Treatment with Glycyrrhiza glabra Extract Induces Anxiolytic Effects Associated with Reduced Salt Preference and Changes in Barrier Protein Gene Expression
Abstract
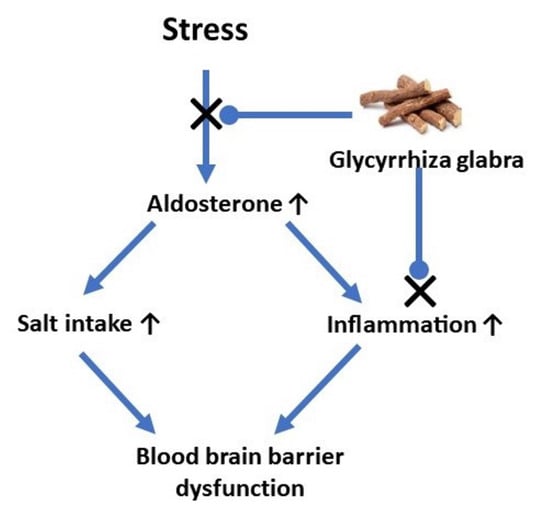
:1. Introduction
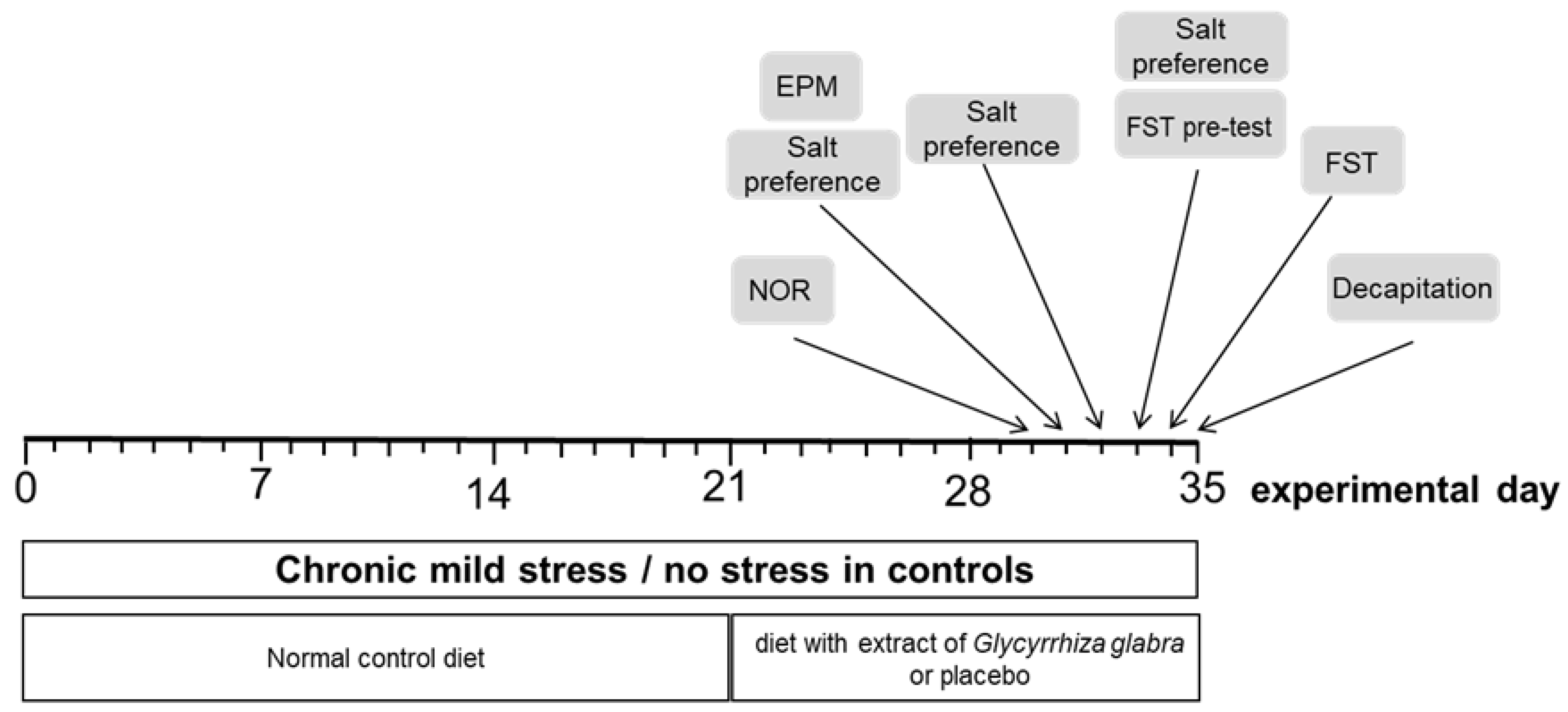
2. Material and Methods
2.1. Animals
2.2. Treatment
2.3. Behavioral Testing
2.3.1. Elevated Plus-Maze Test
2.3.2. Salt Preference Test
2.3.3. Forced Swim Test (FST)
2.3.4. Novel Object Recognition Test
2.4. Organ Collection
2.5. Reverse Transcription and Quantitative Real-Time Polymerase Chain Reaction (RT-PCR)
2.6. Glial Fibrillary Acidic Protein (GFAP) Quantification by ELISA
2.7. Statistical Analysis
3. Results
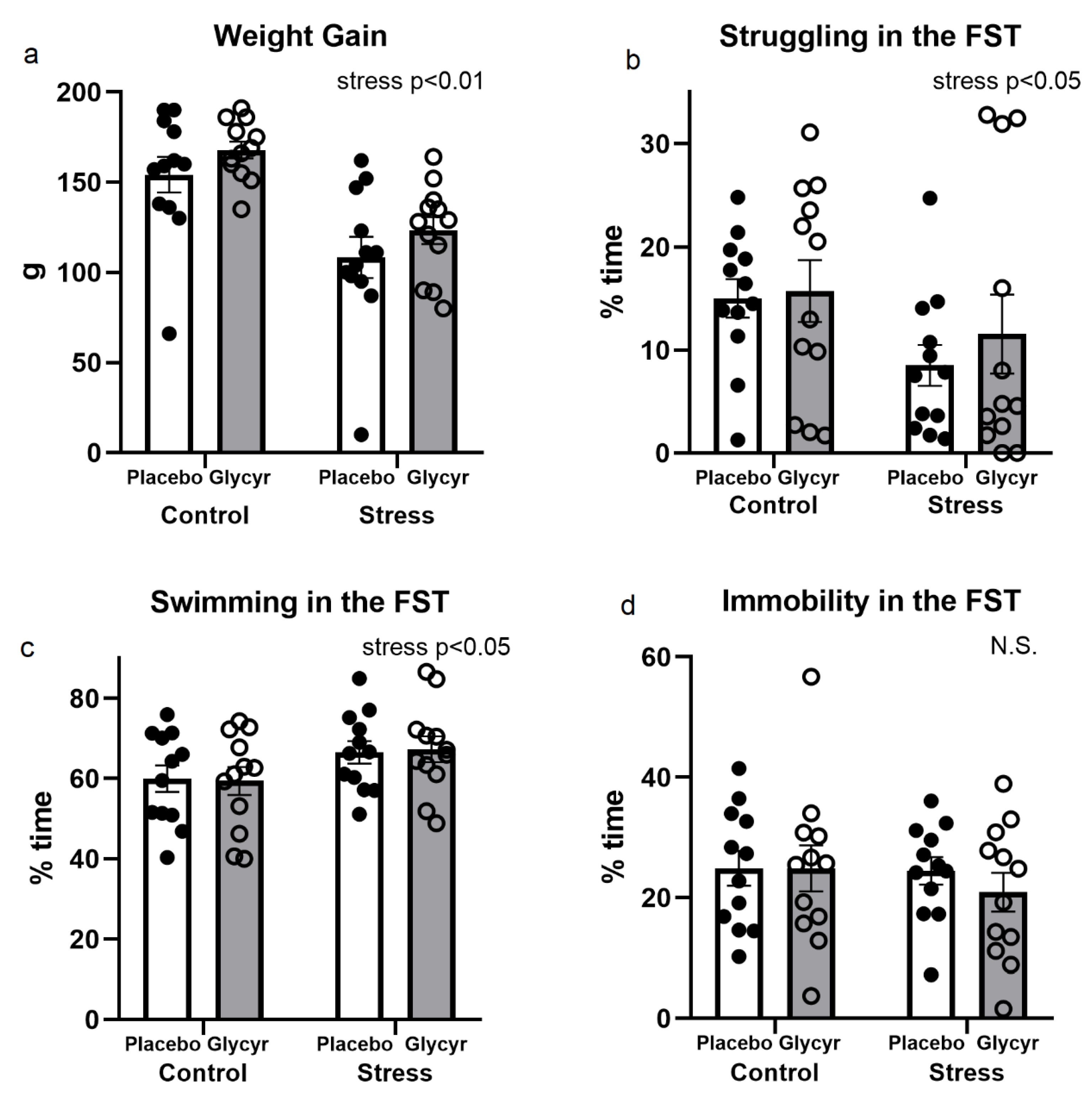
3.1. Stressfulness of the Model Used
3.2. Anxiety Behavior
3.3. Cognitive Testing
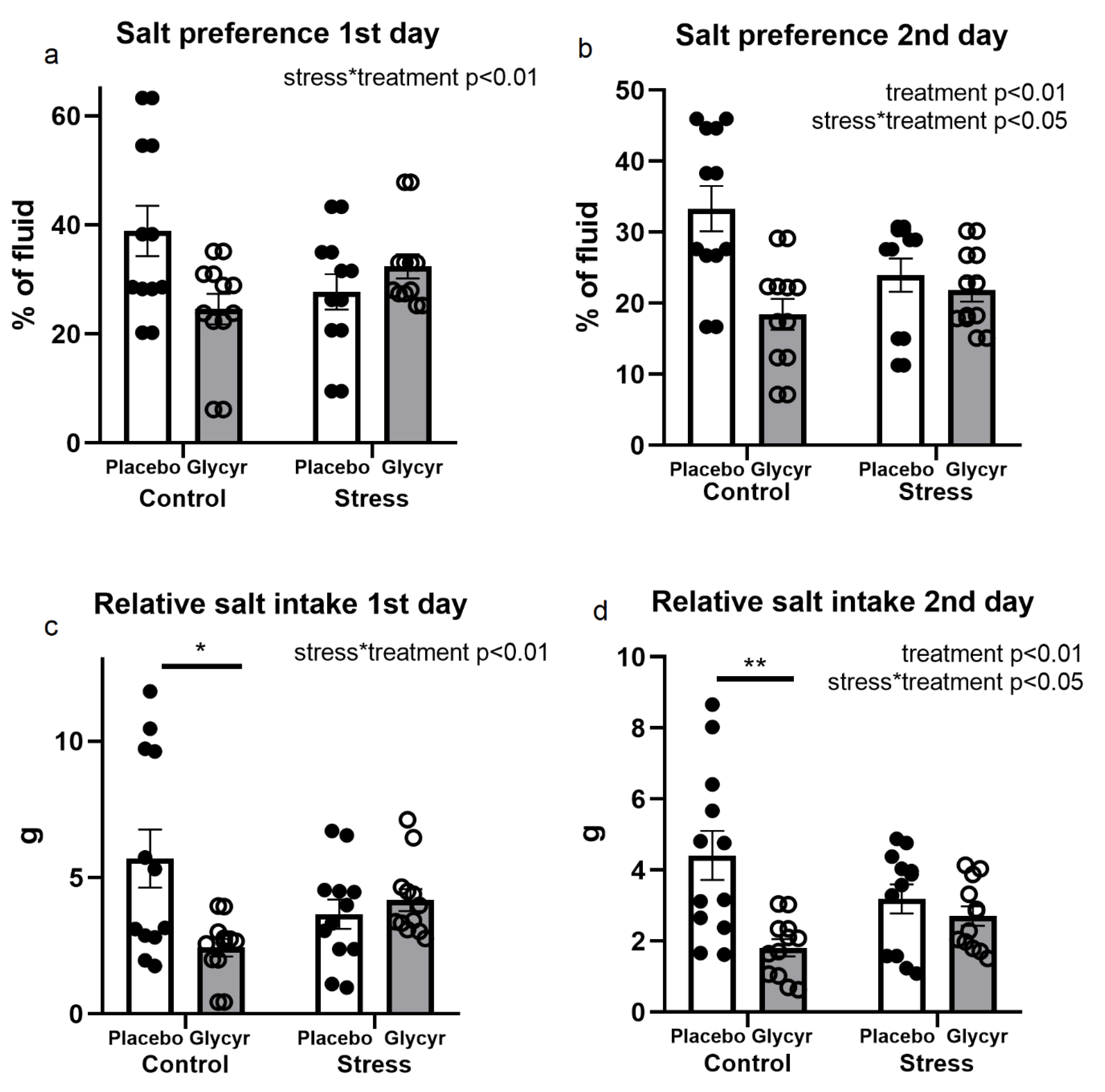
3.4. Salt Preference
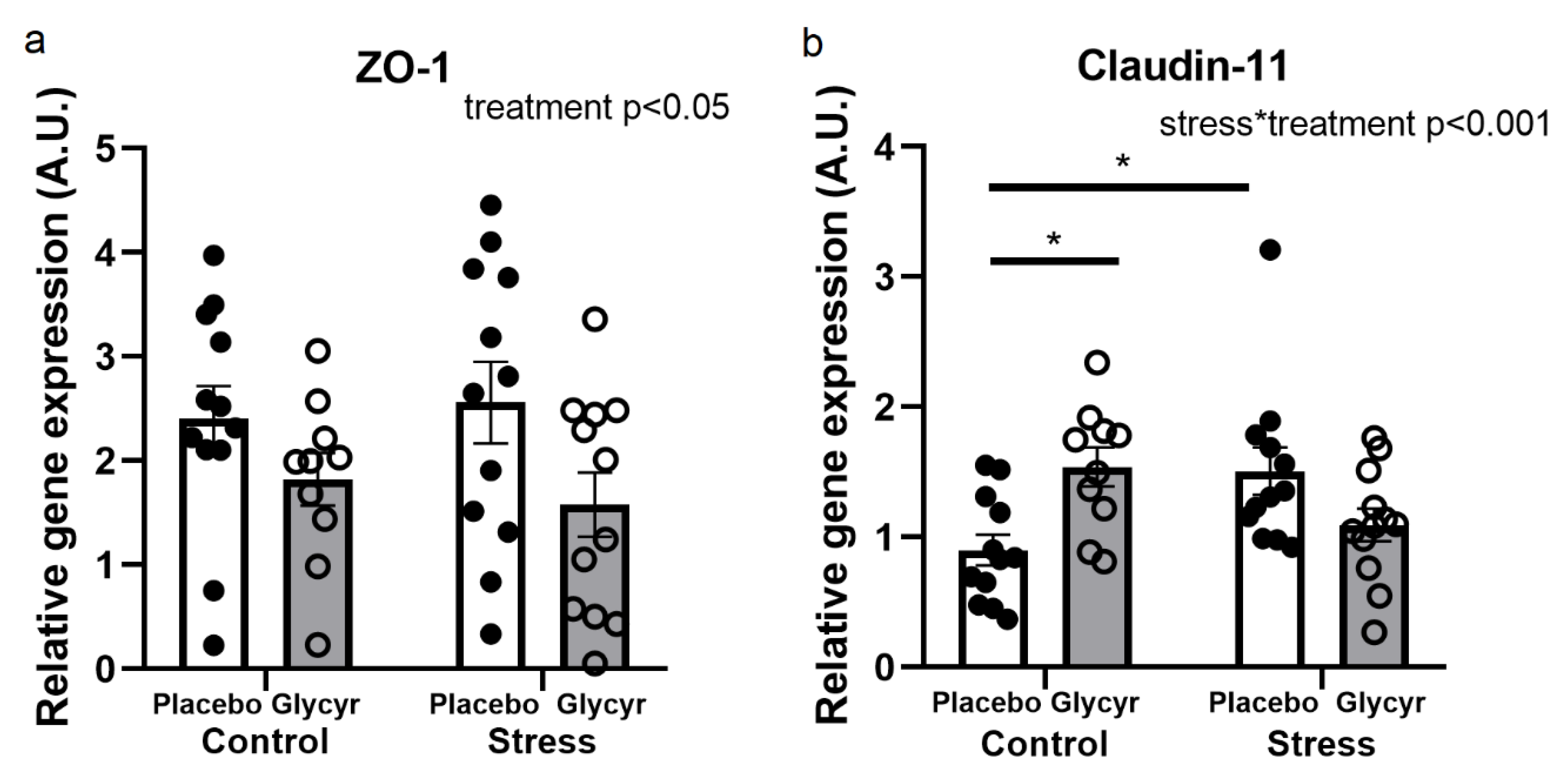
3.5. Gene Expression of Barrier Proteins
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Izakova, L.; Hlavacova, N.; Segeda, V.; Kapsdorfer, D.; Morovicsova, E.; Jezova, D. Salivary aldosterone, cortisol and their morning to evening slopes in patients with depressive disorder and healthy subjects: Acute episode and follow up six months after reaching remission. Neuroendocrinology 2020, 110, 1001–1009. [Google Scholar] [CrossRef]
- Murck, H.; Uhr, M.; Ziegenbein, M.; Kunzel, H.; Held, K.; Antonijevic, I.A.; Schussler, P.; Steiger, A. Renin-angiotensin-aldosterone system, HPA-axis and sleep-EEG changes in unmedicated patients with depression after total sleep deprivation. Pharmacopsychiatry 2006, 39, 23–29. [Google Scholar] [CrossRef]
- Emanuele, E.; Geroldi, D.; Minoretti, P.; Coen, E.; Politi, P. Increased plasma aldosterone in patients with clinical depression. Arch. Med. Res. 2005, 36, 544–548. [Google Scholar] [CrossRef]
- Nowacki, J.; Wingenfeld, K.; Kaczmarczyk, M.; Chae, W.R.; Salchow, P.; Abu-Tir, I.; Piber, D.; Hellmann-Regen, J.; Otte, C. Cardiovascular risk and steroid hormone secretion after stimulation of mineralocorticoid and NMDA receptors in depressed patients. Transl. Psychiatry 2020, 10, 109. [Google Scholar] [CrossRef]
- Buttner, M.; Jezova, D.; Greene, B.; Konrad, C.; Kircher, T.; Murck, H. Target-based biomarker selection—Mineralocorticoid receptor-related biomarkers and treatment outcome in major depression. J. Psychiatr. Res. 2015, 66–67, 24–37. [Google Scholar] [CrossRef]
- Engelmann, J.; Murck, H.; Wagner, S.; Zillich, L.; Streit, F.; Herzog, D.P.; Braus, D.F.; Tadic, A.; Lieb, K.; Muller, M.B. Routinely accessible parameters of mineralocorticoid receptor function, depression subtypes and response prediction: A post-hoc analysis from the early medication change trial in major depressive disorder. World J. Biol. Psychiatry 2022, 23, 631–642. [Google Scholar] [CrossRef] [PubMed]
- de Kloet, E.R.; Joels, M. Brain mineralocorticoid receptor function in control of salt balance and stress-adaptation. Physiol. Behav. 2017, 178, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Adolf, C.; Schneider, H.; Heinrich, D.A.; Handgriff, L.; Reincke, M. Salt Appetite and Its Effects on Cardiovascular Risk in Primary Aldosteronism. Horm. Metab. Res. 2020, 52, 386–393. [Google Scholar] [CrossRef]
- Ileri-Gurel, E.; Pehlivanoglu, B.; Dogan, M. Effect of acute stress on taste perception: In relation with baseline anxiety level and body weight. Chem. Senses 2013, 38, 27–34. [Google Scholar] [CrossRef]
- Volkov, V.S.; Poseliugina, O.B.; Rokkina, S.A.; Nilova, S.A. Consumption of table salt and psychological status in patients with arterial hypertension. Klin. Med. 2012, 90, 56–58. [Google Scholar]
- Arab, A.; Zabibah, R.S.; Alshahrani, S.H.; Karimi, E.; Askari, G.; Khorvash, F. Is there a relationship between 24-hour urinary sodium and potassium and mental health in migraine patients?: A cross-sectional study. Medicine 2022, 101, e31037. [Google Scholar] [CrossRef] [PubMed]
- Heath, T.P.; Melichar, J.K.; Nutt, D.J.; Donaldson, L.F. Human taste thresholds are modulated by serotonin and noradrenaline. J. Neurosci. 2006, 26, 12664–12671. [Google Scholar] [CrossRef] [PubMed]
- Murck, H.; Fava, M.; Cusin, C.; Fatt, C.C.; Trivedi, M. Brain ventricle and choroid plexus morphology as predictor of treatment response in major depression: Findings from the EMBARC study. Brain Behav. Immun. Health 2024, 35, 100717. [Google Scholar] [CrossRef]
- Althubaity, N.; Schubert, J.; Martins, D.; Yousaf, T.; Nettis, M.A.; Mondelli, V.; Pariante, C.; Harrison, N.A.; Bullmore, E.T.; Dima, D.; et al. Choroid plexus enlargement is associated with neuroinflammation and reduction of blood brain barrier permeability in depression. Neuroimage Clin. 2022, 33, 102926. [Google Scholar] [CrossRef]
- Doney, E.; Cadoret, A.; Dion-Albert, L.; Lebel, M.; Menard, C. Inflammation-driven brain and gut barrier dysfunction in stress and mood disorders. Eur. J. Neurosci. 2022, 55, 2851–2894. [Google Scholar] [CrossRef]
- Serna-Rodriguez, M.F.; Bernal-Vega, S.; de la Barquera, J.A.O.; Camacho-Morales, A.; Perez-Maya, A.A. The role of damage associated molecular pattern molecules (DAMPs) and permeability of the blood-brain barrier in depression and neuroinflammation. J. Neuroimmunol. 2022, 371, 577951. [Google Scholar] [CrossRef]
- Skultetyova, I.; Tokarev, D.; Jezova, D. Stress-induced increase in blood-brain barrier permeability in control and monosodium glutamate-treated rats. Brain Res. Bull. 1998, 45, 175–178. [Google Scholar] [CrossRef]
- Vermette, D.; Hu, P.; Canarie, M.F.; Funaro, M.; Glover, J.; Pierce, R.W. Tight junction structure, function, and assessment in the critically ill: A systematic review. Intensive Care Med. Exp. 2018, 6, 37. [Google Scholar] [CrossRef]
- Tikiyani, V.; Babu, K. Claudins in the brain: Unconventional functions in neurons. Traffic 2019, 20, 807–814. [Google Scholar] [CrossRef]
- Seckl, J. 11β-Hydroxysteroid dehydrogenase and the brain: Not (yet) lost in translation. J. Intern. Med. 2024, 295, 20–37. [Google Scholar] [CrossRef]
- Makino, T. Exploration for the real causative agents of licorice-induced pseudoaldosteronism. J. Nat. Med. 2021, 75, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Zhang, Y.; Liu, Y.; Wang, X.; Guo, Z.; Liang, X.; Lai, W. Effects of glycyrrhizin on production of vascular aldosterone and corticosterone. Horm. Res. 1999, 51, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Cosmetic Ingredient Review Expert Panel. Final report on the safety assessment of Glycyrrhetinic Acid, Potassium Glycyrrhetinate, Disodium Succinoyl Glycyrrhetinate, Glyceryl Glycyrrhetinate, Glycyrrhetinyl Stearate, Stearyl Glycyrrhetinate, Glycyrrhizic Acid, Ammonium Glycyrrhizate, Dipotassium Glycyrrhizate, Disodium Glycyrrhizate, Trisodium Glycyrrhizate, Methyl Glycyrrhizate, and Potassium Glycyrrhizinate. Int. J. Toxicol. 2007, 26 (Suppl. S2), 79–112. [Google Scholar] [CrossRef]
- Epstein, M.T.; Espiner, E.A.; Donald, R.A.; Hughes, H. Effect of eating liquorice on the renin-angiotensin aldosterone axis in normal subjects. Br. Med. J. 1977, 1, 488–490. [Google Scholar] [CrossRef] [PubMed]
- Murck, H.; Lehr, L.; Hahn, J.; Braunisch, M.C.; Jezova, D.; Zavorotnyy, M. Adjunct Therapy with Glycyrrhiza Glabra Rapidly Improves Outcome in Depression—A Pilot Study to Support 11-Beta-Hydroxysteroid Dehydrogenase Type 2 Inhibition as a New Target. Front. Psychiatry 2020, 11, 605949. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.Y.; Liu, Y.Z.; Li, J.M.; Ruan, Y.M.; Yan, W.J.; Zhong, S.Y.; Zhang, T.; Liu, L.L.; Wu, R.; Wang, B.; et al. Glycyrrhizic acid as an adjunctive treatment for depression through anti-inflammation: A randomized placebo-controlled clinical trial. J. Affect Disord. 2020, 265, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Schrofelbauer, B.; Raffetseder, J.; Hauner, M.; Wolkerstorfer, A.; Ernst, W.; Szolar, O.H. Glycyrrhizin, the main active compound in liquorice, attenuates pro-inflammatory responses by interfering with membrane-dependent receptor signalling. Biochem. J. 2009, 421, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, Y.; Weng, Z.; Zhou, T.; Feng, T.; Lin, Y. Glycyrrhizin protects brain against ischemia-reperfusion injury in mice through HMGB1-TLR4-IL-17A signaling pathway. Brain Res. 2014, 1582, 176–186. [Google Scholar] [CrossRef]
- Uchida, Y.; Sumiya, T.; Tachikawa, M.; Yamakawa, T.; Murata, S.; Yagi, Y.; Sato, K.; Stephan, A.; Ito, K.; Ohtsuki, S.; et al. Involvement of Claudin-11 in Disruption of Blood-Brain, -Spinal Cord, and -Arachnoid Barriers in Multiple Sclerosis. Mol. Neurobiol. 2019, 56, 2039–2056. [Google Scholar] [CrossRef]
- Uchida, Y. Quantitative Proteomics-Based Blood-Brain Barrier Study. Biol. Pharm. Bull. 2021, 44, 465–473. [Google Scholar] [CrossRef]
- Tezuka, K.; Suzuki, M.; Sato, R.; Kawarada, S.; Terasaki, T.; Uchida, Y. Activation of Annexin A2 signaling at the blood-brain barrier in a mouse model of multiple sclerosis. J. Neurochem. 2022, 160, 662–674. [Google Scholar] [CrossRef]
- Tian, J.; Li, X.; Zhao, L.; Shen, P.; Wang, Z.; Zhu, L.; Li, C.; Su, C.; Zhang, Y. Glycyrrhizic acid promotes neural repair by directly driving functional remyelination. Food Funct. 2020, 11, 992–1005. [Google Scholar] [CrossRef]
- Wolburg, H.; Wolburg-Buchholz, K.; Liebner, S.; Engelhardt, B. Claudin-1, claudin-2 and claudin-11 are present in tight junctions of choroid plexus epithelium of the mouse. Neurosci. Lett. 2001, 307, 77–80. [Google Scholar] [CrossRef]
- Kratzer, I.; Vasiljevic, A.; Rey, C.; Fevre-Montange, M.; Saunders, N.; Strazielle, N.; Ghersi-Egea, J.F. Complexity and developmental changes in the expression pattern of claudins at the blood-CSF barrier. Histochem. Cell Biol. 2012, 138, 861–879. [Google Scholar] [CrossRef] [PubMed]
- Jezova, D.; Karailiev, P.; Karailievova, L.; Puhova, A.; Murck, H. Food Enrichment with Glycyrrhiza glabra Extract Suppresses ACE2 mRNA and Protein Expression in Rats-Possible Implications for COVID-19. Nutrients 2021, 13, 2321. [Google Scholar] [CrossRef]
- Chakravarthi, K.K.; Avadhani, R. Beneficial effect of aqueous root extract of Glycyrrhiza glabra on learning and memory using different behavioral models: An experimental study. J. Nat. Sci. Biol. Med. 2013, 4, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Hlavacova, N.; Jezova, D. Chronic treatment with the mineralocorticoid hormone aldosterone results in increased anxiety-like behavior. Horm. Behav. 2008, 54, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Inui-Yamamoto, C.; Yamamoto, T.; Ueda, K.; Nakatsuka, M.; Kumabe, S.; Inui, T.; Iwai, Y. Taste preference changes throughout different life stages in male rats. PLoS ONE 2017, 12, e0181650. [Google Scholar] [CrossRef] [PubMed]
- Flynn, F.W.; Schulkin, J.; Havens, M. Sex differences in salt preference and taste reactivity in rats. Brain Res. Bull. 1993, 32, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Porsolt, R.D.; Bertin, A.; Jalfre, M. Behavioral despair in mice: A primary screening test for antidepressants. Arch. Int. Pharmacodyn. Ther. 1977, 229, 327–336. [Google Scholar] [PubMed]
- Hlavacova, N.; Wes, P.D.; Ondrejcakova, M.; Flynn, M.E.; Poundstone, P.K.; Babic, S.; Murck, H.; Jezova, D. Subchronic treatment with aldosterone induces depression-like behaviours and gene expression changes relevant to major depressive disorder. Int. J. Neuropsychopharmacol. 2012, 15, 247–265. [Google Scholar] [CrossRef] [PubMed]
- Zamberletti, E.; Prini, P.; Speziali, S.; Gabaglio, M.; Solinas, M.; Parolaro, D.; Rubino, T. Gender-dependent behavioral and biochemical effects of adolescent delta-9-tetrahydrocannabinol in adult maternally deprived rats. Neuroscience 2012, 204, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Karailiev, P.; Hlavacova, N.; Chmelova, M.; Homer, N.Z.M.; Jezova, D. Tight junction proteins in the small intestine and prefrontal cortex of female rats exposed to stress of chronic isolation starting early in life. Neurogastroenterol. Motil. 2021, 33, e14084. [Google Scholar] [CrossRef] [PubMed]
- Hrivikova, K.; Zelena, D.; Graban, J.; Puhova, A.; Miklya, I.; Balazsfi, D.; Jezova, D. Chronic treatment with enhancer drugs modifies the gene expression of selected parameters related to brain plasticity in rats under stress conditions. Neurochem. Int. 2022, 159, 105404. [Google Scholar] [CrossRef] [PubMed]
- Gasparini, S.; Resch, J.M.; Narayan, S.V.; Peltekian, L.; Iverson, G.N.; Karthik, S.; Geerling, J.C. Aldosterone-sensitive HSD2 neurons in mice. Brain Struct. Funct. 2019, 224, 387–417. [Google Scholar] [CrossRef] [PubMed]
- Geerling, J.C.; Loewy, A.D. Aldosterone in the brain. Am. J. Physiol. Ren. Physiol. 2009, 297, F559–F576. [Google Scholar] [CrossRef]
- Chapman, K.; Holmes, M.; Seckl, J. 11β-hydroxysteroid dehydrogenases: Intracellular gate-keepers of tissue glucocorticoid action. Physiol. Rev. 2013, 93, 1139–1206. [Google Scholar] [CrossRef]
- Johnson, A.K.; Grippo, A.J. Sadness and broken hearts: Neurohumoral mechanisms and co-morbidity of ischemic heart disease and psychological depression. J. Physiol. Pharmacol. 2006, 57 (Suppl. S11), 5–29. [Google Scholar]
- Grippo, A.J.; Francis, J.; Beltz, T.G.; Felder, R.B.; Johnson, A.K. Neuroendocrine and cytokine profile of chronic mild stress-induced anhedonia. Physiol. Behav. 2005, 84, 697–706. [Google Scholar] [CrossRef]
- Johnson, A.K.; Thunhorst, R.L. The neuroendocrinology of thirst and salt appetite: Visceral sensory signals and mechanisms of central integration. Front. Neuroendocr. 1997, 18, 292–353. [Google Scholar] [CrossRef]
- Geerling, J.C.; Loewy, A.D. Central regulation of sodium appetite. Exp. Physiol. 2008, 93, 177–209. [Google Scholar] [CrossRef]
- Geerling, J.C.; Engeland, W.C.; Kawata, M.; Loewy, A.D. Aldosterone target neurons in the nucleus tractus solitarius drive sodium appetite. J. Neurosci. 2006, 26, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Murck, H.; Buttner, M.; Kircher, T.; Konrad, C. Genetic, molecular and clinical determinants for the involvement of aldosterone and its receptors in major depression. Nephron Physiol. 2014, 128, 17–25. [Google Scholar] [CrossRef]
- Forstenpointner, J.; Maallo, A.M.S.; Elman, I.; Holmes, S.; Freeman, R.; Baron, R.; Borsook, D. The solitary nucleus connectivity to key autonomic regions in humans. Eur. J. Neurosci. 2022, 56, 3938–3966. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, K.K. Glial fibrillary acidic protein: From intermediate filament assembly and gliosis to neurobiomarker. Trends Neurosci. 2015, 38, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Molendijk, M.L.; de Kloet, E.R. Forced swim stressor: Trends in usage and mechanistic consideration. Eur. J. Neurosci. 2022, 55, 2813–2831. [Google Scholar] [CrossRef]
- Molendijk, M.L.; de Kloet, E.R. Immobility in the forced swim test is adaptive and does not reflect depression. Psychoneuroendocrinology 2015, 62, 389–391. [Google Scholar] [CrossRef] [PubMed]
- Fava, M.; Rush, A.J.; Alpert, J.E.; Balasubramani, G.K.; Wisniewski, S.R.; Carmin, C.N.; Biggs, M.M.; Zisook, S.; Leuchter, A.; Howland, R.; et al. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: A STAR*D report. Am. J. Psychiatry 2008, 165, 342–351. [Google Scholar] [CrossRef]
- Dhingra, D.; Sharma, A. Antidepressant-like activity of Glycyrrhiza glabra L. in mouse models of immobility tests. Prog. Neuropsychopharmacol. Biol. Psychiatry 2006, 30, 449–454. [Google Scholar] [CrossRef]
- Jiang, Q.; Li, C.R.; Zeng, W.F.; Xu, H.J.; Li, J.M.; Zhang, T.; Deng, G.H.; Wang, Y.X. Inhibition of Connexin 36 attenuates HMGB1-mediated depressive-like behaviors induced by chronic unpredictable mild stress. Brain Behav. 2022, 12, e2470. [Google Scholar] [CrossRef]
- Hisaoka-Nakashima, K.; Tomimura, Y.; Yoshii, T.; Ohata, K.; Takada, N.; Zhang, F.F.; Nakamura, Y.; Liu, K.; Wake, H.; Nishibori, M.; et al. High-mobility group box 1-mediated microglial activation induces anxiodepressive-like behaviors in mice with neuropathic pain. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 92, 347–362. [Google Scholar] [CrossRef]
- Wang, B.; Lian, Y.J.; Dong, X.; Peng, W.; Liu, L.L.; Su, W.J.; Gong, H.; Zhang, T.; Jiang, C.L.; Li, J.S.; et al. Glycyrrhizic acid ameliorates the kynurenine pathway in association with its antidepressant effect. Behav. Brain Res. 2018, 353, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.J.; Na, E.S.; Johnson, A.K. Mineralocorticoid receptor antagonism prevents hedonic deficits induced by a chronic sodium appetite. Behav. Neurosci. 2010, 124, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Grippo, A.J.; Moffitt, J.A.; Beltz, T.G.; Johnson, A.K. Reduced hedonic behavior and altered cardiovascular function induced by mild sodium depletion in rats. Behav. Neurosci. 2006, 120, 1133–1143. [Google Scholar] [CrossRef]
- Morris, M.J.; Na, E.S.; Grippo, A.J.; Johnson, A.K. The effects of deoxycorticosterone-induced sodium appetite on hedonic behaviors in the rat. Behav. Neurosci. 2006, 120, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Leshem, M. Low dietary sodium is anxiogenic in rats. Physiol. Behav. 2011, 103, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Blank, M.C.; Bedarf, J.R.; Russ, M.; Grosch-Ott, S.; Thiele, S.; Unger, J.K. Total body Na+-depletion without hyponatraemia can trigger overtraining-like symptoms with sleeping disorders and increasing blood pressure: Explorative case and literature study. Med. Hypotheses 2012, 79, 799–804. [Google Scholar] [CrossRef]
- Fan, S.S.; Lin, L.F.; Chen, V.C.; Hsieh, C.W.; Hsiao, H.P.; McIntyre, R.S.; Iacobucci, M.; Coles, A.S.; Tsai, D.J.; Weng, J.C.; et al. Effects of Lower Past-Year Serum Sodium and Hyponatremia on Depression Symptoms and Cognitive Impairments in Patients with Hemodialysis. Ther. Apher. Dial. 2020, 24, 169–177. [Google Scholar] [CrossRef]
- Graudal, N.A.; Hubeck-Graudal, T.; Jurgens, G. Effects of low sodium diet versus high sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride. Cochrane Database Syst. Rev. 2020, 12, CD004022. [Google Scholar] [CrossRef]
- Vivas, L.; Godino, A.; Dalmasso, C.; Caeiro, X.E.; Macchione, A.F.; Cambiasso, M.J. Neurochemical Circuits Subserving Fluid Balance and Baroreflex: A Role for Serotonin, Oxytocin, and Gonadal Steroids. In Neurobiology of Body Fluid Homeostasis: Transduction and Integration; De Luca, L.A., Jr., Menani, J.V., Johnson, A.K., Eds.; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Saavedra, J.M.; Benicky, J.; Zhou, J. Angiotensin II: Multitasking in the brain. J. Hypertens. Suppl. 2006, 24, S131–S137. [Google Scholar] [CrossRef]
- Saavedra, J.M.; Sanchez-Lemus, E.; Benicky, J. Blockade of brain angiotensin II AT1 receptors ameliorates stress, anxiety, brain inflammation and ischemia: Therapeutic implications. Psychoneuroendocrinology 2011, 36, 1–18. [Google Scholar] [CrossRef]
- Turkheimer, F.E.; Althubaity, N.; Schubert, J.; Nettis, M.A.; Cousins, O.; Dima, D.; Mondelli, V.; Bullmore, E.T.; Pariante, C.; Veronese, M. Increased serum peripheral C-reactive protein is associated with reduced brain barriers permeability of TSPO radioligands in healthy volunteers and depressed patients: Implications for inflammation and depression. Brain Behav. Immun. 2021, 91, 487–497. [Google Scholar] [CrossRef]
- Dantzer, R. Cytokine-induced sickness behavior: Mechanisms and implications. Ann. N. Y. Acad. Sci. 2001, 933, 222–234. [Google Scholar] [CrossRef]
- Murck, H.; Lehr, L.; Jezova, D. A viewpoint on aldosterone and BMI related brain morphology in relation to treatment outcome in patients with major depression. J. Neuroendocr. 2022, 35, e13219. [Google Scholar] [CrossRef]
- Bravi, B.; Melloni, E.M.T.; Paolini, M.; Palladini, M.; Calesella, F.; Servidio, L.; Agnoletto, E.; Poletti, S.; Lorenzi, C.; Colombo, C.; et al. Choroid plexus volume is increased in mood disorders and associates with circulating inflammatory cytokines. Brain Behav. Immun. 2023, 116, 52–61. [Google Scholar] [CrossRef]
- Cao, Y.; Lizano, P.; Deng, G.; Sun, H.; Zhou, X.; Xie, H.; Zhan, Y.; Mu, J.; Long, X.; Xiao, H.; et al. Brain-derived subgroups of bipolar II depression associate with inflammation and choroid plexus morphology. Psychiatry Clin. Neurosci. 2023, 77, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, V.; Gonzalez-Escamilla, G.; Ciolac, D.; Albrecht, P.; Kury, P.; Gruchot, J.; Dietrich, M.; Hecker, C.; Muntefering, T.; Bock, S.; et al. Translational value of choroid plexus imaging for tracking neuroinflammation in mice and humans. Proc. Natl. Acad. Sci. USA 2021, 118, e2025000118. [Google Scholar] [CrossRef] [PubMed]
- Lizano, P.; Lutz, O.; Ling, G.; Lee, A.M.; Eum, S.; Bishop, J.R.; Kelly, S.; Pasternak, O.; Clementz, B.; Pearlson, G.; et al. Association of Choroid Plexus Enlargement with Cognitive, Inflammatory, and Structural Phenotypes Across the Psychosis Spectrum. Am. J. Psychiatry 2019, 176, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Morita, K.; Sasaki, H.; Fujimoto, K.; Furuse, M.; Tsukita, S. Claudin-11/OSP-based tight junctions of myelin sheaths in brain and Sertoli cells in testis. J. Cell Biol. 1999, 145, 579–588. [Google Scholar] [CrossRef]
- Wakayama, K.; Shimamura, M.; Yoshida, S.; Hayashi, H.; Ju, N.; Nakagami, H.; Morishita, R. Prevention of vascular dementia via immunotherapeutic blockade of renin-angiotensin system in a rat model. Brain Res. 2021, 1772, 147667. [Google Scholar] [CrossRef] [PubMed]
- Kolbasi, B.; Bulbul, M.V.; Karabulut, S.; Altun, C.E.; Cakici, C.; Ulfer, G.; Mudok, T.; Keskin, I. Chronic unpredictable stress disturbs the blood-testis barrier affecting sperm parameters in mice. Reprod. Biomed. Online 2021, 42, 983–995. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.W.; Wang, X.; Zhao, Y.; Sun, Z.X.; Wu, Y.H.; Hu, H.; Zhang, L.; Wang, S.D.; Li, F.; Wei, A.J.; et al. Blood-brain barrier dysfunction mediated by the EZH2-Claudin-5 axis drives stress-induced TNF-α infiltration and depression-like behaviors. Brain Behav. Immun. 2024, 115, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, K.; Chiba, H.; Fujita, H.; Kojima, T.; Saito, T.; Endo, T.; Sawada, N. Possible involvement of gap junctions in the barrier function of tight junctions of brain and lung endothelial cells. J. Cell. Physiol. 2006, 208, 123–132. [Google Scholar] [CrossRef]
- Yi, R.; Xiao-Ping, G.; Hui, L. Atorvastatin prevents angiotensin II-induced high permeability of human arterial endothelial cell monolayers via ROCK signaling pathway. Biochem. Biophys. Res. Commun. 2015, 459, 94–99. [Google Scholar] [CrossRef]
- Yang, L.; Chen, Z.; Wan, X.; Liu, M.; Wu, J.; Chen, Y.; Zhang, G.; Fan, Z. Angiotensin II type 1 receptor deficiency protects against the impairment of blood-brain barrier in a mouse model of traumatic brain injury. Int. J. Neurosci. 2023, 133, 604–611. [Google Scholar] [CrossRef]
- Brocca, M.E.; Pietranera, L.; de Kloet, E.R.; De Nicola, A.F. Mineralocorticoid Receptors, Neuroinflammation and Hypertensive Encephalopathy. Cell Mol. Neurobiol. 2019, 39, 483–492. [Google Scholar] [CrossRef]
- Cooper, J.N.; Tepper, P.; Barinas-Mitchell, E.; Woodard, G.A.; Sutton-Tyrrell, K. Serum aldosterone is associated with inflammation and aortic stiffness in normotensive overweight and obese young adults. Clin. Exp. Hypertens. 2012, 34, 63–70. [Google Scholar] [CrossRef]
- Duprez, D.A. Role of the renin-angiotensin-aldosterone system in vascular remodeling and inflammation: A clinical review. J. Hypertens. 2006, 24, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Felder, R.B. Mineralocorticoid receptors, inflammation and sympathetic drive in a rat model of systolic heart failure. Exp. Physiol. 2010, 95, 19–25. [Google Scholar] [CrossRef]
- Fukuda, S.; Horimai, C.; Harada, K.; Wakamatsu, T.; Fukasawa, H.; Muto, S.; Itai, A.; Hayashi, M. Aldosterone-induced kidney injury is mediated by NFκB activation. Clin. Exp. Nephrol. 2011, 15, 41–49. [Google Scholar] [CrossRef]
- Gomez-Sanchez, E.P. Mineralocorticoid receptors in the brain and cardiovascular regulation: Minority rule? Trends Endocrinol. Metab. 2011, 22, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Rocha, R.; Rudolph, A.E.; Frierdich, G.E.; Nachowiak, D.A.; Kekec, B.K.; Blomme, E.A.; McMahon, E.G.; Delyani, J.A. Aldosterone induces a vascular inflammatory phenotype in the rat heart. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H1802–H1810. [Google Scholar] [CrossRef] [PubMed]
- Dinh, Q.N.; Young, M.J.; Evans, M.A.; Drummond, G.R.; Sobey, C.G.; Chrissobolis, S. Aldosterone-induced oxidative stress and inflammation in the brain are mediated by the endothelial cell mineralocorticoid receptor. Brain Res. 2016, 1637, 146–153. [Google Scholar] [CrossRef]
- Bay-Richter, C.; Hallberg, L.; Ventorp, F.; Janelidze, S.; Brundin, L. Aldosterone synergizes with peripheral inflammation to induce brain IL-1β expression and depressive-like effects. Cytokine 2012, 60, 749–754. [Google Scholar] [CrossRef]


| Stressor | Stressor Description |
|---|---|
| Social isolation | Alone in the cage |
| Unknown cage-mate | Sharing the cage with an unknown rat |
| Stroboscopic light | Light flashes with a frequency of 5 flashes/s |
| Cage tilt | Cages tilted by 45 degrees |
| Wet cage | Water surface 2 cm above the bottom of the cage |
| Continuous lighting | Lights on for 24 h |
| Water deprivation | Without water for 12 h |
| White noise | Sound of 90 dB for 12 h |
| Uncomfortable cage | Cage without bedding |
| Gene | Sense | Sequence 5′ → 3′ |
|---|---|---|
| ZO-1 | Forward | CATGAGAAGCAGACACCCACT |
| Reverse | CAGTTTCATGCTGGGCCTAA | |
| Claudin-5 | Forward | CGCTTGTGGCACTCTTTGT |
| Reverse | ACTCCCGGACTACGATGTTG | |
| Claudin-11 | Forward | ATTGGCATCATCGTCACAAC |
| Reverse | ATGTCCACCAGGGGCTTG | |
| HPRT1 | Forward | CGTCGTGATTAGTGATGATGAAC |
| Reverse | CAAGTCTTTCAGTCCTGTCCATAA | |
| TfR1 | Forward | ATACGTTCCCCGTTGTTGAGG |
| Reverse | GGCGGAAACTGAGTATGGTTGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murck, H.; Karailiev, P.; Karailievova, L.; Puhova, A.; Jezova, D. Treatment with Glycyrrhiza glabra Extract Induces Anxiolytic Effects Associated with Reduced Salt Preference and Changes in Barrier Protein Gene Expression. Nutrients 2024, 16, 515. https://doi.org/10.3390/nu16040515
Murck H, Karailiev P, Karailievova L, Puhova A, Jezova D. Treatment with Glycyrrhiza glabra Extract Induces Anxiolytic Effects Associated with Reduced Salt Preference and Changes in Barrier Protein Gene Expression. Nutrients. 2024; 16(4):515. https://doi.org/10.3390/nu16040515
Chicago/Turabian StyleMurck, Harald, Peter Karailiev, Lucia Karailievova, Agnesa Puhova, and Daniela Jezova. 2024. "Treatment with Glycyrrhiza glabra Extract Induces Anxiolytic Effects Associated with Reduced Salt Preference and Changes in Barrier Protein Gene Expression" Nutrients 16, no. 4: 515. https://doi.org/10.3390/nu16040515
APA StyleMurck, H., Karailiev, P., Karailievova, L., Puhova, A., & Jezova, D. (2024). Treatment with Glycyrrhiza glabra Extract Induces Anxiolytic Effects Associated with Reduced Salt Preference and Changes in Barrier Protein Gene Expression. Nutrients, 16(4), 515. https://doi.org/10.3390/nu16040515