The Protective Impact of Aronia melanocarpa L. Berries Extract against Prooxidative Cadmium Action in the Brain—A Study in an In Vivo Model of Current Environmental Human Exposure to This Harmful Element
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cadmium in the Diet
2.2. The Extract from A. melanocarpa Berries
2.3. Experimental Animals
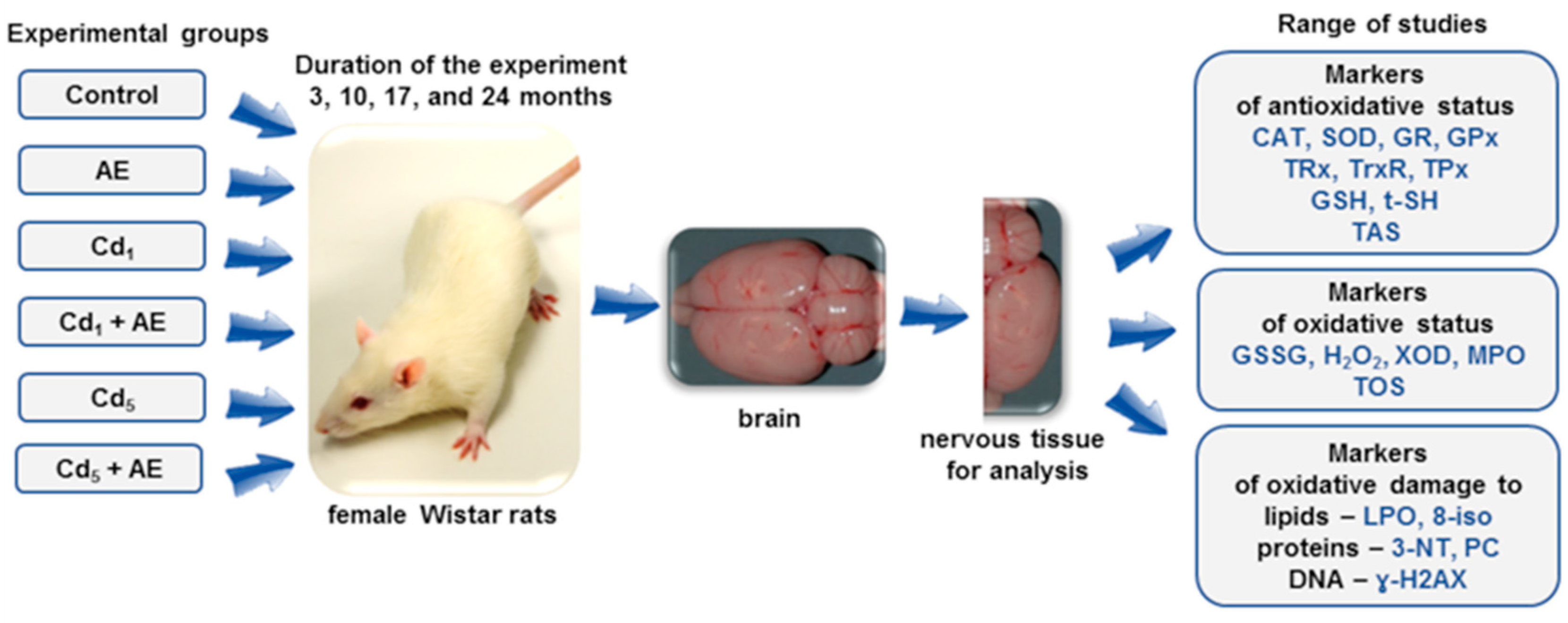
2.4. Design of the Study
2.5. Analytical Procedures
2.5.1. Preparation of the Brain Homogenates
2.5.2. Establishment of the Markers of Oxidative–Antioxidative Balance and the Extent of Oxidative Stress and Oxidative Damage to Macromolecules in the Brain
Antioxidative Enzymes
Indices of Trx-Dependent Antioxidative System
GSH, GSSG, and t-SH
TOS and TAS
H2O2, MPO, and XOD
Biomarkers of Oxidative Modifications of Lipids, Proteins, and DNA
2.5.3. Statistical Analysis
3. Results
3.1. Macroscopic Picture and Weight of the Brain
3.2. Antioxidative Barrier of the Brain
3.2.1. Antioxidative Enzymes (SOD, CAT, GPx, and GR)
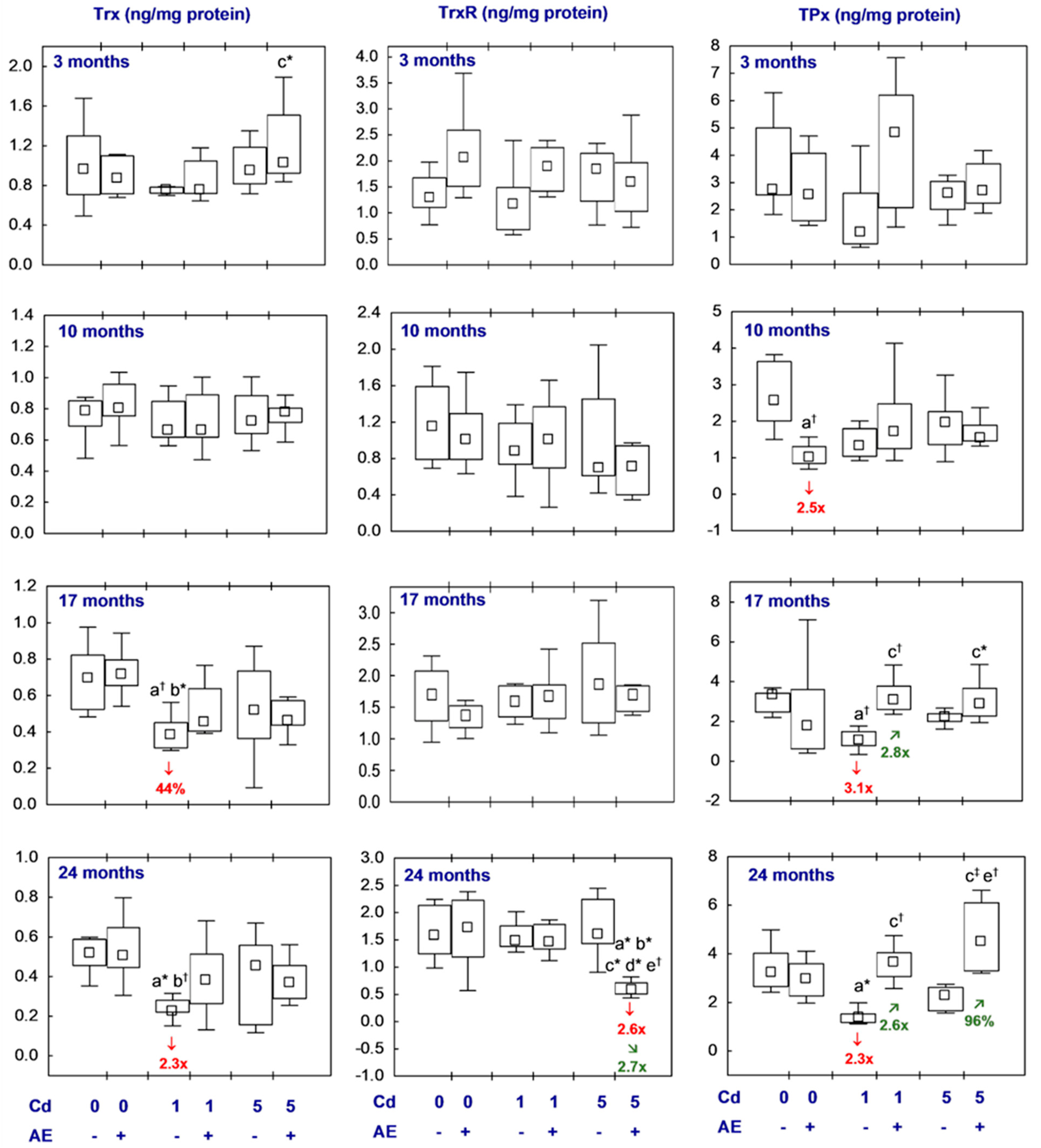
3.2.2. Trx-Dependent Antioxidative System
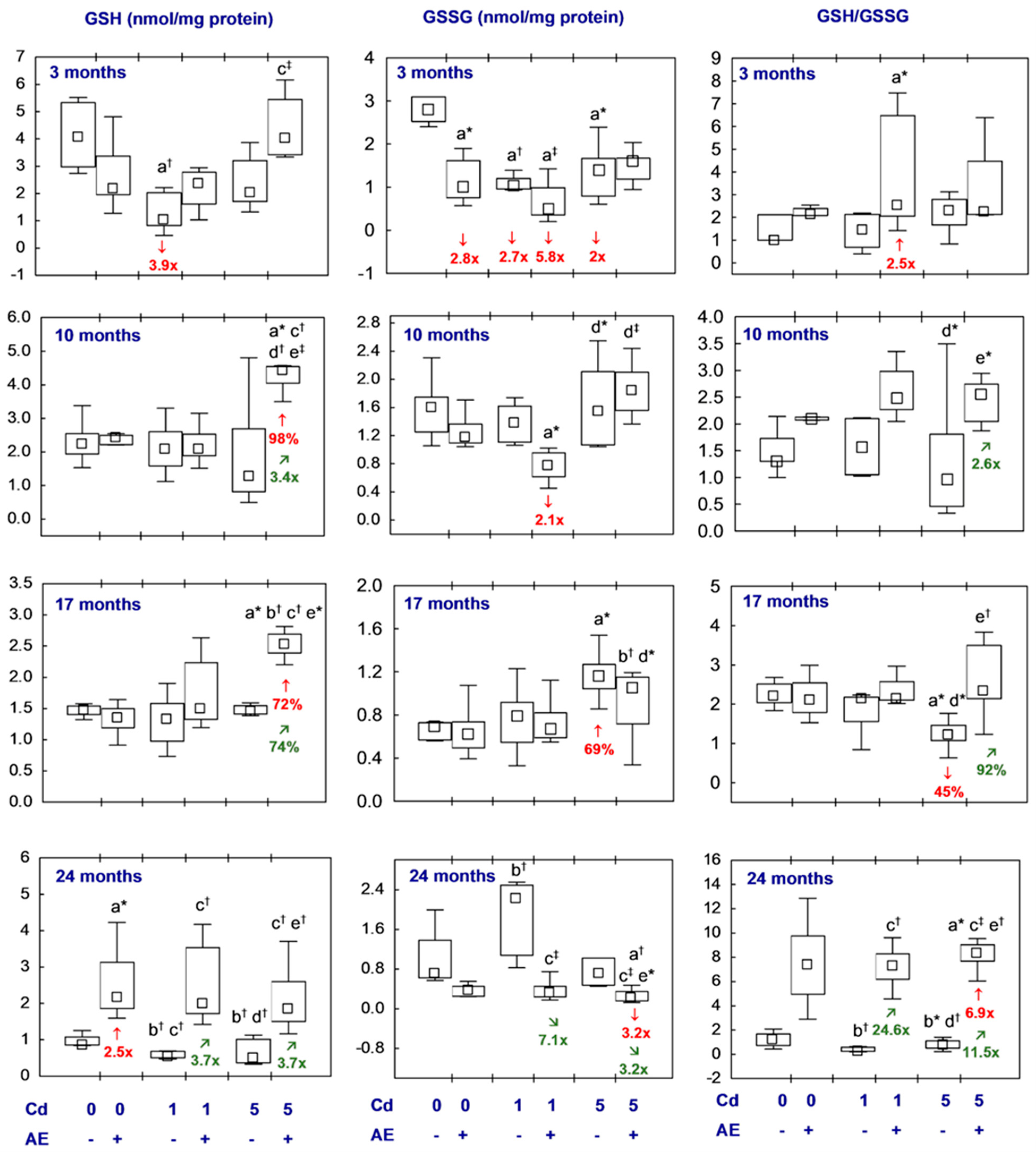
3.2.3. Glutathione Homeostasis
3.2.4. t-SH
3.3. TAS, TOS, and OSI in the Brain
3.4. Concentrations of H2O2, MPO, and XOD in the Brain
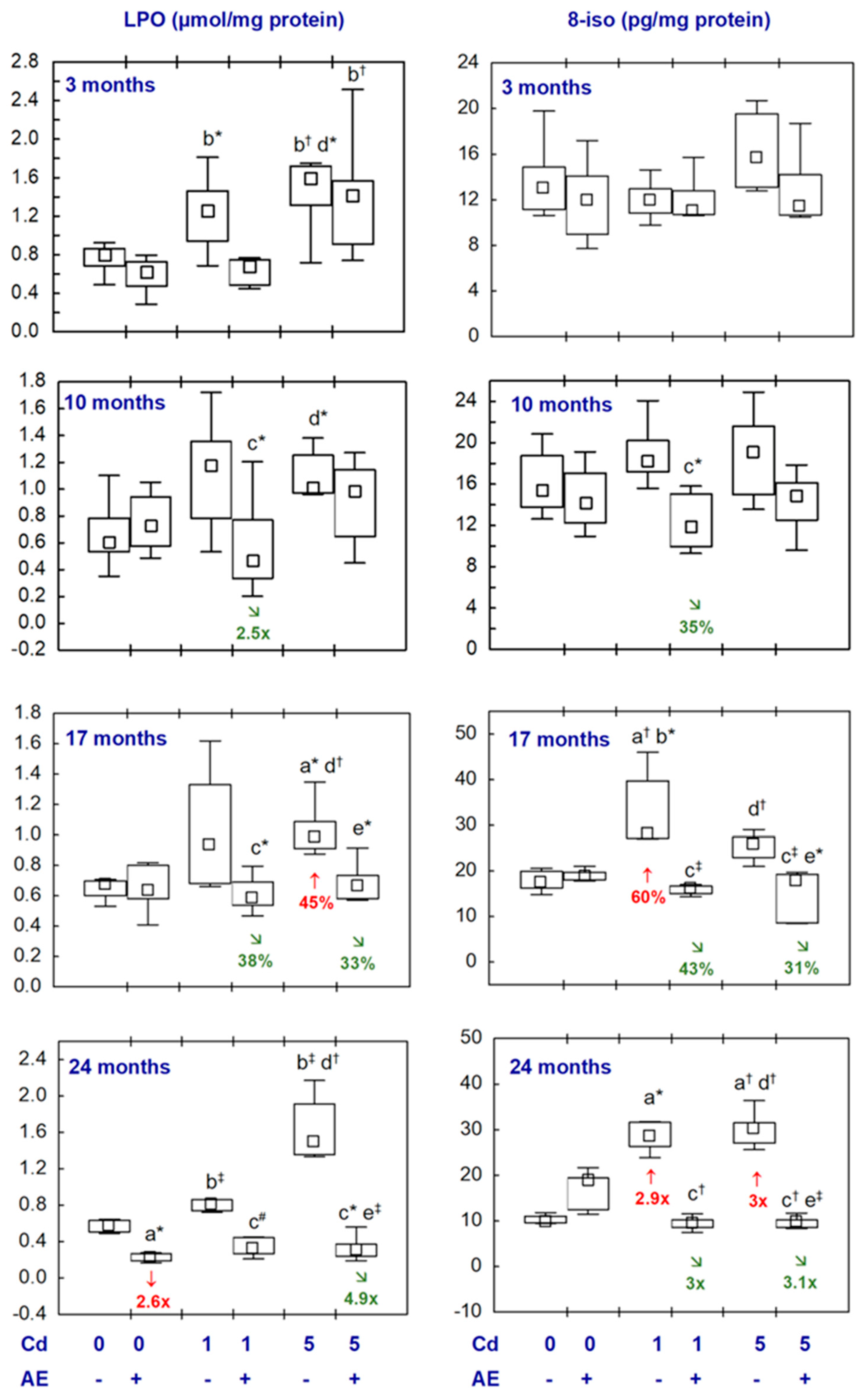
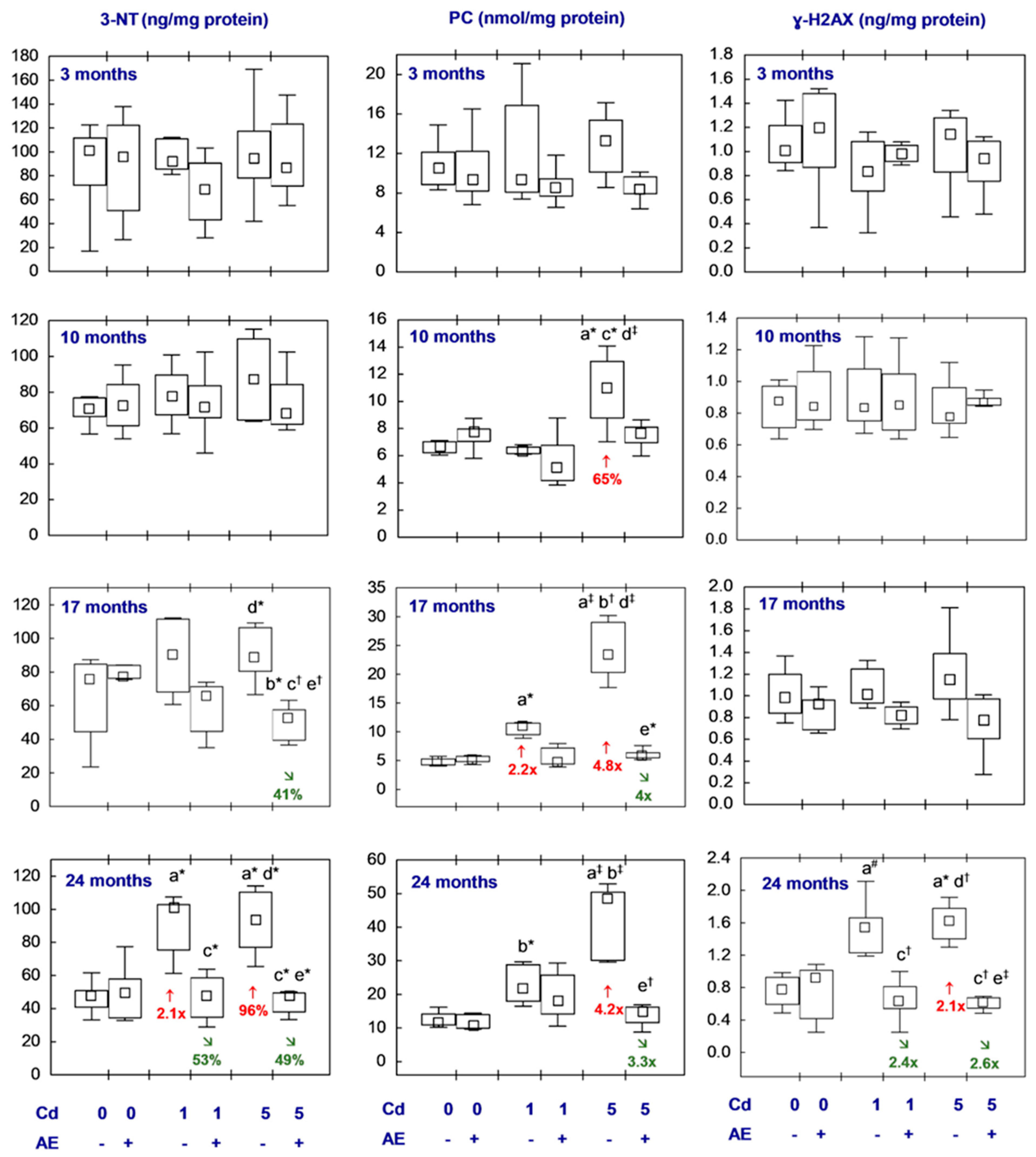
3.5. Biomarkers of Oxidative Damage to Lipids, Proteins, and DNA in the Brain
3.6. Relationships between the Examined Parameters in the Brain
3.7. Relationships between Investigated Parameters and Cadmium Concentration in the Brain, Blood, and Urine
4. Discussion
Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AE | Aronia melanocarpa L. berries extract |
| b.w. | body weight |
| CAT | catalase |
| Cd | cadmium |
| CV | coefficient of variation |
| DNA | deoxyribonucleic acid |
| ELISA | enzyme-linked immunosorbent assay |
| GPx | glutathione peroxidase |
| GR | glutathione reductase |
| GSH | reduced glutathione |
| GSSG | oxidized glutathione |
| GSH/GSSG | the ratio of reduced glutathione and oxidized glutathione |
| H2O2 | hydrogen peroxide |
| MDA | malondialdehyde |
| MnSOD | manganese-dependent superoxide dismutase |
| MPO | myeloperoxidase |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| LPO | lipid peroxides |
| OSI | oxidative stress index |
| PC | protein carbonyl groups |
| ROS | reactive oxygen species |
| SOD | superoxide dismutase |
| TAS | total antioxidative status |
| TOS | total oxidative status |
| TPx | thioredoxin peroxidase |
| Trx | thioredoxin |
| TrxR | thioredoxin reductase |
| t-SH | total thiol groups |
| XOD | xanthine oxidase |
| ɣ-H2AX | ɣ-H2A histone family member X |
| 3-NT | 3-nitrotyrosine |
| 8-iso | 8-isoprostane |
References
- WHO. The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 11 October 2023).
- Min, J.Y.; Min, K.B. Blood cadmium levels and Alzheimer’s disease mortality risk in older US adults. Environ. Health 2016, 15, 69. [Google Scholar] [CrossRef] [PubMed]
- Kincaid, H.J.; Nagpal, R.; Yadav, H. Diet-microbiota-brain axis in Alzheimer’s disease. Ann. Nutr. Metab. 2021, 77 (Suppl. S2), 21–27. [Google Scholar] [CrossRef]
- Mir, R.H.; Sawhney, G.; Pottoo, F.H.; Mohi-Ud-Din, R.; Madishetti, S.; Jachak, S.M.; Ahmed, Z.; Masoodi, M.H. Role of environmental pollutants in Alzheimer’s disease: A review. Environ. Sci. Pollut. Res. Int. 2020, 27, 44724–44742. [Google Scholar] [CrossRef]
- Ruczaj, A.; Brzóska, M.M. Environmental exposure of the general population to cadmium as a risk factor of the damage to the nervous system: A critical review of current data. J. Appl. Toxicol. 2023, 43, 66–88. [Google Scholar] [CrossRef]
- Stefaniak, O.; Dobrzyńska, M.; Drzymała-Czyż, S.; Przysławski, J. Diet in the prevention of Alzheimer’s disease: Current knowledge and future research requirements. Nutrients 2022, 14, 4564. [Google Scholar] [CrossRef]
- Ren, Y.; Frank, T.; Meyer, G.; Lei, J.; Grebenc, J.R.; Slaughter, R.; Gao, Y.G.; Kinghorn, A.D. Potential benefits of black chokeberry (Aronia melanocarpa) fruits and their constituents in improving human health. Molecules 2022, 27, 7823. [Google Scholar] [CrossRef]
- Calella, P.; Di Dio, M.; Cerullo, G.; Di Onofrio, V.; Gallé, F.; Liguori, G. Antioxidant, immunomodulatory, and anti-inflammatory effects of Spirulina in disease conditions: A systematic review. Int. J. Food Sci. Nutr. 2022, 73, 1047–1056. [Google Scholar] [CrossRef]
- Torres-Mendoza, B.M.G.; Ortiz, G.G.; Sánchez-Romero, L.; Delgado-Lara, D.L.C.; García Martínez, M.T.; Mireles-Ramírez, M.A.; Cruz Serrano, J.A.; Pacheco Moisés, F.P. Dietary fish oil increases catalase activity in patients with probable Alzheimer’s disease. Nutr. Hosp. 2022, 39, 1364–1368. [Google Scholar] [CrossRef]
- Garza-Lombó, C.; Posadas, Y.; Quintanar, L.; Gonsebatt, M.E.; Franco, R. Neurotoxicity linked to dysfunctional metal ion homeostasis and xenobiotic metal exposure: Redox signaling and oxidative stress. Antioxid. Redox Signal. 2018, 28, 1669–1703. [Google Scholar] [CrossRef]
- Nordberg, G.F.; Bernard, A.; Diamond, G.L.; Duffus, J.H.; Illing, P.; Nordberg, M.; Bergdahl, I.; Jin, T.; Skerfving, S. Risk assessment of effects of cadmium on human health (IUPAC Technical Report). Pure App. Chem. 2018, 90, 755–808. [Google Scholar] [CrossRef]
- Peng, Q.; Bakulski, K.M.; Nan, B.; Park, S.K. Cadmium and Alzheimer’s disease mortality in U.S. adults: Updated evidence with a urinary biomarker and extended follow-up time. Environ. Res. 2017, 157, 44–51. [Google Scholar] [CrossRef]
- Sola, E.; Moyano, P.; Flores, A.; García, J.M.; García, J.; Anadon, M.J.; Frejo, M.T.; Pelayo, A.; de la Cabeza Fernandez, M.; Del Pino, J. Cadmium-promoted thyroid hormones disruption mediates ROS, inflammation, Aβ and Tau proteins production, gliosis, spongiosis and neurodegeneration in rat basal forebrain. Chem. Biol. Interact. 2023, 375, 110428. [Google Scholar] [CrossRef]
- Martins, A.C.; Urbano, M.R.; Almeida Lopes, A.C.B.; Carvalho, M.F.H.; Buzzo, M.L.; Docea, A.O.; Mesas, A.E.; Aschner, M.; Silva, A.M.R.; Silbergeld, E.K.; et al. Blood cadmium levels and sources of exposure in an adult urban population in southern Brazil. Environ. Res. 2020, 187, 109618. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aleem, G.A.; Khaleel, E.F. Rutin hydrate ameliorates cadmium chloride-induced spatial memory loss and neural apoptosis in rats by enhancing levels of acetylcholine, inhibiting JNK and ERK1/2 activation and activating mTOR signalling. Arch. Physiol. Biochem. 2018, 124, 367–377. [Google Scholar] [CrossRef] [PubMed]
- El-Kott, A.F.; Bin-Meferij, M.M.; Eleawa, S.M.; Alshehri, M.M. Kaempferol protects against cadmium chloride-induced memory loss and hippocampal apoptosis by increased intracellular glutathione stores and activation of PTEN/AMPK induced inhibition of Akt/mTOR signaling. Neurochem. Res. 2020, 45, 295–309. [Google Scholar] [CrossRef]
- Namgyal, D.; Ali, S.; Mehta, R.; Sarwat, M. The neuroprotective effect of curcumin against Cd-induced neurotoxicity and hippocampal neurogenesis promotion through CREB-BDNF signaling pathway. Toxicology 2020, 442, 152542. [Google Scholar] [CrossRef]
- Qu, Y.; Liu, Y.; Chen, L.; Zhu, Y.; Xiao, X.; Wang, D.; Zhu, Y. Nobiletin prevents cadmium-induced neuronal apoptosis by inhibiting reactive oxygen species and modulating JNK/ERK1/2 and Akt/mTOR networks in rats. Neurol. Res. 2018, 40, 211–220. [Google Scholar] [CrossRef]
- Shati, A.A. Resveratrol protects against cadmium chloride-induced hippocampal neurotoxicity by inhibiting ER stress and GAAD 153 and activating sirtuin 1/AMPK/Akt. Environ. Toxicol. 2019, 34, 1340–1353. [Google Scholar] [CrossRef]
- Al Omairi, N.E.; Radwan, O.K.; Alzahrani, Y.A.; Kassab, R.B. Neuroprotective efficiency of Mangifera indica leaves extract on cadmium-induced cortical damage in rats. Metab. Brain Dis. 2018, 33, 1121–1130. [Google Scholar] [CrossRef]
- Chouit, Z.; Djellal, D.; Haddad, S.; Hanfer, M.; Hachemi, M.; Lakroun, Z.; Chafaa, S.; Fetoui, H.; Kebieche, M.; Soulimani, R. Potentiation of the apoptotic signaling pathway in both the striatum and hippocampus and neurobehavioral impairment in rats exposed chronically to a low-dose of cadmium. Environ. Sci. Pollut. Res. 2021, 28, 3307–3317. [Google Scholar] [CrossRef]
- Elkhadragy, M.F.; Kassab, R.B.; Metwally, D.; Almeer, R.S.; Abdel-Gaber, R.; Al-Olayan, E.M.; Essawy, E.A.; Amin, H.K.; Abdel Moneim, A.E. Protective effects of Fragaria ananassa methanolic extract in a rat model of cadmium chloride-induced neurotoxicity. Biosci. Rep. 2018, 38, BSR20180861. [Google Scholar] [CrossRef]
- El-Tarras, A.; Attia, H.F.; Soliman, M.M.; El Awady, M.A.; Amin, A.A. Neuroprotective effect of grape seed extract against cadmium toxicity in male albino rats. Int. J. Immunopathol. Pharmacol. 2016, 29, 398–407. [Google Scholar] [CrossRef]
- Fadaei, H.; Mirhosseini Ardakani, S.; Faraj Zadeh, A.; Aghayan, S.S.; Jafarisani, M.; Garmabi, B. Aqueous-alcoholic Ferulla extract reduces memory impairments in rats exposed to cadmium chloride. Brain Behav. 2021, 11, e2285. [Google Scholar] [CrossRef]
- Gupta, R.; Shukla, R.K.; Chandravanshi, L.P.; Srivastava, P.; Dhuriya, Y.K.; Shanker, J.; Singh, M.P.; Pant, A.B.; Khanna, V.K. Protective role of quercetin in cadmium-induced cholinergic dysfunctions in rat brain by modulating mitochondrial integrity and MAP kinase signaling. Mol. Neurobiol. 2017, 54, 4560–4583. [Google Scholar] [CrossRef]
- Maodaa, S.N.; Allam, A.A.; Ajarem, J.; Abdel-Maksoud, M.A.; Al-Basher, G.I.; Wang, Z.Y. Effect of parsley (Petroselinum crispum, Apiaceae) juice against cadmium neurotoxicity in albino mice (Mus musculus). Behav. Brain Funct. 2016, 12, 6. [Google Scholar] [CrossRef]
- Montes, S.; Juárez-Rebollar, D.; Nava-Ruíz, C.; Sánchez-García, A.; Heras-Romero, Y.; Rios, C.; Méndez-Armenta, M. Immunohistochemical study of Nrf2-antioxidant response element as indicator of oxidative stress induced by cadmium in developing rats. Oxid. Med. Cell Longev. 2015, 2015, 570650. [Google Scholar] [CrossRef]
- Adefegha, S.A.; Oboh, G.; Omojokun, O.S.; Adefegha, O.M. Alterations of Na+/K+-ATPase, cholinergic and antioxidant enzymes activity by protocatechuic acid in cadmium-induced neurotoxicity and oxidative stress in Wistar rats. Biomed. Pharmacother. 2016, 83, 559–568. [Google Scholar] [CrossRef]
- Al-Brakati, A.; Albarakati, A.; Lokman, M.S.; Theyab, A.; Algahtani, M.; Menshawi, S.; AlAmri, O.D.; Al Omairi, N.E.; Essawy, E.A.; Kassab, R.B.; et al. Possible role of kaempferol in reversing oxidative damage, inflammation, and apoptosis-mediated cortical injury following cadmium exposure. Neurotox. Res. 2021, 39, 198–209. [Google Scholar] [CrossRef]
- Allam, A.A.; Maodaa, S.N.; Abo-Eleneen, R.; Ajarem, J. Protective effect of parsley juice (Petroselinum crispum, Apiaceae) against cadmium deleterious changes in the developed albino mice newborns (Mus musculus). Oxid. Med. Cell Longev. 2016, 2016, 2646840. [Google Scholar] [CrossRef]
- Shagirtha, K.; Bashir, N.; Milton Prabu, S. Neuroprotective efficacy of hesperetin against cadmium induced oxidative stress in the brain of rats. Toxicol. Ind. Health 2017, 33, 454–468. [Google Scholar] [CrossRef]
- Shati, A.A.; Alfaifi, M.Y. Trans-resveratrol inhibits tau phosphorylation in the brains of control and cadmium chloride-treated rats by activating PP2A and PI3K/Akt induced-inhibition of GSK3β. Neurochem. Res. 2019, 44, 357–373. [Google Scholar] [CrossRef]
- Brzóska, M.M.; Kozłowska, M.; Rogalska, J.; Gałażyn-Sidorczuk, M.; Roszczenko, A.; Smereczański, N.M. Enhanced zinc intake protects against oxidative stress and its consequences in the brain: A study in an in vivo rat model of cadmium exposure. Nutrients 2021, 13, 478. [Google Scholar] [CrossRef]
- Branca, J.J.V.; Fiorillo, C.; Carrino, D.; Paternostro, F.; Taddei, N.; Gulisano, M.; Pacini, A.; Becatti, M. Cadmium-induced oxidative stress: Focus on the central nervous system. Antioxidants 2020, 9, 492. [Google Scholar] [CrossRef]
- Dionísio, P.A.; Amaral, J.D.; Rodrigues, C.M.P. Oxidative stress and regulated cell death in Parkinson’s disease. Ageing Res. Rev. 2021, 67, 101263. [Google Scholar] [CrossRef]
- Ionescu-Tucker, A.; Cotman, C.W. Emerging roles of oxidative stress in brain aging and Alzheimer’s disease. Neurobiol. Aging 2021, 107, 86–95. [Google Scholar] [CrossRef]
- Schaefer, H.R.; Flannery, B.M.; Crosby, L.; Jones-Dominic, O.E.; Punzalan, C.; Middleton, K. A systematic review of adverse health effects associated with oral cadmium exposure. Regul. Toxicol. Pharmacol. 2022, 134, 105243. [Google Scholar] [CrossRef]
- Cobley, J.N.; Fiorello, M.L.; Bailey, D.M. 13 reasons why the brain is susceptible to oxidative stress. Redox Biol. 2018, 15, 490–503. [Google Scholar] [CrossRef]
- Brzóska, M.M.; Gałażyn-Sidorczuk, M.; Jurczuk, M.; Tomczyk, M. Protective effect of Aronia melanocarpa polyphenols on cadmium accumulation in the body: A study in a rat model of human exposure to this metal. Curr. Drug Targets 2015, 16, 1470–1487. [Google Scholar] [CrossRef]
- Brzóska, M.M.; Gałażyn-Sidorczuk, M.; Kozłowska, M.; Smereczański, N.M. The body status of manganese and activity of this element-dependent mitochondrial superoxide dismutase in a rat model of human exposure to cadmium and co-administration of Aronia melanocarpa L. extract. Nutrients 2022, 14, 4773. [Google Scholar] [CrossRef]
- Brzóska, M.M.; Rogalska, J.; Gałażyn-Sidorczuk, M.; Jurczuk, M.; Roszczenko, A.; Tomczyk, M. Protective effect of Aronia melanocarpa polyphenols against cadmium-induced disorders in bone metabolism: A study in a rat model of lifetime human exposure to this heavy metal. Chem. Biol. Interact. 2015, 229, 132–146. [Google Scholar] [CrossRef]
- Brzóska, M.M.; Rogalska, J.; Roszczenko, A.; Gałażyn-Sidorczuk, M.; Tomczyk, M. The mechanism of the osteoprotective action of a polyphenol-rich Aronia melanocarpa extract during chronic exposure to cadmium is mediated by the oxidative defense system. Planta Med. 2016, 82, 621–631. [Google Scholar] [CrossRef]
- Mężyńska, M.; Brzóska, M.M.; Rogalska, J.; Galicka, A. Extract from Aronia melanocarpa L. berries protects against cadmium-induced lipid peroxidation and oxidative damage to proteins and DNA in the liver: A study in a rat model of environmental human exposure to this xenobiotic. Nutrients 2019, 11, 758. [Google Scholar] [CrossRef]
- Mężyńska, M.; Brzóska, M.M.; Rogalska, J.; Piłat-Marcinkiewicz, B. Extract from Aronia melanocarpa L. berries prevents cadmium-induced oxidative stress in the liver: A study in a rat model of low-level and moderate lifetime human exposure to this toxic metal. Nutrients 2019, 11, 21. [Google Scholar] [CrossRef]
- Smereczański, N.M.; Brzóska, M.M.; Rogalska, J. Protective effect of the extract from Aronia melanocarpa L. berries against cadmium-induced oxidative stress in the kidney: A study in an in vivo experimental model. Acta Pol. Pharm. 2023, 80, 627–648. [Google Scholar] [CrossRef]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef]
- Olechno, E.; Puścion-Jakubik, A.; Zujko, M.E. Chokeberry (A. melanocarpa (Michx.) Elliott)—A natural product for metabolic disorders? Nutrients 2022, 14, 2688. [Google Scholar] [CrossRef]
- Sidor, A.; Gramza-Michałowska, A. Black chokeberry Aronia melanocarpa L.—A qualitative composition, phenolic profile and antioxidant potential. Molecules 2019, 24, 3710. [Google Scholar] [CrossRef]
- Denev, P.; Číž, M.; Kratchanova, M.; Blazheva, D. Black chokeberry (Aronia melanocarpa) polyphenols reveal different antioxidant, antimicrobial and neutrophil-modulating activities. Food Chem. 2019, 284, 108–117. [Google Scholar] [CrossRef]
- Dąbrowska, Z.; Dąbrowska, E.; Onopiuk, B.; Onopiuk, P.; Orywal, K.; Mroczko, B.; Pieruska, M. The protective impact of black chokeberry fruit extract (Aronia melanocarpa L.) on the oxidoreductive system of the parotid gland of rats exposed to cadmium. Oxid. Med. Cell Longev. 2019, 2019, 3403264. [Google Scholar] [CrossRef]
- Snoj Tratnik, J.; Falnoga, I.; Mazej, D.; Kocman, D.; Fajon, V.; Jagodic, M.; Stajnko, A.; Trdin, A.; Šlejkovec, Z.; Jeran, Z.; et al. Results of the first national human biomonitoring in Slovenia: Trace elements in men and lactating women, predictors of exposure and reference values. Int. J. Hyg. Environ. Health 2019, 222, 563–582. [Google Scholar] [CrossRef] [PubMed]
- Del Bo’, C.; Bernardi, S.; Marino, M.; Porrini, M.; Tucci, M.; Guglielmetti, S.; Cherubini, A.; Carrieri, B.; Kirkup, B.; Kroon, P.; et al. Systematic review on polyphenol intake and health outcomes: Is there sufficient evidence to define a health-promoting polyphenol-rich dietary pattern? Nutrients 2019, 11, 1355. [Google Scholar] [CrossRef]
- Toro-Román, V.; Robles-Gil, M.C.; Muñoz, D.; Bartolomé, I.; Grijota, F.J.; Maynar-Mariño, M. Sex differences in cadmium and lead concentrations in different biological matrices in athletes. Relationship with iron status. Environ. Toxicol. Pharmacol. 2023, 99, 104107. [Google Scholar] [CrossRef]
- Andreollo, N.A.; Santos, E.F.; Araújo, M.R.; Lopes, L.R. Rat’s age versus human’s age: What is the relationship? Arq. Bras. Cir. Dig. 2012, 25, 49–51. [Google Scholar] [CrossRef]
- Sengupta, P. The laboratory rat: Relating its age with human’s. Int. J. Prev. Med. 2013, 4, 624–630. [Google Scholar]
- Aebi, H.E. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Gawdi, R.; Shumway, K.R.; Emmady, P.D. Physiology, Blood Brain Barrier; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Szyguła-Jurkiewicz, B.; Szczurek-Wasilewicz, W.; Osadnik, T.; Frycz-Kurek, A.M.; Macioł-Skurk, K.; Małyszek-Tumidajewicz, J.; Skrzypek, M.; Romuk, E.; Gąsior, M.; Banach, M.; et al. Oxidative stress markers in hypertrophic cardiomyopathy. Medicina 2021, 58, 31. [Google Scholar] [CrossRef]
- Mostafa, D.G.; Khaleel, E.F.; Badi, R.M.; Abdel-Aleem, G.A.; Abdeen, H.M. Rutin hydrate inhibits apoptosis in the brains of cadmium chloride-treated rats via preserving the mitochondrial integrity and inhibiting endoplasmic reticulum stress. Neurol. Res. 2019, 41, 594–608. [Google Scholar] [CrossRef]
- Bjørklund, G.; Zou, L.; Peana, M.; Chasapis, C.T.; Hangan, T.; Lu, J.; Maes, M. The role of the thioredoxin system in brain diseases. Antioxidants 2022, 11, 2161. [Google Scholar] [CrossRef] [PubMed]
- Al-Sowayan, N.S.; Abdullah Almarzougi, R. Vitamin E reduces oxidative stress in brains of male albino male rats undergoing immobilization. Saudi J. Biol. Sci. 2024, 31, 103900. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Han, L.; Zhang, X.; Xie, L.; Pan, P.; Chen, F. Selenium alleviates cerebral ischemia/reperfusion injury by regulating oxidative stress, mitochondrial fusion and ferroptosis. Neurochem. Res. 2022, 47, 2992–3002. [Google Scholar] [CrossRef]
- Alonso-Garrido, M.; Frangiamone, M.; Font, G.; Cimbalo, A.; Manyes, L. In vitro blood brain barrier exposure to mycotoxins and carotenoids pumpkin extract alters mitochondrial gene expression and oxidative stress. Food Chem. Toxicol. 2021, 153, 112261. [Google Scholar] [CrossRef]
- Luís, Â.; Duarte, A.P.; Pereira, L.; Domingues, F. Interactions between the major bioactive polyphenols of berries: Effects on antioxidant properties. Eur. Food Res. Technol. 2017, 244, 175–185. [Google Scholar] [CrossRef]
- Fabre, G.; Bayach, I.; Berka, K.; Paloncýová, M.; Starok, M.; Rossi, C.; Duroux, J.L.; Otyepka, M.; Trouillas, P. Synergism of antioxidant action of vitamins E, C and quercetin is related to formation of molecular associations in biomembranes. Chem. Commun. 2015, 51, 7713–7716. [Google Scholar] [CrossRef]
- Zhang, L.; Guan, Q.; Jiang, J.; Khan, M.S. Tannin complexation with metal ions and its implication on human health, environment and industry: An overview. Int. J. Biol. Macromol. 2023, 253, 127485. [Google Scholar] [CrossRef]
- Kumar, G.; Paliwal, P.; Mukherjee, S.; Patnaik, N.; Krishnamurthy, S.; Patnaik, R. Pharmacokinetics and brain penetration study of chlorogenic acid in rats. Xenobiotica 2019, 49, 339–345. [Google Scholar] [CrossRef]
- Jin, Z.; Ke, J.; Guo, P.; Wang, Y.; Wu, H. Quercetin improves blood-brain barrier dysfunction in rats with cerebral ischemia reperfusion via Wnt signaling pathway. Am. J. Transl. Res. 2019, 11, 4683–4695. [Google Scholar]
- Zingg, J.M. Vitamin E: Regulatory role on signal transduction. IUBMB Life 2019, 71, 456–478. [Google Scholar] [CrossRef]





| Parameter | Regression Analysis | TAS | TOS | OSI | |||
|---|---|---|---|---|---|---|---|
| Without AE | With AE | Without AE | With AE | Without AE | With AE | ||
| SOD | βp R2 | NS | NS | −0.440 * 0.157 | NS | NS | NS |
| CAT | βp R2 | 0.725 ‡ 0.502 | NS | −0.780 ‡ 0.591 | NS | −0.500 * 0.214 | NS |
| GPx | βp R2 | 0.514 * 0.227 | NS | −0.650 ‡ 0.399 | NS | −0.470 * 0.187 | NS |
| GR | βp R2 | NS | 0.577 † 0.302 | 0.493 * 0.205 | NS | NS | NS |
| Trx | βp R2 | NS | NS | NS | NS | NS | NS |
| TrxR | βp R2 | NS | NS | NS | NS | NS | NS |
| TPx | βp R2 | 0.650 † 0.394 | NS | −0.510 * 0.220 | NS | NS | 0.502 * 0.216 |
| GSH | βp R2 | 0.471 * 0.182 | NS | −0.410 # 0.129 | NS | NS | NS |
| GSSG | βp R2 | NS | NS | NS | NS | NS | NS |
| GSH/GSSG | βp R2 | 0.424 * 0.138 | NS | NS | NS | NS | NS |
| t-SH | βp R2 | 0.742 ‡ 0.528 | NS | −0.735 ‡ 0.517 | NS | −0.505 * 0.218 | NS |
| H2O2 | βp R2 | NS | NS | 0.561 * 0.281 | −0.500 * 0.213 | NS | −0.520 † 0.237 |
| XOD | βp R2 | −0.480 * 0.193 | NS | 0.454 * 0.166 | NS | NS | NS |
| MPO | βp R2 | −0.740 ‡ 0.528 | NS | 0.687 ‡ 0.446 | NS | 0.488 * 0.200 | NS |
| LPO | βp R2 | −0.630 † 0.368 | NS | 0.743 ‡ 0.529 | NS | 0.676 ‡ 0.430 | NS |
| 8-iso | βp R2 | −0.740 ‡ 0.524 | NS | 0.767 ‡ 0.568 | NS | 0.516 * 0.230 | NS |
| 3-NT | βp R2 | −0.660 ‡ 0.401 | NS | 0.722 ‡ 0.497 | NS | NS | NS |
| PC | βp R2 | −0.450 * 0.163 | 0.473 * 0.187 | 0.765 ‡ 0.565 | NS | NS | NS |
| ɣ-H2AX | βp R2 | −0.590 † 0.320 | NS | 0.743 ‡ 0.530 | NS | NS | −0.430 * 0.144 |
| Parameter | Regression Analysis | Cd in the Brain | Cd in the Blood | Cd in the Urine | |||
|---|---|---|---|---|---|---|---|
| Without AE | With AE | Without AE | With AE | Without AE | With AE | ||
| SOD | βp R2 | −0.351 ‡ 0.114 | NS | −0.268 † 0.062 | NS | −0.283 † 0.070 | NS |
| CAT | βp R2 | −0.418 ‡ 0.166 | NS | −0.423 ‡ 0.170 | NS | −0.366 ‡ 0.125 | NS |
| GPx | βp R2 | −0.304 † 0.823 | NS | −0.433 ‡ 0.179 | NS | −0.351 ‡ 0.114 | NS |
| GR | βp R2 | NS | 0.407 ‡ 0.156 | NS | NS | NS | NS |
| Trx | βp R2 | NS | NS | NS | NS | NS | NS |
| TrxR | βp R2 | NS | NS | NS | −0.218 * 0.037 | NS | NS |
| TPx | βp R2 | NS | NS | NS | NS | NS | NS |
| GSH | βp R2 | NS | 0.346 ‡ 0.110 | NS | 0.389 ‡ 0.142 | NS | 0.399 ‡ 0.150 |
| GSSG | βp R2 | NS | NS | NS | 0.257 * 0.056 | NS | 0.255 * 0.055 |
| GSH/GSSG | βp R2 | NS | NS | NS | NS | NS | NS |
| t-SH | βp R2 | −0.273 † 0.064 | NS | −0.325 † 0.096 | 0.205 * 0.031 | −0.280 † 0.068 | NS |
| TAS | βp R2 | NS | NS | NS | NS | NS | NS |
| TOS | βp R2 | 0.363 ‡ 0.122 | 0.437 ‡ 0.182 | 0.513 ‡ 0.255 | 0.304 † 0.083 | 0.432 ‡ 0.178 | 0.387 ‡ 0.141 |
| OSI | βp R2 | 0.378 ‡ 0.134 | 0.384 ‡ 0.138 | 0.495 ‡ 0.237 | 0.338 ‡ 0.105 | 0.432 ‡ 0.178 | 0.432 ‡ 0.178 |
| H2O2 | βp R2 | NS | −0.413 ‡ 0.161 | NS | −0.461 ‡ 0.204 | NS | −0.462 ‡ 0.205 |
| XOD | βp R2 | 0.233 * 0.044 | NS | NS | NS | NS | NS |
| MPO | βp R2 | 0.299 † 0.795 | NS | 0.332 † 0.101 | NS | 0.353 ‡ 0.115 | NS |
| LPO | βp R2 | 0.555 ‡ 0.301 | 0.221 * 0.039 | 0.530 * 0.273 | 0.251 * 0.052 | 0.464 ‡ 0.207 | 0.398 ‡ 0.150 |
| 8-iso | βp R2 | NS | −0.328 † 0.098 | 0.316 † 0.090 | NS | 0.232 * 0.044 | NS |
| 3-NT | βp R2 | 0.213 * 0.035 | NS | 0.229 * 0.042 | NS | 0.229 * 0.042 | NS |
| PC | βp R2 | 0.364 ‡ 0.123 | NS | 0.538 ‡ 0.282 | NS | 0.429 ‡ 0.175 | NS |
| ɣ-H2AX | βp R2 | 0.272 † 0.064 | NS | 0.263 * 0.059 | −0.229 * 0.042 | NS | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruczaj, A.; Brzóska, M.M.; Rogalska, J. The Protective Impact of Aronia melanocarpa L. Berries Extract against Prooxidative Cadmium Action in the Brain—A Study in an In Vivo Model of Current Environmental Human Exposure to This Harmful Element. Nutrients 2024, 16, 502. https://doi.org/10.3390/nu16040502
Ruczaj A, Brzóska MM, Rogalska J. The Protective Impact of Aronia melanocarpa L. Berries Extract against Prooxidative Cadmium Action in the Brain—A Study in an In Vivo Model of Current Environmental Human Exposure to This Harmful Element. Nutrients. 2024; 16(4):502. https://doi.org/10.3390/nu16040502
Chicago/Turabian StyleRuczaj, Agnieszka, Małgorzata M. Brzóska, and Joanna Rogalska. 2024. "The Protective Impact of Aronia melanocarpa L. Berries Extract against Prooxidative Cadmium Action in the Brain—A Study in an In Vivo Model of Current Environmental Human Exposure to This Harmful Element" Nutrients 16, no. 4: 502. https://doi.org/10.3390/nu16040502
APA StyleRuczaj, A., Brzóska, M. M., & Rogalska, J. (2024). The Protective Impact of Aronia melanocarpa L. Berries Extract against Prooxidative Cadmium Action in the Brain—A Study in an In Vivo Model of Current Environmental Human Exposure to This Harmful Element. Nutrients, 16(4), 502. https://doi.org/10.3390/nu16040502






