Formulated Palmitoylethanolamide Supplementation Improves Parameters of Cognitive Function and BDNF Levels in Young, Healthy Adults: A Randomised Cross-Over Trial
Abstract
1. Introduction
2. Materials and Methods
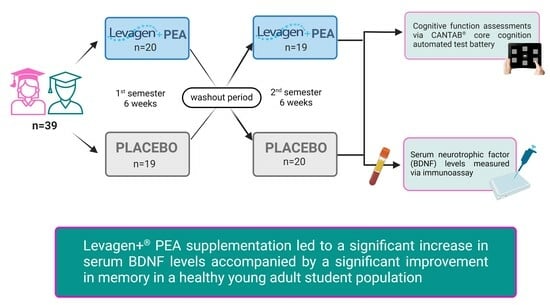
2.1. Study Design
2.2. Participants
2.3. Intervention
2.4. Blood Sampling and Sample Handling
2.5. BDNF Analysis
2.6. Cognitive Function Assessment
2.7. Statistical Analysis
3. Results
3.1. Demographics
3.2. BDNF Measurement
3.3. CANTAB® Cognition Function Assessments
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harvey, P.D. Domains of Cognition and Their Assessment. Dialogues Clin. Neurosci. 2019, 21, 227–237. [Google Scholar] [CrossRef]
- Roy, T.; Saroka, K.S.; Hossack, V.L.; Dotta, B.T. The Effects of Exam-Induced Stress on EEG Profiles and Memory Scores. Behav. Sci. 2023, 13, 373. [Google Scholar] [CrossRef]
- Thompson, J.J.; Blair, M.R.; Henrey, A.J. Over the Hill at 24: Persistent Age-Related Cognitive-Motor Decline in Reaction Times in an Ecologically Valid Video Game Task Begins in Early Adulthood. PLoS ONE 2014, 9, e94215. [Google Scholar] [CrossRef]
- Salthouse, T.A. When Does Age-Related Cognitive Decline Begin? Neurobiol. Aging 2009, 30, 507–514. [Google Scholar] [CrossRef]
- Sharif, S.; Guirguis, A.; Fergus, S.; Schifano, F. The Use and Impact of Cognitive Enhancers among University Students: A Systematic Review. Brain Sci. 2021, 11, 355. [Google Scholar] [CrossRef] [PubMed]
- Maggi, S.; Ticinesi, A.; Limongi, F.; Noale, M.; Ecarnot, F. The Role of Nutrition and the Mediterranean Diet on the Trajectories of Cognitive Decline. Exp. Gerontol. 2023, 173, 112110. [Google Scholar] [CrossRef] [PubMed]
- Puri, S.; Shaheen, M.; Grover, B. Nutrition and Cognitive Health: A Life Course Approach. Front. Public Health 2023, 11, 1023907. [Google Scholar] [CrossRef]
- Lam, L.F.; Lawlis, T.R. Feeding the Brain—The Effects of Micronutrient Interventions on Cognitive Performance among School-Aged Children: A Systematic Review of Randomized Controlled Trials. Clin. Nutr. 2017, 36, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Abrego-Guandique, D.M.; Bonet, M.L.; Caroleo, M.C.; Cannataro, R.; Tucci, P.; Ribot, J.; Cione, E. The Effect of Beta-Carotene on Cognitive Function: A Systematic Review. Brain Sci. 2023, 13, 1468. [Google Scholar] [CrossRef]
- Denniss, R.J.; Barker, L.A.; Day, C.J. Improvement in Cognition Following Double-Blind Randomized Micronutrient Interventions in the General Population. Front. Behav. Neurosci. 2019, 13, 447445. [Google Scholar] [CrossRef]
- Gonzalez, A.; Moya-Alvarado, G.; Gonzalez-Billaut, C.; Bronfman, F.C. Cellular and Molecular Mechanisms Regulating Neuronal Growth by Brain-Derived Neurotrophic Factor. Cytoskeleton 2016, 73, 612–628. [Google Scholar] [CrossRef]
- DiSabato, D.J.; Quan, N.; Godbout, J.P. Neuroinflammation: The Devil Is in the Details. J. Neurochem. 2016, 139 (Suppl. 2), 136–153. [Google Scholar] [CrossRef]
- Chen, X.; Chen, C.; Fan, S.; Wu, S.; Yang, F.; Fang, Z.; Fu, H.; Li, Y. Omega-3 Polyunsaturated Fatty Acid Attenuates the Inflammatory Response by Modulating Microglia Polarization through SIRT1-Mediated Deacetylation of the HMGB1/NF-ΚB Pathway Following Experimental Traumatic Brain Injury. J. Neuroinflamm. 2018, 15, 116. [Google Scholar] [CrossRef] [PubMed]
- Winiarska-Mieczan, A.; Kwiecień, M.; Jachimowicz-Rogowska, K.; Donaldson, J.; Tomaszewska, E.; Baranowska-Wójcik, E. Anti-Inflammatory, Antioxidant, and Neuroprotective Effects of Polyphenols-Polyphenols as an Element of Diet Therapy in Depressive Disorders. Int. J. Mol. Sci. 2023, 24, 2258. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.; Morici, J.F.; Zanoni, M.B.; Bekinschtein, P. Brain-Derived Neurotrophic Factor: A Key Molecule for Memory in the Healthy and the Pathological Brain. Front. Cell Neurosci. 2019, 13, 472800. [Google Scholar] [CrossRef]
- Tran, P.H.L.; Tran, T.T.D. Blueberry Supplementation in Neuronal Health and Protective Technologies for Efficient Delivery of Blueberry Anthocyanins. Biomolecules 2021, 11, 102. [Google Scholar] [CrossRef] [PubMed]
- Sarraf, P.; Parohan, M.; Javanbakht, M.H.; Ranji-Burachaloo, S.; Djalali, M. Short-Term Curcumin Supplementation Enhances Serum Brain-Derived Neurotrophic Factor in Adult Men and Women: A Systematic Review and Dose-Response Meta-Analysis of Randomized Controlled Trials. Nutr. Res. 2019, 69, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lorinczova, H.T.; Fitzsimons, O.; Mursaleen, L.; Renshaw, D.; Begum, G.; Zariwala, M.G. Co-Administration of Iron and a Bioavailable Curcumin Supplement Increases Serum BDNF Levels in Healthy Adults. Antioxidants 2020, 9, 645. [Google Scholar] [CrossRef] [PubMed]
- Rankin, L.; Fowler, C.J. The Basal Pharmacology of Palmitoylethanolamide. Int. J. Mol. Sci. 2020, 21, 7942. [Google Scholar] [CrossRef]
- Petrosino, S.; Di Marzo, V. The Pharmacology of Palmitoylethanolamide and First Data on the Therapeutic Efficacy of Some of Its New Formulations. Br. J. Pharmacol. 2017, 174, 1349–1365. [Google Scholar] [CrossRef]
- Clayton, P.; Hill, M.; Bogoda, N.; Subah, S.; Venkatesh, R. Palmitoylethanolamide: A Natural Compound for Health Management. Int. J. Mol. Sci. 2021, 22, 5305. [Google Scholar] [CrossRef] [PubMed]
- Artamonov, M.; Zhukov, O.; Shuba, I.; Storozhuk, L.; Khmel, T.; Klimashevsky, V.; Mikosha, A.; Gula, N. Incorporation of Labelled N-Acylethanolamine (NAE) into Rat Brain Regions in Vivo and Adaptive Properties of Saturated NAE under X-ray Irradiation. Ukr. Biokhim. Zh. 2005, 77, 51–62. [Google Scholar]
- Lo Verme, J.; Fu, J.; Astarita, G.; La Rana, G.; Russo, R.; Calignano, A.; Piomelli, D. The Nuclear Receptor Peroxisome Proliferator-Activated Receptor-α Mediates the Anti-Inflammatory Actions of Palmitoylethanolamide. Mol. Pharmacol. 2005, 67, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Hesselink, J.M.K. Professor Rita Levi-Montalcini on Nerve Growth Factor, Mast Cells and Palmitoylethanolamide, an Endogenous Anti-Inflammatory and Analgesic Compound. J. Pain Relief 2013, 2, 1–4. [Google Scholar] [CrossRef]
- Ho, W.S.V.; Barrett, D.A.; Randall, M.D. ‘Entourage’ Effects of N-Palmitoylethanolamide and N-Oleoylethanolamide on Vasorelaxation to Anandamide Occur through TRPV1 Receptors. Br. J. Pharmacol. 2008, 155, 837. [Google Scholar] [CrossRef]
- Hill, M.N.; Campolongo, P.; Yehuda, R.; Patel, S. Integrating Endocannabinoid Signaling and Cannabinoids into the Biology and Treatment of Posttraumatic Stress Disorder. Neuropsychopharmacology 2017, 43, 80–102. [Google Scholar] [CrossRef]
- Silva-Cardoso, G.K.; Lazarini-Lopes, W.; Primini, E.O.; Hallak, J.E.; Crippa, J.A.; Zuardi, A.W.; Garcia-Cairasco, N.; Leite-Panissi, C.R.A. Cannabidiol Modulates Chronic Neuropathic Pain Aversion Behavior by Attenuation of Neuroinflammation Markers and Neuronal Activity in the Corticolimbic Circuit in Male Wistar Rats. Behav. Brain Res. 2023, 452, 114588. [Google Scholar] [CrossRef]
- Rossi, G.N.; Rocha, J.M.; Osório, F.L.; Bouso, J.C.; Ona, G.; Silveira, G.D.O.; Yonamine, M.; Bertozi, G.; Crevelin, E.J.; Queiroz, M.E.; et al. Interactive Effects of Ayahuasca and Cannabidiol in Social Cognition in Healthy Volunteers: A Pilot, Proof-of-Concept, Feasibility, Randomized-Controlled Trial. J. Clin. Psychopharmacol. 2023, 43, 339–349. [Google Scholar] [CrossRef]
- Fernández-Moncada, I.; Rodrigues, R.S.; Fundazuri, U.B.; Bellocchio, L.; Marsicano, G. Type-1 Cannabinoid Receptors and Their Ever-Expanding Roles in Brain Energy Processes. J. Neurochem. 2023. [Google Scholar] [CrossRef]
- Lang-Illievich, K.; Klivinyi, C.; Lasser, C.; Brenna, C.T.A.; Szilagyi, I.S.; Bornemann-Cimenti, H. Palmitoylethanolamide in the Treatment of Chronic Pain: A Systematic Review and Meta-Analysis of Double-Blind Randomized Controlled Trials. Nutrients 2023, 15, 1350. [Google Scholar] [CrossRef]
- Petrosino, S.; Moriello, A.S. Palmitoylethanolamide: A Nutritional Approach to Keep Neuroinflammation within Physiological Boundaries—A Systematic Review. Int. J. Mol. Sci. 2020, 21, 9526. [Google Scholar] [CrossRef]
- Chirchiglia, D.; Paventi, S.; Seminara, P.; Cione, E.; Gallelli, L. N-Palmitoyl Ethanol Amide Pharmacological Treatment in Patients With Nonsurgical Lumbar Radiculopathy. J. Clin. Pharmacol. 2018, 58, 733–739. [Google Scholar] [CrossRef]
- Clayton, P.; Subah, S.; Venkatesh, R.; Hill, M.; Bogoda, N. Palmitoylethanolamide: A Potential Alternative to Cannabidiol. J. Diet. Suppl. 2023, 20, 505–530. [Google Scholar] [CrossRef]
- D’aloia, A.; Molteni, L.; Gullo, F.; Bresciani, E.; Artusa, V.; Rizzi, L.; Ceriani, M.; Meanti, R.; Lecchi, M.; Coco, S.; et al. Palmitoylethanolamide Modulation of Microglia Activation: Characterization of Mechanisms of Action and Implication for Its Neuroprotective Effects. Int. J. Mol. Sci. 2021, 22, 3054. [Google Scholar] [CrossRef]
- Antonucci, N.; Cirillo, A.; Siniscalco, D. Beneficial Effects of Palmitoylethanolamide on Expressive Language, Cognition, and Behaviors in Autism: A Report of Two Cases. Case Rep. Psychiatry 2015, 2015, 325061. [Google Scholar] [CrossRef] [PubMed]
- Caltagirone, C.; Cisari, C.; Schievano, C.; Di Paola, R.; Cordaro, M.; Bruschetta, G.; Esposito, E.; Cuzzocrea, S.; Ventura, F.; Casaleggio, M.; et al. Co-Ultramicronized Palmitoylethanolamide/Luteolin in the Treatment of Cerebral Ischemia: From Rodent to Man. Transl. Stroke Res. 2016, 7, 54–69. [Google Scholar] [CrossRef]
- Mittleman, M.A.; Mostofsky, E. Exchangeability in the Case-Crossover Design. Int. J. Epidemiol. 2014, 43, 1645. [Google Scholar] [CrossRef]
- Garg, R. Methodology for Research I. Indian. J. Anaesth. 2016, 60, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Qu, S. Cognition and Academic Performance: Mediating Role of Personality Characteristics and Psychology Health. Front. Psychol. 2021, 12, 774548. [Google Scholar] [CrossRef] [PubMed]
- Barbayannis, G.; Bandari, M.; Zheng, X.; Baquerizo, H.; Pecor, K.W.; Ming, X. Academic Stress and Mental Well-Being in College Students: Correlations, Affected Groups, and COVID-19. Front. Psychol. 2022, 13, 886344. [Google Scholar] [CrossRef]
- Khanna, D.; Khanna, S.; Khanna, P.; Kahar, P.; Patel, B.M. Obesity: A Chronic Low-Grade Inflammation and Its Markers. Cureus 2022, 14, e22711. [Google Scholar] [CrossRef]
- Lee, J.; Spratling, R. Recruiting Mothers of Children With Developmental Disabilities: Adaptations of the Snowball Sampling Technique Using Social Media. J. Pediatr. Health Care 2019, 33, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Briskey, D.; Mallard, A.R.; Rao, A. Increased Absorption of Palmitoylethanolamide Using a Novel Dispersion Technology System (LipiSperse). J. Nutraceuticals Food Sci. 2020, 5, 1–6. [Google Scholar] [CrossRef]
- Pickering, E.; Steels, E.L.; Steadman, K.J.; Rao, A.; Vitetta, L. A Randomized Controlled Trial Assessing the Safety and Efficacy of Palmitoylethanolamide for Treating Diabetic-Related Peripheral Neuropathic Pain. Inflammopharmacology 2022, 30, 2063–2077. [Google Scholar] [CrossRef] [PubMed]
- Scuteri, D.; Guida, F.; Boccella, S.; Palazzo, E.; Maione, S.; Rodríguez-Landa, J.F.; Martínez-Mota, L.; Tonin, P.; Bagetta, G.; Corasaniti, M.T. Effects of Palmitoylethanolamide (PEA) on Nociceptive, Musculoskeletal and Neuropathic Pain: Systematic Review and Meta-Analysis of Clinical Evidence. Pharmaceutics 2022, 14, 1672. [Google Scholar] [CrossRef] [PubMed]
- Ghazizadeh-Hashemi, M.; Ghajar, A.; Shalbafan, M.R.; Ghazizadeh-Hashemi, F.; Afarideh, M.; Malekpour, F.; Ghaleiha, A.; Ardebili, M.E.; Akhondzadeh, S. Palmitoylethanolamide as Adjunctive Therapy in Major Depressive Disorder: A Double-Blind, Randomized and Placebo-Controlled Trial. J. Affect. Disord. 2018, 232, 127–133. [Google Scholar] [CrossRef]
- Polyakova, M.; Schlögl, H.; Sacher, J.; Schmidt-Kassow, M.; Kaiser, J.; Stumvoll, M.; Kratzsch, J.; Schroeter, M.L. Stability of BDNF in Human Samples Stored Up to 6 Months and Correlations of Serum and EDTA-Plasma Concentrations. Int. J. Mol. Sci. 2017, 18, 1189. [Google Scholar] [CrossRef]
- Polacchini, A.; Metelli, G.; Francavilla, R.; Baj, G.; Florean, M.; Mascaretti, L.G.; Tongiorgi, E. A Method for Reproducible Measurements of Serum BDNF: Comparison of the Performance of Six Commercial Assays. Sci. Rep. 2015, 5, 17989. [Google Scholar] [CrossRef]
- Lenehan, M.E.; Summers, M.J.; Saunders, N.L.; Summers, J.J.; Vickers, J.C. Does the Cambridge Automated Neuropsychological Test Battery (CANTAB) Distinguish Between Cognitive Domains in Healthy Older Adults? Assessment 2016, 23, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Sahakian, B.J.; Owen, A.M. Computerized Assessment in Neuropsychiatry Using CANTAB: Discussion Paper. J. R. Soc. Med. 1992, 85, 399–402. [Google Scholar] [PubMed]
- Forbes, S.C.; Holroyd-Leduc, J.M.; Poulin PhD, M.J.; Hogan, D.B. Effect of Nutrients, Dietary Supplements and Vitamins on Cognition: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Can. Geriatr. J. 2015, 18, 231. [Google Scholar] [CrossRef]
- Berding, K.; Long-Smith, C.M.; Carbia, C.; Bastiaanssen, T.F.S.; van de Wouw, M.; Wiley, N.; Strain, C.R.; Fouhy, F.; Stanton, C.; Cryan, J.F.; et al. A Specific Dietary Fibre Supplementation Improves Cognitive Performance—An Exploratory Randomised, Placebo-Controlled, Crossover Study. Psychopharmacology 2021, 238, 149–163. [Google Scholar] [CrossRef]
- Agh, F.; Hasani, M.; Khazdouz, M.; Amiri, F.; Heshmati, J.; Aryaeian, N. The Effect of Zinc Supplementation on Circulating Levels of Brain-Derived Neurotrophic Factor (BDNF): A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int. J. Prev. Med. 2022, 13, 117. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.L.; Yanes, J.A.; Reid, M.A.; Murphy, J.E.; Busler, J.N.; Mumford, P.W.; Young, K.C.; Pietrzkowski, Z.J.; Nemzer, B.V.; Hunter, J.M.; et al. Neurophysiological Effects of Whole Coffee Cherry Extract in Older Adults with Subjective Cognitive Impairment: A Randomized, Double-Blind, Placebo-Controlled, Cross-Over Pilot Study. Antioxidants 2021, 10, 144. [Google Scholar] [CrossRef]
- Colizzi, M.; Bortoletto, R.; Colli, C.; Bonomo, E.; Pagliaro, D.; Maso, E.; Di Gennaro, G.; Balestrieri, M. Therapeutic Effect of Palmitoylethanolamide in Cognitive Decline: A Systematic Review and Preliminary Meta-Analysis of Preclinical and Clinical Evidence. Front. Psychiatry 2022, 13, 1038122. [Google Scholar] [CrossRef] [PubMed]
- Paterniti, I.; Cordaro, M.; Campolo, M.; Siracusa, R.; Cornelius, C.; Navarra, M.; Cuzzocrea, S.; Esposito, E. Neuroprotection by Association of Palmitoylethanolamide with Luteolin in Experimental Alzheimer’s Disease Models: The Control of Neuroinflammation. CNS Neurol. Disord. Drug Targets 2014, 13, 1530–1541. [Google Scholar] [CrossRef]
- Dehghani, F.; Abdollahi, S.; Shidfar, F.; Clark, C.C.T.; Soltani, S. Probiotics Supplementation and Brain-Derived Neurotrophic Factor (BDNF): A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutr. Neurosci. 2023, 26, 942–952. [Google Scholar] [CrossRef]
- Ammar, A.; Trabelsi, K.; Boukhris, O.; Bouaziz, B.; Müller, P.; Glenn, J.M.; Chamari, K.; Müller, N.; Chtourou, H.; Driss, T.; et al. Moderators of the Impact of (Poly)Phenols Interventions on Psychomotor Functions and BDNF: Insights from Subgroup Analysis and Meta-Regression. Nutrients 2020, 12, 2872. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Jaiswal, V.; Lee, H.J. Dietary Intake of Flavonoids and Carotenoids Is Associated with Anti-Depressive Symptoms: Epidemiological Study and In Silico—Mechanism Analysis. Antioxidants 2022, 11, 53. [Google Scholar] [CrossRef] [PubMed]
- Gravesteijn, E.; Mensink, R.P.; Plat, J. Effects of Nutritional Interventions on BDNF Concentrations in Humans: A Systematic Review. Nutr. Neurosci. 2022, 25, 1425–1436. [Google Scholar] [CrossRef]
- Stringham, N.T.; Holmes, P.V.; Stringham, J.M. Effects of Macular Xanthophyll Supplementation on Brain-Derived Neurotrophic Factor, pro-Inflammatory Cytokines, and Cognitive Performance. Physiol. Behav. 2019, 211, 112650. [Google Scholar] [CrossRef]
- Angoa-Pérez, M.; Anneken, J.H.; Kuhn, D.M. The Role of Brain-Derived Neurotrophic Factor in the Pathophysiology of Psychiatric and Neurological Disorders. J. Psychiatry Psychiatr. Disord. 2017, 1, 252. [Google Scholar] [CrossRef]
- Keservani, R.K.; Sharma, A.K.; Kesharwani, R.K. Medicinal Effect of Nutraceutical Fruits for the Cognition and Brain Health. Scientifica 2016, 2016, 3109254. [Google Scholar] [CrossRef]
- Zhang, L.; Fang, Y.; Xu, Y.; Lian, Y.; Xie, N.; Wu, T.; Zhang, H.; Sun, L.; Zhang, R.; Wang, Z. Curcumin Improves Amyloid β-Peptide (1–42) Induced Spatial Memory Deficits through BDNF-ERK Signaling Pathway. PLoS ONE 2015, 10, e0131525. [Google Scholar] [CrossRef]
- Martin, A.; Stillman, J.; Miguez, M.-J.; McDaniel, H.R.; Konefal, J.; Woolger, J.M.; Lewis, J.E. The Effect of Dietary Supplementation on Brain-Derived Neurotrophic Factor and Cognitive Functioning in Alzheimer’s Dementia. J. Clin. Transl. Res. 2018, 3, 337. [Google Scholar] [CrossRef]
- Rahmati-Ahmadabad, S.; Azarbayjani, M.A.; Broom, D.R.; Nasehi, M. Effects of High-Intensity Interval Training and Flaxseed Oil Supplement on Learning, Memory and Immobility: Relationship with BDNF and TrkB Genes. Comp. Exerc. Physiol. 2020, 17, 273–283. [Google Scholar] [CrossRef]
- Mizoguchi, Y.; Yao, H.; Imamura, Y.; Hashimoto, M.; Monji, A. Lower Brain-Derived Neurotrophic Factor Levels Are Associated with Age-Related Memory Impairment in Community-Dwelling Older Adults: The Sefuri Study. Sci. Rep. 2020, 10, 16442. [Google Scholar] [CrossRef]
- Siuda, J.; Patalong-Ogiewa, M.; Żmuda, W.; Targosz-Gajniak, M.; Niewiadomska, E.; Matuszek, I.; Jędrzejowska-Szypułka, H.; Rudzińska-Bar, M. Cognitive Impairment and BDNF Serum Levels. Neurol. Neurochir. Pol. 2017, 51, 24–32. [Google Scholar] [CrossRef]
- Yurko-Mauro, K.; McCarthy, D.; Rom, D.; Nelson, E.B.; Ryan, A.S.; Blackwell, A.; Salem, N.; Stedman, M. Beneficial Effects of Docosahexaenoic Acid on Cognition in Age-Related Cognitive Decline. Alzheimers Dement. 2010, 6, 456–464. [Google Scholar] [CrossRef]



| Endpoint Tested | Attention and Psychomotor Speed | Memory | Executive Function |
|---|---|---|---|
| Test | Rapid Visual Information Processing (RVP) | Paired Associates Learning (PAL) | Spatial Working Memory (SWM) |
| Outcome Measure | RVPA: The sensitivity to the target regardless of response tendency. RVPMDL: The median response latency on trials where the subject responded correctly. Calculated across all assessed trials. RVPFA: The number of sequence presentations that were false alarms divided by the number of sequence presentations that were false alarms plus the number of sequence presentations that were correct rejections: (false alarms ÷ (false alarms + correct rejections)). | PALFAMS: The frequency with which participants chose the correct box on their first attempt. PALTEA: The number of trials required to locate the pattern(s) correctly and the memory scores and stages completed. | SWMS: The possibility of the participant using a certain searching strategy. SWMBE: The number of times an individual incorrectly revisited an emptied box. |
| Task Format | A white box was shown in the centre of the screen, inside which digits from 2 to 9 appeared in a pseudo-random order, at the rate of 100 digits per minute. Participants were requested to detect target sequences of digits (for example, 2-4-6, 3-5-7, 4-6-8). When the participant saw the target sequence, they had to respond by selecting the button in the centre of the screen as quickly as possible. The level of difficulty varied with either one or three target sequences that the participant had to watch for at the same time. | Boxes were displayed on the screen and were “opened” in a randomised order. One or more of them contained a pattern. The patterns were then displayed in the middle of the screen, one at a time, and the participant had to select the box in which the pattern was originally located. If the participant made an error, the boxes were opened in sequence again to remind the participant of the locations of the patterns. Increased difficulty levels were used to test high-functioning, healthy individuals. | The test began with several coloured squares (boxes) shown on the screen. The aim of this test was that, by selecting the boxes and using a process of elimination, the participant should find one yellow ‘token’ in each of several boxes and use it to fill up an empty column on the right-hand side of the screen. Depending on the difficulty level used for this test, the number of boxes could be gradually increased until a maximum of 12 boxes were shown for the participants to search. The colour and position of the boxes used were changed from trial to trial to discourage the use of stereotyped search strategies. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, N.; Parolin, B.; Renshaw, D.; Deb, S.K.; Zariwala, M.G. Formulated Palmitoylethanolamide Supplementation Improves Parameters of Cognitive Function and BDNF Levels in Young, Healthy Adults: A Randomised Cross-Over Trial. Nutrients 2024, 16, 489. https://doi.org/10.3390/nu16040489
Kim N, Parolin B, Renshaw D, Deb SK, Zariwala MG. Formulated Palmitoylethanolamide Supplementation Improves Parameters of Cognitive Function and BDNF Levels in Young, Healthy Adults: A Randomised Cross-Over Trial. Nutrients. 2024; 16(4):489. https://doi.org/10.3390/nu16040489
Chicago/Turabian StyleKim, Nadia, Brenda Parolin, Derek Renshaw, Sanjoy K. Deb, and Mohammed Gulrez Zariwala. 2024. "Formulated Palmitoylethanolamide Supplementation Improves Parameters of Cognitive Function and BDNF Levels in Young, Healthy Adults: A Randomised Cross-Over Trial" Nutrients 16, no. 4: 489. https://doi.org/10.3390/nu16040489
APA StyleKim, N., Parolin, B., Renshaw, D., Deb, S. K., & Zariwala, M. G. (2024). Formulated Palmitoylethanolamide Supplementation Improves Parameters of Cognitive Function and BDNF Levels in Young, Healthy Adults: A Randomised Cross-Over Trial. Nutrients, 16(4), 489. https://doi.org/10.3390/nu16040489