Dynamics of Gut Microbiota and Short-Chain Fatty Acids during a Cycling Grand Tour Are Related to Exercise Performance and Modulated by Dietary Intake
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Anthropometric Measurements
2.3. Collection of Faecal Samples
2.4. Analysis of Gut Microbiota Populations Using 16S rRNA Sequencing
2.5. Short-Chain Fatty Acid Analysis
2.6. Performance, Fatigue Perception, and Recovery
2.7. Dietary Assessment and Recording of Probiotic Supplement Use
2.8. Statistical Analysis
3. Results
3.1. Gut Microbiota and SCFA Composition Varies throughout La Vuelta
3.2. Gut Microbiota Dynamics during La Vuelta Predicts Performance
3.3. Dietary Intake Modifies Microbiota Composition to Modulate Performance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Kitai, T.; Hazen, S.L. Gut Microbiota in Cardiovascular Health and Disease. Circ. Res. 2017, 120, 1183–1196. [Google Scholar] [CrossRef]
- Hawley, J.A.; Hargreaves, M.; Joyner, M.J.; Zierath, J.R. Integrative biology of exercise. Cell 2014, 159, 738–749. [Google Scholar] [CrossRef]
- Bermon, S.; Petriz, B.; Kajėnienė, A.; Prestes, J.; Castell, L.; Franco, O.L. The microbiota: An exercise immunology perspective. Exerc. Immunol. Rev. 2015, 21, 70–79. [Google Scholar]
- A Gilbert, J.; Blaser, M.J.; Caporaso, J.G.; Jansson, J.K.; Lynch, S.V.; Knight, R. Current understanding of the human microbiome. Nat. Med. 2018, 24, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.M.; Miller, M.E.B.; Pence, B.D.; Whitlock, K.; Nehra, V.; Gaskins, H.R.; White, B.A.; Fryer, J.D.; Woods, J.A. Voluntary and forced exercise differentially alters the gut microbiome in C57BL/6J mice. J. Appl. Physiol. 2015, 118, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.M.; Mailing, L.J.; Cohrs, J.; Salmonson, C.; Fryer, J.D.; Nehra, V.; Hale, V.L.; Kashyap, P.; White, B.A.; Woods, J.A. Exercise training-induced modification of the gut microbiota persists after microbiota colo-nization and attenuates the response to chemically-induced colitis in gnotobiotic mice. Gut Microbes 2018, 9, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Barton, W.; Penney, N.C.; Cronin, O.; Garcia-Perez, I.; Molloy, M.G.; Holmes, E.; Shanahan, F.; Cotter, P.D.; O’Sullivan, O. The microbiome of professional athletes differs from that of more sedentary subjects in composition and particularly at the functional metabolic level. Gut 2018, 67, 625–633. [Google Scholar] [CrossRef]
- Fernández, J.; Fernández-Sanjurjo, M.; Iglesias-Gutiérrez, E.; Martínez-Camblor, P.; Villar, C.J.; Tomás-Zapico, C.; Fernández-García, B.; Lombó, F. Resistance and Endurance Exercise Training Induce Differential Changes in Gut Microbiota Composition in Murine Models. Front. Physiol. 2021, 12, 748854. [Google Scholar] [CrossRef]
- Okamoto, T.; Morino, K.; Ugi, S.; Nakagawa, F.; Lemecha, M.; Ida, S.; Ohashi, N.; Sato, D.; Fujita, Y.; Maegawa, H. Microbiome potentiates endurance exercise through intestinal acetate production. Am. J. Physiol. Metab. 2019, 316, E956–E966. [Google Scholar] [CrossRef] [PubMed]
- Scheiman, J.; Luber, J.M.; Chavkin, T.A.; MacDonald, T.; Tung, A.; Pham, L.D.; Wibowo, M.C.; Wurth, R.C.; Punthambaker, S.; Tierney, B.T.; et al. Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Nat. Med. 2019, 25, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.J.; Chiu, C.C.; Li, Y.P.; Huang, W.C.; Huang, Y.T.; Huang, C.C.; Chuang, H.L. Effect of intestinal microbiota on exercise performance in mice. J. Strength Cond. Res. 2015, 29, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Maughan, R.J.; Shirreffs, S.M.; Vernec, A. Making Decisions About Supplement Use. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Zeppa, S.D.; Agostini, D.; Gervasi, M.; Annibalini, G.; Amatori, S.; Ferrini, F.; Sisti, D.; Piccoli, G.; Barbieri, E.; Sestili, P.; et al. Mutual Interactions among Exercise, Sport Supplements and Microbiota. Nutrients 2019, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Chicharro, J.L.; Hoyos, J.; Bandrés, F.; Terrados, N.; Fernández, B.; Lucía, A. Thyroid hormone levels during a 3-week professional road cycling competition. Horm. Res. Paediatr. 2001, 56, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Lucía, A.; Díaz, B.; Hoyos, J.; Fernández, C.; Villa, G.; Bandrés, F.; Chicharro, J.L. Hormone levels of world class cyclists during the Tour of Spain stage race. Br. J. Sports Med. 2001, 35, 424–430. [Google Scholar] [CrossRef][Green Version]
- Yang, W.; Liu, Y.; Yang, G.; Meng, B.; Yi, Z.; Chen, M.; Hou, P.; Wang, H.; Xu, X. Moderate-Intensity Physical Exercise Affects the Exercise Performance and Gut Microbiota of Mice. Front. Cell. Infect. Microbiol. 2021, 11, 712381. [Google Scholar] [CrossRef]
- Moris, G.; Arboleya, S.; Mancabelli, L.; Milani, C.; Ventura, M.; de los Reyes-Gavilán, C.G.; Gueimonde, M. Fecal microbiota profile in a group of myasthenia gravis patients. Sci. Rep. 2018, 8, 14384. [Google Scholar] [CrossRef]
- Borg, G. Psychophysical scaling with applications in physical work and the perception of exertion. Scand. J. Work Environ. Health 1990, 16 (Suppl. S1), 55–58. [Google Scholar] [CrossRef]
- Kenttä, G.; Hassmén, P. Overtraining and recovery. Sports Med. 1998, 26, 1–16. [Google Scholar] [CrossRef]
- Rodríguez, I.T.; Ballart, J.F.; Pastor, G.C.; Jordà, E.B.; Val, V.A. Validation of a short questionnaire on frequency of dietary intake: Reproducibility and validity. Nutr. Hosp. 2008, 23, 242–252. [Google Scholar]
- García-Rovés, P.M.; Terrados, N.; Fernández, S.; Patterson, A.M. Comparison of dietary intake and eating behavior of professional road cyclists during training and competition. Int. J. Sport Nutr. Exerc. Metab. 2000, 10, 82–98. [Google Scholar] [CrossRef]
- Alonso, M.R.; Fernández-García, B. Evolution of the use of sports supplements. PharmaNutrition 2020, 14, 100239. [Google Scholar] [CrossRef]
- Hughes, R.L. A Review of the Role of the Gut Microbiome in Personalized Sports Nutrition. Front. Nutr. 2019, 6, 191. [Google Scholar] [CrossRef]
- Bongiovanni, T.; Yin, M.O.L.; Heaney, L.M. The Athlete and Gut Microbiome: Short-chain Fatty Acids as Potential Ergogenic Aids for Exercise and Training. Int. J. Sports Med. 2021, 42, 1143–1158. [Google Scholar]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Luo, L.; Li, R.; Wang, G.; Chen, J.; Chen, L.; Qin, L.-Q.; Yu, Z.-L.; Wan, Z. Age-dependent effects of a high-fat diet combined with dietary advanced glycation end products on cognitive function and protection with voluntary exercise. Food Funct. 2022, 13, 4445–4458. [Google Scholar] [CrossRef]
- Lamoureux, E.V.; Grandy, S.A.; Langille, M.G.I. Moderate Exercise Has Limited but Distinguishable Effects on the Mouse Microbiome. mSystems 2017, 2, e00006-17. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, Z.; Hu, B.; Huang, W.; Yuan, C.; Zou, L. Response of Gut Microbiota to Metabolite Changes Induced by Endurance Exercise. Front. Microbiol. 2018, 9, 765. [Google Scholar] [CrossRef] [PubMed]
- Jäger, R.; Purpura, M.; Stone, J.D.; Turner, S.M.; Anzalone, A.J.; Eimerbrink, M.J.; Pane, M.; Amoruso, A.; Rowlands, D.S.; Oliver, J.M. Probiotic Streptococcus thermophilus FP4 and Bifidobacterium breve BR03 Supplementation Attenuates Performance and Range-of-Motion Decrements Following Muscle Damaging Exercise. Nutrients 2016, 8, 642. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-C.; Hsu, Y.-J.; Huang, C.-C.; Liu, H.-C.; Lee, M.-C. Exercise Training Combined with Bifidobacterium longum OLP-01 Supplementation Improves Exercise Physiological Adaption and Performance. Nutrients 2020, 12, 1145. [Google Scholar] [CrossRef] [PubMed]
- Estaki, M.; Pither, J.; Baumeister, P.; Little, J.P.; Gill, S.K.; Ghosh, S.; Ahmadi-Vand, Z.; Marsden, K.R.; Gibson, D.L. Cardiorespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions. Microbiome 2016, 4, 42. [Google Scholar] [CrossRef]
- Resende, A.S.; Leite, G.S.F.; Junior, A.H.L. Changes in the Gut Bacteria Composition of Healthy Men with the Same Nutritional Profile Undergoing 10-Week Aerobic Exercise Training: A Randomized Controlled Trial. Nutrients 2021, 13, 2839. [Google Scholar] [CrossRef] [PubMed]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.-H.; Sperandio, M.; Di Ciaula, A. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int. J. Mol. Sci. 2022, 23, 1105. [Google Scholar] [CrossRef]
- Deleu, S.; Machiels, K.; Raes, J.; Verbeke, K.; Vermeire, S. Short chain fatty acids and its producing organisms: An overlooked therapy for IBD? EBio-Medicine 2021, 66, 103293. [Google Scholar] [CrossRef] [PubMed]
- Frampton, J.; Murphy, K.G.; Frost, G.; Chambers, E.S. Short-chain fatty acids as potential regulators of skeletal muscle metabolism and function. Nat. Metab. 2020, 2, 840–848. [Google Scholar] [CrossRef]
- Ríos-Covián, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; De Los Reyes-Gavilán, C.G.; Salazar, N. Intestinal Short Chain Fatty Acids and their Link with Diet and Human Health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef]
- Dziewiecka, H.; Buttar, H.S.; Kasperska, A.; Ostapiuk–Karolczuk, J.; Domagalska, M.; Cichoń, J.; Skarpańska-Stejnborn, A. Physical activity induced alterations of gut microbiota in humans: A systematic review. BMC Sports Sci. Med. Rehabil. 2022, 14, 1–22. [Google Scholar] [CrossRef]
- D’Souza, G.; Shitut, S.; Preussger, D.; Yousif, G.; Waschina, S.; Kost, C. Ecology and evolution of metabolic cross-feeding interactions in bacteria. Nat. Prod. Rep. 2018, 35, 455–488. [Google Scholar] [CrossRef]
- Vacca, M.; Celano, G.; Calabrese, F.M.; Portincasa, P.; Gobbetti, M.; De Angelis, M. The Controversial Role of Human Gut Lachnospiraceae. Microorganisms 2020, 8, 573. [Google Scholar] [CrossRef] [PubMed]
- Jeukendrup, A.E. Training the Gut for Athletes. Sports Med. 2017, 47, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Petersen, L.M.; Bautista, E.J.; Nguyen, H.; Hanson, B.M.; Chen, L.; Lek, S.H.; Sodergren, E.; Weinstock, G.M. Community characteristics of the gut microbiomes of competitive cyclists. Microbiome 2017, 5, 98. [Google Scholar] [CrossRef] [PubMed]
- Wiącek, J.; Szurkowska, J.; Kryściak, J.; Galecka, M.; Karolkiewicz, J. No changes in the abundance of selected fecal bacteria during increased carbohydrates consumption period associated with the racing season in amateur road cyclists. PeerJ 2023, 11, e14594. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Chen, Y.H.; Chuang, H.L.; Chiu, C.C.; Huang, C.C. Investigation of the Effects of Microbiota on Exercise Physiological Adaption, Performance, and Energy Utilization Using a Gnotobiotic Animal Model. Front. Microbiol. 2019, 10, 1906. [Google Scholar] [CrossRef]

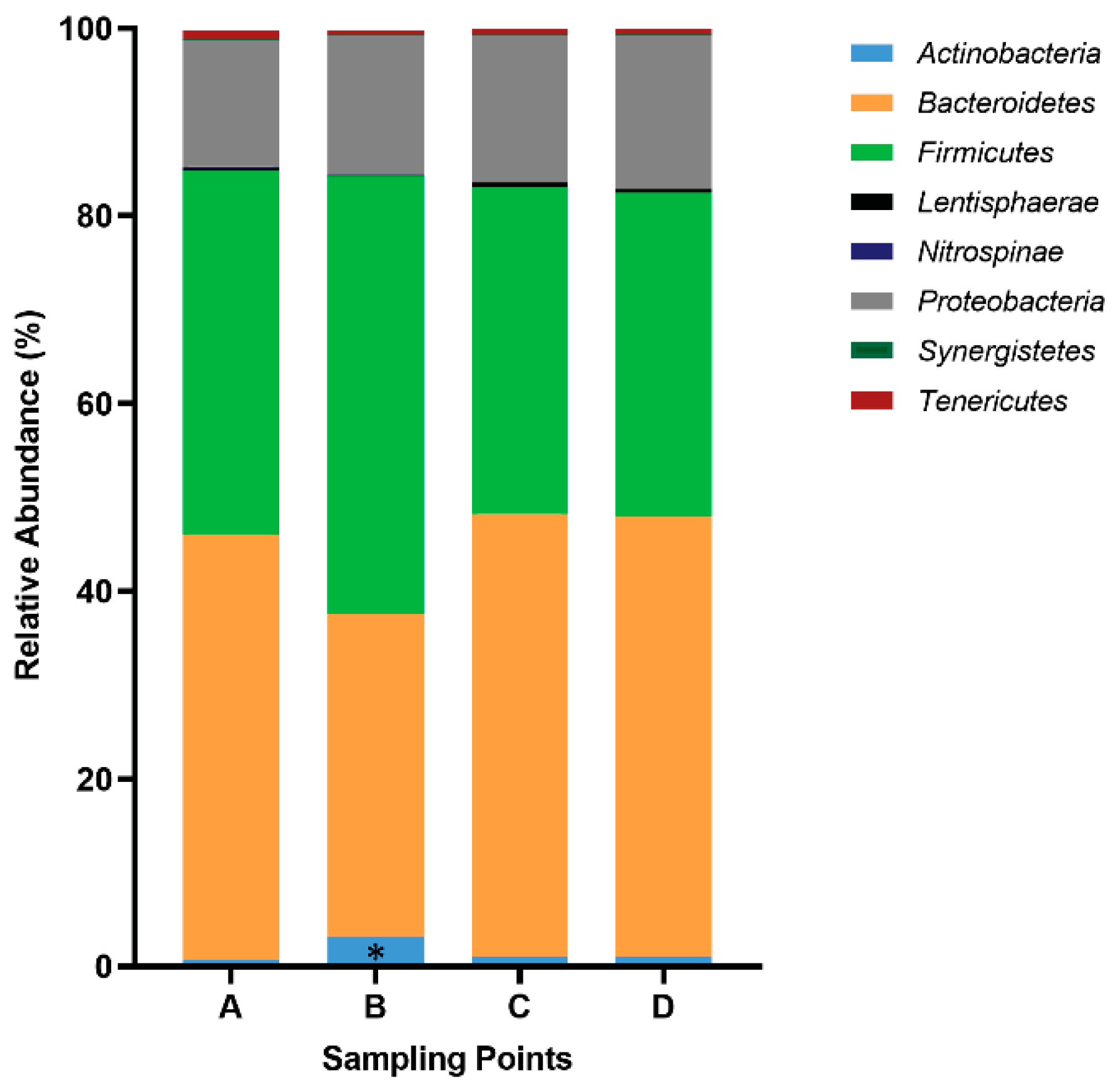
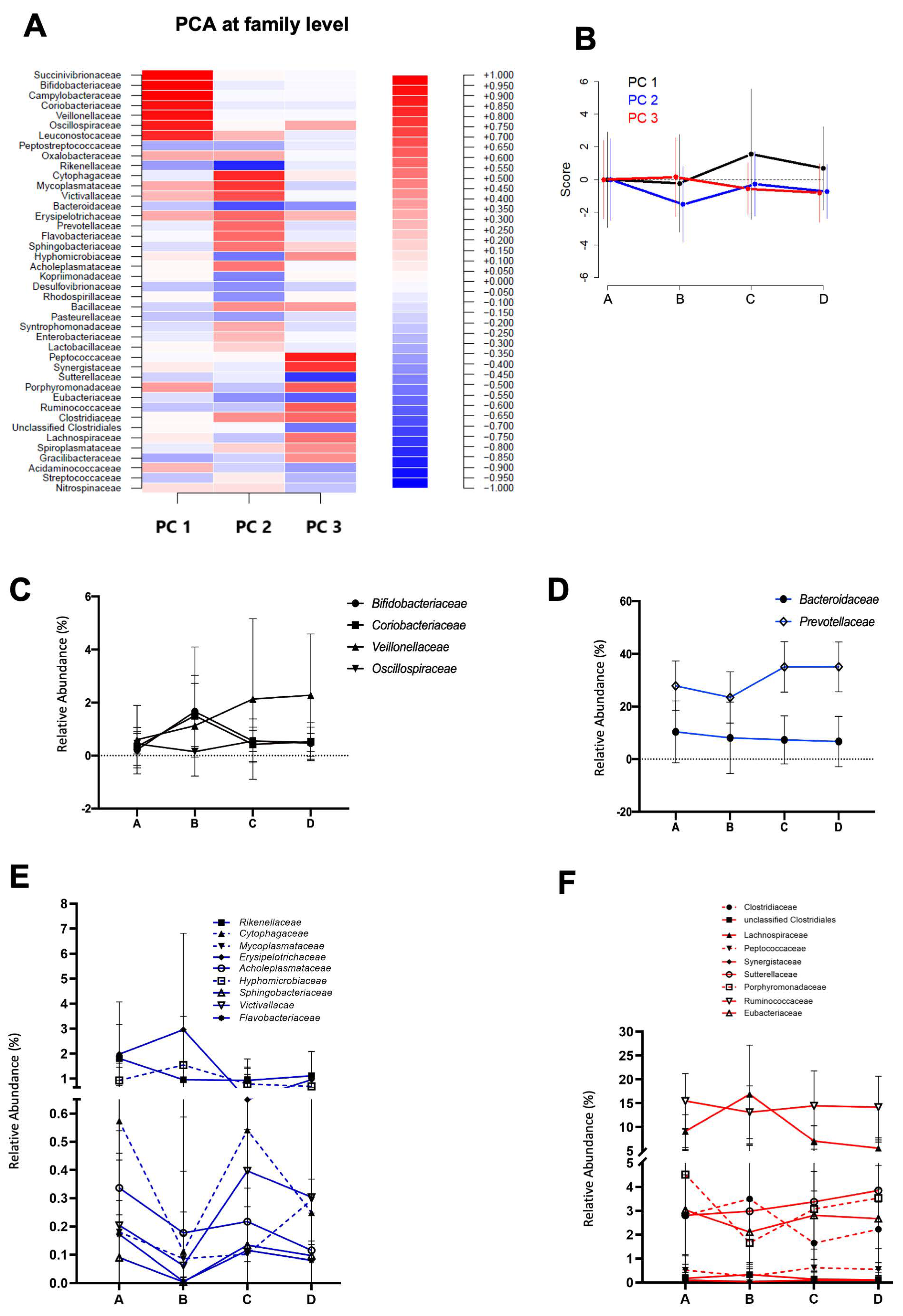


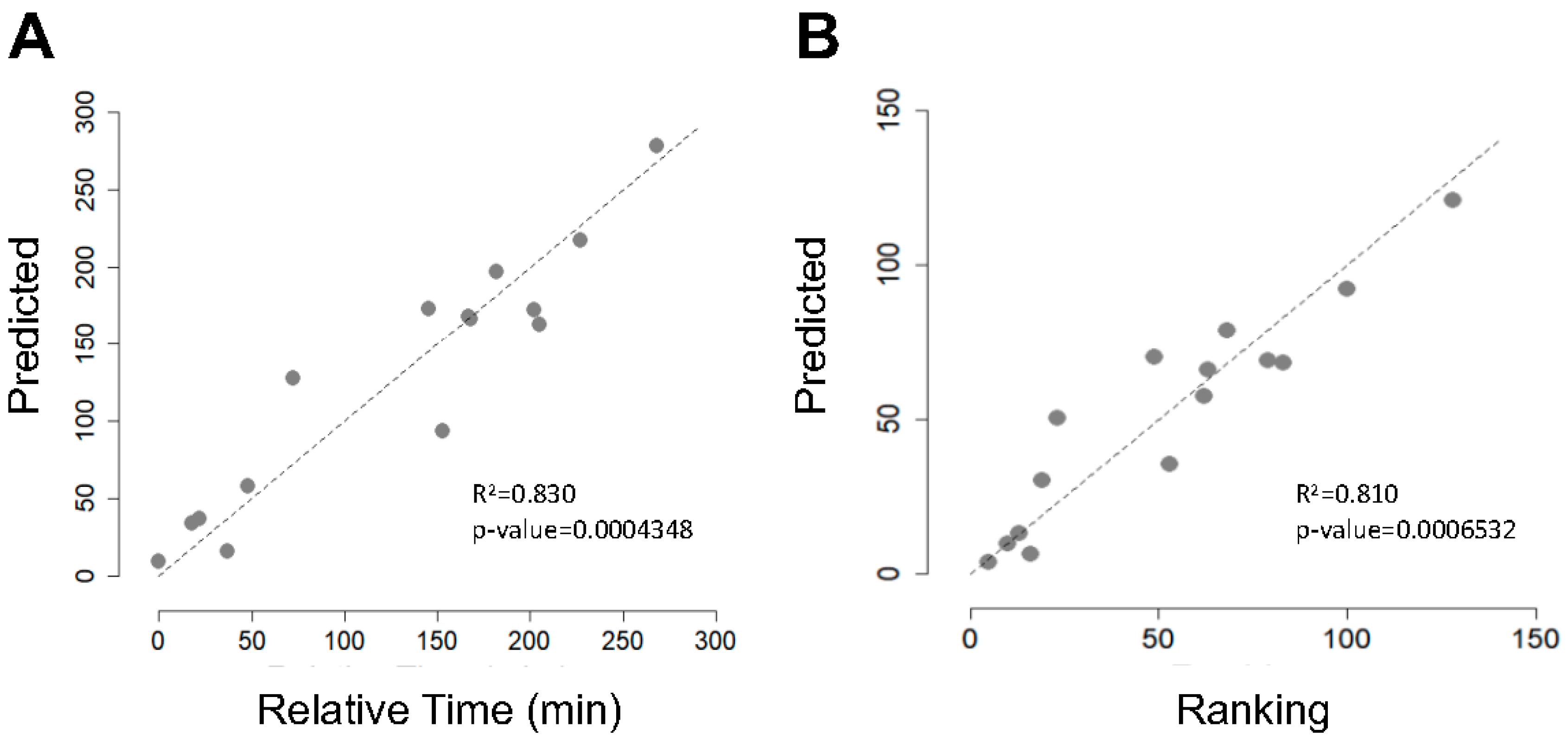
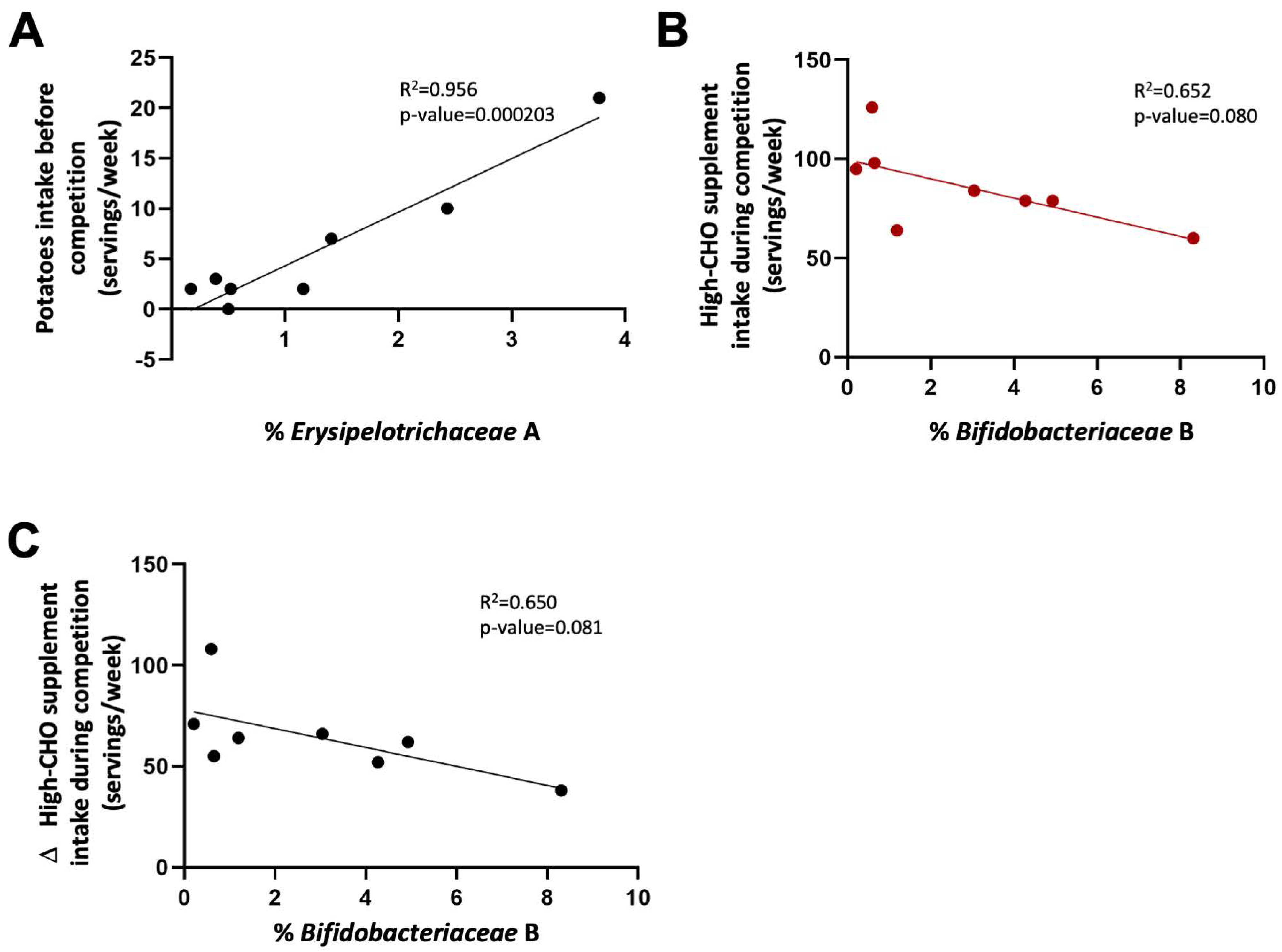
| Characteristics | Mean ± SD |
|---|---|
| Age (years) | 30.2 ± 3.4 |
| Height (cm) | 178.4 ± 7.3 |
| Initial weight (kg) | 66.8 ± 5.0 |
| Final weight (kg) | 66.7 ± 5.0 |
| Initial BMI (kg/m2) | 21.0 ± 0.8 |
| Final BMI (kg/m2) | 21.0 ± 0.9 |
| Parameter | Mean ± SD |
|---|---|
| TQR, A | 17.7 ± 1.7 |
| TQR, D | 12.7 ± 7.3 * |
| RPE, D | 16.3 ± 1.5 |
| Classification position, B | 67 ± 47 † |
| Classification position, C | 56 ± 40 |
| Classification position, D | 51 ± 37 |
| Accumulated time (min), B | 2172.2 ± 38.2 |
| Accumulated time (min), C | 3829.3 ± 63.7 |
| Accumulated time (min), D | 5119.5 ± 86.7 |
| Average power-to-weight ratio per stage (W·kg−1), A–B | 4.2 ± 0.4 ‡ |
| Average power-to-weight ratio per stage (W·kg−1), B–C | 3.9 ± 0.3 |
| Average power-to-weight ratio per stage (W·kg−1), C–D | 3.8 ± 0.4 |
| Average power-to-weight ratio per stage (W·kg−1), Total | 4.0 ± 0.3 |
| Food Item | Before Competition (Portions per Week) | During Competition (Portions per Week) | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Supplements | |||||||
| Carbohydrate drinks | 3.2 | ± | 3.0 | 18.5 | ± | 14.5 | 0.012 |
| Gels | 0.8 | ± | 1.5 | 15.9 | ± | 6.3 | 0.011 |
| Energy bars | 4.4 | ± | 4.1 | 18.1 | ± | 8.5 | 0.018 |
| Sport snacks | 12.8 | ± | 11.1 | 33.1 | ± | 12.6 | 0.028 |
| Protein bars | 1.1 | ± | 1.6 | 1.9 | ± | 2.8 | 0.285 |
| Foods | |||||||
| Breakfast cereals | 4.6 | ± | 7.2 | 10.0 | ± | 11.5 | 0.027 |
| Wholegrain breakfast cereals | 3.1 | ± | 3.4 | 4.4 | ± | 5.2 | 0.109 |
| Bread | 11.1 | ± | 9.1 | 18.4 | ± | 10.9 | 0.046 |
| Wholegrain bread | 2.0 | ± | 3.2 | 5.2 | ± | 6.2 | 0.102 |
| Rice | 3.9 | ± | 3.4 | 9.0 | ± | 4.5 | 0.011 |
| Brown rice | 0.4 | ± | 1.1 | 0.0 | ± | 0.0 | 0.317 |
| Pasta | 2.5 | ± | 1.8 | 7.6 | ± | 2.7 | 0.012 |
| Wholegrain pasta | 0.2 | ± | 0.7 | 0.0 | ± | 0.0 | 0.317 |
| Biscuits | 2.5 | ± | 4.2 | 8.1 | ± | 6.2 | 0.016 |
| Pastry products | 3.4 | ± | 7.3 | 4.9 | ± | 7.4 | 0.109 |
| Chocolate | 3.6 | ± | 6.9 | 4.8 | ± | 12.3 | 1.000 |
| Fruits | 12.5 | ± | 11.0 | 11.4 | ± | 7.5 | 0.500 |
| Fresh fruit juices | 1.1 | ± | 2.5 | 2.5 | ± | 2.9 | 0.141 |
| Commercial fruit juices | 0.4 | ± | 1.1 | 0.0 | ± | 0.0 | 0.317 |
| Leafy vegetables | 8.0 | ± | 4.7 | 9.1 | ± | 4.9 | 0.396 |
| Other vegetables | 8.8 | ± | 3.6 | 9.4 | ± | 4.2 | 0.705 |
| Potatoes | 5.9 | ± | 6.9 | 5.8 | ± | 5.3 | 0.344 |
| Legumes | 3.9 | ± | 4.8 | 5.0 | ± | 6.6 | 0.343 |
| Nuts | 2.1 | ± | 2.4 | 3.0 | ± | 3.3 | 0.414 |
| Whole milk | 3.4 | ± | 3.2 | 3.6 | ± | 3.2 | 0.414 |
| Semi-skimmed milk | 1.8 | ± | 3.2 | 2.6 | ± | 5.2 | 0.317 |
| Skimmed milk | 0.9 | ± | 2.5 | 0.9 | ± | 2.5 | 1.000 |
| Yoghurt | 13.0 | ± | 13.1 | 10.8 | ± | 5.9 | 0.865 |
| Other fermented milks | 0.9 | ± | 2.5 | 0.8 | ± | 2.1 | 0.317 |
| Fresh cheese | 3.2 | ± | 3.3 | 5.1 | ± | 4.7 | 0.102 |
| Mature cheese | 1.1 | ± | 2.5 | 2.0 | ± | 4.9 | 0.317 |
| Dairy desserts | 0.5 | ± | 0.9 | 1.8 | ± | 2.8 | 0.180 |
| Ice cream | 1.0 | ± | 2.4 | 0.8 | ± | 1.2 | 0.655 |
| Lean meat | 4.8 | ± | 1.7 | 5.1 | ± | 1.6 | 0.180 |
| Red meat | 2.4 | ± | 2.3 | 2.8 | ± | 2.4 | 0.102 |
| Meat products | 0.4 | ± | 0.5 | 0.2 | ± | 0.5 | 0.317 |
| Lean fish | 2.1 | ± | 3.1 | 2.6 | ± | 3.0 | 0.180 |
| Oily fish | 3.4 | ± | 2.4 | 3.2 | ± | 2.6 | 1.000 |
| Shellfish | 0.4 | ± | 0.7 | 0.2 | ± | 0.7 | 0.317 |
| Eggs | 13.5 | ± | 8.2 | 13.5 | ± | 5.5 | 0.892 |
| Olive oil | 9.8 | ± | 8.2 | 10.9 | ± | 10.2 | 0.180 |
| Other vegetable oils | 0.0 | ± | 0.0 | 0.0 | ± | 0.0 | 1.000 |
| Butter | 7.1 | ± | 6.1 | 8.1 | ± | 8.3 | 0.343 |
| Margarine | 0.0 | ± | 0.0 | 0.0 | ± | 0.0 | 1.000 |
| Salty snacks | 1.2 | ± | 2.4 | 0.9 | ± | 2.5 | 0.180 |
| Precooked food | 0.0 | ± | 0.0 | 0.0 | ± | 0.0 | 1.000 |
| Soft drinks | 1.4 | ± | 2.1 | 5.2 | ± | 5.2 | 0.043 |
| Diet drinks | 0.9 | ± | 2.5 | 0.0 | ± | 0.0 | 0.317 |
| Wine | 1.2 | ± | 1.6 | 1.6 | ± | 1.4 | 0.180 |
| Beer | 1.6 | ± | 3.5 | 0.8 | ± | 1.5 | 0.180 |
| Distilled alcoholic beverages | 0.0 | ± | 0.0 | 0.0 | ± | 0.0 | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandez-Sanjurjo, M.; Fernandez, J.; Martinez-Camblor, P.; Rodriguez-Alonso, M.; Ortolano-Rios, R.; Pinto-Hernandez, P.; Castilla-Silgado, J.; Coto-Vilcapoma, A.; Ruiz, L.; Villar, C.J.; et al. Dynamics of Gut Microbiota and Short-Chain Fatty Acids during a Cycling Grand Tour Are Related to Exercise Performance and Modulated by Dietary Intake. Nutrients 2024, 16, 661. https://doi.org/10.3390/nu16050661
Fernandez-Sanjurjo M, Fernandez J, Martinez-Camblor P, Rodriguez-Alonso M, Ortolano-Rios R, Pinto-Hernandez P, Castilla-Silgado J, Coto-Vilcapoma A, Ruiz L, Villar CJ, et al. Dynamics of Gut Microbiota and Short-Chain Fatty Acids during a Cycling Grand Tour Are Related to Exercise Performance and Modulated by Dietary Intake. Nutrients. 2024; 16(5):661. https://doi.org/10.3390/nu16050661
Chicago/Turabian StyleFernandez-Sanjurjo, Manuel, Javier Fernandez, Pablo Martinez-Camblor, Manuel Rodriguez-Alonso, Raquel Ortolano-Rios, Paola Pinto-Hernandez, Juan Castilla-Silgado, Almudena Coto-Vilcapoma, Lorena Ruiz, Claudio J. Villar, and et al. 2024. "Dynamics of Gut Microbiota and Short-Chain Fatty Acids during a Cycling Grand Tour Are Related to Exercise Performance and Modulated by Dietary Intake" Nutrients 16, no. 5: 661. https://doi.org/10.3390/nu16050661
APA StyleFernandez-Sanjurjo, M., Fernandez, J., Martinez-Camblor, P., Rodriguez-Alonso, M., Ortolano-Rios, R., Pinto-Hernandez, P., Castilla-Silgado, J., Coto-Vilcapoma, A., Ruiz, L., Villar, C. J., Tomas-Zapico, C., Margolles, A., Fernandez-Garcia, B., Iglesias-Gutierrez, E., & Lombó, F. (2024). Dynamics of Gut Microbiota and Short-Chain Fatty Acids during a Cycling Grand Tour Are Related to Exercise Performance and Modulated by Dietary Intake. Nutrients, 16(5), 661. https://doi.org/10.3390/nu16050661








