Timing and Nutrient Type of Isocaloric Snacks Impacted Postprandial Glycemic and Insulinemic Responses of the Subsequent Meal in Healthy Subjects
Abstract
1. Introduction
2. Materials and Methods
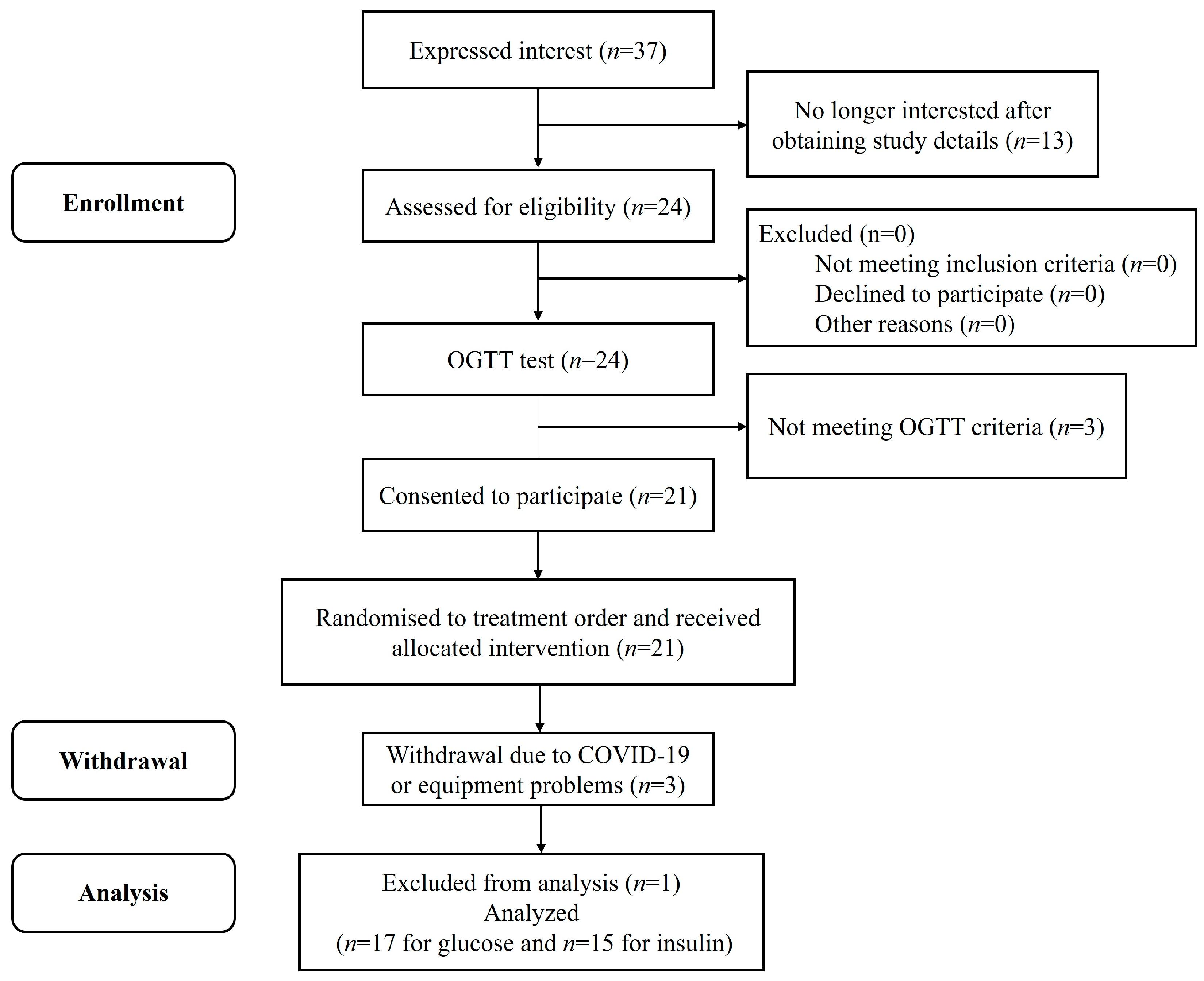
2.1. Participants and Ethics
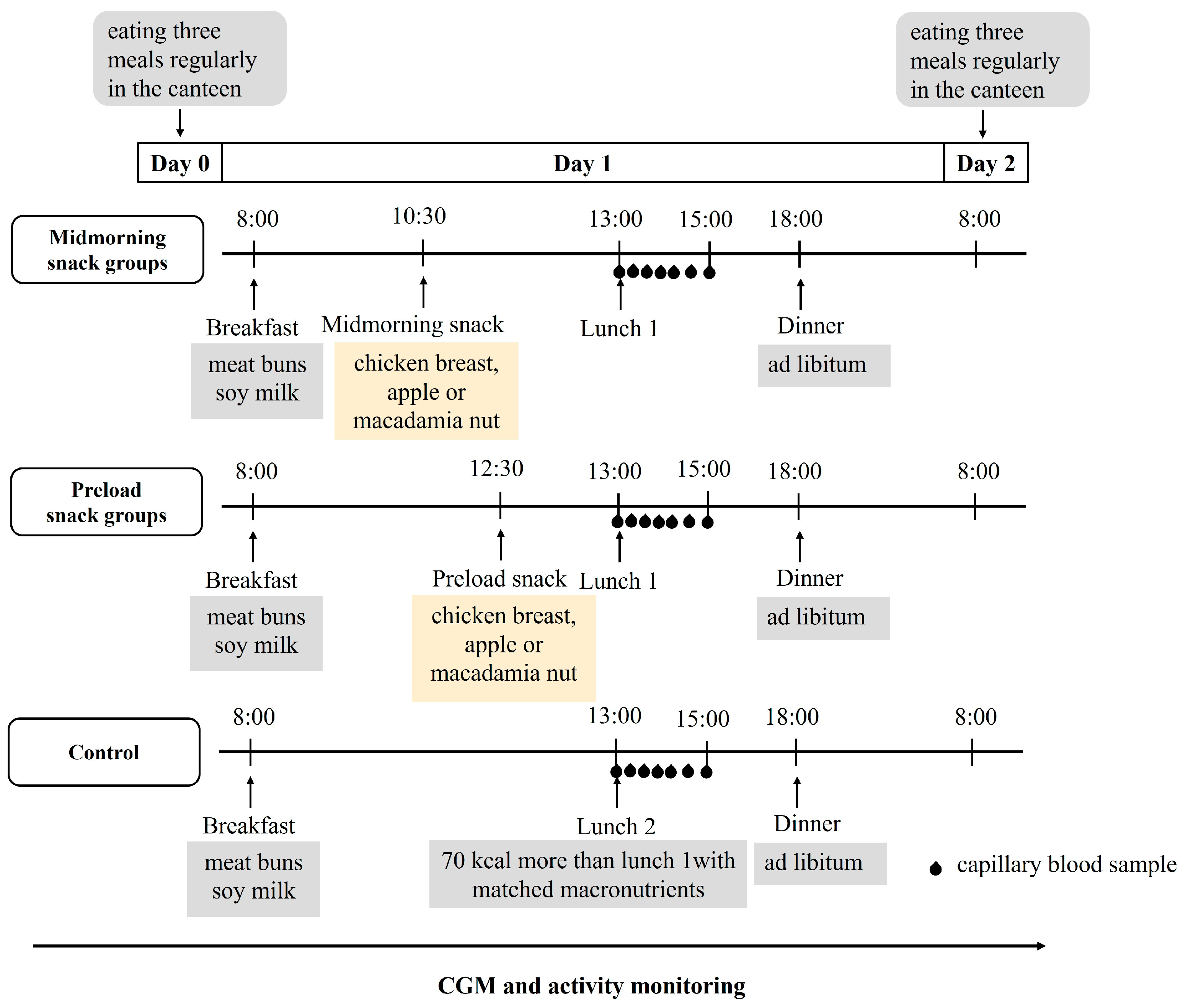
2.2. Study Design and Procedures
2.3. Test Meal Challenge
2.4. Continuous Glucose Monitoring
2.5. Blood Collection and Analysis
2.6. Data Processing and Statistical Analysis
3. Results
3.1. Baseline Characteristics of Participants
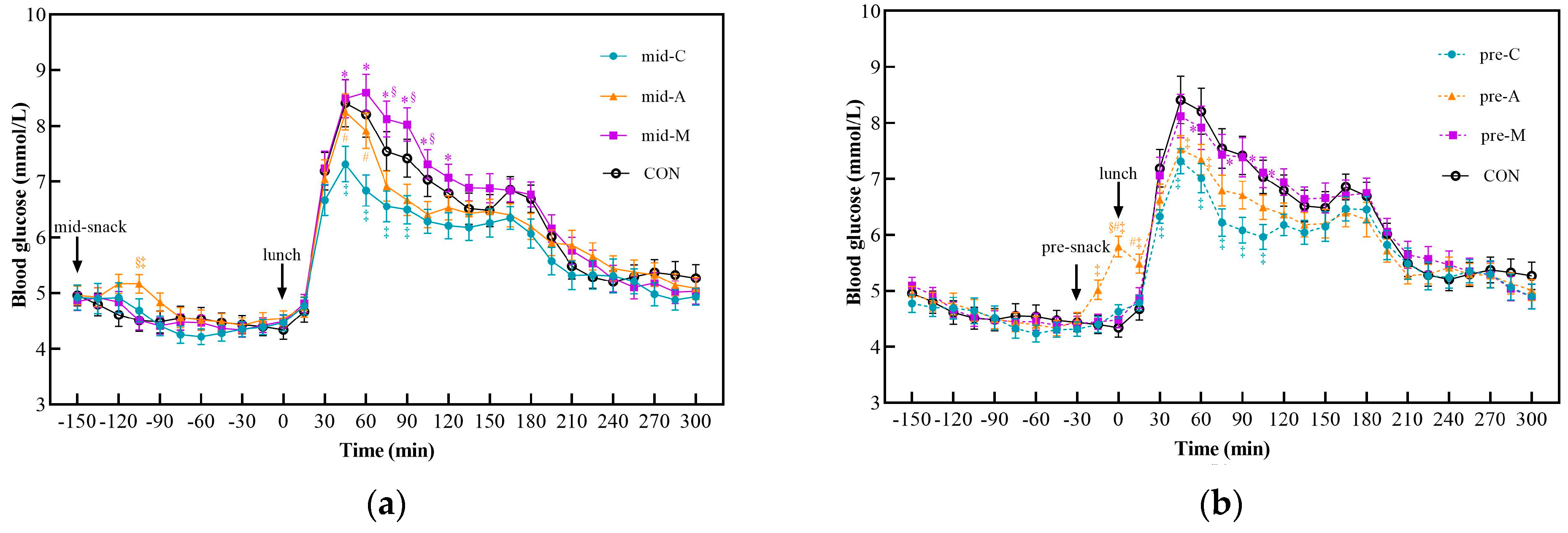
3.2. Postprandial Glycemic Response after Snacks and Lunch
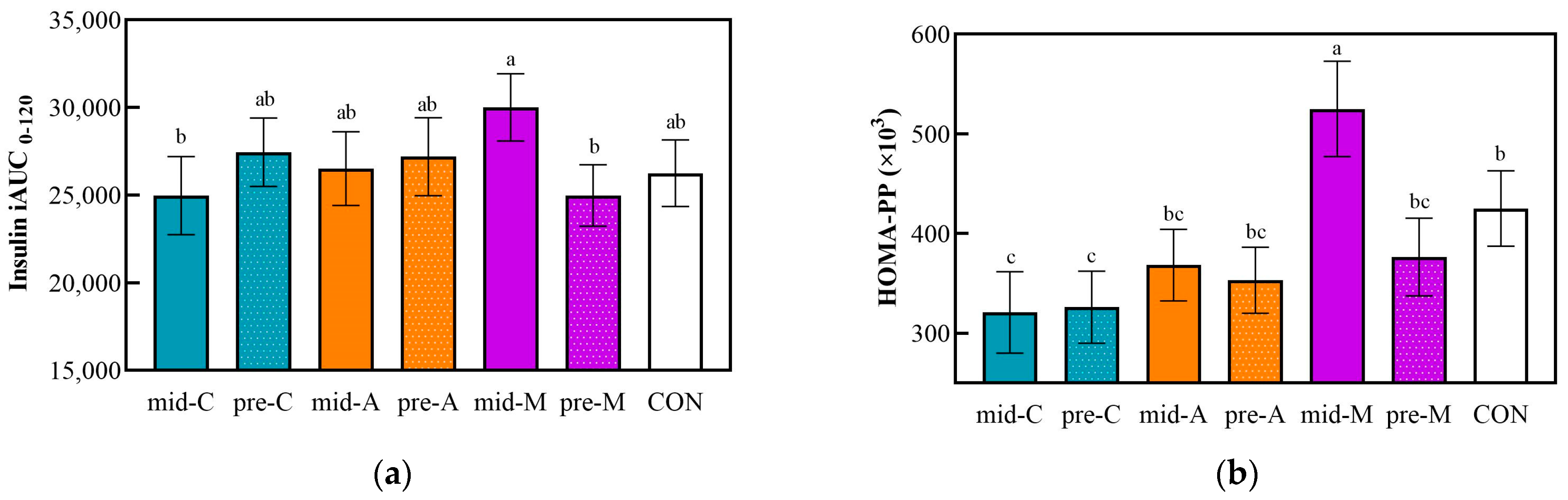
3.3. Postprandial Insulinemic Response after Lunch
3.4. Energy Intake and Postprandial Glycemic Response at Dinner
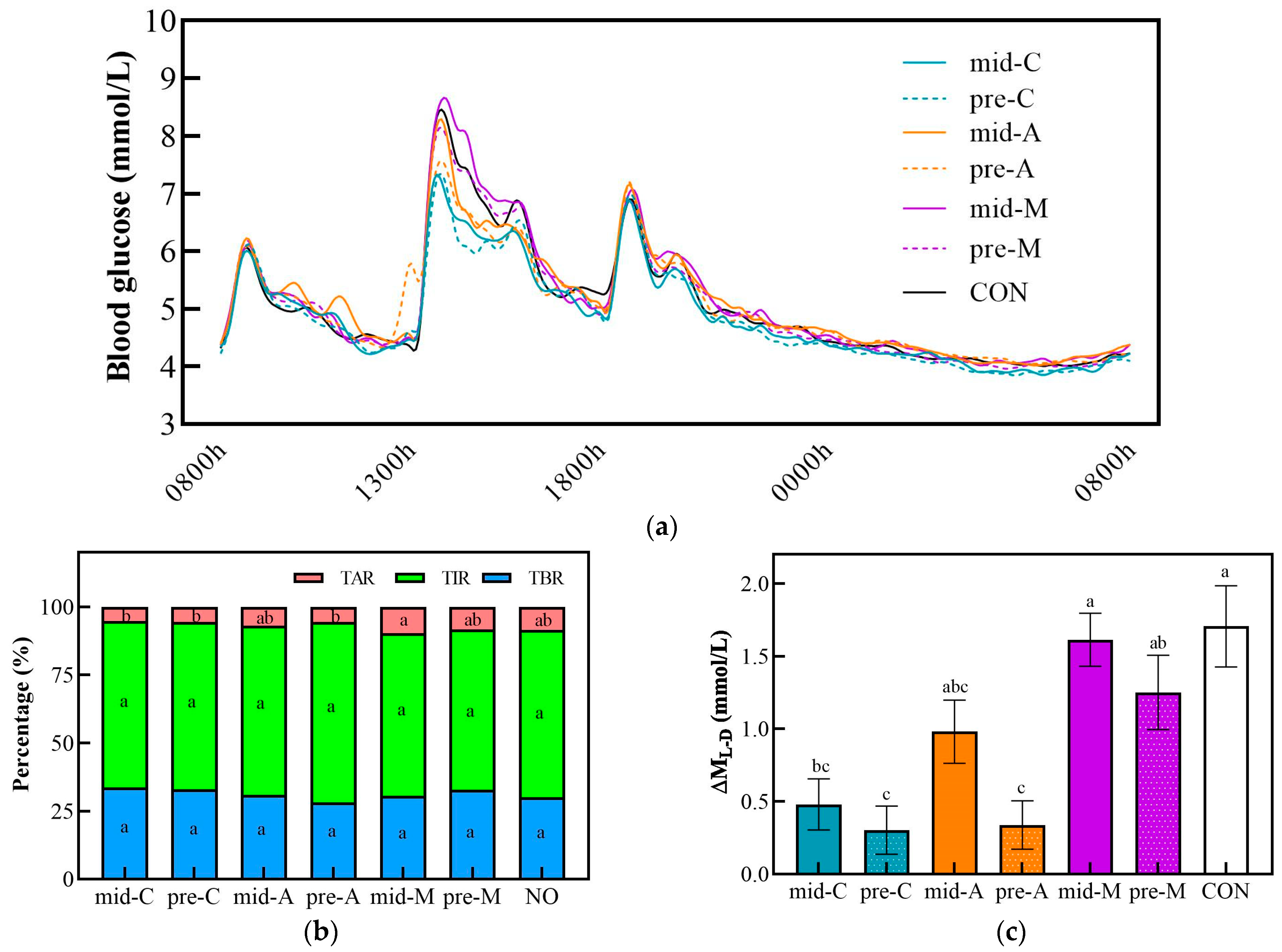
3.5. 24 h Glucose Trace
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heller, T.; Kloos, C.; Kessler, D.; Müller, N.; Thierbach, R.; Wolf, G.; Müller, U.A. Use of snacks in insulin-treated people with diabetes mellitus and association with HbA1c, weight and quality of life: A cross sectional study. Diabet. Med. 2015, 32, 353–358. [Google Scholar] [CrossRef]
- Almoraie, N.M.; Saqaan, R.; Alharthi, R.; Alamoudi, A.; Badh, L.; Shatwan, I.M. Snacking patterns throughout the life span: Potential implications on health. Nutr. Res. 2021, 91, 81–94. [Google Scholar] [CrossRef]
- Hartmann, C.; Siegrist, M.; van der Horst, K. Snack frequency: Associations with healthy and unhealthy food choices. Public Health Nutr. 2013, 16, 1487–1496. [Google Scholar] [CrossRef] [PubMed]
- Leidy, H.J.; Campbell, W.W. The effect of eating frequency on appetite control and food intake: Brief synopsis of controlled feeding studies. J. Nutr. 2011, 141, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Reister, E.J.; Leidy, H.J. An afternoon hummus snack affects diet quality, appetite, and glycemic control in healthy adults. J. Nutr. 2020, 150, 2214–2222. [Google Scholar] [CrossRef] [PubMed]
- Imai, S.; Kajiyama, S.; Hashimoto, Y.; Nitta, A.; Miyawaki, T.; Matsumoto, S.; Ozasa, N.; Tanaka, M.; Kajiyama, S.; Fukui, M. Consuming snacks mid-afternoon compared with just after lunch improves mean amplitude of glycaemic excursions in patients with type 2 diabetes: A randomized crossover clinical trial. Diabetes Metab. 2018, 44, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Nitta, A.; Imai, S.; Kajiyama, S.; Miyawaki, T.; Matsumoto, S.; Ozasa, N.; Kajiyama, S.; Hashimoto, Y.; Tanaka, M.; Fukui, M. Impact of different timing of consuming sweet snack on postprandial glucose excursions in healthy women. Diabetes Metab. 2019, 45, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Masutomi, H.; Mineshita, Y.; Ishihara, K.; Hirao, K.; Shibata, S.; Furutani, A. Effects of intake of four types of snack with different timings on postprandial glucose levels after dinner. Eur. J. Nutr. 2023, 62, 2217–2231. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.J.; Lu, J.C.; Fan, Z.H.; Liu, A.S.; Zhao, W.Q.; Wu, Y.X.; Zhu, R.X. Both isocarbohydrate and hypercarbohydrate fruit preloads curbed postprandial glycemic excursion in healthy subjects. Nutrients 2021, 13, 2470. [Google Scholar] [CrossRef] [PubMed]
- Nesti, L.; Mengozzi, A.; Tricò, D. Impact of nutrient type and sequence on glucose tolerance: Physiological insights and therapeutic implications. Front. Endocrinol. 2019, 10, 144. [Google Scholar] [CrossRef]
- Tricò, D.; Baldi, S.; Tulipani, A.; Frascerra, S.; Macedo, M.P.; Mari, A.; Ferrannini, E.; Natali, A. Mechanisms through which a small protein and lipid preload improves glucose tolerance. Diabetologia 2015, 58, 2503–2512. [Google Scholar] [CrossRef]
- Jovanovic, A.; Gerrard, J.; Taylor, R. The second-meal phenomenon in type 2 diabetes. Diabetes Care 2009, 32, 1199–1201. [Google Scholar] [CrossRef]
- Chen, M.J.; Jovanovic, A.; Taylor, R. Utilizing the second-meal effect in type 2 diabetes: Practical use of a soya-yogurt snack. Diabetes Care 2010, 33, 2552–2554. [Google Scholar] [CrossRef]
- Bandin, C.; Scheer, F.A.J.L.; Luque, A.J.; Avila-Gandia, V.; Zamora, S.; Madrid, J.A.; Gomez-Abellan, P.; Garaulet, M. Meal timing affects glucose tolerance, substrate oxidation and circadian-related variables: A randomized, crossover trial. Int. J. Obes. 2015, 39, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Mattes, R.D. Snacking: A cause for concern. Physiol. Behav. 2018, 193, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Papakonstantinou, E.; Galanopoulos, K.; Kapetanakou, A.E.; Gkerekou, M.; Skandamis, P.N. Short-term effects of traditional Greek meals: Lentils with lupins, Trahana with tomato sauce and Halva with currants and dried figs on postprandial glycemic responses—A randomized clinical trial in healthy humans. Int. J. Environ. Res. Public Health 2022, 19, 11502. [Google Scholar] [CrossRef]
- Hoss, U.; Budiman, E.S. Factory-calibrated continuous glucose sensors: The science behind the technology. Diabetes Technol. Ther. 2017, 19, S44–S50. [Google Scholar] [CrossRef]
- Banholzer, N.; Herzig, D.; Piazza, C.; Álvarez-Martínez, M.; Nakas, C.T.; Kosinski, C.; Feuerriegel, S.; Hovorka, R.; Bally, L. Effect of nutrition on postprandial glucose control in hospitalized patients with type 2 diabetes receiving fully automated closed-loop insulin therapy. Diabetes Obes. Metab. 2021, 23, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Brouns, F.; Bjorck, I.; Frayn, K.N.; Gibbs, A.L.; Lang, V.; Slama, G.; Wolever, T.M.S. Glycaemic index methodology. Nutr. Res. Rev. 2005, 18, 145–171. [Google Scholar] [CrossRef]
- McDonnell, C.M.; Donath, S.M.; Vidmar, S.I.; Werther, G.A.; Cameron, F.J. A novel approach to continuous glucose analysis utilizing glycemic variation. Diabetes Technol. Ther. 2005, 7, 253. [Google Scholar] [CrossRef]
- Lu, X.J.; Fan, Z.H.; Liu, A.S.; Liu, R.; Lou, X.L.; Hu, J.H. Extended inter-meal interval negatively impacted the glycemic and insulinemic responses after both lunch and dinner in healthy subjects. Nutrients 2022, 14, 3617. [Google Scholar] [CrossRef]
- Brynes, A.E.; Edwards, C.M.; Ghatei, M.A.; Dornhorst, A.; Morgan, L.M.; Bloom, S.R.; Frost, G.S. A randomised four-intervention crossover study investigating the effect of carbohydrates on daytime profiles of insulin, glucose, non-esterified fatty acids and triacylglycerols in middle-aged men. Br. J. Nutr. 2003, 89, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Marty, L.; Evans, R.; Sheen, F.; Humphreys, G.; Jones, A.; Boyland, E.; Robinson, E. The energy and nutritional content of snacks sold at supermarkets and coffee shops in the UK. J. Hum. Nutr. Diet. 2021, 34, 1035–1041. [Google Scholar] [CrossRef]
- Angelico, F.; Baratta, F.; Coronati, M.; Ferro, D.; Del Ben, M. Diet and metabolic syndrome: A narrative review. Intern. Emerg. Med. 2023, 18, 1007–1017. [Google Scholar] [CrossRef]
- Wu, L.; Wang, Z.; Zhu, J.J.; Murad, A.L.; Prokop, L.J.; Murad, M.H. Nut consumption and risk of cancer and type 2 diabetes: A systematic review and meta-analysis. Nutr. Rev. 2015, 73, 409–425. [Google Scholar] [CrossRef] [PubMed]
- Arabi, S.M.; Bahrami, L.S.; Milkarizi, N.; Nematy, M.; Kalmykov, V.; Sahebkar, A. Impact of walnut consumption on cardio metabolic and anthropometric parameters in metabolic syndrome patients: GRADE-assessed systematic review and dose-response meta-analysis of data from randomized controlled trials. Pharmacol. Res. 2022, 178, 106190. [Google Scholar] [CrossRef]
- Zhao, C.N.; Meng, X.; Li, Y.; Li, S.; Liu, Q.; Tang, G.Y.; Li, H.B. Fruits for prevention and treatment of cardiovascular diseases. Nutrients 2017, 9, 598. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Sun, S.; Su, Y.W.; Ying, C.F.; Luo, H. Effect of fruit on glucose control in diabetes mellitus: A meta-analysis of nineteen randomized controlled trials. Front. Endocrinol. 2023, 14, 1174545. [Google Scholar] [CrossRef]
- Bondonno, N.P.; Davey, R.J.; Murray, K.; Radavelli-Bagatini, S.; Bondonno, C.P.; Blekkenhorst, L.C.; Sim, M.; Magliano, D.J.; Daly, R.M.; Shaw, J.E.; et al. Associations between fruit intake and risk of diabetes in the AusDiab cohort. J. Clin. Endocrinol. 2021, 106, E4097–E4108. [Google Scholar] [CrossRef]
- Wu, Q.J.; Wu, L.; Zheng, L.Q.; Xu, X.; Ji, C.; Gong, T.T. Consumption of fruit and vegetables reduces risk of pancreatic cancer: Evidence from epidemiological studies. Eur. J. Cancer Prev. 2016, 25, 196–205. [Google Scholar] [CrossRef]
- Kim, Y.; Keogh, J.B.; Clifton, P.M. Benefits of nut consumption on insulin resistance and cardiovascular risk factors: Multiple potential mechanisms of actions. Nutrients 2017, 9, 1271. [Google Scholar] [CrossRef]
- Meng, H.C.; Matthan, N.R.; Ausman, L.M.; Lichtenstein, A.H. Effect of prior meal macronutrient composition on postprandial glycemic responses and glycemic index and glycemic load value determinations. Am. J. Clin. Nutr. 2017, 106, 1246–1256. [Google Scholar] [CrossRef]
- Ma, J.; Stevens, J.E.; Cukier, K.; Maddox, A.F.; Wishart, J.M.; Jones, K.L.; Clifton, P.M.; Horowitz, M.; Rayner, C.K. Effects of a protein preload on gastric emptying, glycemia, and gut hormones after a carbohydrate meal in diet-controlled type 2 diabetes. Diabetes Care 2009, 32, 1600–1602. [Google Scholar] [CrossRef]
- Jakubowicz, D.; Froy, O.; Ahrén, B.; Boaz, M.; Landau, Z.; Bar-Dayan, Y.; Ganz, T.; Barnea, M.; Wainstein, J. Incretin, insulinotropic and glucose-lowering effects of whey protein pre-load in type 2 diabetes: A randomised clinical trial. Diabetologia 2014, 57, 1807–1811. [Google Scholar] [CrossRef] [PubMed]
- Ton, W.T.S.; de Almeida, C.D.; Cardoso, L.D.; Girondoli, Y.M.; Pereira, P.F.; Schitini, J.K.V.G.; Cândido, F.G.; Arbex, P.M.; Alfenas, R.D.G. Effect of different protein types on second meal postprandial glycaemia in normal weight and normoglycemic subjects. Nutr. Hosp. 2014, 29, 553–558. [Google Scholar]
- Skalkos, S.; Moschonis, G.; Thomas, C.J.; McMillan, J.; Kouris-Blazos, A. Effect of lupin-enriched biscuits as substitute mid-meal snacks on post-prandial interstitial glucose excursions in post-surgical hospital patients with type 2 diabetes. Nutrients 2020, 12, 1239. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.T.; Hao, G.X.; Lai, D.; Tian, Y.; Long, M.; Lai, F.; Xiong, Y.M.; Ji, C.F.; Zang, Y. Effect of oyster meat preload on postmeal glycemic control in healthy young adults. J. Am. Coll. Nutr. 2020, 39, 511–517. [Google Scholar] [CrossRef]
- Sun, L.; Tan, K.W.J.; Han, C.M.S.; Leow, M.K.; Henry, C.J. Impact of preloading either dairy or soy milk on postprandial glycemia, insulinemia and gastric emptying in healthy adults. Eur. J. Nutr. 2017, 56, 77–87. [Google Scholar] [CrossRef]
- Collier, G.; O’Dea, K. The effect of coingestion of fat on the glucose, insulin, and gastric-inhibitory polypeptide responses to carbohydrate and protein. Am. J. Clin. Nutr. 1983, 37, 941–944. [Google Scholar] [CrossRef]
- Ando, T.; Nakae, S.; Usui, C.; Yoshimura, E.; Nishi, N.; Takimoto, H.; Tanaka, S. Effect of diurnal variations in the carbohydrate and fat composition of meals on postprandial glycemic response in healthy adults: A novel insight for the second-meal phenomenon. Am. J. Clin. Nutr. 2018, 108, 332–342. [Google Scholar] [CrossRef]
- Mihai, B.M.; Mihai, C.; Cijevschi-Prelipcean, C.; Grigorescu, E.D.; Dranga, M.; Drug, V.; Sporea, I.; Lacatușu, C.M. Bidirectional relationship between gastric emptying and plasma glucose control in normoglycemic individuals and diabetic patients. J. Diabetes Res. 2018, 2018, 1736959. [Google Scholar] [CrossRef]
- Crouch, M.A.; Slater, R.T. Almond “appetizer” effect on glucose tolerance test (GTT) results. J. Am. Board Fam. Med. 2016, 29, 759–766. [Google Scholar] [CrossRef]
- Gulati, S.; Misra, A.; Tiwari, R.; Sharma, M.; Pandey, R.M.; Upadhyay, A.D.; Sati, H.C. Beneficial effects of premeal almond load on glucose profile on oral glucose tolerance and continuous glucose monitoring: Randomized crossover trials in Asian Indians with prediabetes. Eur. J. Clin. Nutr. 2023, 77, 586–595. [Google Scholar] [CrossRef]
- Kasuya, N.; Ohta, S.; Takanami, Y.; Kawai, Y.; Inoue, Y.; Murata, I.; Kanamoto, I. Effect of low glycemic index food and postprandial exercise on blood glucose level, oxidative stress and antioxidant capacity. Exp. Ther. Med. 2015, 9, 1201–1204. [Google Scholar] [CrossRef]
- Katsarou, V.; Tsolaki, M. Trends in Personalized Nutrition. In Personalized Nutrition by Predicting Glycemic Responses; Galanakis, C.M., Ed.; Academic Press Ltd.: London, UK, 2019; pp. 55–79. [Google Scholar]
- Gentilcore, D.; Chaikomin, R.; Jones, K.L.; Russo, A.; Feinle-Bisset, C.; Wishart, J.M.; Rayner, C.K.; Horowitz, M. Effects of fat on gastric emptying of and the glycemic, insulin, and incretin responses to a carbohydrate meal in type 2 diabetes. J. Clin. Endocrinol. 2006, 91, 2062–2067. [Google Scholar] [CrossRef]
- Hakim, B.N.A.; Yahya, H.M.; Shahar, S.; Abdul Manaf, Z.; Damanhuri, H. Effect of sequence of fruit intake in a meal on satiety. Int. J. Environ. 2019, 16, 4464. [Google Scholar]
- Lubransky, A.; Monro, J.; Mishra, S.; Yu, H.; Haszard, J.J.; Venn, B.J. Postprandial glycaemic, hormonal and satiety responses to rice and kiwifruit preloads in Chinese adults: A randomised controlled crossover trial. Nutrients 2018, 10, 1110. [Google Scholar] [CrossRef]
- Lu, J.C.; Zhao, W.Q.; Wang, L.L.; Fan, Z.H.; Zhu, R.X.; Wu, Y.X.; Zhou, Y. Apple preload halved the postprandial glycaemic response of rice meal in healthy subjects. Nutrients 2019, 11, 2912. [Google Scholar] [CrossRef]
- Pullicin, A.J.; Glendinning, J.I.; Lim, J. Cephalic phase insulin release: A review of its mechanistic basis and variability in humans. Physiol. Behav. 2021, 239, 113514. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.F.; Laurent, D.; Yu, C.L.; Cline, G.W.; Shulman, G.I. Stimulating effects of low-dose fructose on insulin-stimulated hepatic glycogen synthesis in humans. Diabetes 2001, 50, 1263–1268. [Google Scholar] [CrossRef]
- Sievenpiper, J.L.; Chiavaroli, L.; de Souza, R.J.; Mirrahimi, A.; Cozma, A.I.; Ha, V.; Wang, D.; Yu, M.E.; Carleton, A.J.; Beyene, J.; et al. ‘Catalytic’ doses of fructose may benefit glycaemic control without harming cardiometabolic risk factors: A small meta-analysis of randomised controlled feeding trials. Br. J. Nutr. 2012, 108, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; McLaughlin, A.; Monro, J. Food order and timing effects on glycaemic and satiety responses to partial fruit-for-cereal carbohydrate exchange: A randomized cross-over human intervention study. Nutrients 2023, 15, 3269. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Wang, Y.T.; Xiao, J.B. Dietary polyphenols and type 2 diabetes: Human study and clinical trial. Free. Radic. Biol. Med. 2017, 112, 158. [Google Scholar] [CrossRef]
- Raphaelli, C.D.; Pereira, E.D.; Camargo, T.M.; Vinholes, J.; Rombaldi, C.V.; Vizzotto, M.; Nora, L. Apple phenolic extracts strongly inhibit α-glucosidase activity. Plant Food Hum. Nutr. 2019, 74, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Hanhineva, K.; Törrönen, R.; Bondia-Pons, I.; Pekkinen, J.; Kolehmainen, M.; Mykkänen, H.; Poutanen, K. Impact of dietary polyphenols on carbohydrate metabolism. Int. J. Mol. Sci. 2010, 11, 1365–1402. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.F.; Yang, B.; Tang, J.; Jiang, J.J.; Li, D. Apple and pear consumption and type 2 diabetes mellitus risk: A meta-analysis of prospective cohort studies. Food Funct. 2017, 8, 927–934. [Google Scholar] [CrossRef]
- Ismaiel, M.; Yang, H.; Min, C. Dietary fiber role in type 2 diabetes prevention. Brit. Food J. 2016, 118, 961–975. [Google Scholar] [CrossRef]
- Paddon-Jones, D.; Westman, E.; Mattes, R.D.; Wolfe, R.R.; Astrup, A.; Westerterp-Plantenga, M. Protein, weight management, and satiety. Am. J. Clin. Nutr. 2008, 87, 1558S–1561S. [Google Scholar] [CrossRef]
- Lim, J.J.; Sequeira, I.R.; Yip, W.C.Y.; Lu, L.W.; Barnett, D.; Cameron-Smith, D.; Poppitt, S.D. Postprandial glycine as a biomarker of satiety: A dose-rising randomised control trial of whey protein in overweight women. Appetite 2022, 169, 105871. [Google Scholar] [CrossRef]
- Ingves, S.; Vilhelmsson, N.; Ström, E.; Fredrikson, M.; Guldbrand, H.; Nystrom, F.H. A randomized cross-over study of the effects of macronutrient composition and meal frequency on GLP-1, ghrelin and energy expenditure in humans. Peptides 2017, 93, 20–26. [Google Scholar] [CrossRef]
- Baer, D.J.; Dalton, M.; Blundell, J.; Finlayson, G.; Hu, F.B. Nuts, energy balance and body weight. Nutrients 2023, 15, 1162. [Google Scholar] [CrossRef] [PubMed]
- Rock, C.L.; Flatt, S.W.; Barkai, H.S.; Pakiz, B.; Heath, D.D. A walnut-containing meal had similar effects on early satiety, CCK, and PYY, but attenuated the postprandial GLP-1 and insulin response compared to a nut-free control meal. Appetite 2017, 117, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Zhong, V.T.W.; Van Horn, L.; Greenland, P.; Carnethon, M.R.; Ning, H.Y.; Wilkins, J.T.; Lloyd-Jones, D.M.; Allen, N.B. Associations of processed meat, unprocessed red meat, poultry, or fish intake with incident cardiovascular disease and all-cause mortality. JAMA Intern. Med. 2020, 180, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Cava, E.; Yeat, N.C.; Mittendorfer, B. Preserving healthy muscle during weight loss. Adv. Nutr. 2017, 8, 511–519. [Google Scholar] [CrossRef]

| Snack Group | C Chicken Breast | A Apple | M Macadamia Nut |
|---|---|---|---|
| Weight (g) 2 | 53.0 | 137.0 | 9.5 |
| Energy (kcal) | 70 | 70 | 70 |
| Carbohydrate (g) | 3.6 | 17.1 | 1.7 |
| Protein (g) | 12.9 | 0 | 0.8 |
| Fat (g) | 0.3 | 0 | 6.7 |
| Test Meals | Carbohydrate (g) | Protein (g) | Fat (g) | Energy (kcal) | Detail Content 2 |
|---|---|---|---|---|---|
| Lunch 1 3 | 96.1 | 23.3 | 17.7 | 637 | uncooked rice 100 g, roasted sesame dressing 25 mL, chicken patty 90 g, cherry tomato 100 g, cucumber 30 g, romaine lettuce 20 g, sesame oil 4 g |
| Lunch 2 4 | 106.4 | 25.9 | 19.8 | 707 | uncooked rice 112 g, roasted sesame dressing 25 mL, chicken patty 101 g, cherry tomato 100 g, cucumber 30 g, romaine lettuce 20 g, sesame oil 5.7 g |
| Characteristics | Mean ± SD |
|---|---|
| Age (years) | 21.8 ± 1.4 |
| BMI (kg/m2) | 21.2 ± 1.7 |
| Waist:hip ratio | 0.7 ± 0.0 |
| Fat mass (%) | 26.6 ± 2.8 |
| Visceral fat index | 2.7 ± 1.0 |
| Basal metabolic rate (BMR) (kcal/day) | 1259.4 ± 107.0 |
| Systolic blood pressure (mmHg) | 101.6 ± 7.3 |
| Diastolic blood pressure (mmHg) | 62.8 ± 5.7 |
| Before Lunch (10:30~13:00) | After Lunch (13:00~15:00) | |||||
|---|---|---|---|---|---|---|
| Test Meals | tAUC1 (mmol·min/L) | tAUC2 (mmol·min/L) | Max (mmol/L) | LAGE (mmol/L) | CV (%) | J-Index |
| mid-C | 676.4 ± 22.2 a | 754.1 ± 22.5 cd | 7.5 ± 0.3 cd | 3.1 ± 0.2 bc | 17.1 ± 1.1 bc | 17.4 ± 1.2 cd |
| pre-C | 672.1 ± 20.8 a | 736.4 ± 17.3 d | 7.4 ± 0.2 d | 2.9 ± 0.2 cd | 16.1 ± 1.1 c | 16.2 ± 0.9 d |
| mid-A | 710.1 ± 21.1 a | 802.4 ± 26.2 bc | 8.3 ± 0.3 abc | 3.8 ± 0.3 ab | 20.5 ± 1.3 ab | 20.7 ± 1.4 bc |
| pre-A | 700.7 ± 21.5 a | 795.8 ± 24.8 bc | 7.7 ± 0.2 bcd | 2.3 ± 0.2 d | 12.1 ± 0.6 d | 17.9 ± 1.1 cd |
| mid-M | 681.3 ± 19.6 a | 875.7 ± 26.7 a | 8.9 ± 0.3 a | 4.4 ± 0.3 a | 22.5 ± 1.0 a | 25.3 ± 1.6 a |
| pre-M | 682.4 ± 18.8 a | 834.2 ± 29.5 ab | 8.5 ± 0.3 ab | 4.0 ± 0.3 a | 20.8 ± 1.2 a | 22.7 ± 1.8 ab |
| CON | 681.5 ± 26.0 a | 840.6 ± 32.6 ab | 8.8 ± 0.4 a | 4.5 ± 0.3 a | 22.6 ± 1.3 a | 23.6 ± 2.0 ab |
| Test Meals | 24 h Mean (mmol/L) | 24 h tAUC (mmol·min/L) | 24 h Max (mmol/L) | 24 h LAGE (mmol/L) | 24 h SD | 24 h CV (%) |
|---|---|---|---|---|---|---|
| mid-C | 4.9 ± 0.1 ab | 7018.2 ± 143.1 ab | 7.7 ± 0.3 c | 4.2 ± 0.3 b | 1.0 ± 0.1 bc | 20.0 ± 1.4 bc |
| pre-C | 4.8 ± 0.1 b | 6998.7 ± 141.1 b | 7.7 ± 0.2 c | 4.1 ± 0.2 b | 1.0 ± 0.0 c | 20.1 ± 0.9 bc |
| mid-A | 5.1 ± 0.1 a | 7330.1 ± 170.6 a | 8.5 ± 0.3 ab | 4.7 ± 0.3 ab | 1.0 ± 0.1 bc | 20.5 ± 0.9 abc |
| pre-A | 5.0 ± 0.1 ab | 7241.1 ± 178.7 ab | 8.0 ± 0.2 bc | 4.2 ± 0.2 b | 1.0 ± 0.0 c | 19.4 ± 1.1 c |
| mid-M | 5.1 ± 0.1 a | 7374.8 ± 146.4 a | 9.0 ± 0.3 a | 5.2 ± 0.3 a | 1.2 ± 0.1 a | 23.3 ± 1.3 a |
| pre-M | 5.0 ± 0.1 ab | 7255.7 ± 202.4 ab | 8.6 ± 0.3 ab | 4.8 ± 0.3 ab | 1.1 ± 0.1 ab | 22.6 ± 1.7 ab |
| CON | 5.0 ± 0.1 ab | 7253.3 ± 215.6 ab | 8.9 ± 0.3 a | 5.1 ± 0.3 a | 1.1 ± 0.1 ab | 22.4 ± 1.5 abc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lou, X.; Fan, Z.; Wei, J.; Peng, X.; Hu, J.; Lu, X.; Liu, A. Timing and Nutrient Type of Isocaloric Snacks Impacted Postprandial Glycemic and Insulinemic Responses of the Subsequent Meal in Healthy Subjects. Nutrients 2024, 16, 535. https://doi.org/10.3390/nu16040535
Lou X, Fan Z, Wei J, Peng X, Hu J, Lu X, Liu A. Timing and Nutrient Type of Isocaloric Snacks Impacted Postprandial Glycemic and Insulinemic Responses of the Subsequent Meal in Healthy Subjects. Nutrients. 2024; 16(4):535. https://doi.org/10.3390/nu16040535
Chicago/Turabian StyleLou, Xinling, Zhihong Fan, Jinjie Wei, Xiyihe Peng, Jiahui Hu, Xuejiao Lu, and Anshu Liu. 2024. "Timing and Nutrient Type of Isocaloric Snacks Impacted Postprandial Glycemic and Insulinemic Responses of the Subsequent Meal in Healthy Subjects" Nutrients 16, no. 4: 535. https://doi.org/10.3390/nu16040535
APA StyleLou, X., Fan, Z., Wei, J., Peng, X., Hu, J., Lu, X., & Liu, A. (2024). Timing and Nutrient Type of Isocaloric Snacks Impacted Postprandial Glycemic and Insulinemic Responses of the Subsequent Meal in Healthy Subjects. Nutrients, 16(4), 535. https://doi.org/10.3390/nu16040535






