Abstract
Omega-3 Long-Chain Polyunsaturated Fatty Acids (n-3 LCPUFAs) play a key role in early neurodevelopment, but evidence from observational and clinical studies remains inconsistent. This study investigates the association between maternal n-3 LCPUFA, Docosahexaenoic Acid (DHA), and eicosapentaenoic acid (EPA) concentrations during pregnancy and infant development functioning at 40 days. This study includes 348 mother–infant pairs. Maternal serum concentrations were assessed in the first and third trimesters alongside sociodemographic, clinical, nutritional, psychological, and obstetrical data. At 40 days, the Bayley Scales of Infant and Toddler Development, Third Edition (BSID-III) was administered. An adjusted analysis revealed that lower first-trimester n-3 LCPUFA and DHA concentrations are associated with better infant motor development. These results underscore the potential significance of the maternal n-3 LCPUFA status in early pregnancy for influencing fetal neurodevelopment. However, the complexity of these associations necessitates further investigation, emphasizing the urgent need for additional studies to comprehensively elucidate the nuanced interplay between the maternal n-3 LCPUFA status and infant neurodevelopment.
Keywords:
serum concentrations; omega-3; DHA; EPA; infant neurodevelopment; cognitive development; pregnancy 1. Introduction
The first 1000 days of life have been recognized as a critical period for the optimal growth and development of an individual. During this period, nutrition is the non-genetic factor with the most influence [1]. The developing brain is highly susceptible to nutritional influences, and adequate nutrient status during pregnancy is vital for promoting optimal cognitive and neurological outcomes in offspring [2]. Within this context, n-3 LCPUFAs (including alpha-linolenic acid (ALA), DHA, and EPA) play a vital role in early neurodevelopment, as they are crucial for the formation and functioning of neuronal membranes, synapses, and neurotransmitter release and for regulating overall brain function and development [3,4,5]. To carry out their function, n-3 LCPUFA and, concretely, DHA, are accumulated in human tissues during gestation and the first two years of life [6,7,8], which is a period of rapid brain development in humans [9]. Therefore, in humans, while it is widely recognized that the maternal prenatal intake of essential n-3 LCPUFA is crucial for the optimal development and functioning of the central nervous system (CNS) in infants [10], studies present conflicting results. Most existing evidence regarding the association between n-3 LCPUFA and children’s neurodevelopment stems from Randomized Controlled Trial (RCT) studies of maternal supplementation during pregnancy. While some studies conducted in developed countries found that DHA supplementation during pregnancy [11,12,13] did not have an effect on infant cognitive development in 9- and 18-month-old and 5-year-old infants, others observed a positive effect [14]. In this sense, Cochrane reviews and meta-analyses of these RCTs have shown that the results are inconclusive and that there is insufficient evidence to support that n-3 LCPUFA supplementation during gestation has a positive impact on neurodevelopment [15,16,17,18,19]. Another source of evidence comes from observational studies about the maternal prenatal intake of DHA-rich foods, which have shown associations with better infant development [20,21,22,23,24,25,26,27].
Studies about maternal prenatal concentrations are limited in number and have yielded less conclusive findings. Rioux et al. [28] assessed the maternal prenatal DHA status in the third trimester of pregnancy, and assessed infant cognitive development at six months using the Bayley Scales of Infant Development (BSID) in a cohort of 63 mother–infant pairs, and found that there is no significant association between DHA and infant performance. In older Dutch children, Brouwer-Brolsma et al. [29] examined the maternal concentrations of arachidonic acid (AA), DHA, and EPA in plasma phospholipids in the third trimester of pregnancy and child cognitive function at seven years without finding significant associations. However, there are additional studies that have established links between n-3 LCPUFA and infant cognitive performance. For example, a study conducted in Seychelles investigated the connections between maternal PUFAs, methylmercury, and infant cognitive development at 9 and 30 months of age [30]. The results demonstrated a positive correlation between psychomotor development in 9-month-old infants and total n-3 LCPUFA levels during the third trimester. Similarly, a study conducted on 12-month-old Norwegian children found that maternal DHA concentrations in the third trimester were associated positively with infants’ problem-solving abilities [31]. As can be observed, few studies have assessed maternal n-3 LCPUFA concentrations during the whole gestation, and the results are inconclusive and difficult to compare due to variations in n-3 LCPUFA measurements across different tissues and the assessment of cognitive abilities at different ages when other significant factors may have influences.
Most of the studies presented (observational and RCT), which investigate the impact of several n-3 LCPUFAs on child neurodevelopment, have been carried out mainly in older infants and children, and they hardly provide data on the maternal serum levels of fatty acids resulting from supplementation or observation. Given the complexity of early fetal brain development, it is essential to understand which n-3 LCPUFA serum levels are related to neurodevelopment and at which stage of pregnancy, including early pregnancy, and to expand this knowledge to cover very young infants. Thus, the main aim of this study is to analyze the association of the maternal n-3 LCPUFA concentration during pregnancy with early neurodevelopment in infants, adjusting the relationship for other related factors in a population of women from the Mediterranean region of Spain.
2. Materials and Methods
2.1. Study Design and Procedure
This is a prospective follow-up study of pregnant women from the first trimester to 40 days postpartum. This study is part of the ECLIPSES data research project in Tarragona, Catalonia, Spain [32,33]. Before the 12th week of pregnancy, individuals were chosen from primary care institutions, and they were closely monitored at weeks 12, 24, and 36 of pregnancy, as well as on the 40th day after giving birth. Eligible participants were at least 18 years old, within their first 12 weeks of pregnancy, and free from anemia (Hb > 110 g/L). Multiple pregnancies, previous severe diseases such as immunosuppression, or any chronic disease that can affect nutritional development (malabsorption syndrome, diabetes, cancer, or hepatopathies) were the exclusion criteria in this study. Samples of blood and data related to sociodemographic, clinical, and psychosocial factors were gathered. Serum concentrations of n-3 LCPUFA, DHA, and EPA were determined in the 12th and 36th weeks of gestation in a random subsample of 450 participants. Out of the initial 450 participants, 348 took part in the postpartum evaluation, during which their infants were examined. As a result, this study’s ultimate sample size was 348 mother–infant pairs. A flow chart of participants and measurements is provided in Figure 1.
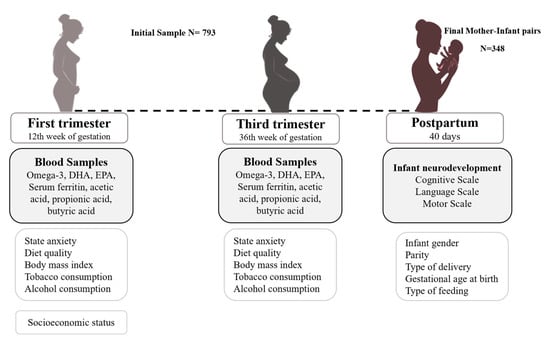
Figure 1.
Flow chart of the study design, measurements, and population.
The Clinical Research Ethics Committee of the Jordi Gol Institute for Primary Care Research (IDIAP), the Pere Virgili Institute for Health Research (IISPV) (REF.NO. 118/2017, date: 2017), and the Spanish Agency for Medicines and Medical Devices (AEMPS) approved all procedures involving human subjects. Every woman who took part in the study gave her informed permission. Also, this clinical trial was registered at www.clinicaltrialsregister.eu, accessed on 21 May 2013 with EudraCT number 2012-005480-28, and at www.clinicaltrials.gov, accessed on 23 June 2017 with identification number NCT03196882. All participants signed an informed consent form. The study complies with the tenets of the Declaration of Helsinki.
2.2. Instruments and Data Collection
2.2.1. Main Measurements
Blood samples were obtained from pregnant women who were fasting. After centrifugation, the serum was kept cold—at −80 °C—until further examination. LC-MS/MS was used to measure serum concentrations of n-3 LCPUFA, DHA, and EPA. Briefly, a standard internal mixture was mixed with 20 μL of serum in methanol to precipitate proteins. The supernatant was mixed with water, O-Benzylhydroxylamine (BHA, Sigma-Aldrich, St. Louis, MO, USA), and N-(3 Dimethylaminopropyl)-N0-ethyl carbodiimide (EDC, Sigma-Aldrich) to obtain n-3 LCPUFA derivatives. n-3 LCPUFA derivatives were purified by liquid–liquid extraction using diethyl ether and by ultra-performance liquid chromatography-mass spectrometry (UHLC-MS/MS) using a UHPLC 1290 Infinity II Series coupled to a QqQ 6470 Series® (Agilent Technologies Inc., Santa Clara, CA, USA) and analyzed. Chromatographic separation was performed by gradient elution using a ternary mobile phase containing water, methanol, and isopropanol with ammonium formate on a Kinetex Polar C18 analytical column (2.6 μm, 2.1 × 100 mm) (Phenomenex, Torrance, CA, USA). The mass spectrometer was operated in multiple reaction monitoring (MRM) modes, and PUFAs were ionized by positive electrospray. The UHPLC-MS/MS system was monitored by an Agilent MassHunter® workstation (Agilent Technologies Inc., Santa Clara, CA, USA).
Infants’ neurodevelopment was assessed using the BSID-III [34], an individually administered assessment designed to assess the current developmental status of language and motor skills and four specific subscales (expressive language, receptive language, fine motor, and gross motor). At 40 days old, the cognitive scale focused on assessing infants’ sensorimotor development, visual attention, exploration, self-regulation ability, and habituation to environmental infants aged 0 to 42 months. The BSID-III comprises three general scales (cognitive, stimuli, and recognition of the primary caregiver). The receptive language subscale examined an infant’s capacity for pre-verbal communication skills, sound differentiation, attention to and perception of environmental stimuli, as well as orientation towards social and environmental stimuli. Meanwhile, the expressive language subscale assessed pre-verbal communication, encompassing behaviors such as smiles, early vocalizations, and babbling. The motor scale included the fine motor subscale, evaluating an infant’s hand position, visual object tracking, and response to tactile stimuli, and the gross motor subscale appraised an infant’s muscular tone, limb and torso movement, static positioning, and cephalic control. Two trained psychologists conducted the BSID-III assessment at the 40-day postpartum visit in the presence of both parents.
2.2.2. Adjustment Measurements
Prenatal Psychological Distress
The State-Trait Anxiety Inventory (STAI) in Spanish was used to assess the symptoms of mother anxiety [35], which includes 40 items that assess state anxiety (transient and situational anxiety level) and trait anxiety (dispositional and stable trait anxiety level). In this study, the state anxiety score in the first and third trimesters was used.
Sociodemographic Data
The women’s socioeconomic statuses (SESs) were calculated using the Hollingshead index [36] by merging data about the mothers’ and couples’ (if the mothers had a partner) levels of education and profession, classified according to the Catalan classification of occupations (CCO-2011), to obtain a total score representing the family SES [37].
Lifestyle Habits
The diet quality scores of pregnant women were estimated in the first and third trimesters according to their adherence to the Mediterranean diet and by using a Food Intake Frequency Questionnaire (FFQ) [38,39]. The process used to obtain the scores was as follows: The FFQ, which contains 45 food and beverage items, was completed according to the amount of each item consumed per week or month. Based on the daily gram of consumption for each substance, nine groups were formed: fruits (seeds, nuts, and fruits, but not fruit juices), legumes, vegetables, cereals (wholemeal and refined flour, rice, pasta, other grains, and bread), fresh fish (fish and seafood), all meat (fresh meat and processed meat), olive oil, dairy products (milk, cheese, creamy desserts, and yogurt), and alcoholic beverages. Each group was stated in grams per 1000 kcal/day, and tertiles were obtained to assign a categorical score related to low, medium, and high intakes (0, 1, and 2, respectively). To calculate the total rMED score, the nine scores were added together, taking into account that six groups scored positively (fruit, legumes, vegetables, cereals, olive oil, and fresh fish) and three components scored negatively (total meat, dairy products, and alcohol); thus, the total rMED score ranged from 0 points (minimum adherence to the Mediterranean diet) to 18 points (maximum adherence to the Mediterranean diet). The FFQs were carried out by qualified midwives and nutritionists who monitored the reviewing and entering of the food data and the data scrubbing and analysis.
Smoking during pregnancy was assessed using the Fagerström questionnaire (Fagerström_Q) [40], and women were divided into two groups: smokers and non-smokers.
Alcohol use during pregnancy was evaluated based on the mothers’ responses to the alcoholic beverages item in the FFQ questionnaire, and it was categorized as either “yes” or “no” depending on the response.
Clinical Data
The mothers’ anthropometric measures were weight (kg) and height (cm), and Body Mass Index (BMI) was calculated from these measures (weight (kg)/height (m2)). BMI was computed at the first and third trimesters, and BMI change from the first to the third trimester was determined.
The number of alive sons (parity) was obtained from clinical records.
Fasting serum samples were collected throughout the first and final trimesters of pregnancy. Serum ferritin levels were measured through immune chemiluminescence.
Serum short-chain fatty acids (SCFAs) (acetic acid, propionic acid, and butyric acid) were quantified by liquid chromatography– mass spectrometry (LC-MS/MS). Then, the supernatants were mixed with water, o-Benzylhydroxylamine (BHA, Sigma Aldrich, St. Louis, MO, USA), and N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide (EDC, Sigma Aldrich) to obtain amount of SCFAs derived from AGCC. SCFA derivatives were purified by liquid–liquid extraction using diethyl ether, and SCFA quantification was performed via LC-MS/MS using the Series 1290 Infinity II Ultra High-Performance Liquid Chromatography (UHPLC) coupled to a QqQ 6470 Series® (Agilent Technologies Inc., Santa Clara, CA, USA). Chromatographic separation was performed with gradient elution using a ternary mobile phase containing water, methanol, and isopropanol with ammonium formate on the Kinetex Polar C18 analytical column (2.6 μm 2.1 × 100 mm) (Phenomenex, Torrance, CA, USA). The mass spectrometer operates in multiple reaction monitoring (MRM) mode, and SCFAs were ionized by positive electrospray. The UHPLC-MS/MS system was controlled by the Agilent MassHunter® workstation (Agilent Technologies Inc., Santa Clara, CA, USA). Samples were analyzed in duplicate, and the average of the two values was calculated.
Obstetrical and Birth Data
Gestational age at birth and type of delivery were collected from the babies’ health cards. The mothers were also asked about the type of feeding they used. The genders of the babies were also recorded.
2.3. Statistical Analysis
The description of the community was expressed using descriptive statistics, in which the data are expressed as mean and standard deviation (SD) for quantitative variables and number of cases and percentage (%) for qualitative variables.
Tertiles of n-3 LCPUFA, DHA, and EPA serum concentrations at the first and third trimesters were computed to obtain groups at each trimester. After that, ANCOVA analyses were performed to assess the differences in infant cognitive development scores at 40 days old according to maternal n-3 LCPUFA, DHA, and EPA tertiles in the first and third trimesters. The adjustment variables used were the following: family socioeconomic status (low/mid/high); mother’s state anxiety (total score) in the first/third trimesters; tobacco consumption (yes/no); alcohol consumption (yes/no); BMI change from first to third trimester (kg/m2); quality of diet in the first/third trimesters (total score); serum ferritin (μg/L) in the first/third trimesters; SCFAs (acetic acid, propionic acid, and butyric acid) in the first/third trimesters; parity (nulliparous/multiparous); gestational age at birth (weeks); infant’s gender (boy/girl); type of delivery (eutocic/dystocic); and type of feeding (formula/breastfeeding). We calculated the estimated adjusted means and utilized the Bonferroni post hoc analysis to assess significant differences between the n-3 LCPUFA, DHA, and EPA level groups.
To test the predictive and continuous association between n-3 LCPUFA and DHA in the first trimester and the 40-day-old infant neurodevelopment scores, multiple linear regression models using the enter method were performed. The variables entered in the model to adjust the relationship were the same as the ANCOVA adjustment variables.
The significance level was set at 0.05. All the statistical analyses were run by the statistical software package SPSS version 29.0 for Windows (SPSS, Chicago, IL, USA).
3. Results
3.1. General Characteristics of the Sample
The characteristics of the participants are shown in Table 1. Overall, the mean age of the pregnant women was 38.8 years (SD = 5.1), and approximately 60% had a middle to high socioeconomic status.

Table 1.
Descriptive data of the mothers and offspring: sociodemographic, lifestyle, nutrition, and psychological data (n = 348).
3.2. Infant Cognitive Development According to Mothers’ n-3 LCPUFA, DHA, and EPA Levels in First and Third Trimesters
Infant cognitive development mean scores and estimated mean scores after adjusting for covariables according to mothers’ n-3 LCPUFA, DHA, and EPA tertile groups in first and third trimesters are shown in Table 2, Table 3, and Table 4, respectively.

Table 2.
n-3 LCPUFA levels at the first and third trimesters of pregnancy and neurodevelopmental offspring data.

Table 3.
Docosahexaenoic acid levels at the first and third trimesters of pregnancy and neurodevelopmental offspring data.

Table 4.
Eicosapentaenoic acid levels at the first and third trimesters of pregnancy and neurodevelopmental offspring data.
Regarding n-3 LCPUFA, the infants of mothers in tertile 1 in the first trimester showed significantly higher scores in the motor index (adjusted mean = 110.165) and the gross motor index (adjusted mean = 11.679) than the infants of mothers in tertile 3 (adjusted mean = 105.736 and 10.744, respectively). In the third trimester, no significant relationship was observed (Table 2).
Similar results were found regarding DHA (Table 3). The infants of mothers in tertile 1 showed significantly higher scores in motor index (adjusted mean = 109.710) and gross motor index (adjusted mean = 11.669) than the infants of mothers in tertile 3 (adjusted mean = 105.208 and 10.595, respectively).
Concerning the EPA, Table 4 shows no significant difference.
3.3. Predictive Relationship between n-3 LCPUFA and DHA Serum Levels at First Trimester and Infant Motor and Gross Motor Development
The findings of the multivariate-adjusted linear regression models examining the associations between the maternal levels of n-3 LCPUFA and DHA in the first trimester and infant cognitive development are shown in Table 5. Both the maternal n-3 LCPUFA and DHA concentrations in the first trimester showed significant negative relationships with the motor index scores (β = −0.015, p = 0.034 and β = −0.021, p = 0.029, respectively) and gross motor scores (β = −0.004, p = 0.012 and β = −0.005, p = 0.003, respectively). The infants’ motor index and gross motor scores slightly decreased with greater maternal n-3 LCPUFA and DHA levels during the first trimester of pregnancy.

Table 5.
Multivariate-adjusted linear regression models for the associations of maternal n-3 LCPUFA, and DHA levels and infants’ neurodevelopment in the first trimester.
4. Discussion
This study assessed the maternal serum concentrations of circulating n-3 LCPUFA, DHA, and EPA during the first and third trimesters of pregnancy in a large sample of healthy women from a Mediterranean region of Spain. We investigated the impact of these concentrations on the neurodevelopment of their infants at forty days of age. Our findings indicate that lower maternal levels of n-3 LCPUFA and DHA during the first trimester are related to better motor scores in infants, particularly in gross motor skills, while there is no relationship between EPA and infant neurodevelopment scores.
To date, there are no established reference ranges for LCPUFA concentrations. Nevertheless, the n-3 LCPUFA concentrations observed in our sample resembles those reported in other samples of healthy pregnant women from diverse countries, including the Netherlands, Hungary, Finland, England, and Ecuador [41], as well as those documented in the Human Metabolome Database [42]. Considering this, the results of our study introduce a paradox by questioning the conventional belief that high maternal levels of n-3 LCPUFA can improve infant neurodevelopment. However, findings from specific studies examining maternal concentrations during pregnancy and infant neurodevelopment also differ from this widely accepted idea. To our knowledge, the Maastricht Essential Fatty Acid Cohort (MEFAB) study is the only one that has specifically examined associations between maternal concentrations of AA, DHA, and EPA in plasma phospholipids in each trimester of pregnancy and child cognitive and academic performances at the age of 7 [29,43]. Their results showed that there was no significant relationship with school cognitive performance and a negative association between prenatal DHA in the first trimester and child arithmetic skills, indicating that higher levels were detrimental to the development of these areas. The study concluded that the negative associations observed call for prudence when considering DHA supplementation during pregnancy [43]. Regarding studies assessing n-3 LCPUFA concentrations in the third trimester and at the end of pregnancy, mixed findings have been reported. While some studies reported improved psychomotor development in 9-month-old infants, higher problem-solving abilities in 12-month-old infants, and better language skills in 5-year-old children of mothers with higher concentrations of n-3 LCPUFA and DHA [30,31,44], others did not find significant associations with cognitive performance in 6-month-old babies [28]. Additionally, there has not been a reported association between EPA and infant neurodevelopment. These findings highlight the complexity of interpreting the relationship between maternal n-3 LCPUFA concentrations and infant neurodevelopment, given that crucial factors influencing this association remain inadequately studied. Such factors include the timing of pregnancy examined, the methods employed for assessing infant neurodevelopment, and the lack of comprehensive data on the PUFA serum concentrations in most studies, including variations in measurement methods and expressions (% of total fatty acids or serum concentrations), as well as the assessment of the confounding factors of the relationship.
The age of the child and the methodology employed for neurodevelopmental assessment can significantly contribute to variability in the results. In this context, the motor development domain assessed by the BSID-III, which is used in this study, assesses indicators of muscular tone and maturity, encompassing limb and torso movement, static positioning, and cephalic control—all linked to the maturity of the CNS [45,46]. Thus, our results suggest that infants born to mothers in the low tertile of n-3 LCPUFA and DHA during the first trimester may exhibit a more mature CNS.
Given the limited existing evidence and the intricate interpretation of the association between maternal fatty acid concentrations and child neurodevelopment, it is noteworthy to consider the extensive research conducted in supplementation studies during pregnancy, with a primary focus on DHA supplementation or dietary supplements like fish oil. These studies indirectly allow us to observe whether an increase in the serum levels of fatty acids has a positive effect on infant neurodevelopment. However, these studies have also yielded contradictory results. On the one hand, recent reviews suggest that beneficial effects are discernible primarily in specific subgroups, such as preterm infants or pregnant women with low n-3 LCPUFA levels, genetic predisposition, or unfavorable socioeconomic and educational characteristics [10,47,48,49]. However, in many cases, high-dose DHA supplementation had null or even adverse effects on children’s neurodevelopment [10]. The authors of a recent review [10] indicate that these adverse effects have been poorly mentioned in previous reviews and meta-analyses, probably because it is difficult to discern the mechanisms by which DHA supplementation may have adverse effects. Although intervention studies of supplementation do not provide information on maternal serum levels or the specific trimester in which the effects on infant neurodevelopment are occurring, they reported the harm caused by high doses of n-3 LCPUFA, which supports our findings.
Concerning our results and the evidence presented, we can hypothesize that in the first trimester, when the embryo is small in size, lower amounts of n-3 LCPUFA may adequately meet the fetal requirements for proper development, while higher doses may be detrimental, probably due to oxidation and inflammation processes.
Once fetal fatty acid needs are met, excess fatty acids can undergo various metabolic pathways that contribute to the production of Reactive Oxygen Species (ROS) that overwhelm the cellular antioxidant and immune systems, leading to an imbalance known as oxidative stress [50]. This oxidative stress can result in cellular damage, including lipid peroxidation, protein oxidation, and DNA damage, ultimately affecting cellular function [51]. In the context of neurodevelopment, oxidative balance is fundamental to all cellular and neuronal processes, including neurogenesis, synaptogenesis, neuronal migration, and pruning [52]. Disruptions to this balance can have detrimental effects on the CNS, leading to neurodevelopmental dysfunctions [53]. Animal studies have demonstrated the detrimental oxidative effects of high levels of fatty acids, with overfed mice receiving DHA showing decreased lifespans, likely due to oxidative stress and reduced cellular function [54]. Nevertheless, further human studies are needed to confirm these suggestions.
The development of neurological circuits during the embryonic and fetal phases is a highly complex process influenced by multiple genetic, biological, metabolic, and environmental factors, among which maternal diet during pregnancy, nutritional status, the presence of viral infections, stress, medication use, and even the maternal microbiota stand out [55]. In our study, we have adjusted the multivariate analysis for several of these factors, such as sociodemographic characteristics (family socioeconomic status and parity); psychological distress (mother’s anxiety symptoms); clinical aspects (body mass index during pregnancy); nutritional factors (mother’s serum ferritin levels and quality of diet during pregnancy); lifestyle habits (tobacco and alcohol use); obstetric outcomes (gestational age at birth, type of delivery, and infant’s gender); mother’s acetic, propionic, and butyric acid levels (as indicators of mother microbiota); and infant breastfeeding at the moment of cognitive assessment. This adjustment has allowed us to isolate the effect of maternal n-3 LCPUFA concentrations on fetal neurodevelopment from the rest of the mentioned adjusting factors.
Various strengths of our study have been mentioned previously, such as the assessment of the n-3 LCPUFA concentration in the serum at the beginning and end of pregnancy, as well as the exhaustive control of confounders in our analysis. However, the limitations of our study need to be contextualized to interpret our results with caution. Of the group of omega-3 fatty acids, α-linolenic acid (ALA) could not be incorporated in this work because only trace levels were detected. We assessed infant neurodevelopment using the BSID-III. Although it is a widely recognized and internationally used tool specifically designed and validated for assessing cognitive development from birth to 42 months, it is crucial to acknowledge the inherent limitations of early childhood neurodevelopment assessment, which tends to exhibit notable variability [56,57]. Recognizing this inherent variability is essential for interpreting our results cautiously. Nevertheless, it is important to note that in our previous studies, we have identified associations between various maternal prenatal nutritional states and other environmental exposures and neurodevelopment assessed at this stage using the same tool [58,59]. This highlights the complexity and multifactorial nature of early neurodevelopment, underscoring the need for careful consideration when interpreting our findings in the broader context of infant development. On the other hand, the inclusion of a substantial number of participants enhances the generalizability of our findings to similar populations but limits the generalizability to other populations with different dietary patterns and genetic backgrounds. Therefore, we posit that these findings may be applicable to Mediterranean populations in southern Europe sharing similar sociocultural and environmental characteristics. Consequently, the replication of our study across diverse populations would help validate the observed associations and explore potential variations. Moreover, further research is needed to explore the long-term implications of maternal fatty acid levels on cognitive and neurological performance in children. Overall, these findings contribute to our understanding and can guide professionals in supporting optimal prenatal nutrition and neurodevelopmental outcomes.
5. Conclusions
In conclusion, our study findings indicate that, in a community sample of well-nourished, healthy pregnant women from a Mediterranean area, lower serum levels of n-3 LCPUFA and DHA in the first trimester of pregnancy are associated with improved neurodevelopmental scores in infants 40 days after birth. The levels observed in this sample may be adequate during early embryonic stages, while the higher levels observed might potentially induce adverse effects by fostering oxidation and inflammation processes, which could negate the beneficial effects. Although these results seem to partially support the evidence from some previous studies, it is crucial to acknowledge that further studies are needed to confirm and expand upon these findings. The replication of our study in larger and more diverse populations, as well as longitudinal studies assessing long-term neurodevelopmental outcomes, would provide a stronger evidence base.
Author Contributions
B.S. conducted the conceptualization and formal analysis and wrote the original draft. C.H.-M. contributed to the conceptualization, data curation, formal analysis, investigation, and methodology and participated in writing, reviewing, and editing. N.V. was involved in the investigation and methodology and participated in writing, reviewing, and editing. J.C. guided the conceptualization, methodology, and supervision and contributed to writing, reviewing, and editing. V.A. was responsible for the conceptualization, formal analysis, funding acquisition, methodology, project administration, resource management, and supervision and participated in writing, reviewing, and editing. All authors have read and agreed to the published version of the manuscript.
Funding
The ECLIPSES trial received financial support from the Health Research Fund of the Ministry of Health and Consumption (Madrid, Spain) through grants PI12/02777 and PI17/01754, as well as from the European Union (ERDF/ESF, “A way to make Europe”/“Investing in your future”).
Institutional Review Board Statement
This research was registered at both ClinicalTrials.gov (with identification number NCT03196882, approval on 21 May 2013) and the EU Clinical Trials Register (EUCTR-2012-005480-28, approval on 23 June 2017). Approval for this study was obtained from the Ethical Committee of the Jordi Gol Institute for Primary Care Research and the Pere Virgili Institute for Health Research (ref. no. 118/2017, date: 28 September 2017), and it adhered to the principles outlined in the Helsinki Declaration.
Informed Consent Statement
All participants provided their signature on an informed consent document.
Data Availability Statement
The datasets generated and/or analyzed during the study are not publicly accessible due to considerations of subject confidentiality. However, they can be obtained from the corresponding author upon reasonable request.
Acknowledgments
We express our gratitude to the volunteers and staff involved in the ECLIPSES trial for their valuable contributions. We would also like to thank the Jordi Gol Research Institute in Primary Care [Instituto de Investigación en Atención Primaria; IDIAP] for their support. N.V. is acknowledged as a Serra Hunter Fellow in Spain.
Conflicts of Interest
The authors declare no conflicts of interest. The funders played no role in the study’s design, data collection, analysis, manuscript writing, or in the decision to publish.
References
- Colombo, J.; Gustafson, K.M.; Carlson, S.E. Critical and Sensitive Periods in Development and Nutrition. Ann. Nutr. Metab. 2019, 75, 34–42. [Google Scholar] [CrossRef]
- Cusick, S.E.; Georgieff, M.K. The Role of Nutrition in Brain Development: The Golden Opportunity of the “First 1000 Days” Brain Development in Late Fetal and Early Postnatal Life. J. Pediatr. 2016, 175, 16–21. [Google Scholar] [CrossRef]
- Büyükuslu, N.; Ovalı, S.; Altuntaş, Ş.L.; Batırel, S.; Yiğit, P.; Garipağaoğlu, M. Supplementation of Docosahexaenoic Acid (DHA) / Eicosapentaenoic Acid (EPA) in a Ratio of 1/1.3 during the Last Trimester of Pregnancy Results in EPA Accumulation in Cord Blood. Prostaglandins Leukot Essent Fat. Acids 2017, 125, 32–36. [Google Scholar] [CrossRef]
- Martinat, M.; Rossitto, M.; Di Miceli, M.; Layé, S. Perinatal Dietary Polyunsaturated Fatty Acids in Brain Development, Role in Neurodevelopmental Disorders. Nutrients 2021, 13, 1185. [Google Scholar] [CrossRef]
- Dinicolantonio, J.J.; O’keefe, J.H. The Importance of Marine OMEGA-3S for Brain Development and the Prevention and Treatment of Behavior, Mood, and Other Brain Disorders. Nutrients 2020, 12, 2333. [Google Scholar] [CrossRef]
- Clandinin, M.T.; Chappell, J.E.; Leong, S.; Heim, T.; Swyer, P.R.; Chance, G.W. Intrauterine Fatty Acid Accretion in Infant Brain: Implications for Fatty Acid Requirements. Early Hum. Dev. 1980, 4, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Clandinin, M.T.; Chappell, J.E.; Leong, S.; Heim, T.; Swyer, P.R.; Chance, G.W. Extrauterine Fatty Acid Accretion in Infant Brain: Implications for Fatty Acid Requirements. Early Hum. Dev. 1980, 4, 131–138. [Google Scholar] [CrossRef]
- Martinez, M. Tissue Levels of Polyunsaturated Fatty Acids during Early Human Development. J. Pediatr. 1992, 120, S129–S138. [Google Scholar] [CrossRef] [PubMed]
- Georgieff, M.K.; Ramel, S.E.; Cusick, S.E. Nutritional Influences on Brain Development. Acta Paediatr. Int. J. Paediatr. 2018, 107, 1310–1321. [Google Scholar] [CrossRef] [PubMed]
- Gould, J.F.; Roberts, R.M.; Makrides, M. The Influence of Omega-3 Long-Chain Polyunsaturated Fatty Acid, Docosahexaenoic Acid, on Child Behavioral Functioning: A Review of Randomized Controlled Trials of Dha Supplementation in Pregnancy, the Neonatal Period and Infancy. Nutrients 2021, 13, 415. [Google Scholar] [CrossRef] [PubMed]
- Judge, M.P.; Harel, O.; Lammi-Keefe, C.J. Maternal Consumption of a Docosahexaenoic Acid-Containing Functional Food during Pregnancy: Benefit for Infant Performance on Problem-Solving but Not on Recognition Memory Tasks at Age 9 Mo. Am. J. Clin. Nutr. 2007, 85, 1572–1577. [Google Scholar] [CrossRef]
- Makrides, M.; Gibson, R.A.; McPhee, A.J.; Yelland, L.; Quinlivan, J.; Ryan, P.; Doyle, L.W.; Anderson, P.; Else, P.L.; Meyer, B.J.; et al. Effect of DHA Supplementation during Pregnancy on Maternal Depression and Neurodevelopment of Young Children: A Randomized Controlled Trial. JAMA 2010, 304, 1675–1683. [Google Scholar] [CrossRef]
- Colombo, J.; Jill Shaddy, D.; Gustafson, K.; Gajewski, B.J.; Thodosoff, J.M.; Kerling, E.; Carlson, S.E. The Kansas University DHA Outcomes Study (KUDOS) Clinical Trial: Long-Term Behavioral Follow-up of the Effects of Prenatal DHA Supplementation. Am. J. Clin. Nutr. 2019, 109, 1380–1392. [Google Scholar] [CrossRef]
- Mulder, K.A.; King, D.J.; Innis, S.M. Omega-3 Fatty Acid Deficiency in Infants before Birth Identified Using a Randomized Trial of Maternal DHA Supplementation in Pregnancy. PLoS ONE 2014, 9, 19–22. [Google Scholar] [CrossRef]
- Gould, J.F.; Smithers, L.G.; Makrides, M. The Effect of Maternal Omega-3 (N23) LCPUFA Supplementation during Pregnancy on Early Childhood Cognitive and Visual Development: A Systematic Review and Meta-Analysis of Randomized Controlled Trials1-3. Am. J. Clin. Nutr. 2013, 97, 531–544. [Google Scholar] [CrossRef]
- Middleton, P.; Gomersall, J.C.; Gould, J.F.; Shepherd, E.; Olsen, S.F.; Makrides, M. Omega-3 Fatty Acid Addition during Pregnancy. Cochrane Database Syst. Rev. 2018, 2018, CD003402. [Google Scholar] [CrossRef] [PubMed]
- Moon, K.; Rao, S.C.; Schulzke, S.M.; Patole, S.K.; Simmer, K. Longchain Polyunsaturated Fatty Acid Supplementation in Preterm Infants. Cochrane Database Syst. Rev. 2016, 2016, CD000375. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Noguera, M.F.; Calvache, J.A.; Bonfill Cosp, X.; Kotanidou, E.P.; Galli-Tsinopoulou, A. Supplementation with Long Chain Polyunsaturated Fatty Acids (LCPUFA) to Breastfeeding Mothers for Improving Child Growth and Development. Cochrane Database Syst. Rev. 2015, 2015, CD007901. [Google Scholar] [CrossRef] [PubMed]
- Jasani, B.; Simmer, K.; Patole, S.K.; Rao, S.C. Long Chain Polyunsaturated Fatty Acid Supplementation in Infants Born at Term. Cochrane Database Syst. Rev. 2017, 2017, CD000376. [Google Scholar] [CrossRef] [PubMed]
- Spiller, P.; Hibbeln, J.R.; Myers, G.; Vannice, G.; Golding, J.; Crawford, M.A.; Strain, J.J.; Connor, S.L.; Brenna, J.T.; Kris-Etherton, P.; et al. An Abundance of Seafood Consumption Studies Presents New Opportunities to Evaluate Effects on Neurocognitive Development. Prostaglandins Leukot Essent Fat. Acids 2019, 151, 8–13. [Google Scholar] [CrossRef]
- Hibbeln, C.J.R.; Spiller, P.; Brenna, J.T.; Golding, J.; Holub, B.J.; Harris, W.S.; Kris-Etherton, P.; Lands, B.; Connor, S.L.; Myers, G.; et al. Relationships between Seafood Consumption during Pregnancy and Childhood and Neurocognitive Development: Two Systematic Reviews. Prostaglandins Leukot Essent Fat. Acids 2019, 151, 14–36. [Google Scholar] [CrossRef] [PubMed]
- Mendez, M.A.; Torrent, M.; Julvez, J.; Ribas-Fitó, N.; Kogevinas, M.; Sunyer, J. Maternal Fish and Other Seafood Intakes during Pregnancy and Child Neurodevelopment at Age 4 Years. Public Health Nutr. 2009, 12, 1702–1710. [Google Scholar] [CrossRef] [PubMed]
- Daniels, J.L.; Longnecker, M.P.; Rowland, A.S.; Golding, J. Fish Intake during Pregnancy and Early Cognitive Development of Offspring. Epidemiology 2004, 15, 394–402. [Google Scholar] [CrossRef]
- Oken, E.; Radesky, J.S.; Wright, R.O.; Bellinger, D.C.; Amarasiriwardena, C.J.; Kleinman, K.P.; Hu, H.; Gillman, M.W. Maternal Fish Intake during Pregnancy, Blood Mercury Levels, and Child Cognition at Age 3 Years in a US Cohort. Am. J. Epidemiol. 2008, 167, 1171–1181. [Google Scholar] [CrossRef]
- Gale, C.R.; Robinson, S.M.; Godfrey, K.M.; Law, C.M.; Schlotz, W.; O’Callaghan, F.J. Oily Fish Intake during Pregnancy—Association with Lower Hyperactivity but Not with Higher Full-Scale IQ in Offspring. J. Child Psychol. Psychiatry 2008, 49, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Hibbeln, J.R.; Davis, J.M.; Steer, C.; Emmett, P.; Rogers, I.; Williams, C.; Golding, J. Maternal Seafood Consumption in Pregnancy and Neurodevelopmental Outcomes in Childhood (ALSPAC Study): An Observational Cohort Study. Lancet 2007, 369, 578–585. [Google Scholar] [CrossRef]
- Oken, E.; Østerdal, M.L.; Gillman, M.W.; Knudsen, V.K.; Halldorsson, T.I.; Strøm, M.; Bellinger, D.C.; Hadders-Algra, M.; Michaelsen, K.F.; Olsen, S.F. Associations of Maternal Fish Intake during Pregnancy and Breastfeeding Duration with Attainment of Developmental Milestones in Early Childhood: A Study from the Danish National Birth Cohort. Am. J. Clin. Nutr. 2008, 88, 789–796. [Google Scholar] [CrossRef]
- Rioux, F.M.; Bélanger-Plourde, J.; Leblanc, C.P.; Vigneau, F. Relationship Between Maternal DHA and Iron Status: And Infants’ Cognitive Performance. Can. J. Diet. Pract. Res. 2011, 72, e140–e146. [Google Scholar] [CrossRef]
- Brouwer-Brolsma, E.M.; van de Rest, O.; Godschalk, R.; Zeegers, M.P.A.; Gielen, M.; de Groot, R.H.M. Associations between Maternal Long-Chain Polyunsaturated Fatty Acid Concentrations and Child Cognition at 7 Years of Age: The MEFAB Birth Cohort. Prostaglandins Leukot Essent Fat. Acids 2017, 126, 92–97. [Google Scholar] [CrossRef]
- Strain, J.J.; Davidson, P.W.; Bonham, M.P.; Duffy, E.M.; Stokes-Riner, A.; Thurston, S.W.; Wallace, J.M.W.; Robson, P.J.; Shamlaye, C.F.; Georger, L.A.; et al. Associations of Maternal Long-Chain Polyunsaturated Fatty Acids, Methyl Mercury, and Infant Development in the Seychelles Child Development Nutrition Study. Neurotoxicology 2008, 29, 776–782. [Google Scholar] [CrossRef]
- Braarud, H.C.; Markhus, M.W.; Skotheim, S.; Stormark, K.M.; Frøyland, L.; Graff, I.E.; Kjellevold, M. Maternal DHA Status during Pregnancy Has a Positive Impact on Infant Problem Solving: A Norwegian Prospective Observation Study. Nutrients 2018, 10, 529. [Google Scholar] [CrossRef] [PubMed]
- Arija, V.; Fargas, F.; March, G.; Abajo, S.; Basora, J.; Canals, J.; Ribot, B.; Aparicio, E.; Serrat, N.; Hernández-Martínez, C.; et al. Adapting Iron Dose Supplementation in Pregnancy for Greater Effectiveness on Mother and Child Health: Protocol of the ECLIPSES Randomized Clinical Trial. BMC Pregnancy Childbirth 2014, 14, 33. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, L.I.; Arija, V.; Aranda, N.; Aparicio, E.; Serrat, N.; Fargas, F.; Ruiz, F.; Pallejà, M.; Coronel, P.; Gimeno, M.; et al. The Effectiveness of Different Doses of Iron Supplementation and the Prenatal Determinants of Maternal Iron Status in Pregnant Spanish Women: ECLIPSES Study. Nutrients 2019, 11, 2418. [Google Scholar] [CrossRef] [PubMed]
- Baylay, N. Bayley Scales for Infant and Toddler Development, 3rd ed.; Psychological Corporation: San Antonio, TX, USA, 2006. [Google Scholar]
- Spielberger, C.D.; Gorsuch, R.L.; Lushene, R.E. STAI Cuestionario de Ansiedad Estado Rasgo; TEA Ediciones: Madrid, Spain, 1997. [Google Scholar]
- Hollingshead, A.B. Four Factor Index of Social Status. Yale J. Sociol. 2011, 8, 21–52. [Google Scholar]
- Institut d’Estadística de Catalunya. Catalan Classification of Occupations; Institut d’Estadística de Catalunya; Catalonia, Spain: 2011. Available online: https://www.idescat.cat/metodes/classificacions/cco-2011-ca?lang=es/ (accessed on 27 November 2023).
- Validación de Un Cuestionario de Frecuencia de Consumo Alimentario Corto: Reproducibilidad y Validez. Available online: https://scielo.isciii.es/scielo.php?script=sci_arttext&pid=S0212-16112008000300011 (accessed on 25 October 2022).
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean Diet and Survival in a Greek Population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef]
- Fagerström, K.O. Measuring Degree of Physical Dependence to Tobacco Smoking with Reference to Individualization of Treatment. Addict. Behav. 1978, 3, 235–241. [Google Scholar] [CrossRef]
- Otto, S.J.; Houwelingen, A.C.; Antal, M.; Manninen, A.; Godfrey, K.; López-Jaramillo, P.; Hornstra, G. Maternal and Neonatal Essential Fatty Acid Status in Phospholipids: An International Comparative Study. Eur. J. Clin. Nutr. 1997, 51, 232–242. [Google Scholar] [CrossRef]
- Human Metabolome Database. Available online: https://hmdb.ca/ (accessed on 27 November 2023).
- van der Wurff, I.S.M.; Bakker, E.C.; Hornstra, G.; Kirschner, P.A.; Gielen, M.; Godschalk, R.W.L.; Kremers, S.; Zeegers, M.P.; de Groot, R.H.M. Association between Prenatal and Current Exposure to Selected LCPUFAs and School Performance at Age 7. Prostaglandins Leukot Essent Fat. Acids 2016, 108, 22–29. [Google Scholar] [CrossRef]
- Strain, J.J.; Davidson, P.W.; Thurston, S.W.; Harrington, D.; Mulhern, M.S.; McAfee, A.J.; van Wijngaarden, E.; Shamlaye, C.F.; Henderson, J.; Watson, G.E.; et al. Maternal PUFA Status but Not Prenatal Methylmercury Exposure Is Associated with Children’s Language Functions at Age Five Years in the Seychelles. J. Nutr. 2012, 142, 1943–1949. [Google Scholar] [CrossRef] [PubMed]
- Thomason, M.E.; Hect, J.; Waller, R.; Manning, J.H.; Stacks, A.M.; Beeghly, M.; Boeve, J.L.; Wong, K.; Van Den Heuvel, M.I.; Hernandez-Andrade, E.; et al. Prenatal Neural Origins of Infant Motor Development: Associations between Fetal Brain and Infant Motor Development. Dev. Psychopathol. 2018, 30, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Borsani, E.; Della Vedova, A.M.; Rezzani, R.; Rodella, L.F.; Cristini, C. Correlation between Human Nervous System Development and Acquisition of Fetal Skills: An Overview. Brain Dev. 2019, 41, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Shulkin, M.; Pimpin, L.; Bellinger, D.; Kranz, S.; Fawzi, W.; Duggan, C.; Mozaffarian, D. N-3 Fatty Acid Supplementation in Mothers, Preterm Infants, and Term Infants and Childhood Psychomotor and Visual Development: A Systematic Review and Meta-Analysis. J. Nutr. 2018, 148, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Gawlik, N.R.; Anderson, A.J.; Makrides, M.; Kettler, L.; Gould, J.F. The Influence of DHA on Language Development: A Review of Randomized Controlled Trials of DHA Supplementation in Pregnancy, the Neonatal Period, and Infancy. Nutrients 2020, 12, 3106. [Google Scholar] [CrossRef] [PubMed]
- Nevins, J.E.H.; Donovan, S.M.; Snetselaar, L.; Dewey, K.G.; Novotny, R.; Stang, J.; Taveras, E.M.; Kleinman, R.E.; Bailey, R.L.; Raghavan, R.; et al. Omega-3 Fatty Acid Dietary Supplements Consumed during Pregnancy and Lactation and Child Neurodevelopment: A Systematic Review. J. Nutr. 2021, 151, 3483–3494. [Google Scholar] [CrossRef]
- Hussain, T.; Murtaza, G.; Metwally, E.; Kalhoro, D.H.; Kalhoro, M.S.; Rahu, B.A.; Sahito, R.G.A.; Yin, Y.; Yang, H.; Chughtai, M.I.; et al. The Role of Oxidative Stress and Antioxidant Balance in Pregnancy. Mediat. Inflamm. 2021, 2021, 9962860. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Londono Tobon, A.; Diaz Stransky, A.; Ross, D.A.; Stevens, H.E. Effects of Maternal Prenatal Stress: Mechanisms, Implications, and Novel Therapeutic Interventions. Biol. Psychiatry 2016, 80, e85–e87. [Google Scholar] [CrossRef]
- Cattane, N.; Räikkönen, K.; Anniverno, R.; Mencacci, C.; Riva, M.A.; Pariante, C.M.; Cattaneo, A. Depression, Obesity and Their Comorbidity during Pregnancy: Effects on the Offspring’s Mental and Physical Health. Mol. Psychiatry 2021, 26, 462–481. [Google Scholar] [CrossRef]
- Tsuduki, T.; Honma, T.; Nakagawa, K.; Ikeda, I.; Miyazawa, T. Long-Term Intake of Fish Oil Increases Oxidative Stress and Decreases Lifespan in Senescence-Accelerated Mice. Nutrition 2011, 27, 334–337. [Google Scholar] [CrossRef]
- Sajdel-Sulkowska, E.M. The Impact of Maternal Gut Microbiota during Pregnancy on Fetal Gut–Brain Axis Development and Life-Long Health Outcomes. Microorganisms 2023, 11, 2199. [Google Scholar] [CrossRef]
- Del Rosario, C.; Slevin, M.; Molloy, E.J.; Quigley, J.; Nixon, E. How to Use the Bayley Scales of Infant and Toddler Development. Arch. Dis. Child. Educ. Pract. 2021, 106, 108–112. [Google Scholar] [CrossRef]
- Kvestad, I.; Hysing, M.; Ranjitkar, S.; Shrestha, M.; Ulak, M.; Chandyo, R.K.; Strand, T.A. The Stability of the Bayley Scales in Early Childhood and Its Relationship with Future Intellectual Abilities in a Low to Middle Income Country. Early Hum. Dev. 2022, 170, 105610. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Rodríguez, J.; Díaz-López, A.; Canals-Sans, J.; Arija, V. Maternal Vitamin B12 Status during Pregnancy and Early Infant Neurodevelopment: The ECLIPSES Study. Nutrients 2023, 15, 1529. [Google Scholar] [CrossRef] [PubMed]
- Voltas, N.; Canals, J.; Hernández-Martínez, C.; Serrat, N.; Basora, J.; Arija, V. Effect of Vitamin d Status during Pregnancy on Infant Neurodevelopment: The Eclipses Study. Nutrients 2020, 12, 3196. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).