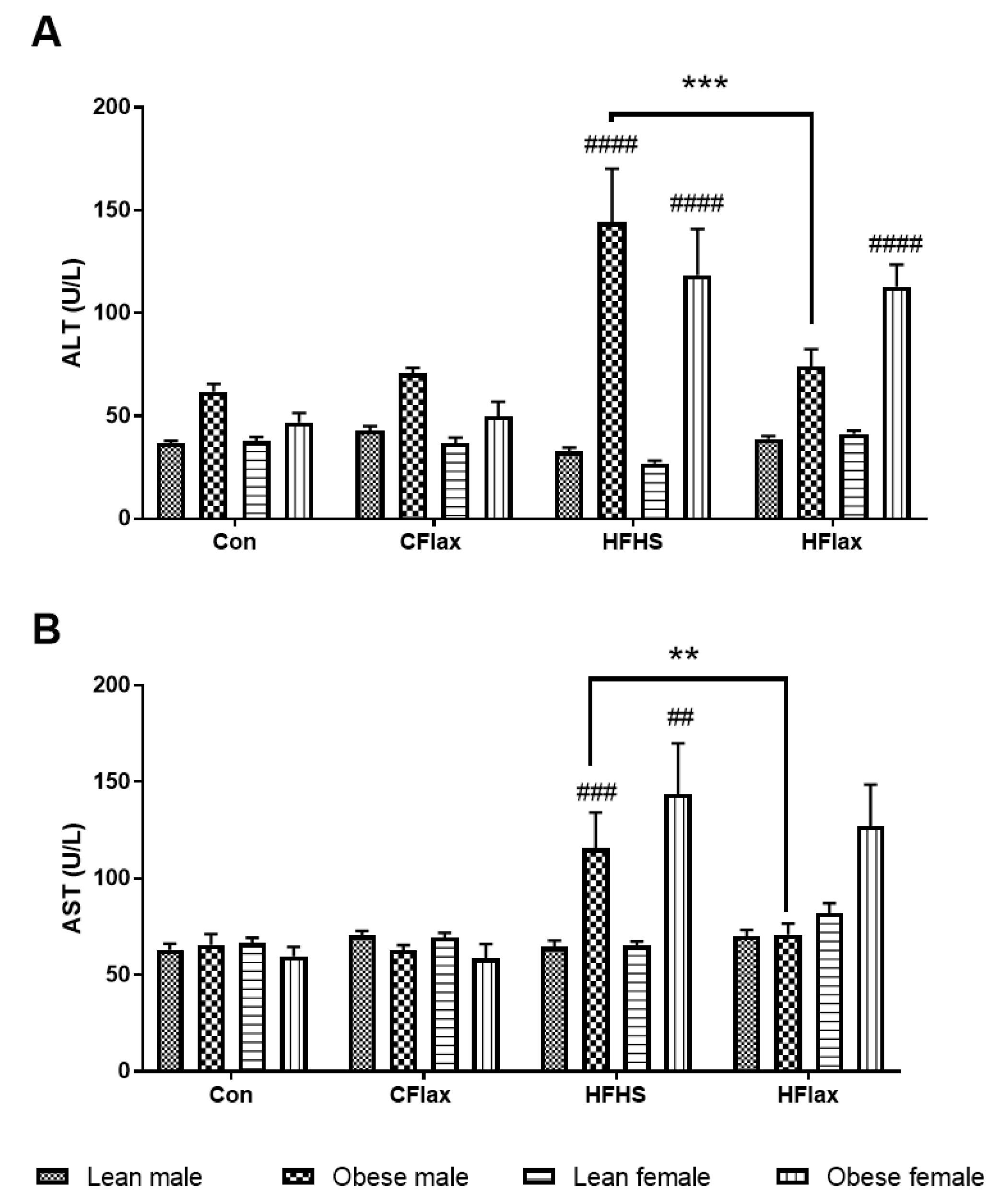
3.2. Plasma Levels of Liver Enzymes
3.3. Plasma Lipid Levels
3.4. Liver Fatty Acid Profile
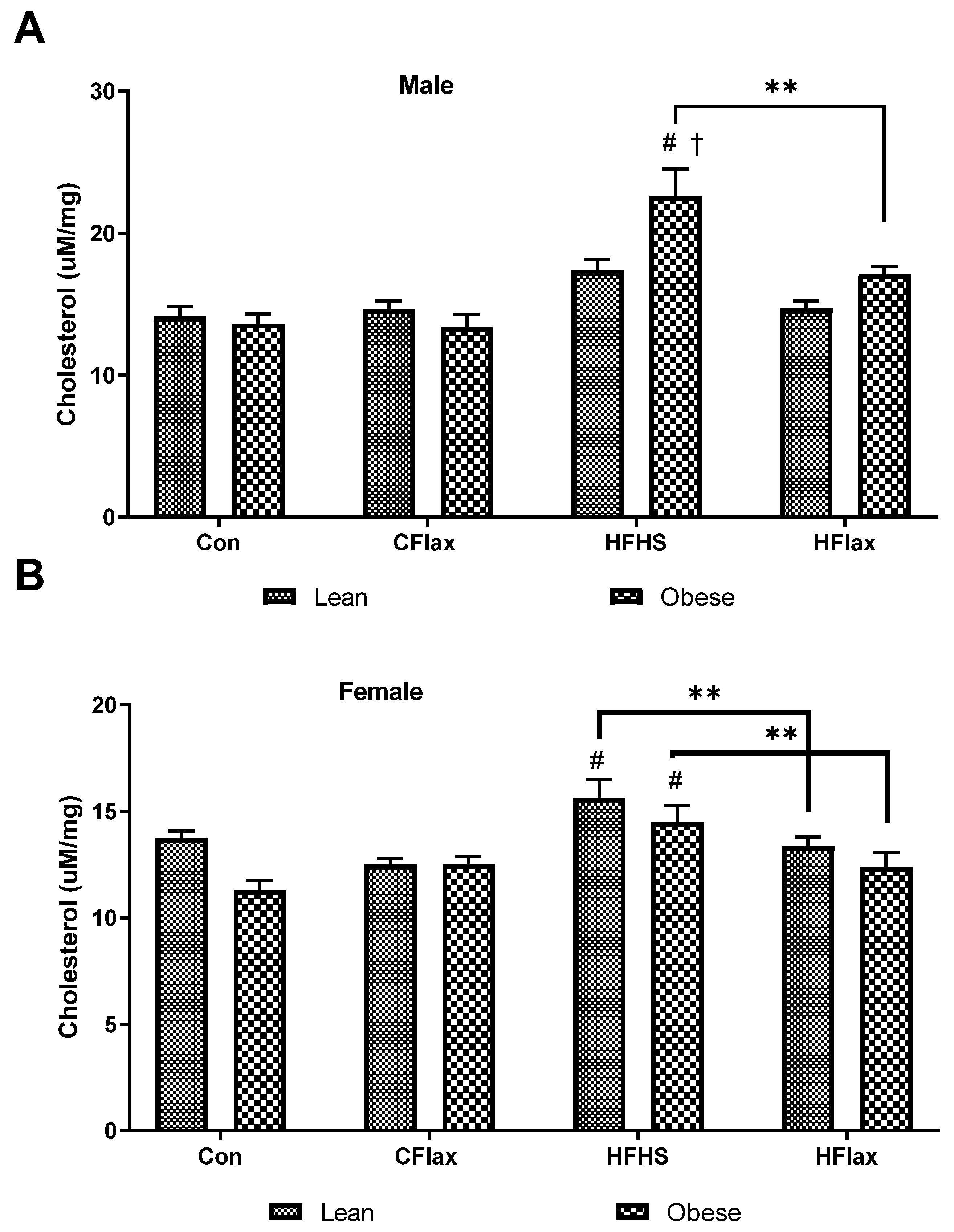
3.5. Liver Cholesterol and Triglyceride Content
3.6. Lipid Metabolism Protein Expression in Liver
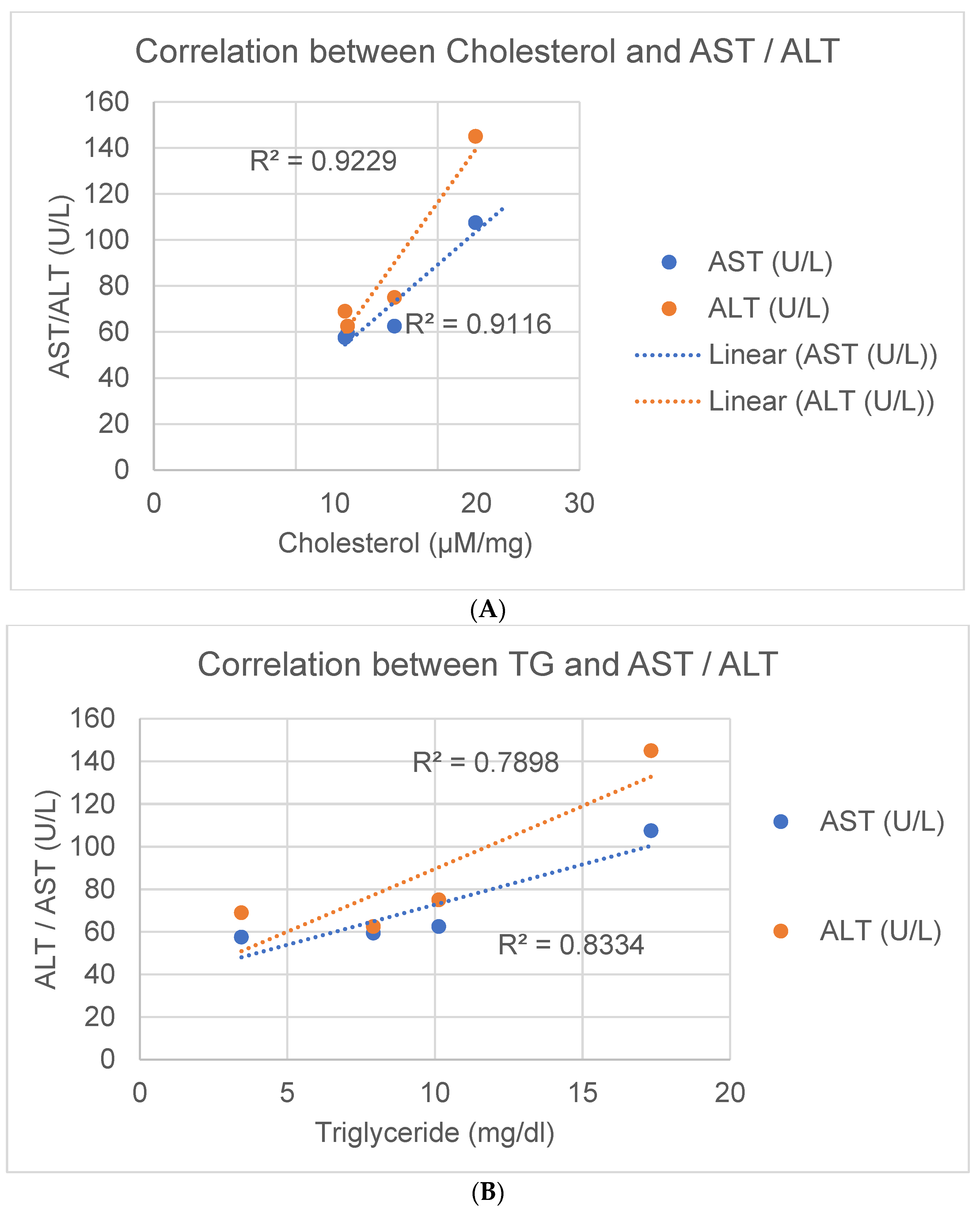
3.7. Correlative Analysis of AST and ALT Changes with Lipid Levels
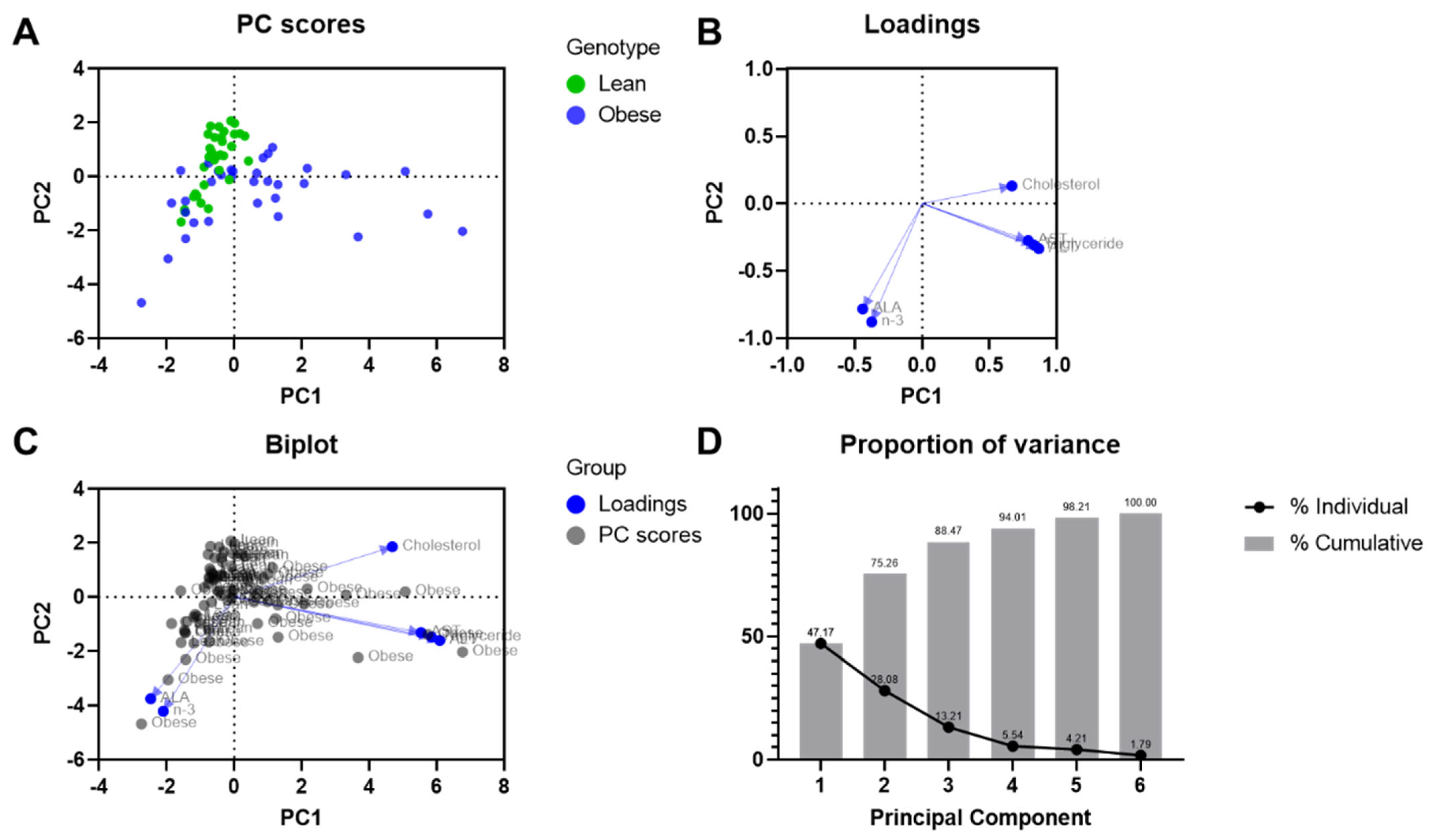
3.8. Principal Component Analysis for the Biochemical Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALT | Alanine aminotransferase |
| ALA | Alpha-linolenic acid |
| AST | aspartate aminotransferase |
| HFHS | high-fat, high-sucrose diet |
| NAFLD | Non-alcoholic fatty liver disease |
References
- Byrne, C.D.; Targher, G. NAFLD: A multisystem disease. J. Hepatol. 2015, 62 (Suppl. S1), S47–S64. [Google Scholar] [CrossRef]
- Teng, M.L.P.; Ng, C.H.; Huang, D.Q.; Chan, K.E.; Tan, D.J.H.; Lim, W.H.; Yang, J.D.; Tan, E.; Muthiah, M.D. Global incidence and prevalence of nonalcoholic fatty liver disease. Clin. Mol. Hepatol. 2023, 29, S32–S42. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): A systematic review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef] [PubMed]
- Riazi, K.; Azhari, H.; Charette, J.H.; Underwood, F.E.; King, J.A.; Afshar, E.E.; Swain, M.G.; Congly, S.E.; Kaplan, G.G.; Shaheen, A.A. The prevalence and incidence of NAFLD worldwide: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2022, 7, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease—Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Amarapurkur, D.; Kamani, P.; Patel, N.; Gupte, P.; Kumar, P.; Agal, S.; Baijal, R.; Lala, S.; Chaudhary, D.; Deshpande, A. Prevalence of nonalcoholic fatty liver disease: Population based study. Ann. Hepatol. 2007, 6, 161–163. [Google Scholar] [CrossRef]
- Vernon, G.; Baranova, A.; Younossi, Z.M. Systematic review: The epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment. Pharmacol. Ther. 2011, 34, 274–285. [Google Scholar] [CrossRef]
- Schwimmer, J.B.; McGreal, N.; Deutsch, R.; Finegold, M.J.; Lavine, J.E. Influence of gender, race and ethnicity on suspected fatty liver in obese adolescents. Pediatrics 2005, 115, e561–e565. [Google Scholar] [CrossRef]
- Estes, C.; Razavi, H.; Loomba, R.; Younossi, Z.; Sanyal, A.J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018, 67, 123–133. [Google Scholar] [CrossRef]
- Riazi, K.; Raman, M.; Taylor, L.; Swain, M.G.; Shaheen, A.A. Dietary Patterns and Components in Nonalcoholic Fatty Liver Disease (NAFLD): What Key Messages Can Health Care Providers Offer? Nutrients 2019, 11, 2878. [Google Scholar] [CrossRef]
- Russell, J.C.; Dolphin, P.J.; Graham, S.E.; Amy, R.M.; Brindley, D.N. Improvement of insulin sensitivity and cardiovascular outcomes in the JCR: LA-cp rat by D-fenfluramine. Diabetologia 1998, 41, 380–389. [Google Scholar] [CrossRef][Green Version]
- Russell, J.C.; Koeslag, D.G.; Dolphin, P.J.; Amy, R.M. Beneficial effects of acarbose in the atherosclerosis-prone JCR: LA-corpulent rat. Metabolism 1993, 42, 218–223. [Google Scholar] [CrossRef]
- Parikh, M.; Kura, B.; Garg, B.; Austria, J.A.; Yu, L.; Maddaford, T.G.; Proctor, S.D.; Netticadan, T.; Pierce, G.N. Dietary flaxseed reduces myocardial ischemic lesions, improves cardiac function and lowers cholesterol levels despite the presence of severe obesity in JCR: LA-cp rats. J. Nutr. Biochem. 2021, 98, 108829. [Google Scholar] [CrossRef]
- Russell, J.C.; Graham, S.E.; Richardson, M. Cardiovascular disease in the JCR: LA-cp rat. Mol. Cell. Biochem. 1998, 188, 113–126. [Google Scholar] [CrossRef]
- Parikh, M.; Netticadan, T.; Pierce, G.N. Flaxseed: Its bioactive components and their cardiovascular benefits. Am. J. Physiol. Heart Circ. Physiol. 2018, 314, H146–H159. [Google Scholar] [CrossRef]
- Goyal, A.; Sharma, V.; Upadhyay, N.; Singh, A.K.; Arora, S.; Lal, D.; Sabikhi, L. Flax and flaxseed oil: An ancient medicine & modern functional food. J. Food Sci. Technol. 2015, 52, 4256–4265. [Google Scholar]
- Edel, A.L.; Rodriguez-Leyva, D.; Maddaford, T.G.; Caligiuri, S.P.B.; Austria, J.A.; Weighell, W.; Guzman, R.; Aliani, M.; Pierce, G.N. Dietary flaxseed independently lowers circulating cholesterol and lowers it beyond the effects of cholesterol-lowering medications alone in patients with peripheral arterial disease. J. Nutr. 2015, 145, 749–757. [Google Scholar] [CrossRef]
- Madduma Hewage, S.; Prashar, S.; O, K.; Siow, Y.L. Lingonberry improves non-alcoholic fatty liver disease by reducing hepatic lipid accumulation, oxidative stress and inflammatory response. Antioxidants 2021, 10, 565. [Google Scholar] [CrossRef] [PubMed]
- Sid, V.; Siow, Y.L.; O, K. Role of folate in nonalcoholic fatty liver disease. Can. J. Physiol. Pharmacol. 2017, 95, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Parikh, M.; Raj, P.; Austria, J.A.; Yu, L.; Garg, B.; Netticadan, T.; Pierce, G.N. Dietary flaxseed protects against ventricular arrhythmias and left ventricular dilation after a myocardial infarction. J. Nutr. Biochem. 2019, 71, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, H.; Gao, Y.; Vafaei, S.; Zhang, X.; Yang, M. Effect of flaxseed supplementation on blood pressure: A systematic review, and dose-response meta-analysis of randomized clinical trials. Food Funct. 2023, 14, 675–690. [Google Scholar] [CrossRef]
- Russell, J.C.; Koeslag, D.G.; Manickavel, V.; Amy, R.M.; Dolphin, P.J. Effects of advancing age and severe food restriction on pathological processes in the insulin resistant JCR: LA-corpulent rat. Diabetes Res. 1990, 15, 53–62. [Google Scholar]
- Russell, J.C.; Amy, R.M.; Dolphin, P.J. Effect of dietary n-3 fatty acids on atherosclerosis prone JCR: LA-corpulent rats. Exp. Mol. Pathol. 1991, 55, 285–293. [Google Scholar] [CrossRef]
- Russell, J.C.; Shillabeer, G.; Bar-Tana, J.; Lau, D.C.; Richardson, M.; Wenzel, L.M.; Graham, S.E.; Dolphin, P.J. Development of insulin resistance in the JCR: LA-cp rat: Role of triacylglycerols and effects of MEDICA 16. Diabetes 1998, 47, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.C.; Ewart, H.S.; Kelly, S.E.; Kralovec, J.; Wright, J.L.; Dolphin, P.J. Improvement of vascular dysfunction and blood lipids of insulin-resistant rats by a marine oil-based phytosterol compound. Lipids 2002, 37, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Brindley, D.N.; Russell, J.C. Animal models of insulin resistance and cardiovascular disease: Some therapeutic approaches using JCR: LA-cp rat. Diabetes Obes. Metab. 2002, 4, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ander, B.P.; Edel, A.L.; McCullough, R.; Rodriguez-Leyva, D.; Rampersad, P.; Gilchrist, J.S.C.; Lukas, A.; Pierce, G.N. Distribution of omega-3 fatty acids in tissues of rabbits fed a flaxseed-supplemented diet. Metabolism 2010, 59, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wong, V.W.-S.; Dufour, J.-F.; Schattenberg, J.M.; et al. A new definition of metabolic dysfunction-associated liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef] [PubMed]



| Measure | Genotype | Sex | Diet | |||
|---|---|---|---|---|---|---|
| Control | CFlax | HFHS | HFlax | |||
| Liver weight, g | Lean | Male | 8.69 ± 0.25 | 8.62 ± 0.19 | 8.17 ± 0.26 | 8.12 ± 0.32 |
| Female | 6.17 ± 0.36 | 6.49 ± 0.40 | 5.10 ± 0.16 | 5.39 ± 0.20 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parikh, M.; Hirst, B.C.; O’Hara, K.A.; Maddaford, T.G.; Austria, J.A.; Stamenkovic, A.; Yu, L.; Kura, B.; Garg, B.; Netticadan, T.; et al. Beneficial Effects of Dietary Flaxseed on Non-Alcoholic Fatty Liver Disease. Nutrients 2024, 16, 466. https://doi.org/10.3390/nu16040466
Parikh M, Hirst BC, O’Hara KA, Maddaford TG, Austria JA, Stamenkovic A, Yu L, Kura B, Garg B, Netticadan T, et al. Beneficial Effects of Dietary Flaxseed on Non-Alcoholic Fatty Liver Disease. Nutrients. 2024; 16(4):466. https://doi.org/10.3390/nu16040466
Chicago/Turabian StyleParikh, Mihir, Broderick C. Hirst, Kimberley A. O’Hara, Thane G. Maddaford, J. Alejandro Austria, Aleksandra Stamenkovic, Liping Yu, Branislav Kura, Bhavana Garg, Thomas Netticadan, and et al. 2024. "Beneficial Effects of Dietary Flaxseed on Non-Alcoholic Fatty Liver Disease" Nutrients 16, no. 4: 466. https://doi.org/10.3390/nu16040466
APA StyleParikh, M., Hirst, B. C., O’Hara, K. A., Maddaford, T. G., Austria, J. A., Stamenkovic, A., Yu, L., Kura, B., Garg, B., Netticadan, T., Proctor, S. D., & Pierce, G. N. (2024). Beneficial Effects of Dietary Flaxseed on Non-Alcoholic Fatty Liver Disease. Nutrients, 16(4), 466. https://doi.org/10.3390/nu16040466







