Improved Cardiovascular Effects of a Novel Pomegranate Byproduct Extract Obtained through Hydrodynamic Cavitation
Abstract
1. Introduction
2. Materials and Methods
2.1. Manufacturing and Chemical Analysis of Pomegranate Extracts
2.1.1. Extraction of Whole Pomegranate Fruit
2.1.2. Extraction of Pomegranate Byproducts
2.1.3. Chemical Analysis of Pomegranate Extracts
2.2. Animals Procedures
2.2.1. In Vivo Acute Evaluation of the Anti-Hypertensive Effect
Data and Statistical Analysis
2.2.2. Pharmacokinetic Profile
2.2.3. Evaluation of Anti-Hypertensive Effects in Chronic Hypertension Rat Model
Endothelium Dysfunction
Histological Analysis
Evaluation of TGF-β1 and IL-6 Content
Data Analysis
3. Results
3.1. Ellagitannins Content of PFE and PPE
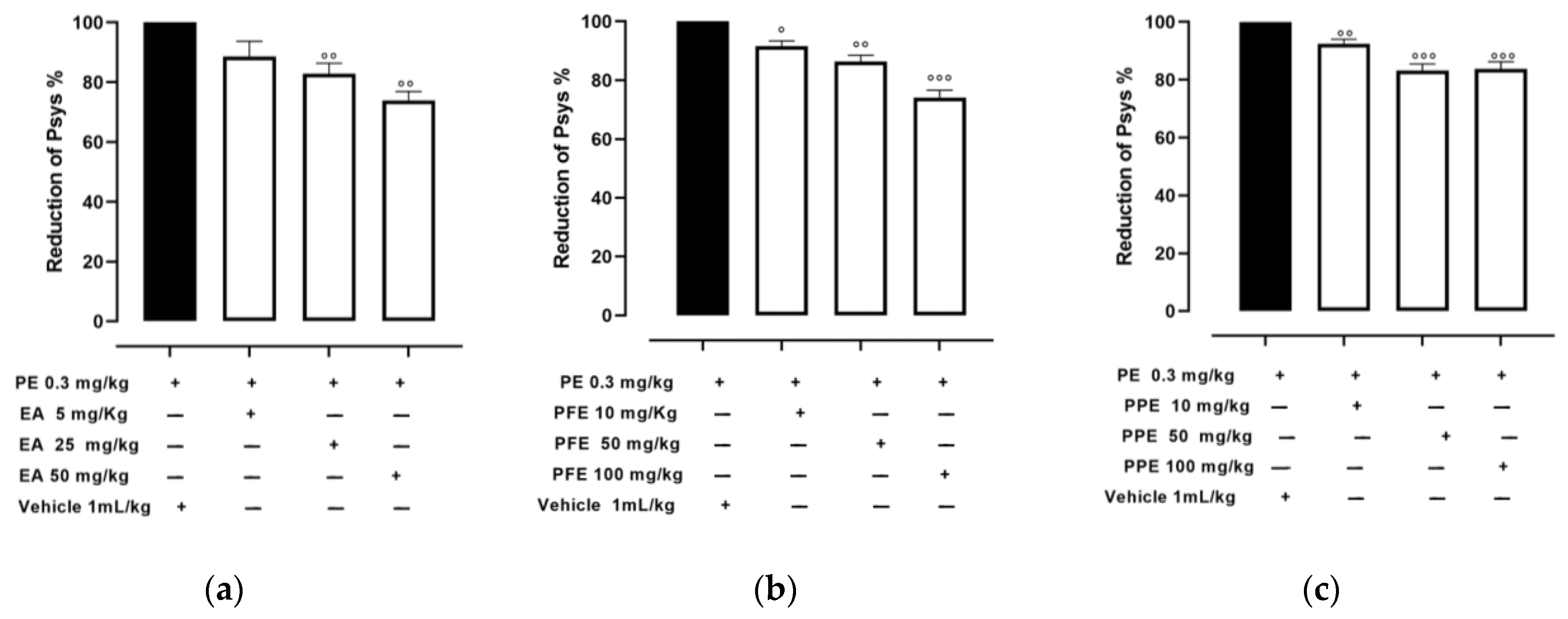
3.2. Effect of PPE and PFE on Psys in PE-Induced Hypertensive Rats
3.3. Pharmacokinetic Profile
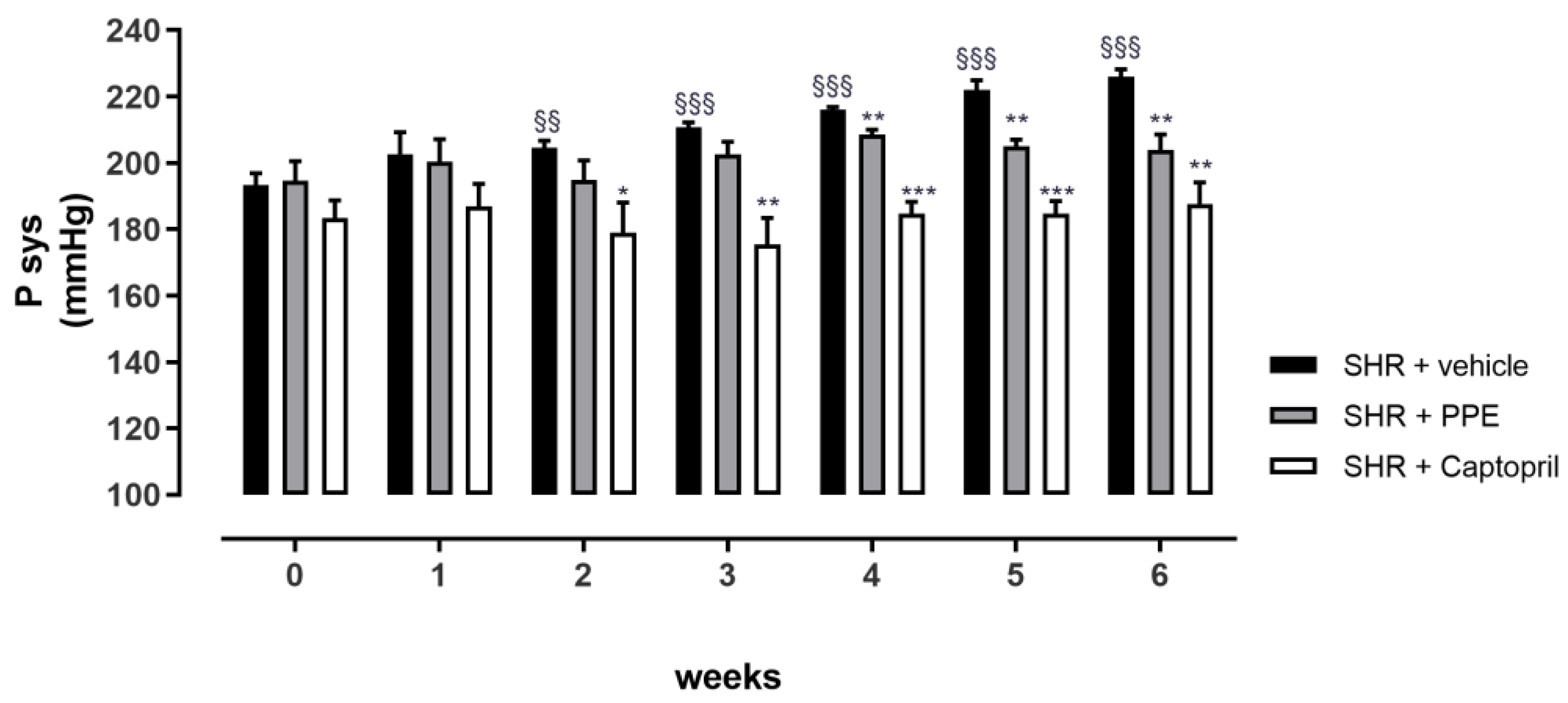
3.4. Anti-Hypertensive Effects of PPE Chronically Administered in SHR Rats
3.4.1. Glycemic and Lipidic Profile
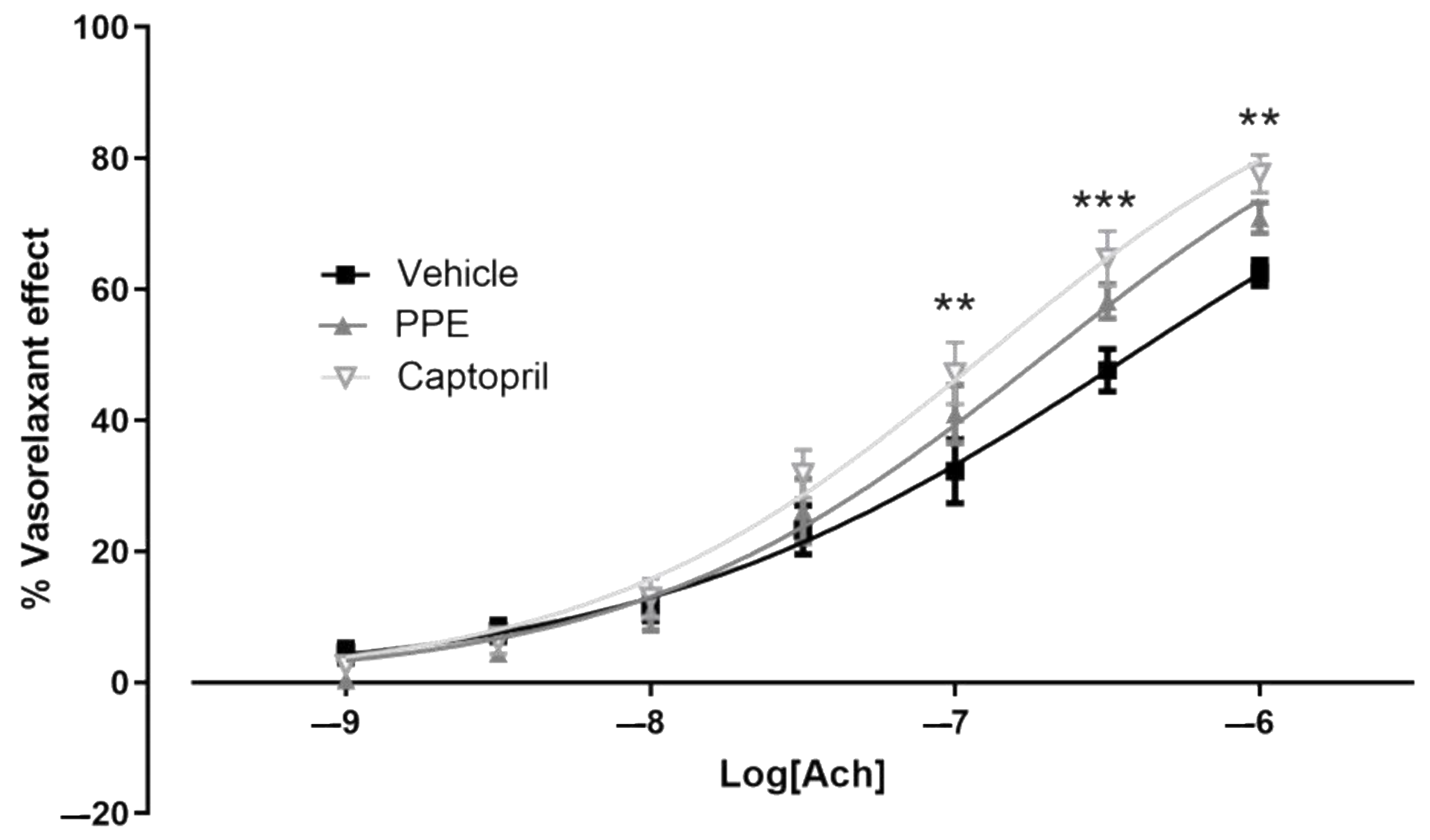
3.4.2. Endothelium Dysfunction
3.4.3. Histological Analyses
3.4.4. Cardiac and Ventricular Hypertrophy
3.4.5. IL6 and TGF-β1 Content
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sarkhosh, A.; Yavari, A.M.; Zamani, Z. The Pomegranate: Botany, Production and Uses; CABI: Wallingford, UK, 2020. [Google Scholar]
- Maphetu, N.; Unuofin, J.O.; Masuku, N.P.; Olisah, C.; Lebelo, S.L. Medicinal uses, pharmacological activities, phytochemistry, and the molecular mechanisms of Punica granatum L. (pomegranate) plant extracts: A review. Biomed. Pharmacother. 2022, 153, 113256. [Google Scholar] [CrossRef]
- Benedetti, G.; Zabini, F.; Tagliavento, L.; Meneguzzo, F.; Calderone, V.; Testai, L. An Overview of the Health Benefits, Extraction Methods and Improving the Properties of Pomegranate. Antioxidants 2023, 12, 1351. [Google Scholar] [CrossRef]
- Ko, K.; Dadmohammadi, Y.; Abbaspourrad, A. Nutritional and bioactive components of pomegranate waste used in food and cosmetic applications: A review. Foods 2021, 10, 657. [Google Scholar] [CrossRef]
- Wang, D.; Özen, C.; Abu-Reidah, I.M.; Chigurupati, S.; Patra, J.K.; Horbanczuk, J.O.; Jóźwik, A.; Tzvetkov, N.T.; Uhrin, P.; Atanasov, A.G. Vasculoprotective Effects of Pomegranate (Punica granatum L.). Front. Pharmacol. 2018, 9, 544. [Google Scholar] [CrossRef]
- Bar-Ya’akov, I.; Tian, L.; Amir, R.; Holland, D. Primary Metabolites, Anthocyanins, and Hydrolyzable Tannins in the Pomegranate Fruit. Front. Plant Sci. 2019, 10, 620. [Google Scholar] [CrossRef] [PubMed]
- García-Villalba, R.; Beltrán, D.; Espín, J.C.; Selma, M.V.; Tomás-Barberán, F.A. Time course production of urolithins from ellagic acid by human gut microbiota. J. Agric. Food Chem. 2013, 61, 8797–8806. [Google Scholar] [CrossRef] [PubMed]
- Mestry, S.N.; Dhodi, J.B.; Kumbhar, S.B.; Juvekar, A.R. Attenuation of diabetic nephropathy in streptozotocin-induced diabetic rats by Punica granatum Linn. leaves extract. J. Tradit. Complement. Med. 2017, 7, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Bhinge, S.D.; Bhutkar, M.A.; Randive, D.S.; Wadkar, G.H.; Todkar, S.S.; Savali, A.S.; Chittapurkar, H.R. Screening of hair growth promoting activity of Punica granatum L. (pomegranate) leaves extracts and its potential to exhibit antidandruff and anti-lice effect. Heliyon 2021, 7, e06903. [Google Scholar] [CrossRef]
- Gracious Ross, R.; Selvasubramanian, S.; Jayasundar, S. Immunomodulatory activity of Punica granatum in rabbits—A preliminary study. J. Ethnopharmacol. 2001, 78, 85–87. [Google Scholar] [CrossRef]
- Marsoul, A.; Ijjaali, M.; Elhajjaji, F.; Taleb, M.; Salim, R.; Boukir, A. Phytochemical screening, total phenolic and flavonoid methanolic extract of pomegranate bark (Punica granatum L.): Evaluation of the inhibitory effect in acidic medium 1 M HCl. Mater. Today Proc. 2020, 27, 3193–3198. [Google Scholar] [CrossRef]
- Khemakhem, M.; Zarroug, Y.; Jabou, K.; Selmi, S.; Bouzouita, N. Physicochemical characterization of oil, antioxidant potential, and phenolic profile of seeds isolated from Tunisian pomegranate (Punica granatum L.) cultivars. J. Food Sci. 2021, 86, 852–859. [Google Scholar] [CrossRef]
- Magangana, T.P.; Makunga, N.P.; Fawole, O.A.; Opara, U.L. Processing factors affecting the phytochemical and nutritional properties of pomegranate (Punica granatum L.) peel waste: A review. Molecules 2020, 25, 4690. [Google Scholar] [CrossRef]
- Kanatt, S.R.; Chander, R.; Sharma, A. Antioxidant and antimicrobial activity of pomegranate peel extract improves the shelf life of chicken products. Int. J. Food Sci. Technol. 2010, 45, 216–222. [Google Scholar] [CrossRef]
- Al-Rawahi, A.S.; Rahman, M.S.; Guizani, N.; Essa, M.M. Chemical composition, water sorption isotherm, and phenolic contents in fresh and dried pomegranate peels. Dry. Technol. 2013, 31, 257–263. [Google Scholar] [CrossRef]
- Lampakis, D.; Skenderidis, P.; Leontopoulos, S. Technologies and extraction methods of polyphenolic compounds derived from pomegranate (Punica granatum) peels. A mini review. Processes 2021, 9, 236. [Google Scholar] [CrossRef]
- El-Shamy, S.; Farag, M.A. Novel trends in extraction and optimization methods of bioactives recovery from pomegranate fruit biowastes: Valorization purposes for industrial applications. Food Chem. 2021, 365, 130465. [Google Scholar] [CrossRef] [PubMed]
- Talekar, S.; Patti, A.F.; Vijayraghavan, R.; Arora, A. Rapid, enhanced and eco-friendly recovery of punicalagin from fresh waste pomegranate peels via aqueous ball milling. J. Clean. Prod. 2019, 228, 1238–1247. [Google Scholar] [CrossRef]
- Albanese, L.; Meneguzzo, F. Hydrodynamic cavitation technologies: A pathway to more sustainable, healthier beverages, and food supply chains. In Processing and Sustainability of Beverages; Elsevier: Amsterdam, The Netherlands, 2019; pp. 319–372. [Google Scholar]
- Albanese, L.; Ciriminna, R.; Meneguzzo, F.; Pagliaro, M. Beer-brewing powered by controlled hydrodynamic cavitation: Theory and real-scale experiments. J. Clean. Prod. 2017, 142, 1457–1470. [Google Scholar] [CrossRef]
- Meneguzzo, F.; Brunetti, C.; Fidalgo, A.; Ciriminna, R.; Delisi, R.; Albanese, L.; Zabini, F.; Gori, A.; dos Santos Nascimento, L.B.; De Carlo, A. Real-scale integral valorization of waste orange peel via hydrodynamic cavitation. Processes 2019, 7, 581. [Google Scholar] [CrossRef]
- Meneguzzo, F.; Albanese, L.; Zabini, F. Hydrodynamic cavitation in beer and other beverage processing. In Innovative Food Processing Technologies: A Comprehensive Review; Elsevier: Amsterdam, The Netherlands, 2021; pp. 369–384. [Google Scholar] [CrossRef]
- Faraloni, C.; Albanese, L.; Chini Zittelli, G.; Meneguzzo, F.; Tagliavento, L.; Zabini, F. New Route to the Production of Almond Beverages Using Hydrodynamic Cavitation. Foods 2023, 12, 935. [Google Scholar] [CrossRef]
- Arya, S.S.; More, P.R.; Ladole, M.R.; Pegu, K.; Pandit, A.B. Non-thermal, energy efficient hydrodynamic cavitation for food processing, process intensification and extraction of natural bioactives: A review. Ultrason. Sonochem. 2023, 98, 106504. [Google Scholar] [CrossRef] [PubMed]
- Minutolo, A.; Gismondi, A.; Chirico, R.; Di Marco, G.; Petrone, V.; Fanelli, M.; D’Agostino, A.; Canini, A.; Grelli, S.; Albanese, L. Antioxidant Phytocomplexes Extracted from Pomegranate (Punica granatum L.) Using Hydrodynamic Cavitation Show Potential Anticancer Activity In Vitro. Antioxidants 2023, 12, 1560. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liao, R.; Zhang, S.; Weng, H.; Liu, Y.; Tao, T.; Yu, F.; Li, G.; Wu, J. Promising Remedies for Cardiovascular Disease: Natural Polyphenol Ellagic Acid and Its Metabolite Urolithins. Phytomedicine 2023, 116, 154867. [Google Scholar] [CrossRef]
- Li, B.; Harich, K.; Wegiel, L.; Taylor, L.S.; Edgar, K.J. Stability and solubility enhancement of ellagic acid in cellulose ester solid dispersions. Carbohydr. Polym. 2013, 92, 1443–1450. [Google Scholar] [CrossRef]
- Tannous, M.; Caldera, F.; Hoti, G.; Dianzani, U.; Cavalli, R.; Trotta, F. Drug-encapsulated cyclodextrin nanosponges. In Supramolecules in Drug Discovery and Drug Delivery: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2021; pp. 247–283. [Google Scholar]
- Nyamba, I.; Lechanteur, A.; Semdé, R.; Evrard, B. Physical formulation approaches for improving aqueous solubility and bioavailability of ellagic acid: A review. Eur. J. Pharm. Biopharm. 2021, 159, 198–210. [Google Scholar] [CrossRef]
- Kawabata, Y.; Wada, K.; Nakatani, M.; Yamada, S.; Onoue, S. Formulation design for poorly water-soluble drugs based on biopharmaceutics classification system: Basic approaches and practical applications. Int. J. Pharm. 2011, 420, 1–10. [Google Scholar] [CrossRef]
- Bala, I.; Bhardwaj, V.; Hariharan, S.; Kharade, S.V.; Roy, N.; Ravi Kumar, M. Sustained release nanoparticulate formulation containing antioxidant-ellagic acid as potential prophylaxis system for oral administration. J. Drug Target. 2006, 14, 27–34. [Google Scholar] [CrossRef]
- Wang, S.-T.; Chou, C.-T.; Su, N.-W. A food-grade self-nanoemulsifying delivery system for enhancing oral bioavailability of ellagic acid. J. Funct. Foods 2017, 34, 207–215. [Google Scholar] [CrossRef]
- Sabzevari, A.G.; Sabahi, H. Montmorillonite an efficient oral nanocarrier for punicalagin-rich pomegranate peel extract: An in vitro study. J. Drug Deliv. Sci. Technol. 2023, 86, 104713. [Google Scholar] [CrossRef]
- Romani, A.; Vignolini, P.; Isolani, L.; Ieri, F.; Heimler, D. HPLC-DAD/MS characterization of flavonoids and hydroxycinnamic derivatives in turnip tops (Brassica rapa L. subsp. sylvestris L.). J. Agric. Food Chem. 2006, 54, 1342–1346. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef]
- Orak, H.H.; Yagar, H.; Isbilir, S.S. Comparison of Antioxidant Activities of Juice, Peel, and Seed of Pomegranate (Punica granatum L.) and Inter-Relationships with Total Phenolic, Tannin, Anthocyanin, and Flavonoid Contents. Food Sci. Biotechnol. 2012, 21, 373–387. [Google Scholar] [CrossRef]
- Takai, S.; Jin, D.; Sakaguchi, M.; Miyazaki, M. Significant target organs for hypertension and cardiac hypertrophy by angiotensin-converting enzyme inhibitors. Hypertens. Res. 2004, 27, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Heckmann, M.; Zimmer, H.G. Effects of triiodothyronine in spontaneously hypertensive rats. Studies on cardiac metabolism, function, and heart weight. Basic Res. Cardiol. 1992, 87, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Ma, H.; Liu, W.; Niesen, D.B.; Shah, N.; Crews, R.; Rose, K.N.; Vattem, D.A.; Seeram, N.P. Pomegranate’s neuroprotective effects against Alzheimer’s disease are mediated by urolithins, its ellagitannin-gut microbial derived metabolites. ACS Chem. Neurosci. 2016, 7, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Long, X.; Yang, J.; Du, L.; Zhang, X.; Li, J.; Hou, C. Pomegranate peel polyphenols reduce chronic low-grade inflammatory responses by modulating gut microbiota and decreasing colonic tissue damage in rats fed a high-fat diet. Food Funct. 2019, 10, 8273–8285. [Google Scholar] [CrossRef] [PubMed]
- García-Villalba, R.; Tomás-Barberán, F.A.; Iglesias-Aguirre, C.E.; Giménez-Bastida, J.A.; González-Sarrías, A.; Selma, M.V.; Espín, J.C. Ellagitannins, urolithins, and neuroprotection: Human evidence and the possible link to the gut microbiota. Mol. Asp. Med. 2023, 89, 101109. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cao, K.; Lv, W.; Feng, Z.; Liu, J.; Gao, J.; Li, H.; Zang, W.; Liu, J. Punicalagin attenuates endothelial dysfunction by activating FoxO1, a pivotal regulating switch of mitochondrial biogenesis. Free Radic Biol. Med. 2019, 135, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.; Kim, C.Y.; Hwang, J.; Jo, K.; Kim, S.; Suh, H.J.; Choi, H.S. Punicalagin, a Pomegranate-Derived Ellagitannin, Suppresses Obesity and Obesity-Induced Inflammatory Responses Via the Nrf2/Keap1 Signaling Pathway. Mol. Nutr. Food Res. 2019, 63, e1900574. [Google Scholar] [CrossRef]
- Seeram, N.P.; Lee, R.; Heber, D. Bioavailability of ellagic acid in human plasma after consumption of ellagitannins from pomegranate (Punica granatum L.) juice. Clin. Chim. Acta 2004, 348, 63–68. [Google Scholar] [CrossRef]
- Yisimayili, Z.; Chao, Z. A review on phytochemicals, metabolic profiles and pharmacokinetics studies of the different parts (juice, seeds, peel, flowers, leaves and bark) of pomegranate (Punica granatum L.). Food Chem. 2022, 395, 133600. [Google Scholar] [CrossRef] [PubMed]
- Cerdá, B.; Llorach, R.; Cerón, J.J.; Espín, J.C.; Tomás-Barberán, F.A. Evaluation of the bioavailability and metabolism in the rat of punicalagin, an antioxidant polyphenol from pomegranate juice. Eur. J. Nutr. 2003, 42, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Konukoglu, D.; Uzun, H. Endothelial Dysfunction and Hypertension. Adv. Exp. Med. Biol. 2017, 956, 511–540. [Google Scholar] [CrossRef] [PubMed]
- Taddei, S.; Virdis, A.; Ghiadoni, L.; Versari, D.; Salvetti, A. Endothelium, aging, and hypertension. Curr. Hypertens. Rep. 2006, 8, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Thuillez, C.; Richard, V. Targeting endothelial dysfunction in hypertensive subjects. J. Hum. Hypertens. 2005, 19 (Suppl. 1), S21–S25. [Google Scholar] [CrossRef]
- Jordão, J.B.R.; Porto, H.K.P.; Lopes, F.M.; Batista, A.C.; Rocha, M.L. Protective Effects of Ellagic Acid on Cardiovascular Injuries Caused by Hypertension in Rats. Planta Med. 2017, 83, 830–836. [Google Scholar] [CrossRef]
- Yang, X.; Nisar, T.; Hou, Y.; Gou, X.; Sun, L.; Guo, Y. Pomegranate peel pectin can be used as an effective emulsifier. Food Hydrocoll. 2018, 85, 30–38. [Google Scholar] [CrossRef]
- Ciriminna, R.; Scurria, A.; Pagliaro, M. Natural Product Extraction via Hydrodynamic Cavitation. Sustain. Chem. Pharm. 2023, 33, 101083. [Google Scholar] [CrossRef]
- Flori, L.; Albanese, L.; Calderone, V.; Meneguzzo, F.; Pagliaro, M.; Ciriminna, R.; Zabini, F.; Testai, L. Cardioprotective effects of grapefruit IntegroPectin extracted via hydrodynamic cavitation from by-products of Citrus fruits industry: Role of mitochondrial potassium channels. Foods 2022, 11, 2799. [Google Scholar] [CrossRef]





| Extract | Time (min) | Temperatures (°C) | Specific Energy (kWh/kg) |
|---|---|---|---|
| PFE | 45 | 21.5–47.0 | 0.720 |
| PPE | 20 | 28.0–39.0 | 0.216 |
| Extract | α + β Punicalagin (mg/g) | Ellagic Acid (mg/g) |
|---|---|---|
| PFE | 38.96 ± 2.71 | 1.03 ± 0.05 |
| PPE | 48.48 ± 5.45 | 3.07 ± 0.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benedetti, G.; Flori, L.; Spezzini, J.; Miragliotta, V.; Lazzarini, G.; Pirone, A.; Meneguzzo, C.; Tagliavento, L.; Martelli, A.; Antonelli, M.; et al. Improved Cardiovascular Effects of a Novel Pomegranate Byproduct Extract Obtained through Hydrodynamic Cavitation. Nutrients 2024, 16, 506. https://doi.org/10.3390/nu16040506
Benedetti G, Flori L, Spezzini J, Miragliotta V, Lazzarini G, Pirone A, Meneguzzo C, Tagliavento L, Martelli A, Antonelli M, et al. Improved Cardiovascular Effects of a Novel Pomegranate Byproduct Extract Obtained through Hydrodynamic Cavitation. Nutrients. 2024; 16(4):506. https://doi.org/10.3390/nu16040506
Chicago/Turabian StyleBenedetti, Giada, Lorenzo Flori, Jacopo Spezzini, Vincenzo Miragliotta, Giulia Lazzarini, Andrea Pirone, Cosimo Meneguzzo, Luca Tagliavento, Alma Martelli, Michele Antonelli, and et al. 2024. "Improved Cardiovascular Effects of a Novel Pomegranate Byproduct Extract Obtained through Hydrodynamic Cavitation" Nutrients 16, no. 4: 506. https://doi.org/10.3390/nu16040506
APA StyleBenedetti, G., Flori, L., Spezzini, J., Miragliotta, V., Lazzarini, G., Pirone, A., Meneguzzo, C., Tagliavento, L., Martelli, A., Antonelli, M., Donelli, D., Faraloni, C., Calderone, V., Meneguzzo, F., & Testai, L. (2024). Improved Cardiovascular Effects of a Novel Pomegranate Byproduct Extract Obtained through Hydrodynamic Cavitation. Nutrients, 16(4), 506. https://doi.org/10.3390/nu16040506












