Exploring the Anti-Diabetic Potential of Quercetagitrin through Dual Inhibition of PTPN6 and PTPN9
Abstract
:1. Introduction
2. Materials and Methods
2.1. PTPN6 and PTPN9 Overexpression and Purification
2.2. Assessment of Enzymatic Activities, Half-Maximal Inhibitory Concentration (IC50) Values, and Hill Coefficients
2.3. Cell Culture and Cell Differentiation
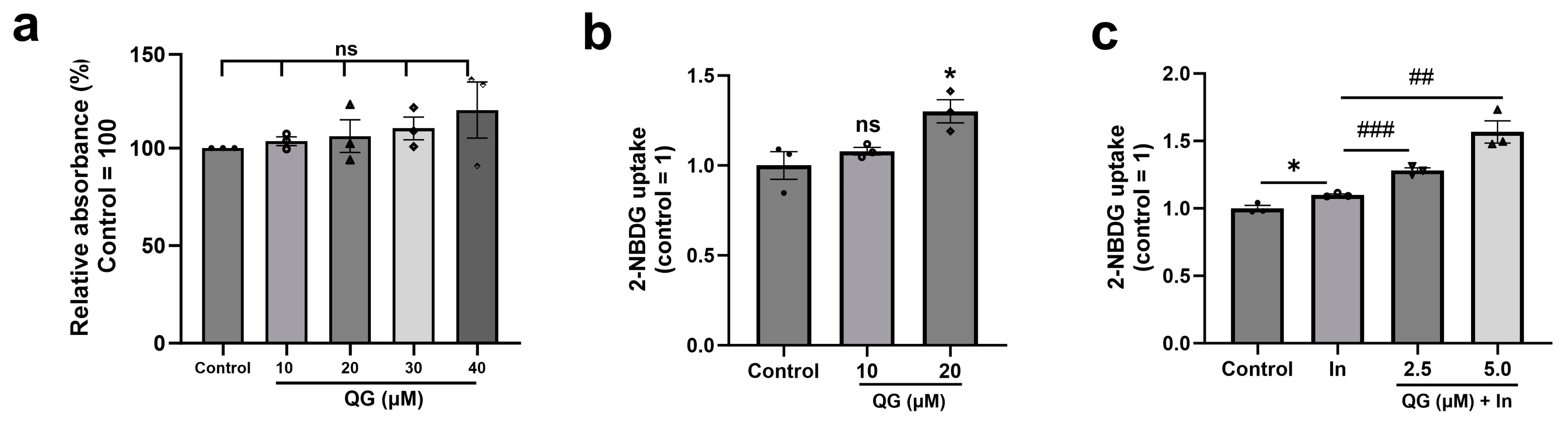
2.4. Cell Cytotoxicity Assay
2.5. Glucose Uptake Assay
2.6. Palmitic Acid-Induced Insulin Resistance in C2C12 Myoblasts
2.7. Western Blotting
2.8. Docking Study of QG on PTPN6 and PTPN9
2.9. Statistical Significance Analysis
3. Results
3.1. Docking Model Predicts the Binding of QG to the Catalytic Sites of PTPN6 and PTPN9
3.2. QG Inhibits Catalytic Activity of PTPN6 and PTPN9 In Vitro
3.3. QG Facilitates Glucose Uptake in C2C12 Myoblasts
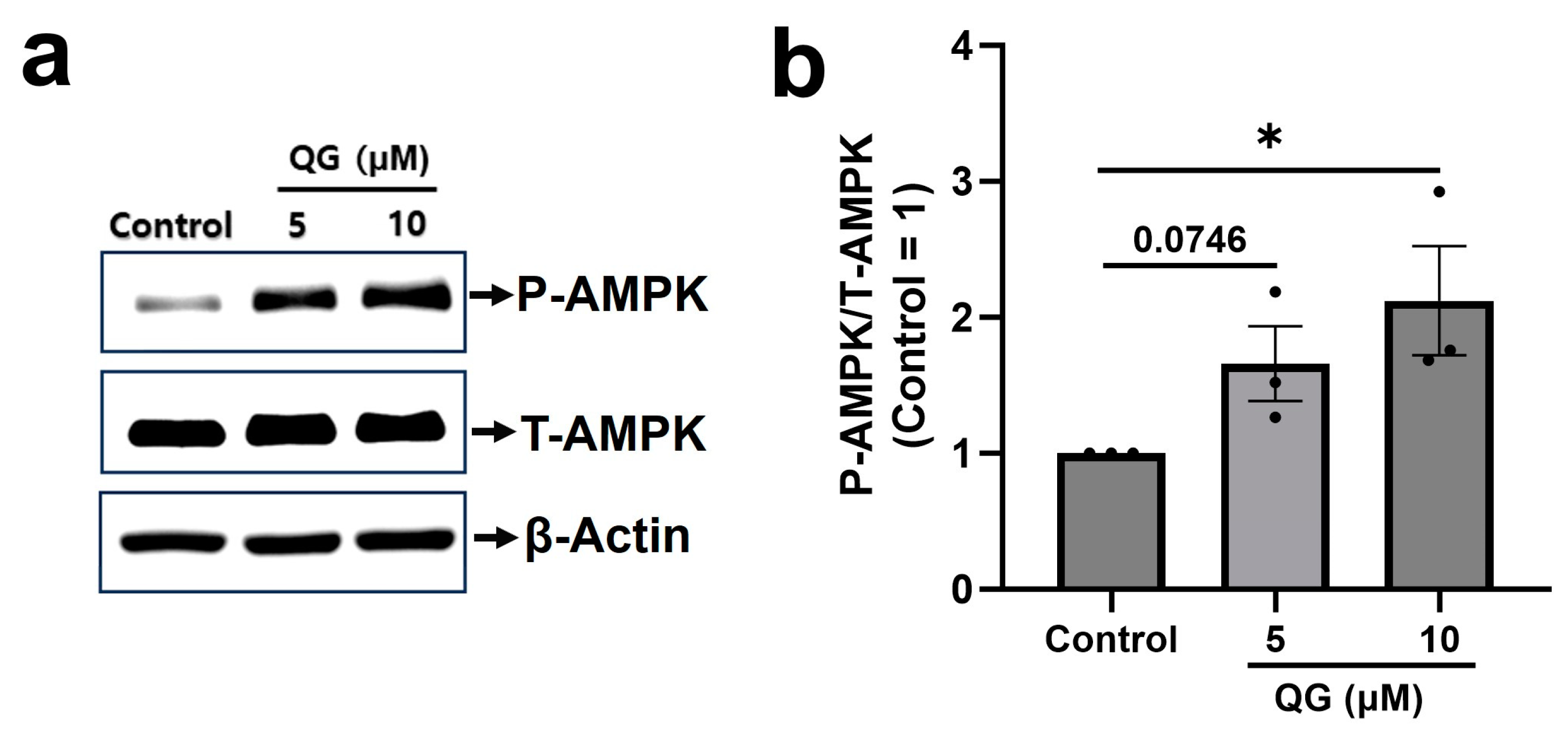
3.4. QG Increases AMPK Phosphorylation in C2C12 Myoblasts
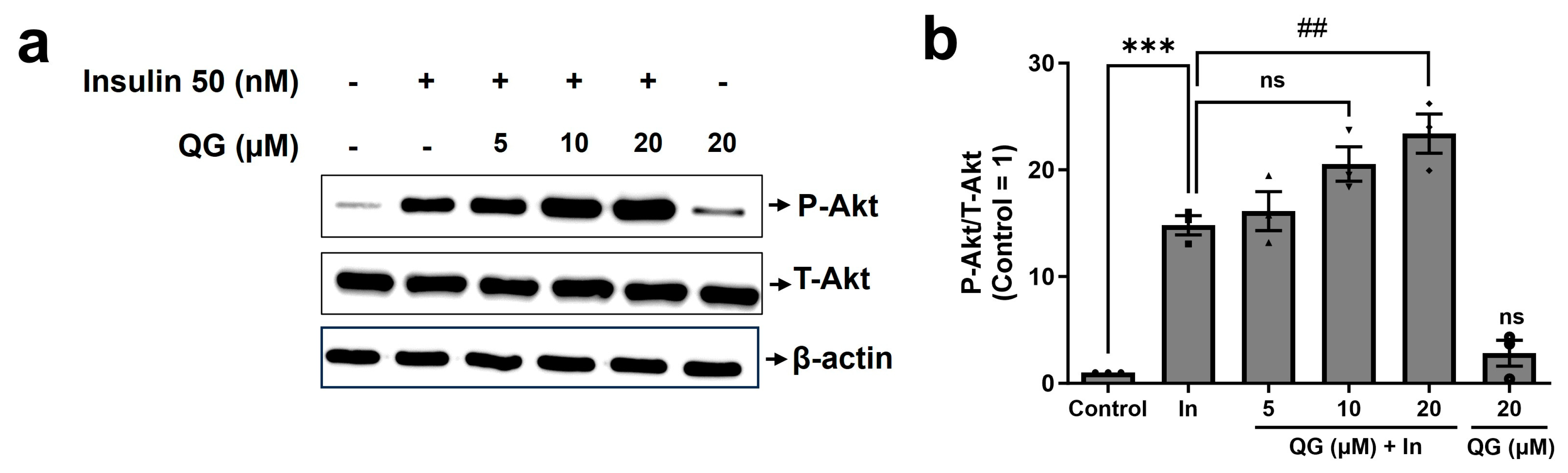
3.5. QG Increases Insulin-Dependent Phosphorylation of Akt in C2C12 Myoblasts
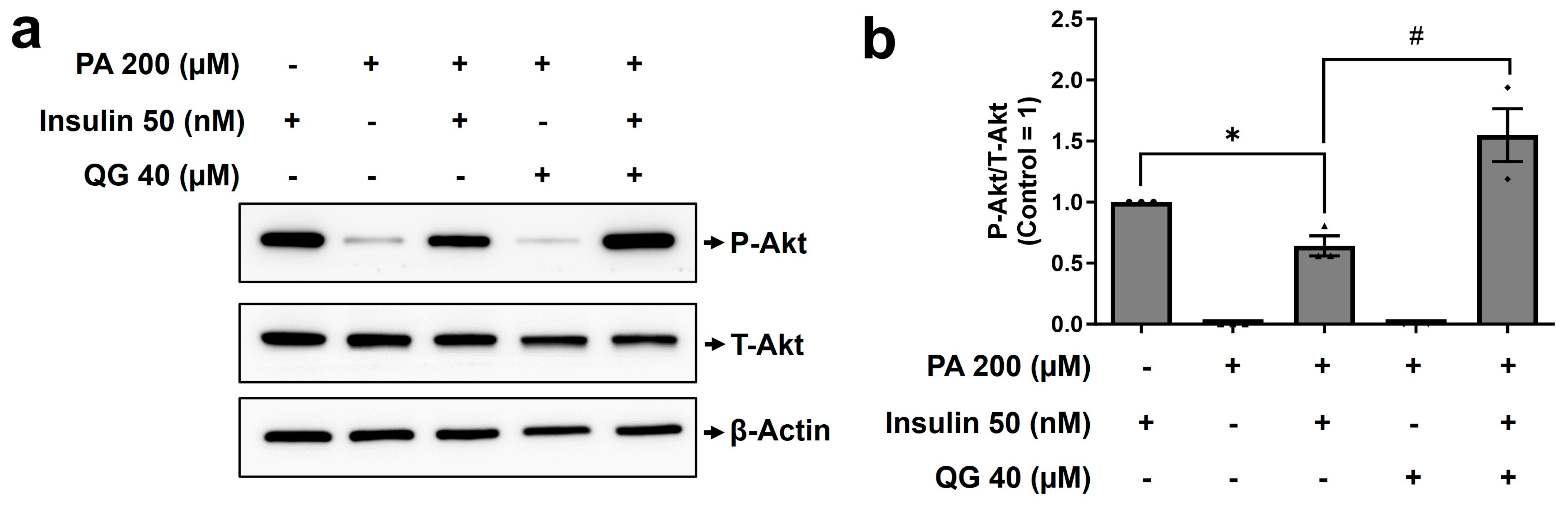
3.6. QG Mitigates Palmitic Acid-Induced Insulin Resistance in C2C12 Myoblasts through the Insulin-Dependent Akt Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Misra, S.; Wagner, R.; Ozkan, B.; Schon, M.; Sevilla-Gonzalez, M.; Prystupa, K.; Wang, C.C.; Kreienkamp, R.J.; Cromer, S.J.; Rooney, M.R.; et al. Precision subclassification of type 2 diabetes: A systematic review. Commun. Med. 2023, 3, 138. [Google Scholar] [CrossRef] [PubMed]
- Ahn, D.; Kwon, J.; Song, S.; Lee, J.; Yoon, S.; Chung, S.J. Methyl Syringate Stimulates Glucose Uptake by Inhibiting Protein Tyrosine Phosphatases Relevant to Insulin Resistance. Life 2023, 13, 1372. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Hossain, K.S.; Das, S.; Kundu, S.; Adegoke, E.O.; Rahman, M.A.; Hannan, M.A.; Uddin, M.J.; Pang, M.G. Role of Insulin in Health and Disease: An Update. Int. J. Mol. Sci. 2021, 22, 6403. [Google Scholar] [CrossRef]
- Vasiljevic, J.; Torkko, J.M.; Knoch, K.P.; Solimena, M. The making of insulin in health and disease. Diabetologia 2020, 63, 1981–1989. [Google Scholar] [CrossRef] [PubMed]
- Ruegsegger, G.N.; Creo, A.L.; Cortes, T.M.; Dasari, S.; Nair, K.S. Altered mitochondrial function in insulin-deficient and insulin-resistant states. J. Clin. Investig. 2018, 128, 3671–3681. [Google Scholar] [CrossRef]
- Bazotte, R.B.; Silva, L.G.; Schiavon, F.P. Insulin resistance in the liver: Deficiency or excess of insulin? Cell Cycle 2014, 13, 2494–2500. [Google Scholar] [CrossRef]
- Yoon, S.Y.; Kang, H.J.; Ahn, D.; Hwang, J.Y.; Kwon, S.J.; Chung, S.J. Identification of chebulinic acid as a dual targeting inhibitor of protein tyrosine phosphatases relevant to insulin resistance. Bioorg. Chem. 2019, 90, 103087. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.Y.; Xiao, L.G.; Liu, H.Y.; Hao, X.J. Hypoglycemic natural products with activities and their mechanisms: A review. Food Sci. Hum. Wellness 2022, 11, 1087–1100. [Google Scholar] [CrossRef]
- Jugran, A.K.; Rawat, S.; Devkota, H.P.; Bhatt, I.D.; Rawal, R.S. Diabetes and plant-derived natural products: From ethnopharmacological approaches to their potential for modern drug discovery and development. Phytother Res. 2021, 35, 223–245. [Google Scholar] [CrossRef]
- Gurzov, E.N.; Stanley, W.J.; Brodnicki, T.C.; Thomas, H.E. Protein tyrosine phosphatases: Molecular switches in metabolism and diabetes. Trends Endocrinol. Metab. 2015, 26, 30–39. [Google Scholar] [CrossRef]
- Crunkhorn, S. Metabolic Disease: Protein tyrosine phosphatase inhibitor reverses diabetes. Nat. Rev. Drug Discov. 2017, 16, 312–313. [Google Scholar] [CrossRef]
- Ruddraraju, K.V.; Zhang, Z.Y. Covalent inhibition of protein tyrosine phosphatases. Mol. Biosyst. 2017, 13, 1257–1279. [Google Scholar] [CrossRef]
- Yoon, S.Y.; Lee, J.H.; Kwon, S.J.; Kang, H.J.; Chung, S.J. Ginkgolic acid as a dual-targeting inhibitor for protein tyrosine phosphatases relevant to insulin resistance. Bioorg. Chem. 2018, 81, 264–269. [Google Scholar] [CrossRef]
- Sharma, C.; Kim, Y.; Ahn, D.; Chung, S.J. Protein tyrosine phosphatases (PTPs) in diabetes: Causes and therapeutic opportunities. Arch. Pharm. Res. 2021, 44, 310–321. [Google Scholar] [CrossRef]
- Ahn, D.; Kim, J.; Nam, G.; Zhao, X.; Kwon, J.; Hwang, J.Y.; Kim, J.K.; Yoon, S.Y.; Chung, S.J. Ethyl Gallate Dual-Targeting PTPN6 and PPARgamma Shows Anti-Diabetic and Anti-Obese Effects. Int. J. Mol. Sci. 2022, 23, 5020. [Google Scholar] [CrossRef]
- Kim, J.; Son, J.; Ahn, D.; Nam, G.; Zhao, X.; Park, H.; Jeong, W.; Chung, S.J. Structure-Activity Relationship of Synthetic Ginkgolic Acid Analogs for Treating Type 2 Diabetes by PTPN9 Inhibition. Int. J. Mol. Sci. 2022, 23, 3927. [Google Scholar] [CrossRef] [PubMed]
- Myers, R.W.; Guan, H.P.; Ehrhart, J.; Petrov, A.; Prahalada, S.; Tozzo, E.; Yang, X.; Kurtz, M.M.; Trujillo, M.; Gonzalez Trotter, D.; et al. Systemic pan-AMPK activator MK-8722 improves glucose homeostasis but induces cardiac hypertrophy. Science 2017, 357, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Esquejo, R.M.; Albuquerque, B.; Sher, A.; Blatnik, M.; Wald, K.; Peloquin, M.; Delmore, J.; Kindt, E.; Li, W.; Young, J.D.; et al. AMPK activation is sufficient to increase skeletal muscle glucose uptake and glycogen synthesis but is not required for contraction-mediated increases in glucose metabolism. Heliyon 2022, 8, e11091. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Liu, X.; Miao, H.; Huang, B.; Liu, Z.; Chen, J.; Quan, X.; Zhu, L.; Dong, H.; Zhang, Z. PEDF increases GLUT4-mediated glucose uptake in rat ischemic myocardium via PI3K/AKT pathway in a PEDFR-dependent manner. Int. J. Cardiol. 2019, 283, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Ke, R.; Xu, Q.; Li, C.; Luo, L.; Huang, D. Mechanisms of AMPK in the maintenance of ATP balance during energy metabolism. Cell Biol. Int. 2018, 42, 384–392. [Google Scholar] [CrossRef]
- Yoon, S.Y.; Ahn, D.; Kim, J.K.; Seo, S.O.; Chung, S.J. Nepetin Acts as a Multi-Targeting Inhibitor of Protein Tyrosine Phosphatases Relevant to Insulin Resistance. Chem. Biodivers. 2022, 19, e202100600. [Google Scholar] [CrossRef] [PubMed]
- Shamsudin, N.F.; Ahmed, Q.U.; Mahmood, S.; Shah, S.A.A.; Sarian, M.N.; Khattak, M.; Khatib, A.; Sabere, A.S.M.; Yusoff, Y.M.; Latip, J. Flavonoids as Antidiabetic and Anti-Inflammatory Agents: A Review on Structural Activity Relationship-Based Studies and Meta-Analysis. Int. J. Mol. Sci. 2022, 23, 12605. [Google Scholar] [CrossRef] [PubMed]
- Stefan, M.I.; Le Novère, N. Cooperative binding. PLoS Comput. Biol. 2013, 9, e1003106. [Google Scholar] [CrossRef] [PubMed]
- Haim, T.E.; Wang, W.; Flagg, T.P.; Tones, M.A.; Bahinski, A.; Numann, R.E.; Nichols, C.G.; Nerbonne, J.M. Palmitate attenuates myocardial contractility through augmentation of repolarizing Kv currents. J. Mol. Cell. Cardiol. 2010, 48, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Gortler, L.; Weininger, S.J. Private Philanthropy and Basic Research in Mid-Twentieth Century America: The Hickrill Chemical Research Foundation. Ambix 2017, 64, 66–94. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, L.; Song, X.; Mo, Y.; Komma, C.; Bellamy, H.D.; Zhao, Z.J.; Zhou, G.W. Crystal structure of human protein tyrosine phosphatase SHP-1 in the open conformation. J. Cell. Biochem. 2011, 112, 2062–2071. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, S.; Tao, R.; Wei, D.; Chen, L.; Shen, W.; Yu, Z.H.; Wang, L.; Jones, D.R.; Dong, X.C.; et al. A highly selective and potent PTP-MEG2 inhibitor with therapeutic potential for type 2 diabetes. J. Am. Chem. Soc. 2012, 134, 18116–18124. [Google Scholar] [CrossRef] [PubMed]
- Tonks, N.K. Protein tyrosine phosphatases: From genes, to function, to disease. Nat. Rev. Mol. Cell Biol. 2006, 7, 833–846. [Google Scholar] [CrossRef]
- Barr, A.J.; Ugochukwu, E.; Lee, W.H.; King, O.N.; Filippakopoulos, P.; Alfano, I.; Savitsky, P.; Burgess-Brown, N.A.; Muller, S.; Knapp, S. Large-scale structural analysis of the classical human protein tyrosine phosphatome. Cell 2009, 136, 352–363. [Google Scholar] [CrossRef]
- Wen, Y.; Yang, S.; Wakabayashi, K.; Svensson, M.N.D.; Stanford, S.M.; Santelli, E.; Bottini, N. RPTPalpha phosphatase activity is allosterically regulated by the membrane-distal catalytic domain. J. Biol. Chem. 2020, 295, 4923–4936. [Google Scholar] [CrossRef]
- Honka, M.J.; Latva-Rasku, A.; Bucci, M.; Virtanen, K.A.; Hannukainen, J.C.; Kalliokoski, K.K.; Nuutila, P. Insulin-stimulated glucose uptake in skeletal muscle, adipose tissue and liver: A positron emission tomography study. Eur. J. Endocrinol. 2018, 178, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, R.; Robaye, B.; Boeynaems, J.M.; Jacobson, K.A. Enhancement of glucose uptake in mouse skeletal muscle cells and adipocytes by P2Y6 receptor agonists. PLoS ONE 2014, 9, e116203. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.Y.; Choi, H.K.; Hwang, J.T. AMPK Activity: A Primary Target for Diabetes Prevention with Therapeutic Phytochemicals. Nutrients 2021, 13, 4050. [Google Scholar] [CrossRef]
- Tiganis, T. PTP1B and TCPTP--nonredundant phosphatases in insulin signaling and glucose homeostasis. FEBS J. 2013, 280, 445–458. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Liu, C.; Wang, X.; Zhao, M.; Zhang, Z.; Zhang, X.; Wang, C.; Song, G. Resveratrol improves palmitic acid-induced insulin resistance via the DDIT4/mTOR pathway in C2C12 cells. Mol. Med. Rep. 2023, 28, 181. [Google Scholar] [CrossRef]
- Zhou, J.; Massey, S.; Story, D.; Li, L. Metformin: An Old Drug with New Applications. Int. J. Mol. Sci. 2018, 19, 2863. [Google Scholar] [CrossRef] [PubMed]
- Fryer, L.G.; Parbu-Patel, A.; Carling, D. The Anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J. Biol. Chem. 2002, 277, 25226–25232. [Google Scholar] [CrossRef]
- Salehi, B.; Ata, A.; Anil Kumar, N.V.; Sharopov, F.; Ramírez-Alarcón, K.; Ruiz-Ortega, A.; Ayatollahi, S.A.; Fokou, P.V.T.; Kobarfard, F.; Zakaria, Z.A.; et al. Antidiabetic Potential of Medicinal Plants and Their Active Components. Biomolecules 2019, 9, 551. [Google Scholar] [CrossRef]
- Prakash Mishra, A.; Sharifi-Rad, M.; Shariati, M.A.; Mabkhot, Y.N.; Al-Showiman, S.S.; Rauf, A.; Salehi, B.; Zupunski, M.; Sharifi-Rad, M.; Gusain, P.; et al. Bioactive compounds and health benefits of edible Rumex species-A review. Cell. Mol. Biol. 2018, 64, 27–34. [Google Scholar] [CrossRef]
- Eidi, A.; Eidi, M.; Esmaeili, E. Antidiabetic effect of garlic (Allium sativum L.) in normal and streptozotocin-induced diabetic rats. Phytomedicine 2006, 13, 624–629. [Google Scholar] [CrossRef]
- Kang, G.J.; Han, S.C.; Ock, J.W.; Kang, H.K.; Yoo, E.S. Anti-Inflammatory Effect of Quercetagetin, an Active Component of Immature Citrus unshiu, in HaCaT Human Keratinocytes. Biomol. Ther. 2013, 21, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xu, H.; Chen, H.; Tai, K.; Liu, F.; Gao, Y. In vitro antioxidant, anti-diabetic and antilipemic potentials of quercetagetin extracted from marigold (Tagetes erecta L.) inflorescence residues. J. Food Sci. Technol. 2016, 53, 2614–2624. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Ye, J.; Deng, Y.; Zhang, M.; Zou, M.; Yao, X.; Xiao, S. Quercetagitrin Inhibits Tau Accumulation and Reverses Neuroinflammation and Cognitive Deficits in P301S-Tau Transgenic Mice. Molecules 2023, 28, 3964. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, K.; Chen, X.; Han, M.; Lu, J.; Zhang, Y. In Vitro and In Vivo Evaluation of Antidiabetic Properties and Mechanisms of Ficus tikoua Bur. Nutrients 2022, 14, 4413. [Google Scholar] [CrossRef] [PubMed]
- Engin, A. The Definition and Prevalence of Obesity and Metabolic Syndrome. Adv. Exp. Med. Biol. 2017, 960, 1–17. [Google Scholar] [PubMed]
- Hossan, T.; Kundu, S.; Alam, S.S.; Nagarajan, S. Epigenetic Modifications Associated with the Pathogenesis of Type 2 Diabetes Mellitus. Endocrine, Metab. Immune Disord. Drug Targets 2019, 19, 775–786. [Google Scholar] [CrossRef]
- Huang, X.; Liu, G.; Guo, J.; Su, Z. The PI3K/AKT pathway in obesity and type 2 diabetes. Int. J. Biol. Sci. 2018, 14, 1483–1496. [Google Scholar] [CrossRef]
- Sharma, B.R.; Kim, H.J.; Rhyu, D.Y. Caulerpa lentillifera extract ameliorates insulin resistance and regulates glucose metabolism in C57BL/KsJ-db/db mice via PI3K/AKT signaling pathway in myocytes. J. Transl. Med. 2015, 13, 62. [Google Scholar] [CrossRef]
- Cao, M.; Wang, J.; Jiang, X.; Sun, Z.; Zhao, L.; Chen, G. Phenolic Constituents from Black Quinoa Alleviate Insulin Resistance in HepG2 Cells via Regulating IRS1/PI3K/Akt/GLUTs Signaling Pathways. J. Agric. Food Chem. 2023, 71, 18780–18791. [Google Scholar] [CrossRef]
- Wong, C.Y.; Al-Salami, H.; Dass, C.R. C2C12 cell model: Its role in understanding of insulin resistance at the molecular level and pharmaceutical development at the preclinical stage. J. Pharm. Pharmacol. 2020, 72, 1667–1693. [Google Scholar] [CrossRef]
- Wu, S.J.; Tung, Y.J.; Ng, L.T. Anti-diabetic effects of Grifola frondosa bioactive compound and its related molecular signaling pathways in palmitate-induced C2C12 cells. J. Ethnopharmacol. 2020, 260, 112962. [Google Scholar] [CrossRef] [PubMed]
- Minge, C.E.; Bennett, B.D.; Norman, R.J.; Robker, R.L. Peroxisome proliferator-activated receptor-gamma agonist rosiglitazone reverses the adverse effects of diet-induced obesity on oocyte quality. Endocrinology 2008, 149, 2646–2656. [Google Scholar] [CrossRef] [PubMed]
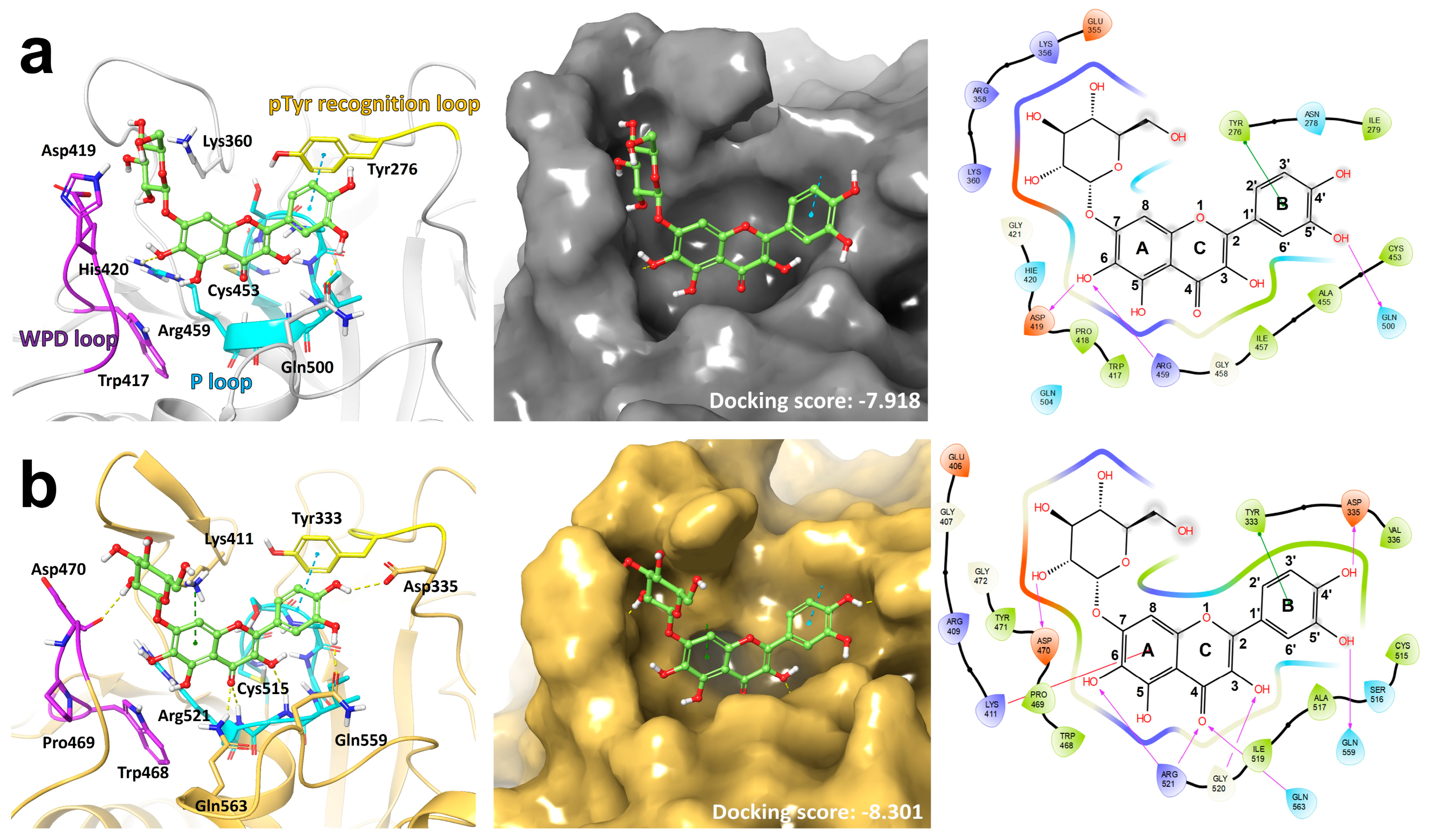

| [E] (nM) | KM (μM) | Vmax (µM min−1) | kcat (min−1) | kcat/KM (µM−1 min−1) | |
|---|---|---|---|---|---|
| PTPN6 | 6 | 187.5 | 10.78 | 1.8 × 103 | 9.7 |
| PTPN9 | 0.05 | 157.4 | 2.316 | 4.6 × 104 | 292.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gone, G.B.; Go, G.; Nam, G.; Jeong, W.; Kim, H.; Lee, S.; Chung, S.J. Exploring the Anti-Diabetic Potential of Quercetagitrin through Dual Inhibition of PTPN6 and PTPN9. Nutrients 2024, 16, 647. https://doi.org/10.3390/nu16050647
Gone GB, Go G, Nam G, Jeong W, Kim H, Lee S, Chung SJ. Exploring the Anti-Diabetic Potential of Quercetagitrin through Dual Inhibition of PTPN6 and PTPN9. Nutrients. 2024; 16(5):647. https://doi.org/10.3390/nu16050647
Chicago/Turabian StyleGone, Geetanjali B., Geonhui Go, Gibeom Nam, Woojoo Jeong, Hyemin Kim, Soah Lee, and Sang J. Chung. 2024. "Exploring the Anti-Diabetic Potential of Quercetagitrin through Dual Inhibition of PTPN6 and PTPN9" Nutrients 16, no. 5: 647. https://doi.org/10.3390/nu16050647
APA StyleGone, G. B., Go, G., Nam, G., Jeong, W., Kim, H., Lee, S., & Chung, S. J. (2024). Exploring the Anti-Diabetic Potential of Quercetagitrin through Dual Inhibition of PTPN6 and PTPN9. Nutrients, 16(5), 647. https://doi.org/10.3390/nu16050647






