CM1, a Chrysin Derivative, Protects from Endotoxin-Induced Lethal Shock by Regulating the Excessive Activation of Inflammatory Responses
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of CM1 and 2
2.2. Mice
2.3. Preparation of Cell
2.4. Reagents and Antibodies
2.5. Cell Viability Assays
2.6. RNA Extraction, RT-PCR and Real-Time Quantitative PCR
2.7. ELISA
2.8. Western Blot Analysis
2.9. NF-κB p65 Nuclear Translocation
2.10. siRNA Transfection
2.11. Mouse Models of Sepsis
2.12. Histology and Immunohistochemistry
2.13. Statistical Analysis
3. Results
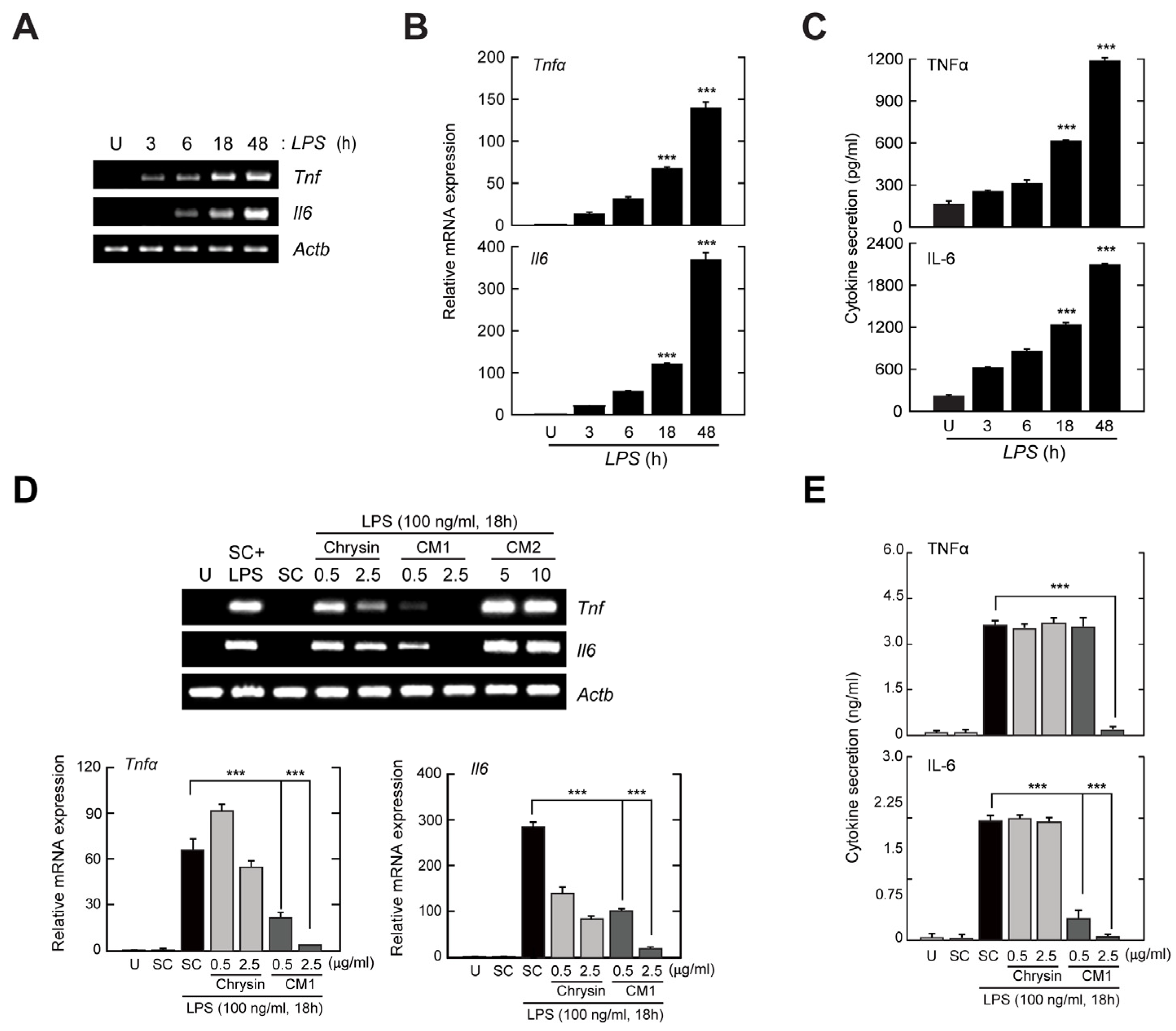
3.1. Cytotoxic Effects of Chrysin and Its Derivatives CM1 and CM2 in Primary Murine Macrophages
3.2. CM1, but Not Chrysin or CM2, Negatively Regulates TLR4-Induced Production of Inflammatory Cytokines in Primary Murine Macrophages
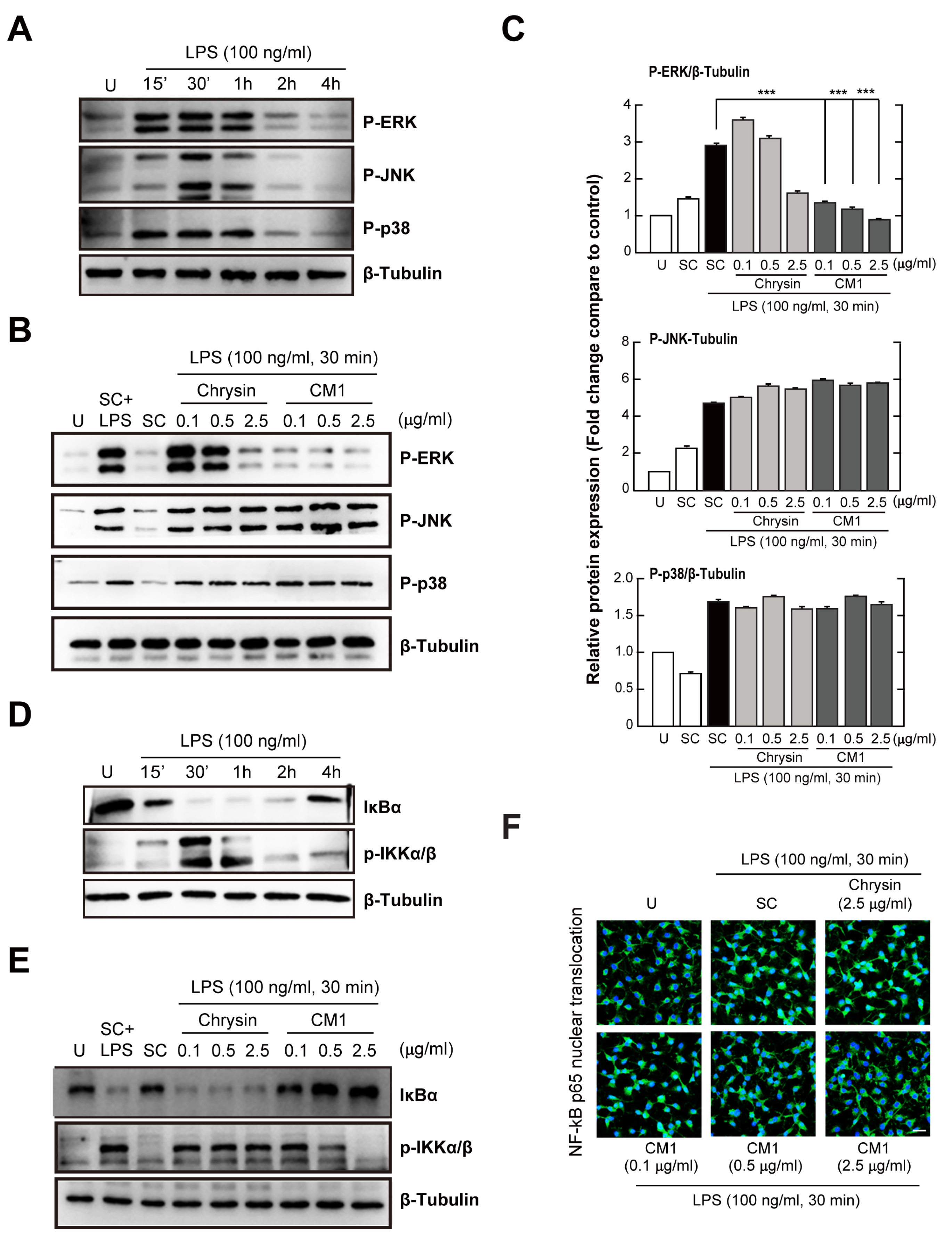
3.3. CM1 Attenuates TLR4-Induced Activation of ERK 1/2 MAPK and the NF-κB Signalling Pathway in Primary Macrophages
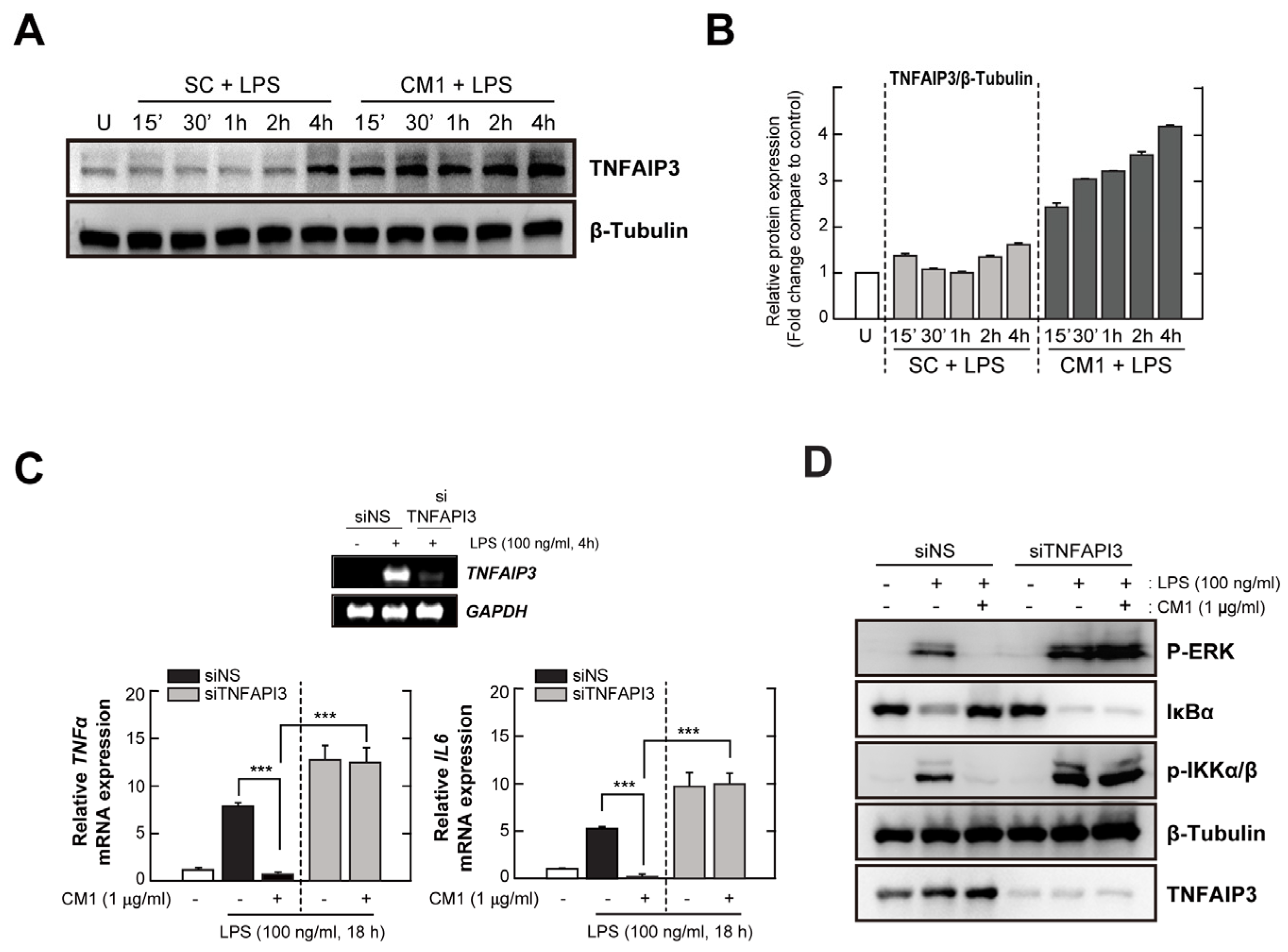
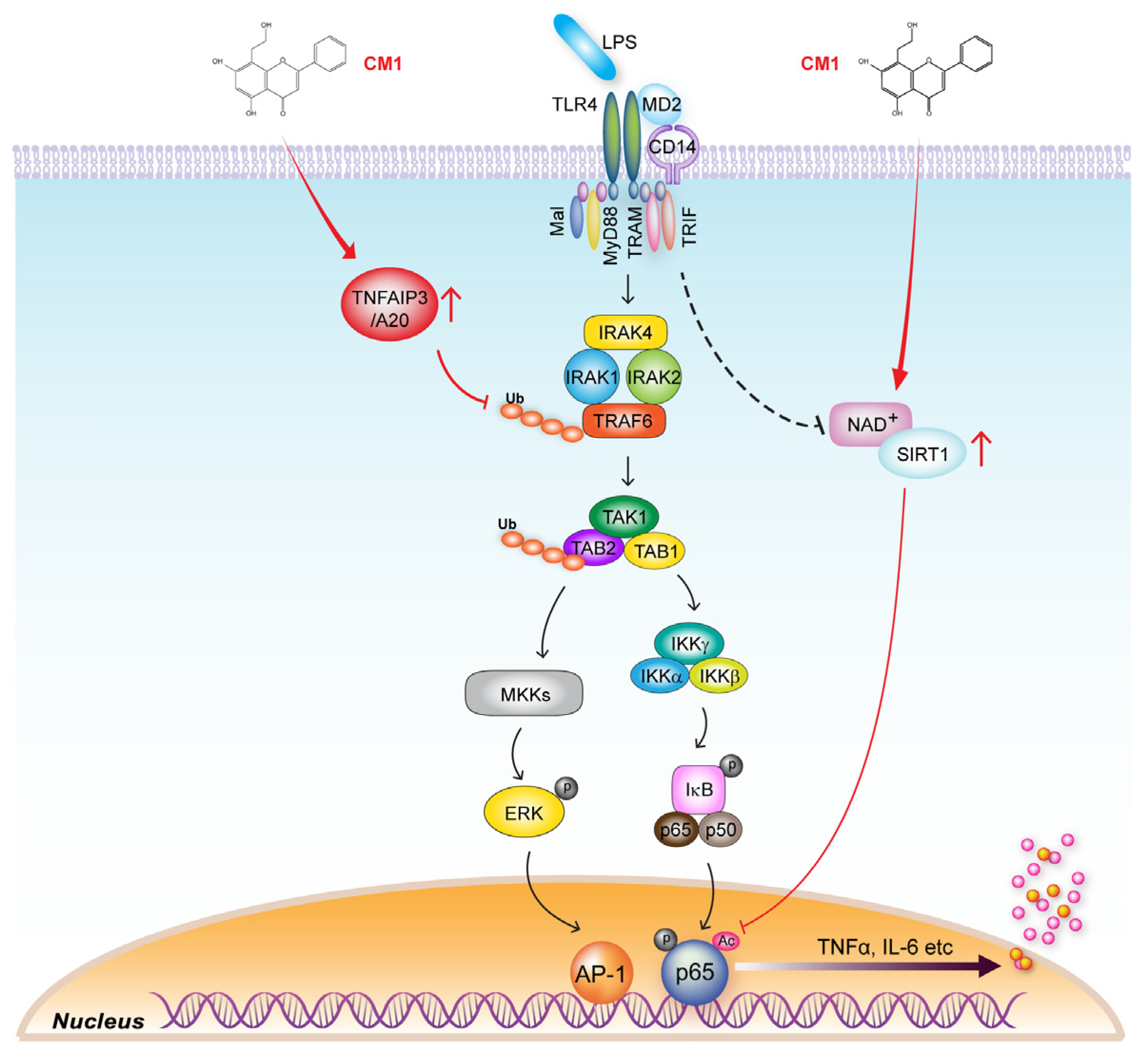
3.4. TNFAIP3 Is Crucial for the CM1-Mediated Anti-Inflammatory Effect in TLR4-Stimuated Cells
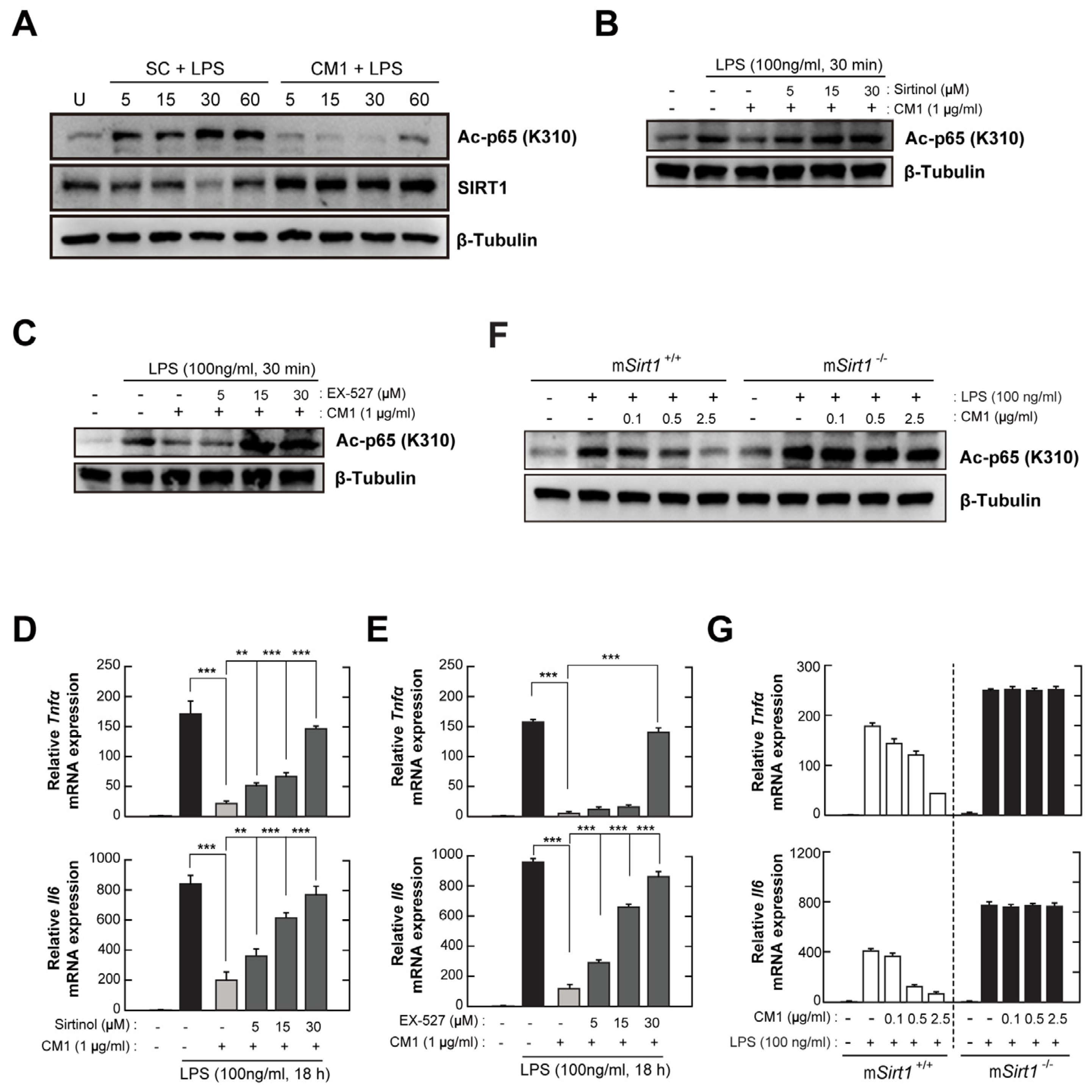
3.5. CM1 Negatively Regulates TLR4-Induced Acetylation of NF-κB p65 by Activating SIRT1
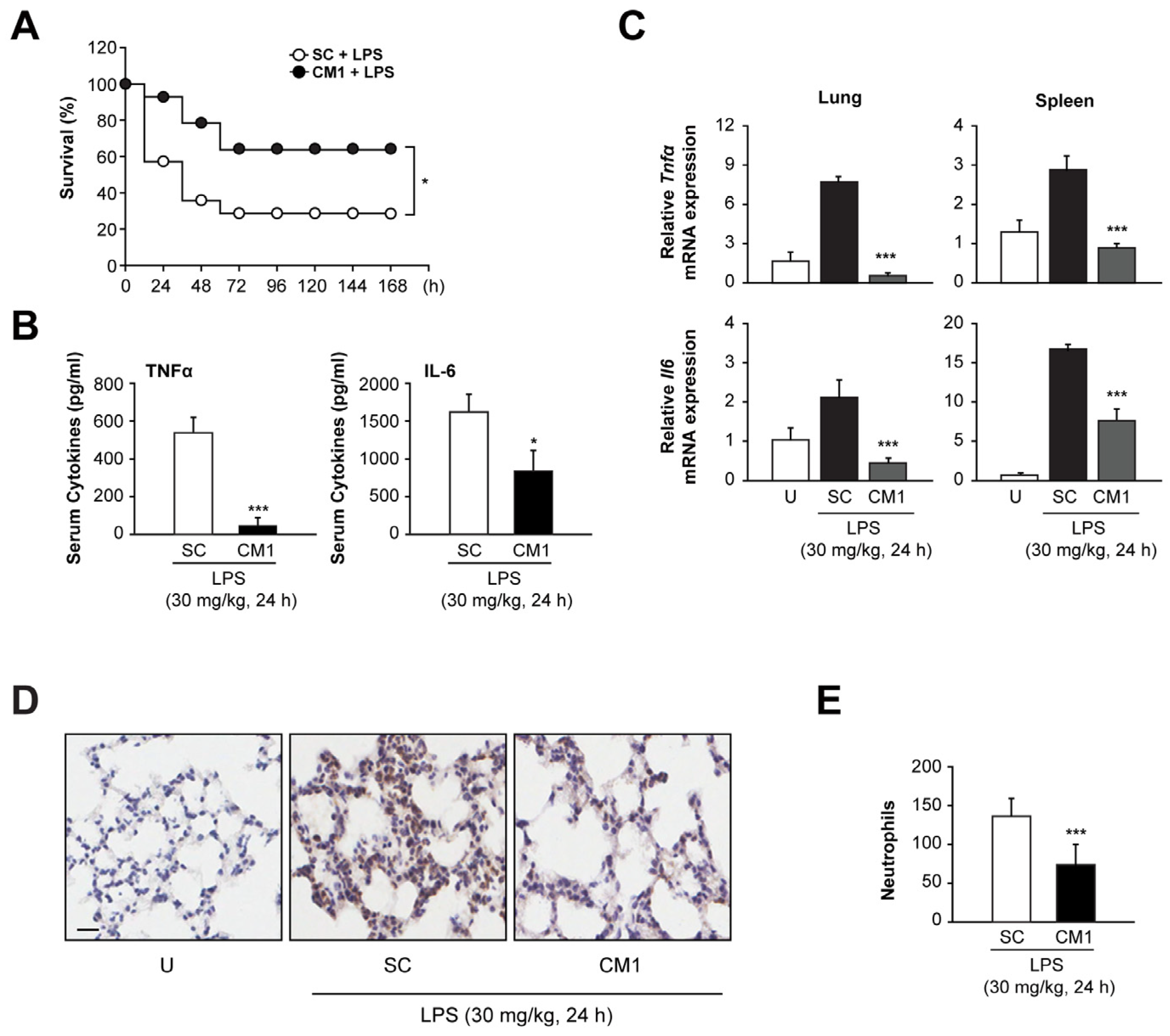
3.6. CM1 Prevents Systemic Inflammation In Vivo, Leading to Decreased Mortality during Endotoxin-Induced Lethal Shock
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cohen, J. The immunopathogenesis of sepsis. Nature 2002, 420, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, R.S.; Monneret, G.; Payen, D. Sepsis-induced immunosuppression: From cellular dysfunctions to immunotherapy. Nat. Rev. Immunol. 2013, 13, 862–874. [Google Scholar] [CrossRef] [PubMed]
- Matera, G.; Quirino, A.; Peronace, C.; Settembre, P.; Marano, V.; Loria, M.T.; Marascio, N.; Galati, L.; Barreca, G.S.; Giancotti, A.; et al. Soluble CD14 Subtype-A New Biomarker in Predicting the Outcome of Critically Ill Septic Patients. Am. J. Med. Sci. 2017, 353, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Hug, H.; Mohajeri, M.H.; La Fata, G. Toll-Like Receptors: Regulators of the Immune Response in the Human Gut. Nutrients 2018, 10, 203. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef]
- Liew, F.Y.; Xu, D.; Brint, E.K.; O’Neill, L.A. Negative regulation of toll-like receptor-mediated immune responses. Nat. Rev. Immunol. 2005, 5, 446–458. [Google Scholar] [CrossRef]
- Boone, D.L.; Turer, E.E.; Lee, E.G.; Ahmad, R.C.; Wheeler, M.T.; Tsui, C.; Hurley, P.; Chien, M.; Chai, S.; Hitotsumatsu, O.; et al. The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat. Immunol. 2004, 5, 1052–1060. [Google Scholar] [CrossRef]
- Kong, S.; McBurney, M.W.; Fang, D. Sirtuin 1 in immune regulation and autoimmunity. Immunol. Cell Biol. 2012, 90, 6–13. [Google Scholar] [CrossRef]
- Yeung, F.; Hoberg, J.E.; Ramsey, C.S.; Keller, M.D.; Jones, D.R.; Frye, R.A.; Mayo, M.W. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004, 23, 2369–2380. [Google Scholar] [CrossRef]
- Yuk, J.M.; Kim, T.S.; Kim, S.Y.; Lee, H.M.; Han, J.; Dufour, C.R.; Kim, J.K.; Jin, H.S.; Yang, C.S.; Park, K.S.; et al. Orphan Nuclear Receptor ERRalpha Controls Macrophage Metabolic Signaling and A20 Expression to Negatively Regulate TLR-Induced Inflammation. Immunity 2015, 43, 80–91. [Google Scholar] [CrossRef]
- Bertelli, A.; Biagi, M.; Corsini, M.; Baini, G.; Cappellucci, G.; Miraldi, E. Polyphenols: From Theory to Practice. Foods 2021, 10, 2595. [Google Scholar] [CrossRef]
- Safe, S.; Jayaraman, A.; Chapkin, R.S.; Howard, M.; Mohankumar, K.; Shrestha, R. Flavonoids: Structure-function and mechanisms of action and opportunities for drug development. Toxicol. Res. 2021, 37, 147–162. [Google Scholar] [CrossRef]
- Rodriguez-Landa, J.F.; German-Ponciano, L.J.; Puga-Olguin, A.; Olmos-Vazquez, O.J. Pharmacological, Neurochemical, and Behavioral Mechanisms Underlying the Anxiolytic- and Antidepressant-like Effects of Flavonoid Chrysin. Molecules 2022, 27, 3551. [Google Scholar] [CrossRef]
- Falbo, F.; Aiello, F. Chrysin: A polyedric flavone as a tool to explore new phytotherapeutic applications and drug design. Arch. Pharm. 2023, 356, e2200347. [Google Scholar] [CrossRef]
- Song, H.Y.; Sik Kim, W.; Kim, J.M.; Bak, D.H.; Moo Han, J.; Lim, S.T.; Byun, E.B. A hydroxyethyl derivative of chrysin exhibits anti-inflammatory activity in dendritic cells and protective effects against dextran sodium salt-induced colitis in mice. Int. Immunopharmacol. 2019, 77, 105958. [Google Scholar] [CrossRef]
- Byun, E.B.; Song, H.Y.; Kim, W.S.; Han, J.M.; Seo, H.S.; Park, W.Y.; Kim, K.; Byun, E.H. Chrysin Derivative CM1 and Exhibited Anti-Inflammatory Action by Upregulating Toll-Interacting Protein Expression in Lipopolysaccharide-Stimulated RAW264.7 Macrophage Cells. Molecules 2021, 26, 1532. [Google Scholar] [CrossRef]
- Prajit, R.; Sritawan, N.; Suwannakot, K.; Naewla, S.; Aranarochana, A.; Sirichoat, A.; Pannangrong, W.; Wigmore, P.; Welbat, J.U. Chrysin Protects against Memory and Hippocampal Neurogenesis Depletion in D-Galactose-Induced Aging in Rats. Nutrients 2020, 12, 1100. [Google Scholar] [CrossRef] [PubMed]
- Song, H.Y.; Kim, W.S.; Mushtaq, S.; Park, J.M.; Choi, S.H.; Cho, J.W.; Lim, S.T.; Byun, E.B. A novel chrysin derivative produced by gamma irradiation attenuates 2,4-dinitrochlorobenzene-induced atopic dermatitis-like skin lesions in Balb/c mice. Food Chem. Toxicol. 2019, 128, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Arthur, J.S.; Ley, S.C. Mitogen-activated protein kinases in innate immunity. Nat. Rev. Immunol. 2013, 13, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 2011, 34, 637–650. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Yao, H.; Caito, S.; Hwang, J.W.; Arunachalam, G.; Rahman, I. Regulation of SIRT1 in cellular functions: Role of polyphenols. Arch. Biochem. Biophys. 2010, 501, 79–90. [Google Scholar] [CrossRef]
- Ulloa, L.; Tracey, K.J. The “cytokine profile”: A code for sepsis. Trends Mol. Med. 2005, 11, 56–63. [Google Scholar] [CrossRef]
- Kondo, T.; Kawai, T.; Akira, S. Dissecting negative regulation of Toll-like receptor signaling. Trends Immunol. 2012, 33, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Anwar, M.A.; Basith, S.; Choi, S. Negative regulatory approaches to the attenuation of Toll-like receptor signaling. Exp. Mol. Med. 2013, 45, e11. [Google Scholar] [CrossRef] [PubMed]
- Trompouki, E.; Hatzivassiliou, E.; Tsichritzis, T.; Farmer, H.; Ashworth, A.; Mosialos, G. CYLD is a deubiquitinating enzyme that negatively regulates NF-kappaB activation by TNFR family members. Nature 2003, 424, 793–796. [Google Scholar] [CrossRef] [PubMed]
- Kawagoe, T.; Takeuchi, O.; Takabatake, Y.; Kato, H.; Isaka, Y.; Tsujimura, T.; Akira, S. TANK is a negative regulator of Toll-like receptor signaling and is critical for the prevention of autoimmune nephritis. Nat. Immunol. 2009, 10, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Yuk, J.M.; Shin, D.M.; Lee, H.M.; Kim, J.J.; Kim, S.W.; Jin, H.S.; Yang, C.S.; Park, K.A.; Chanda, D.; Kim, D.K.; et al. The orphan nuclear receptor SHP acts as a negative regulator in inflammatory signaling triggered by Toll-like receptors. Nat. Immunol. 2011, 12, 742–751. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Jono, H.; Kai, H.; Li, J.D. The tumor suppressor cylindromatosis (CYLD) acts as a negative regulator for toll-like receptor 2 signaling via negative cross-talk with TRAF6 AND TRAF7. J. Biol. Chem. 2005, 280, 41111–41121. [Google Scholar] [CrossRef]
- Xia, M.; Liu, J.; Wu, X.; Liu, S.; Li, G.; Han, C.; Song, L.; Li, Z.; Wang, Q.; Wang, J.; et al. Histone methyltransferase Ash1l suppresses interleukin-6 production and inflammatory autoimmune diseases by inducing the ubiquitin-editing enzyme A20. Immunity 2013, 39, 470–481. [Google Scholar] [CrossRef] [PubMed]
- Wertz, I.E.; O’Rourke, K.M.; Zhou, H.; Eby, M.; Aravind, L.; Seshagiri, S.; Wu, P.; Wiesmann, C.; Baker, R.; Boone, D.L.; et al. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappaB signalling. Nature 2004, 430, 694–699. [Google Scholar] [CrossRef]
- Li, Z.; Chu, S.; He, W.; Zhang, Z.; Liu, J.; Cui, L.; Yan, X.; Li, D.; Chen, N. A20 as a novel target for the anti-neuroinflammatory effect of chrysin via inhibition of NF-kappaB signaling pathway. Brain Behav. Immun. 2019, 79, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.F.; Brown, C.M.; El Gazzar, M.; McPhail, L.; Millet, P.; Rao, A.; Vachharajani, V.T.; Yoza, B.K.; McCall, C.E. Fueling the flame: Bioenergy couples metabolism and inflammation. J. Leukoc. Biol. 2012, 92, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.F.; Yoza, B.K.; El Gazzar, M.; Vachharajani, V.T.; McCall, C.E. NAD+-dependent SIRT1 deacetylase participates in epigenetic reprogramming during endotoxin tolerance. J. Biol. Chem. 2011, 286, 9856–9864. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, J.; Lee, J.H.; Choi, Y.M.; Choi, H.; Cho, H.D.; Cha, G.H.; Lee, Y.H.; Jo, E.K.; Park, B.H.; et al. SIRT1 Promotes Host Protective Immunity against Toxoplasma gondii by Controlling the FoxO-Autophagy Axis via the AMPK and PI3K/AKT Signalling Pathways. Int. J. Mol. Sci. 2022, 23, 13578. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Buechler, N.L.; Woodruff, A.G.; Long, D.L.; Zabalawi, M.; Yoza, B.K.; McCall, C.E.; Vachharajani, V. Sirtuins and Immuno-Metabolism of Sepsis. Int. J. Mol. Sci. 2018, 19, 2738. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhang, W.; Pan, H.; Feldser, H.G.; Lainez, E.; Miller, C.; Leung, S.; Zhong, Z.; Zhao, H.; Sweitzer, S.; et al. SIRT1 activators suppress inflammatory responses through promotion of p65 deacetylation and inhibition of NF-kappaB activity. PLoS ONE 2012, 7, e46364. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, M.C.; Liang, M.; Fu, J. Sirt1 protects against thrombomodulin down-regulation and lung coagulation after particulate matter exposure. Blood 2012, 119, 2422–2429. [Google Scholar] [CrossRef]
- Yang, Z.; Guan, Y.; Li, J.; Li, L.; Li, Z. Chrysin attenuates carrageenan-induced pleurisy and lung injury via activation of SIRT1/NRF2 pathway in rats. Eur. J. Pharmacol. 2018, 836, 83–88. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-H.; Ko, Y.-B.; Choi, Y.-M.; Kim, J.; Cho, H.-D.; Choi, H.; Song, H.-Y.; Han, J.-M.; Cha, G.-H.; Lee, Y.-H.; et al. CM1, a Chrysin Derivative, Protects from Endotoxin-Induced Lethal Shock by Regulating the Excessive Activation of Inflammatory Responses. Nutrients 2024, 16, 641. https://doi.org/10.3390/nu16050641
Lee J-H, Ko Y-B, Choi Y-M, Kim J, Cho H-D, Choi H, Song H-Y, Han J-M, Cha G-H, Lee Y-H, et al. CM1, a Chrysin Derivative, Protects from Endotoxin-Induced Lethal Shock by Regulating the Excessive Activation of Inflammatory Responses. Nutrients. 2024; 16(5):641. https://doi.org/10.3390/nu16050641
Chicago/Turabian StyleLee, Jae-Hyung, Young-Bok Ko, Yong-Min Choi, Jinju Kim, Hwan-Doo Cho, Hyeonil Choi, Ha-Yeon Song, Jeong-Moo Han, Guang-Ho Cha, Young-Ha Lee, and et al. 2024. "CM1, a Chrysin Derivative, Protects from Endotoxin-Induced Lethal Shock by Regulating the Excessive Activation of Inflammatory Responses" Nutrients 16, no. 5: 641. https://doi.org/10.3390/nu16050641
APA StyleLee, J.-H., Ko, Y.-B., Choi, Y.-M., Kim, J., Cho, H.-D., Choi, H., Song, H.-Y., Han, J.-M., Cha, G.-H., Lee, Y.-H., Kim, J.-M., Kim, W.-S., Byun, E.-B., & Yuk, J.-M. (2024). CM1, a Chrysin Derivative, Protects from Endotoxin-Induced Lethal Shock by Regulating the Excessive Activation of Inflammatory Responses. Nutrients, 16(5), 641. https://doi.org/10.3390/nu16050641





