Effects of Kiwifruit Dietary Fibers on Pasting Properties and In Vitro Starch Digestibility of Wheat Starch
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pasting Characteristics
2.2. Fourier Transform Infrared (FT-IR) Spectroscopy
2.3. Scanning Electron Microscopy (SEM)
2.4. Rheological Measurements
2.5. Leached Amylose
2.6. In Vitro Starch Digestion
2.7. Glucose Diffusion Inhibition Index
2.8. Statistical Analysis
3. Results
3.1. Pasting Properties
3.2. FT-IR Spectroscopy
3.3. SEM
3.4. Rheological Characteristics
3.4.1. Dynamic Rheological Characteristics
3.4.2. Static Rheological Characteristics
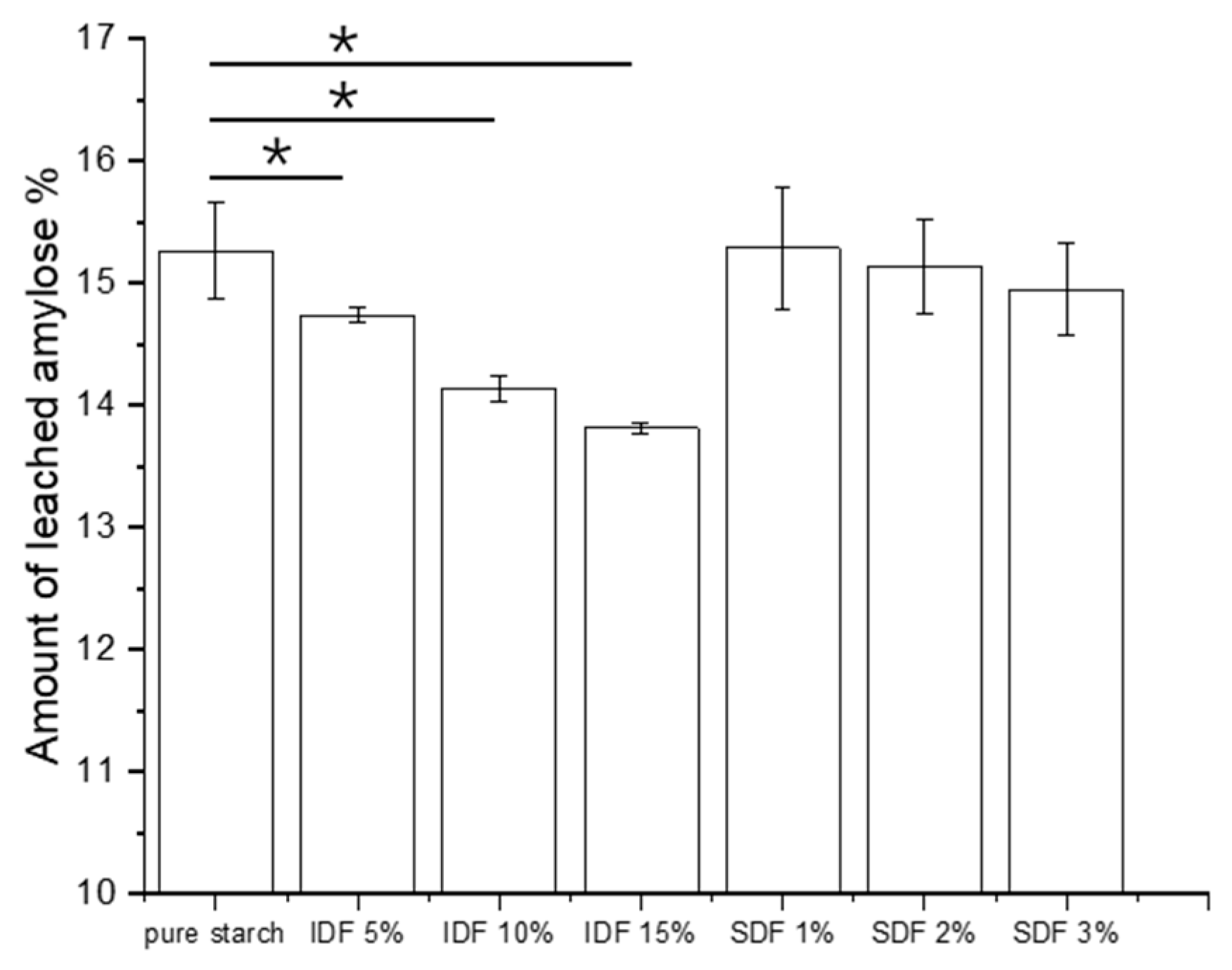
3.5. Amount of Leached Amylose
3.6. In Vitro Starch Digestion
3.7. Glucose Diffusion Inhibition Index Measurements
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, L.; Zhang, H.; McClements, D.J.; Zhang, Z.; Zhang, R.; Jin, Z.; Tian, Y. Effect of dietary fibers on the structure and digestibility of fried potato starch: A comparison of pullulan and pectin. Carbohydr. Polym. 2019, 215, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Butterworth, P.J.; Bajka, B.H.; Edwards, C.H.; Warren, F.J.; Ellis, P.R. Enzyme kinetic approach for mechanistic insight and predictions of in vivo starch digestibility and the glycaemic index of foods. Trends Food Sci. Technol. 2022, 120, 254–264. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, S.; Ai, L. Physical barrier effects of dietary fibers on lowering starch digestibility. Curr. Opin. Food Sci. 2022, 48, 100940. [Google Scholar] [CrossRef]
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46 (Suppl. S2), S33–S50. [Google Scholar] [PubMed]
- Chai, Y.; Wang, M.; Zhang, G. Interaction between Amylose and Tea Polyphenols Modulates the Postprandial Glycemic Response to High-Amylose Maize Starch. J. Agric. Food Chem. 2013, 61, 8608–8615. [Google Scholar] [CrossRef] [PubMed]
- Kawamura-Konishi, Y.; Watanabe, N.; Saito, M.; Nakajima, N.; Sakaki, T.; Katayama, T.; Enomoto, T. Isolation of a New Phlorotannin, a Potent Inhibitor of Carbohydrate-Hydrolyzing Enzymes, from the Brown Alga Sargassum patens. J. Agric. Food Chem. 2012, 60, 5565–5570. [Google Scholar] [CrossRef] [PubMed]
- Sajilata, M.G.; Singhal, R.S.; Kulkarni, P.R. Resistant Starch—A Review. Comprehensive Reviews in Food Science and Food. Safety 2006, 5, 1–17. [Google Scholar]
- Rovalino-Córdova, A.M.; Montesdeoca, V.A.; Capuano, E. A mechanistic model to study the effect of the cell wall on starch digestion in intact cotyledon cells. Carbohydr. Polym. 2021, 253, 117351. [Google Scholar] [CrossRef]
- Korompokis, K.; Verbeke, K.; Delcour, J.A. Structural factors governing starch digestion and glycemic responses and how they can be modified by enzymatic approaches: A review and a guide. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5965–5991. [Google Scholar] [CrossRef]
- Edwards, C.H.; Ryden, P.; Mandalari, G.; Butterworth, P.J.; Ellis, P.R. Structure–function studies of chickpea and durum wheat uncover mechanisms by which cell wall properties influence starch bioaccessibility. Nat. Food 2021, 2, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, Y.; Xu, Z.; Pan, W.; Shen, M.; Han, J.; Sun, X.; Zhang, Y.; Xie, J.; Zhang, X.; et al. Cross-linked corn bran arabinoxylan improves the pasting, rheological, gelling properties of corn starch and reduces its in vitro digestibility. Food Hydrocoll. 2022, 126, 107440. [Google Scholar] [CrossRef]
- Raguzzoni, J.C.; Delgadillo, I.; da Silva, J.A.L. Influence of a cationic polysaccharide on starch functionality. Carbohydr. Polym. 2016, 150, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, C.; Martino, M.N.; Zaritzky, N.E. Effect of hydrocolloids on starch thermal transitions, as measured by DSC. J. Therm. Anal. Calorim. 1996, 47, 1247–1266. [Google Scholar] [CrossRef]
- Liu, S.; Lin, L.; Shen, M.; Wang, W.; Xiao, Y.; Xie, J. Effect of Mesona chinensis polysaccharide on the pasting, thermal and rheological properties of wheat starch. Int. J. Biol. Macromol. 2018, 118, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Heyman, B.; De Vos, W.H.; Depypere, F.; Van der Meeren, P.; Dewettinck, K. Guar and xanthan gum differentially affect shear induced breakdown of native waxy maize starch. Food Hydrocoll. 2014, 35, 546–556. [Google Scholar] [CrossRef]
- Yamazaki, E.; Sago, T.; Kasubuchi, Y.; Imamura, K.; Matsuoka, T.; Kurita, O.; Nambu, H.; Matsumura, Y. Improvement on the freeze–thaw stability of corn starch gel by the polysaccharide from leaves of Corchorus olitorius L. Carbohydr. Polym. 2013, 94, 555–560. [Google Scholar] [CrossRef]
- Sasaki, T.; Kohyama, K. Influence of non-starch polysaccharides on the in vitro digestibility and viscosity of starch suspensions. Food Chem. 2012, 133, 1420–1426. [Google Scholar] [CrossRef]
- Guo, J.; Yuan, Y.; Dou, P.; Yue, T. Multivariate statistical analysis of the polyphenolic constituents in kiwifruit juices to trace fruit varieties and geographical origins. Food Chem. 2017, 232, 552–559. [Google Scholar] [CrossRef]
- Ma, T.; Lan, T.; Ju, Y.; Cheng, G.; Que, Z.; Geng, T.; Fang, Y.; Sun, X. Comparison of the nutritional properties and biological activities of kiwifruit (Actinidia) and their different forms of products: Towards making kiwifruit more nutritious and functional. Food Funct. 2019, 10, 1317–1329. [Google Scholar] [CrossRef]
- Martin-Cabrejas, M.A.; Esteban, R.M.; Lopez-Andreu, F.J.; Waldron, K.; Selvendran, R.R. Dietary Fiber Content of Pear and Kiwi Pomaces. J. Agric. Food Chem. 1995, 43, 662–666. [Google Scholar] [CrossRef]
- Lu, Y.; Du, X.; Lai, L.; Jin, H. Actinidia macrosperma C. F. Liang (a Wild Kiwi): Preliminary Study of Its Antioxidant and Cytotoxic Activities. Evid.-Based Complement. Altern. Med. 2012, 2012, 180262. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Liu, H.; Zhao, T.; Meng, C.; Liu, Z.; Liu, X. Bioactive compounds and in vitro antioxidant activities of peel, flesh and seed powder of kiwifruit. Int. J. Food Sci. Technol. 2018, 53, 2239–2245. [Google Scholar] [CrossRef]
- Wang, K.; Li, M.; Han, Q.; Fu, R.; Ni, Y. Inhibition of α-amylase activity by insoluble and soluble dietary fibers from kiwifruit (Actinidia deliciosa). Food Biosci. 2021, 42, 101057. [Google Scholar] [CrossRef]
- Wang, K.; Wang, Y.; Chen, S.; Gu, J.; Ni, Y. Insoluble and Soluble Dietary Fibers from Kiwifruit (Actinidia deliciosa) Modify Gut Microbiota to Alleviate High-Fat Diet and Streptozotocin-Induced TYPE 2 Diabetes in Rats. Nutrients 2022, 14, 3369. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, S.; Xi, H.; Xu, J.; Deng, D.; Huang, G. Effects of soluble dietary fiber on the crystallinity, pasting, rheological, and morphological properties of corn resistant starch. LWT 2019, 111, 632–639. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, M.; Mo, F.; Zhang, M.; Zheng, J. Effects of Acid and Salt Solutions on the Pasting, Rheology and Texture of Lotus Root Starch–Konjac Glucomannan Mixture. Polymers 2017, 9, 695. [Google Scholar] [CrossRef]
- Bae, I.Y.; Jun, Y.; Lee, S.; Lee, H.G. Characterization of apple dietary fibers influencing the in vitro starch digestibility of wheat flour gel. LWT—Food Sci. Technol. 2016, 65, 158–163. [Google Scholar] [CrossRef]
- Kong, X.-R.; Zhu, Z.-Y.; Zhang, X.-J.; Zhu, Y.-M. Effects of Cordyceps polysaccharides on pasting properties and in vitro starch digestibility of wheat starch. Food Hydrocoll. 2020, 102, 105604. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Zheng, J.; Huang, S.; Zhao, R.; Wang, N.; Kan, J.; Zhang, F. Effect of four viscous soluble dietary fibers on the physicochemical, structural properties, and in vitro digestibility of rice starch: A comparison study. Food Chem. 2021, 362, 130181. [Google Scholar] [CrossRef]
- Wang, N.; Huang, S.; Zhang, Y.; Zhang, F.; Zheng, J. Effect of supplementation by bamboo shoot insoluble dietary fiber on physicochemical and structural properties of rice starch. LWT—Food Sci. Technol. 2020, 129, 109509. [Google Scholar] [CrossRef]
- Liu, T.; Wang, K.; Xue, W.; Wang, L.; Zhang, C.; Zhang, X.; Chen, Z. In vitro starch digestibility, edible quality and microstructure of instant rice noodles enriched with rice bran insoluble dietary fiber. LWT 2021, 142, 111008. [Google Scholar] [CrossRef]
- Ning, Y.; Cui, B.; Yuan, C. Decreasing the digestibility of debranched corn starch by encapsulation with konjac glucomannan. Food Hydrocoll. 2020, 107, 105966. [Google Scholar] [CrossRef]
- Temsiripong, T.; Pongsawatmanit, R.; Ikeda, S.; Nishinari, K. Influence of xyloglucan on gelatinization and retrogradation of tapioca starch. Food Hydrocoll. 2005, 19, 1054–1063. [Google Scholar] [CrossRef]
- Sun, C.; Liu, R.; Liang, B.; Wu, T.; Sui, W.; Zhang, M. Microparticulated whey protein-pectin complex: A texture-controllable gel for low-fat mayonnaise. Food Res. Int. 2018, 108, 151–160. [Google Scholar] [CrossRef]
- Lin, L.; Huang, J.; Zhao, L.; Wang, J.; Wang, Z.; Wei, C. Effect of granule size on the properties of lotus rhizome C-type starch. Carbohydr. Polym. 2015, 134, 448–457. [Google Scholar] [CrossRef]
- Kumar, L.; Brennan, M.; Zheng, H.; Brennan, C. The effects of dairy ingredients on the pasting, textural, rheological, freeze-thaw properties and swelling behaviour of oat starch. Food Chem. 2018, 245, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Keetels, C.J.; Van Vliet, T.; Walstra, P. Gelation and retrogradation of concentrated starch systems: 3. Effect of concentration and heating temperature. Food Hydrocoll. 1996, 10, 363–368. [Google Scholar] [CrossRef]
- Funami, T.; Kataoka, Y.; Omoto, T.; Goto, Y.; Asai, I.; Nishinari, K. Effects of non-ionic polysaccharides on the gelatinization and retrogradation behavior of wheat starch. Food Hydrocoll. 2005, 19, 1–13. [Google Scholar] [CrossRef]
- Sheng, L.; Li, P.; Wu, H.; Liu, Y.; Han, K.; Gouda, M.; Tong, Q.; Ma, M.; Jin, Y. Tapioca starch-pullulan interaction during gelation and retrogradation. LWT 2018, 96, 432–438. [Google Scholar] [CrossRef]
- Zheng, J.; Liu, M.; Zhang, M.; Kan, J.; Zhang, F. Effects of Pectin on the Pasting, Rheological, and Textural Properties of Lotus Root Starch. Starch Staerke 2019, 71, 1700347. [Google Scholar] [CrossRef]
- Kaushal, P.; Kumar, V.; Sharma, H. Comparative study of physicochemical, functional, antinutritional and pasting properties of taro (Colocasia esculenta), rice (Oryza sativa) flour, pigeonpea (Cajanus cajan) flour and their blends. LWT—Food Sci. Technol. 2012, 48, 59–68. [Google Scholar] [CrossRef]
- Wang, R.; Wan, J.; Liu, C.; Xia, X.; Ding, Y. Pasting, thermal, and rheological properties of rice starch partially replaced by inulin with different degrees of polymerization. Food Hydrocoll. 2019, 92, 228–232. [Google Scholar] [CrossRef]
- Liu, D.; Li, Z.; Fan, Z.; Zhang, X.; Zhong, G. Effect of soybean soluble polysaccharide on the pasting, gels, and rheological properties of kudzu and lotus starches. Food Hydrocoll. 2019, 89, 443–452. [Google Scholar] [CrossRef]
- Funami, T.; Nakauma, M.; Noda, S.; Ishihara, S.; Asai, I.; Inouchi, N.; Nishinari, K. Effects of some anionic polysaccharides on the gelatinization and retrogradation behaviors of wheat starch: Soybean-soluble polysaccharide and gum arabic. Food Hydrocoll. 2008, 22, 1528–1540. [Google Scholar] [CrossRef]
- Ek, K.L.; Wang, S.; Brand-Miller, J.; Copeland, L. Properties of starch from potatoes differing in glycemic index. Food Funct. 2014, 5, 2509–2515. [Google Scholar] [CrossRef]
- Yuris, A.; Hardacre, A.K.; Goh, K.K.; Matia-Merino, L. The role of calcium in wheat starch-Mesona chinensis polysaccharide gels: Rheological properties, in vitro digestibility and enzyme inhibitory activities. LWT—Food Sci. Technol. 2019, 99, 202–208. [Google Scholar] [CrossRef]
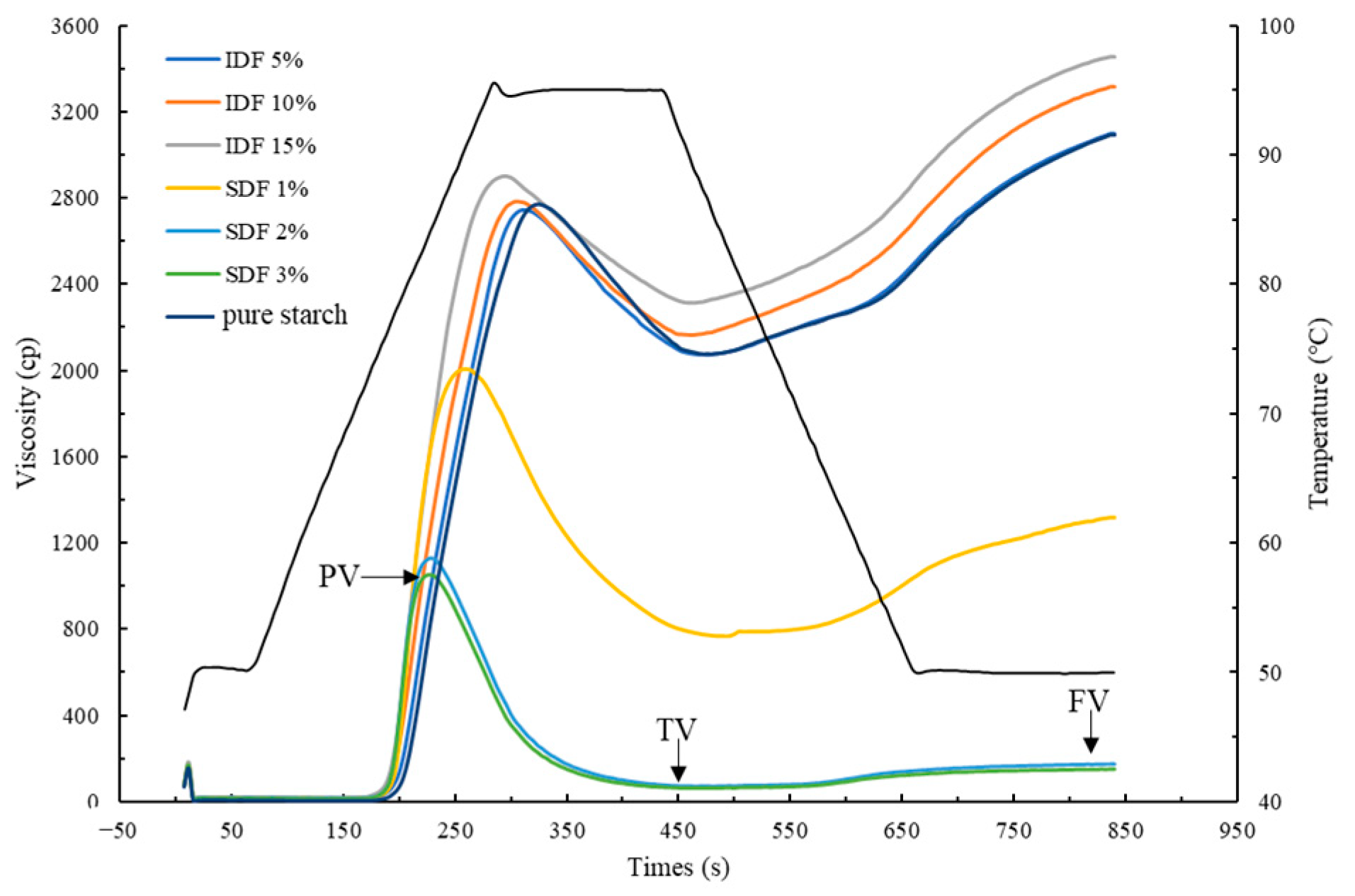
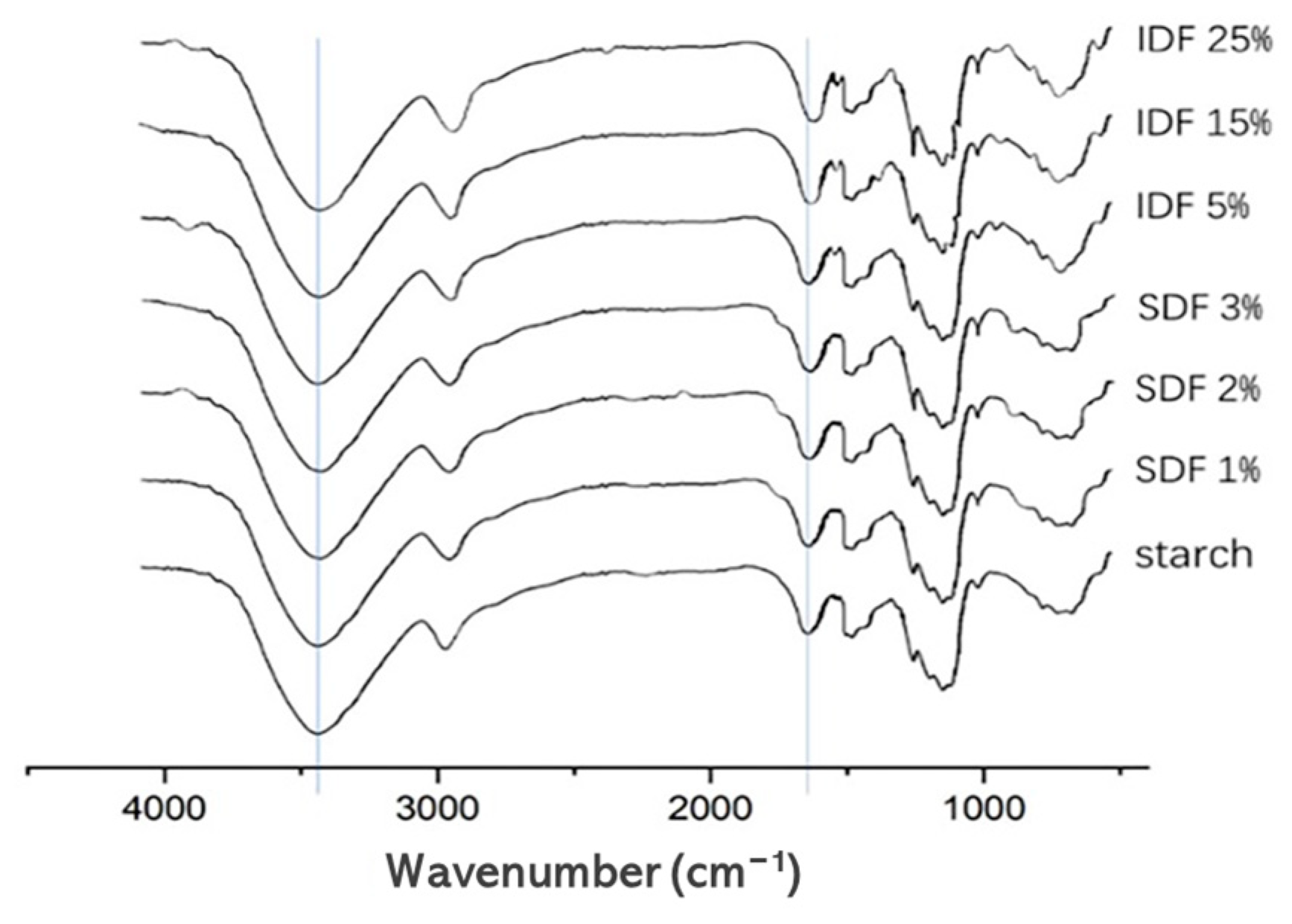

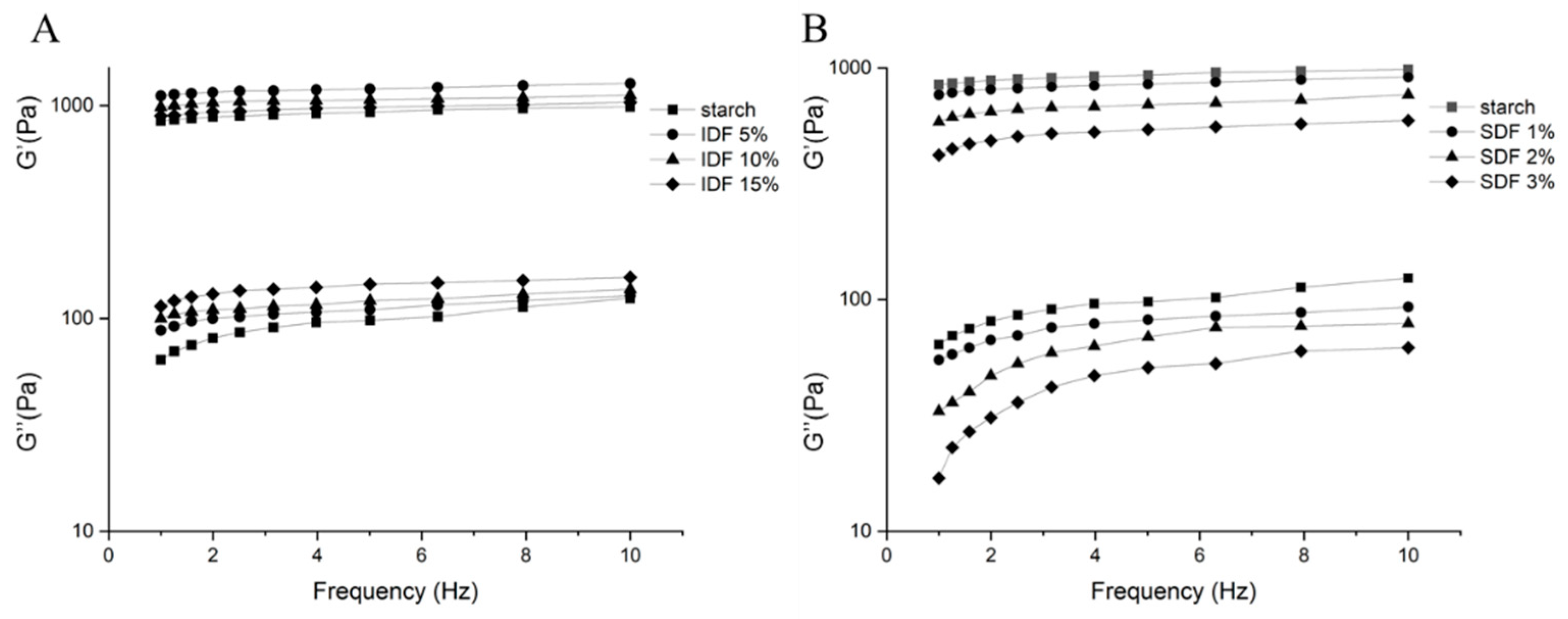
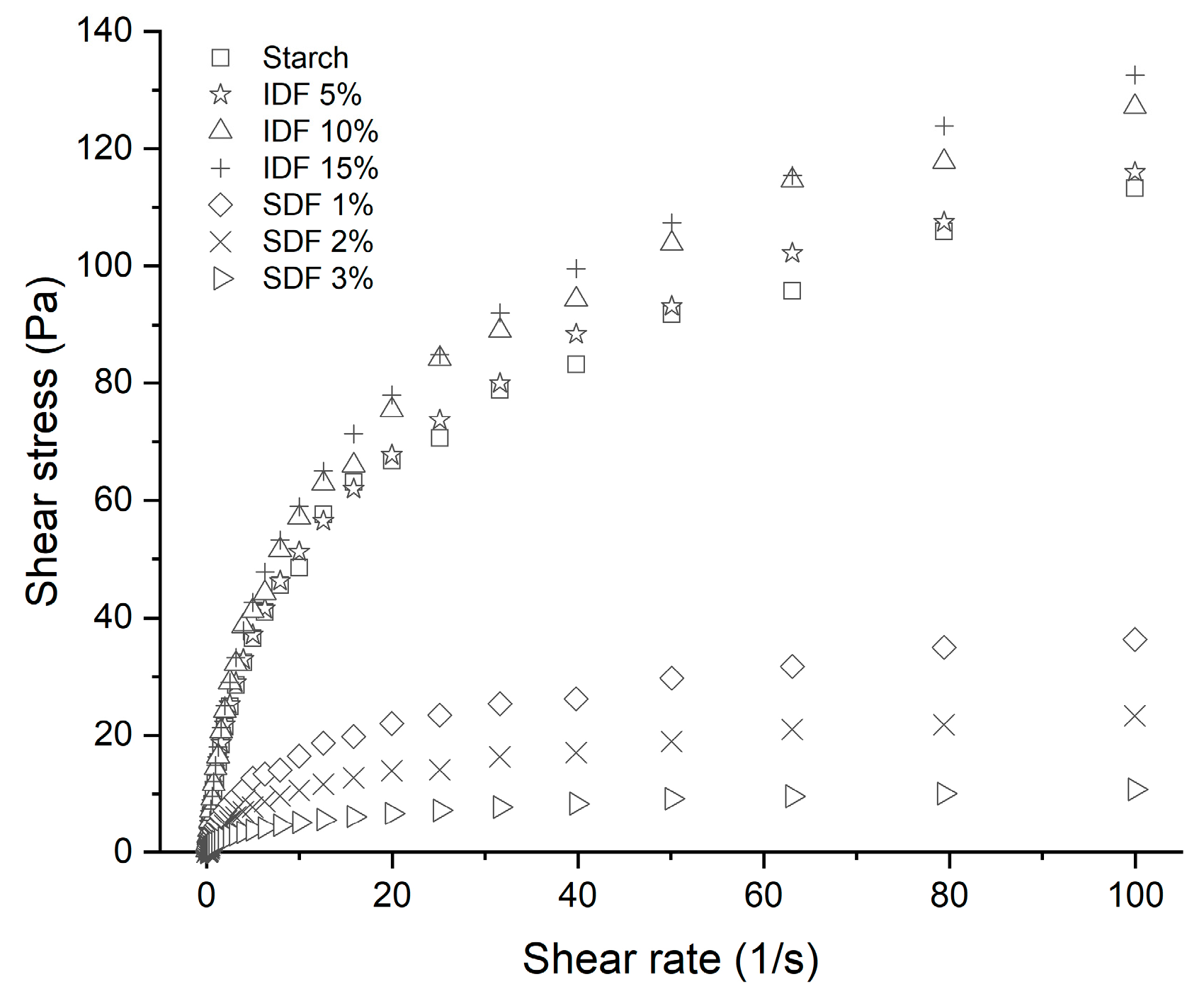
| PV (mPa·s) | TV (mPa·s) | BV (mPa·s) | FV (mPa·s) | SV (mPa·s) | |
|---|---|---|---|---|---|
| Wheat starch | 2781.00 ± 21 d | 2096.33 ± 31.77 c | 684.67 ± 11.68 b | 3096.33 ± 22.19 c | 1000.00 ± 14.73 c |
| IDF 5% | 2764.67 ± 19.76 d | 2068.33 ± 18.45 c | 696.33 ± 20.11 b | 3112.00 ± 17.35 c | 1043.67 ± 14.01 cd |
| IDF 10% | 2857.33 ± 32.59 de | 2218.33 ± 43.68 d | 639.00 ± 11.14 ab | 3395.33 ± 80.01 d | 1177.00 ± 101.06 d |
| IDF 15% | 2979.67 ± 24.85 e | 2383.33 ± 8.39 e | 596.33 ± 33.23 a | 3518.33 ± 61.70 d | 1135.00 ± 69.16 cd |
| SDF 1% | 2004.33 ± 36.07 c | 758.00 ± 37.64 b | 1246.33 ± 4.73 e | 1308.67 ± 60.93 b | 550.67 ± 23.50 b |
| SDF 2% | 1307.33 ± 157.81 b | 138.00 ± 65.78 a | 1169.33 ± 93.38 d | 308.00 ± 124.07 a | 170.00 ± 59.15 a |
| SDF 3% | 980.67 ± 60.05 a | 53.67 ± 9.07 a | 927.00 ± 51.10 c | 126.00 ± 22.72 a | 72.33 ± 13.65 a |
| K (Pa sn) | n (-) | R2 | |
|---|---|---|---|
| Wheat starch | 16.914 ± 0.096 d | 0.429 ± 0.001 d | 0.991 |
| IDF 5% | 17.155 ± 0.045 e | 0.430 ± 0.001 de | 0.99 |
| IDF 10% | 19.052 ± 0.015 f | 0.432 ± 0.001 ef | 0.989 |
| IDF 15% | 19.700 ± 0.021 g | 0.432 ± 0.001 f | 0.991 |
| SDF 1% | 5.634 ± 0.052 c | 0.421 ± 0.001 c | 0.992 |
| SDF 2% | 3.618 ± 0.035 b | 0.418 ± 0.001 b | 0.99 |
| SDF 3% | 1.798 ± 0.010 a | 0.407 ± 0.001 a | 0.992 |
| RDS% | SDS% | RS% | |
|---|---|---|---|
| Wheat starch | 83.15 ± 2.20 d | 10.93 ± 0.65 a | 5.32 ± 2.05 a |
| IDF 5% | 71.49 ± 2.23 c | 17.81 ± 1.85 b | 10.1 ± 4.07 ab |
| IDF 10% | 63.51 ± 1.27 b | 23.13 ± 1.77 cd | 12.76 ± 1.90 bc |
| IDF 15% | 57.06 ± 3.28 a | 25.89 ± 3.34 d | 16.45 ± 0.12 c |
| SDF 1% | 67.34 ± 1.10 bc | 18.87 ± 1.20 bc | 13.19 ± 0.42 bc |
| SDF 2% | 63.2 ± 0.64 b | 18.32 ± 0.82 bc | 17.89 ± 1.12 c |
| SDF 3% | 56.7 ± 1.71 a | 16.29 ± 1.79 b | 26.42 ± 0.43 d |
| Time (min) | 10 | 20 | 30 | 60 | 90 | 120 |
|---|---|---|---|---|---|---|
| Control | 185.32 ± 2.98 c | 484.16 ± 9.37 c | 673.97 ± 17.02 c | 851.89 ± 11.56 c | 969.15 ± 15.23 c | 1025.26 ± 5.50 c |
| SDF | 127.66 ± 1.38 a | 384.84 ± 3.12 a | 526.04 ± 13.31 a | 664.88 ± 5.44 a | 780.94 ± 9.45 a | 832.61 ± 13.57 a |
| IDF | 160.11 ± 6.79 b | 436.86 ± 11.07 b | 593.99 ± 13.18 b | 755.82 ± 6.90 b | 887.48 ± 11.04 b | 955.95 ± 28.73 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Pan, Y.; Zhou, C.; Li, W.; Wang, K. Effects of Kiwifruit Dietary Fibers on Pasting Properties and In Vitro Starch Digestibility of Wheat Starch. Nutrients 2024, 16, 749. https://doi.org/10.3390/nu16050749
Wang Y, Pan Y, Zhou C, Li W, Wang K. Effects of Kiwifruit Dietary Fibers on Pasting Properties and In Vitro Starch Digestibility of Wheat Starch. Nutrients. 2024; 16(5):749. https://doi.org/10.3390/nu16050749
Chicago/Turabian StyleWang, Yaqi, Yaoyi Pan, Chang Zhou, Wenru Li, and Kunli Wang. 2024. "Effects of Kiwifruit Dietary Fibers on Pasting Properties and In Vitro Starch Digestibility of Wheat Starch" Nutrients 16, no. 5: 749. https://doi.org/10.3390/nu16050749
APA StyleWang, Y., Pan, Y., Zhou, C., Li, W., & Wang, K. (2024). Effects of Kiwifruit Dietary Fibers on Pasting Properties and In Vitro Starch Digestibility of Wheat Starch. Nutrients, 16(5), 749. https://doi.org/10.3390/nu16050749






