Abstract
Malnutrition during cancer has a negative impact on prognosis and quality of life. Therefore, it is important to identify those patients at higher nutritional risk to prevent its development. There are nutritional screening tools, such as MUST and NRS-2002, that focus on the patient on admission to hospital. However, most patients will develop malnutrition in the outpatient or ambulatory setting. This study aims to determine which nutritional screening tool is most effective in assessing nutritional risk in the outpatient oncology patient, highlighting the parameters analysed by these tools. Seventeen articles were reviewed, with the most important variables being tumour location, tumour stage, age, and gender, as well as recent weight loss, dietary intake, and digestive disorders. The Nutriscore, NRS-2002, and MUST tools are considered suitable, but the choice varies depending on these parameters. MNA is suitable for elderly patients, while SNAQ was not considered reliable in this population. In conclusion, MUST, NRS-2002, and Nutriscore are suitable tools, but their choice depends on specific characteristics. There is currently no universal tool for nutritional risk assessment in outpatients.
1. Introduction
Cancer is characterised by a rapid proliferation of abnormal cells that can spread, encompassing a group of diseases that share common features, such as their genetic origin, resistance to cell death, and their ability to invade surrounding tissues or metastasise to distant organs [1,2]. In 2020, 19.3 million new cases and 10.0 million deaths were reported globally, with breast, lung, colorectal, prostate, and stomach cancer being the most prevalent, according to data from the International Agency for Research on Cancer (IARC) [3].
Among its consequences are impaired nutritional status, known as disease-related malnutrition (DRE), and cancer cachexia [4,5]. DRE is associated with inflammation and is the preliminary step to cachexia, which represents the most severe state of malnutrition in the cancer patient, characterised by muscle loss (with or without loss of fat mass) that cannot be fully reversed with conventional nutritional support, leading to progressive functional deterioration. It is present in up to 80% of patients and is associated with 20% of cancer mortality [6].
Cachexia is caused by an imbalance between protein and energy intake due to reduced appetite, but also by the effects of inflammation and metabolic alterations induced by the tumour itself [2,7]. Loss of muscle mass has been associated with increased dependency, decreased quality of life, and poorer mental health [7,8]. Furthermore, cachexia increases the risk of infectious complications and limits therapeutic options, as these patients often have poorer tolerance to antineoplastic treatments and lack of response [8].
The aim of any nutritional intervention is therefore to avoid this complication. Screening and early detection tools, which quantify the risk of developing malnutrition (nutritional risk) or are useful in its diagnosis, are available to prevent it. These tools use variables that include the measurement of parameters such as body mass index (BMI), percentage of unintentional weight loss (%WL) [9], or dietary intake [10], among others.
The most developed are nutritional screening tools, which seek to identify those patients who may be at increased nutritional risk and require detailed nutritional assessment [11]. In the hospital and ambulatory field, more than 70 NCs exist. However, there is no international consensus on the best tool to identify malnutrition in specific populations, including oncology outpatients [12]. Among the most relevant tools are the Malnutrition Universal Screening Tool (MUST), Nutritional Risk Screening 2002 (NRS-2002), the Malnutrition Screening Tool (MST), Mini-Nutritional Assessment (MNA), Nutritional Control (CONUT), the Short Nutritional Assessment Questionnaire (SNAQ), and Nutriscore, the latter being the only one specifically designed for oncology patients [13].
Once patients at nutritional risk have been identified, an assessment is performed. Currently, this is conducted following the framework established by the Global Leadership Initiative on Malnutrition (GLIM) [11,12,14]. In addition, a second diagnostic method, the Patient-Generated Subjective Global Assessment (PG-SGA), is considered a secondary reference criterion for identifying malnutrition in oncology patients, according to the GLIM criteria [14,15,16,17].
This study aims to determine which of the current nutritional screening tools is most suitable for assessing nutritional risk in ambulatory oncology patients, according to the factors used for assessment or indicated as predictors of DRE.
2. Materials and Methods
2.1. Literature Research
This study is based on a literature review of information published between 2010 and 2023. This review was carried out according to the PRISMA 2020 statement. The literature search was conducted in the following databases: PubMed, Scopus, Embase, and SciELO, in which a total of 102 articles were found. The search terms used were determined via the PICO method, using the terms “Nutrition Assessment”, “Nutritional Index”, “Mini Nutritional Assessment”, “subjective global assessment”, “malnutrition universal screening tool”, “NRS-2002”, “SNAQ”, “CONUT”, “nutritional screening tools”, “Glim Criteria”, and “Cancer outpatients” in the final route.
2.2. Study Eligibility, Selection and Data Extraction
On the one hand, research articles were selected taking into account the following inclusion criteria: (1) original articles, (2) articles published between 2010 and 2023, (3) those written in English, Spanish, or German, (4) articles whose main objective was to evaluate nutritional screening tools, (5) studies conducted among cancer outpatients. On the other hand, exclusion criteria were as follows: (1) studies that did not specifically address the main objective of the review, (2) articles focusing exclusively on hospitalised patients, (3) those repeated in another database and already included in the review, and (4) systematic reviews, narratives, and meta-analyses.
The first phase of study selection was performed by reviewing titles and abstracts to determine eligibility. Studies that did not meet the inclusion and exclusion criteria at this stage were discarded. Of the remaining studies, a second screening process was carried out on which full articles were reviewed. This process was conducted independently and blindly by two reviewers, who had no access to each other’s decisions, or to reviewer, reference, or author information.
3. Results
After the literature search, 116 articles were identified. After excluding 99 of them, 17 articles that met the inclusion criteria were retained and included in the final review. The screening process can be seen in Figure 1, in a flow diagram of the search process, adapted from PRISMA.
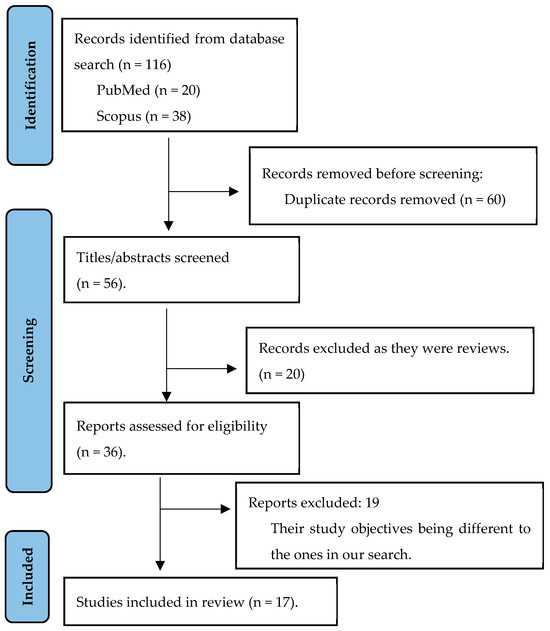
Figure 1.
Study data collection: authors’ own creation.
As shown in Figure 1, 60 articles were identified as duplicates and were therefore not considered in the final selection. After the second screening stage in which titles and abstracts were analysed, 20 articles were excluded. Consequently, 36 references were pre-selected and underwent a second screening process, which involved a detailed analysis of their relevance to the review. Nineteen references were excluded because, despite addressing the nutritional status of outpatient cancer patients, they had different study objectives to those initially proposed. Of these articles, one was discarded because it was a review. Thus, a total of 17 articles selected for the review met the inclusion criteria and are detailed in Table 1.

Table 1.
Summary of studies included in the review.
3.1. Summary of the Studies Included
Prevalence of Malnutrition in the Outpatient Cancer Population
Of the final included articles, eleven addressed the prevalence of undernutrition in the outpatient cancer patient [18,19,21,23,24,27,28,29,30,32,34], while five studies additionally focused on assessing nutritional risk [19,20,26,28,29]. Notably, three of these studies considered both prevalence and nutritional risk, thus allowing comparison of both percentages [19,28,29].
In addition, eight of the studies investigated patients affected by malignancies with a higher incidence of malnutrition, such as tumours of the head and neck, upper and lower digestive tract, and lung [18,19,23,24,25,26,30,34]. In addition, two of them focused on patients with metastatic cancer, whose prevalence of malnutrition often increases [22,25]. In contrast, five studies did not differentiate according to tumour type, but included the entire cancer population without discerning tumour type [20,21,27,31,33]. Of these, one focused specifically on the population over 70 years of age [29].
Also, three studies explored other cancer types in addition to digestive, respiratory, and head and neck tumours, such as gynaecological, breast, prostate, and haemostatic cancers [23,28,34].
3.2. Comparison with Different Screening Tools
Out of the articles reviewed, five of the studies carried out comparisons between various screening tools and the newly established GLIM criteria used as the reference diagnostic method [18,19,29,30,32]. On the other hand, in seven of the articles, the PG-SGA diagnostic method was applied to assess the nutritional status of patients [21,23,24,30,31,32,34], and in four of them, the PG-SGA method was used to assess the validity of the tools and their suitability in this specific population [23,24,31,34]. In addition, two studies focused on examining the validity of the two versions of this diagnostic method in this specific population, compared with the new GLIM criteria [30,33]. Only one study compared the screening tools with the ESPEN criteria [28]. Another study compared two specific tools, NRS-2002 and MUST, without diagnostic methods and looked at the results of each [27].
3.3. Parameters Involved in the Development of Malnutrition
Out of the included articles, seven studies examined the importance of the weight loss parameter as a predictor of undernutrition, highlighting the need to integrate it into screening tools [19,20,22,25,26,27]. In the same context, three of these studies also focused on the importance of reduced intake in nutritional status [19,25,26]. On the other hand, five articles addressed the relevance of body mass index (BMI) in the assessment of nutritional risk [19,20,23,28,31]. In addition, two studies examined how age and sex variables may be associated with nutritional impairment [19,20]. Finally, four studies explored how the nature of the disease, including tumour typology and tumour stage, may contribute to a greater development of malnutrition [21,25,27,28].
4. Discussion
Early detection of nutritional risk or DRE is of vital importance to improve the clinical outcomes and quality of life of these patients. It is necessary to know which tool is best suited to the pathology to detect greater risk and thus avoid DRE. These tools should be used from diagnosis and not only for hospital admissions. For this reason, the objective of this research is to assess which of the current tools are most effective in identifying nutritional risk or the prevalence of DRE in outpatients, as well as which factors are considered most accurate for using one NS tool or another. The studies analysed highlight several influential factors in the prevalence and severity of DRE, including characteristics such as tumour type, location, and stage, as well as gender and age, which will be examined below.
4.1. Malnutrition Prevalence
In relation to the prevalence of DRE, significant figures stand out that range between 40% and 50%, according to the studies by Sobrini et al. (2021) [29], Tob-berup and Thoresen (2020) [32], Auma et al. (2022) [24], Hettiarachchi et al. (2018) [23], and Gascón et al. (2022) [18]. Likewise, a considerable nutritional risk, around 30%, was identified according to studies that analysed the risk of malnutrition such as those by Kaduka et al. (2017) [34], Hauner et al. (2020) [27], and Bozzetti et al. (2012) [26]. These findings show a clear discrepancy between the values obtained through the use of nutritional screening tools and the diagnostic criteria.
Regarding the criteria for diagnosing malnutrition in outpatient cancer patients, despite the consensus among experts on the GLIM criteria, there is a lack of comparative studies that evaluate the validity of their use in screening tools. Five publications were found that used the GLIM criteria [18,19,29,30,32]. It is noteworthy that in the study by Gascón et al. (2022) [19], a general prevalence of malnutrition of 53.3% was revealed, with significant differences between types of cancer. Most articles used the PG-SGA as diagnostic criteria or as a reference to compare other screening tools [21,23,24,30,31,32,34]. There have been similar studies, such as the NUPAC carried out in Spain in 2005, which showed that 52% of patients had severe malnutrition or were at risk of suffering from it [35]; however, Rossi et al. [36] point out that malnutrition can affect up to 75% of patients, associating variability with the influence of factors related to the disease, such as the type of tumour, stage, and treatment, as well as with demographic (age) and social aspects.
It should be indicated that malnutrition has not always been assessed in the most relevant way. Several screening tools use parameters such as BMI [17,23,34,35] and this is a limitation that should be considered in the future, using much more precise tools to assess nutritional status, such as body composition measured via BIA, muscle function, and physical performance [37,38] or GLIM criteria, effective tools for nutrition assessment and survival prediction in older cancer patients [39]. Other authors have included the assessment of body composition or sarcopenia parameters by computed tomography or DEXA [36,38], but these tools are more time-consuming and costly to implement. In fact, the use of anthropometric measures may provide information on alterations in body composition, especially the reduction of muscle mass, but this is not the case with the use of measures such as weight loss or BMI in cancer patients [36].
Novel approaches to the diagnosis of low muscle mass, muscle function, or sarcopenia have been evaluated for use in nutritional screening tools, with the adoption of criteria that include the assessment of body composition, such as BIA.
4.2. Tumour Type
The type and location of the tumour are relevant factors that can affect the development of malnutrition in cancer patients. Several studies have examined the prevalence and risk of malnutrition depending on the type of tumour. For example, Gascón et al. (2022) [19] identified that head and neck tumours, as well as gastrointestinal cancer, have a high incidence of malnutrition (57% and 59% respectively, according to GLIM criteria). Furthermore, other research carried out by Jendretzki et al. (2021) [20] and Hauner et al. (2020) [27] indicates that patients with digestive tumours have the highest risk of malnutrition. In particular, it has been observed that cancers of the stomach, oesophagus, and pancreas show more significant weight loss, with the latter being associated with the greatest deterioration in nutritional status. Furthermore, the study by Hauner et al. (2020) [27] indicates that the specific location of the tumour in the digestive system also has an influence, with tumours located in the upper part of the digestive tract, such as the oesophagus, being more prone to malnutrition than those located in lower parts. In summary, it could be indicated that there are oncological pathologies that relate to weight loss and, therefore, malnutrition. Specifically, it could be said that digestive or pancreatic tumours bring a high risk of weight loss and malnutrition, while other pathologies such as breast cancer do not have this direct relationship with the pathological diagnosis, the risk being associated with the timing of subsequent treatment.
These data are consistent with what has been published by other authors. The NUPAC study revealed that tumours of the oesophagus, stomach, and head and neck are associated with the highest prevalence of weight loss, registering 57%, 50%, and 47%, respectively [35]. Furthermore, the review by Martinovic et al. [40] specifies that patients with head and neck cancer show a prevalence of weight loss that ranges between 25 and 65%, which can increase up to 80% due to various causes such as the interruption of intake due to surgical procedures, as well as the adverse effects of radiotherapy and chemotherapy treatments, such as mucositis, dysphagia, and xerostomia [36,40]. In other studies, the type of malignant disease was not significant for assessing undernutrition using a standardised questionnaire, such as the MNA, because the patients predominantly had diseases such as breast cancer or lymphoma (n = 50), compared with small sample sizes for lung cancer (n = 6) and head and neck cancer (n = 2) [41].
In addition, the risk factors influencing the occurrence of malnutrition in cancer, and more specifically in some types of cancer, are known. In their review, Bossi et al. [36] indicate that this situation is the result of tumour-induced activation of inflammatory pathways, such as TNF-α influence. Other studies report that inflammatory cytokines (i.e., L-1 or IL6) have been proposed as important factors in the development of cancer-associated malnutrition and their variability in different types of tumours, being much more persistent in tumours of the upper digestive tract and less present in tumours such as melanoma or breast tumours [42]. Specific tumours, such as lung and pancreas, present distinct gene expression profiles of inflammatory-inducing factors that may explain why patients with these types of cancer are more susceptible to developing malnutrition, and also asthenia or cachexia [43]. The presence of these pro-inflammatory factors and the symptoms associated with each type of tumour, such as mucositis, dysphagia, or xerostomia, explain the different prevalence of malnutrition in different diseases and the different nutritional risk.
The above data could indicate that the location of the tumour has an impact on the nutritional status of the patient, so the nutritional assessment should discern according to the type of cancer, because some haematological neoplasms have a very significant relationship with malnutrition, such as lung, pancreatic, or upper digestive tract cancer, while others, such as breast cancer, have a less significant relationship with malnutrition.
4.3. Age
In the study by Jendretzki et al. (2021) [20], it was observed that young patients were less likely to experience significant weight loss compared with those over 65 years of age. In contrast, the study by Kaduka et al. (2017) [34] identified age as a predictor of malnutrition and cachexia. These findings suggest that although age is not directly related to weight loss in general, it may influence the risk of significant weight loss in patients. Advanced age is identified as a significant risk factor for the development of malnutrition [44], with an average prevalence of 44.6% in elderly patients [36].
4.4. Gender
Women experience greater weight loss, as revealed in the study by Jendretzki et al. (2021) [20]. This is possibly associated with nutritional difficulties related to treatment. This is linked to the disparity in the incidence of different types of cancer between both sexes, with breast and uterine cancer being more prevalent in women, while prostate, oesophageal, and colorectal cancer are more prevalent in men, according to Opanga et al. (2017) [21]. The prevalence of malnutrition varies significantly between the sexes due to the greater propensity for malnutrition with cancers of the gastrointestinal system compared with those of the reproductive system. As a consequence, among patients, it was observed that a higher percentage of women were well nourished, while 54.7% of men presented severe malnutrition [21]. The relationship between gender and prevalence of DRE seems to be unclear, being mainly influenced by the patient’s type of tumour.
4.5. Tumour Stage
Tumour stage has a significant influence on the nutritional status of cancer patients, according to the study by Opanga et al. (2017) [21]. It was observed that the majority of patients in advanced stages were malnourished, in contrast to those in early stages. This also influences the effectiveness of nutritional treatment. In advanced stages, nutritional support is less effective, and the efficacy of pharmacological treatment plays a more crucial role, according to Bozzetti et al. (2017) [25]. In contrast, in the early stages, dietary intervention is more significant.
Furthermore, Bozzetti et al. (2012) [26] observed that factors other than the nature of the disease and the tumour stage could predict a high degree of malnutrition, according to the NRS-2002 nutritional assessment system.
The reasons why this may occur are varied, but specifically it may stem from an increase in the pro-inflammatory factors and cytokines listed above, the levels of which increase with tumour size [42,45]. In addition, in advanced stages, obstruction (i.e., head and neck or bowel cancer), functionality changes (i.e., asthenia or cachexia), and severe symptoms (i.e., loss of appetite or steatorrhoea in pancreatic cancer) are more common tumour-related mechanisms of malnutrition than are found in early stages [21,26,45].
4.6. Nutritional Status Assessment Factors in Nutritional Screening Tools
It seems relevant to consider decreased intake and recent weight loss (in the last 3–6 months). In this line, Jedretzki et al. (2021) [20] revealed an adequate correlation between results obtained using the MUST tool and variable weight loss, evidencing a weight loss of 44%, while 36% of patients showed malnutrition. However, the patient’s body mass index (BMI), although widely used as a universal parameter, is not an accurate indicator of malnutrition. Hettiaracchi et al. (2018) [23] also noted that BMI misclassified 30 patients, but this error decreased when considering weight loss and performing a full nutritional screening using the MUST tool, misclassifying only 9 patients. Despite its limitations, according to the study by Kaduka et al. (2017) [34], BMI could identify more patients at risk of malnutrition compared with the old ESPEN criteria. Consequently, they concluded that it is important to continuously review the different nutritional screening tools.
However, as stated by Bozzetti et al. (2017) [25], both weight loss and decreased food intake could be key parameters for assessing cancer patients’ nutritional status, along with other clinical variables such as anorexia and digestive disorders. As early as 1980, Dewys et al. (1980) [46] noted that an involuntary reduction of more than 5% of total weight was associated with a decrease in survival, and this weight loss could be used as a prognostic value. In recent years, this factor has been widely used, ranging from 42.25% weight loss associated with cancer-induced anorexia [35] to 74% weight loss between 10–20% of initial weight [47]. Conversely, the review by Bullock et al. (2021) suggested an association between low BMI and unfavourable clinical outcomes, especially in lung cancer patients [48]. It could be suggested that BMI remains the only parameter used to determine nutritional status, despite its acknowledged limitations in adequately identifying malnutrition in these patients.
4.7. Choice of Nutritional Screening Tool
After evaluating the variables analysed with the different NS tools to discern which parameters best predict DRE, we analysed which NS tools would best determine nutritional risk. Studies such as that by Gascón et al. (2022) [19] indicate that Nutriscore is useful in the oncology population, especially in head and neck cancer patients. However, it turns out to be less effective in identifying malnutrition in cases of colorectal cancer. In comparison, Hauner et al. (2020) [27] evaluated MUST and NRS-2002 in outpatient cancer patients, observing that both were adequate, although their effectiveness varied depending on the type of tumour. It was observed that patients with digestive tumours had a higher prevalence of malnutrition according to MUST, reaching 46.6%, while NRS-2002 determined a higher percentage of 63.3% malnutrition in patients with hematopoietic tumours. It seems important to consider in this context that the type of tumour has also an impact on the selection of a more appropriate nutritional screening tool according to its features.
Neither tool, MUST nor NRS-2002, was been designed specifically for oncology patients, which implies in their formulation a lack of a direct approach to aspects related to the nature of the tumour. Despite this, both tools consider essential dietary parameters in the prediction of malnutrition, as previously described, such as weight loss in the case of MUST and decreased intake in the case of NRS-2002, including other relevant factors. Notwithstanding, Nutriscore is designed specifically for cancer patients, incorporating aspects of the nature of the tumour, such as its type and the cancer treatment. In addition, it addresses essential dietary parameters such as weight loss and reduced intake, which are also considered by NRS-2002. However, these tools may be limited as they do not take into account the person’s functionality and age or other aspects of the disease such as tumour stage or disease status.
The consideration of age adds relevance to the choice of specific tools, such as the MNA, which was shown by Sobrini et al. (2021) [29] to be effective in older cancer patients. Despite not being designed exclusively for this population and not addressing specific aspects of oncological pathology, the MNA tool includes essential parameters previously described, such as anorexia, recent weight loss, and patient functionality, and performs a detailed analysis of daily dietary intake, among other aspects.
Furthermore, it is necessary to highlight that the MUST screening tool has been widely used to assess the risk of malnutrition, determining its validity for oncology outpatients. In this regard, multiple authors such as Gascón et al. (2022) [19], Kaduka et al. (2017) [28], Hauner et al. (2020) [27], Hettiarachchi et al. (2018) [23], Jendretzki et al. (2022) [20], and Auma et al. (2022) [24] agree that MUST is a suitable tool for determining undernutrition risk, preferring it over the PG-SGA method due to its ease of application and not requiring specialised training. Although Auma et al. (2022) [24] do not make a clear distinction between PG-SGA and MUST, they emphasise the importance of their implementation in both diagnosis and follow-up, rather than the choice between them.
In the case of the SNAQ questionnaire, Helfenstein et al. (2016) [22] concluded that it is not an adequate predictive tool to identify nutritional risk in ambulatory cancer patients, as it does not take into account specific aspects such as tumour stage and adverse treatment effects.
According to the review by Serón-Arbeloa et al. (2022) [49], given the diversity of the disease, there is no single screening tool that can predict clinical outcomes in all patient groups and care settings. This review also compared different tools specifically addressed to the oncology population, predominantly the use of SGA and PG-SGA, with MNA-SF, MST, MUST, and NRS-2002 having been recognised as being useful. In another analysis focused on elderly oncology patients, it was observed that the most widely used nutritional assessment tool in this specific population was the MNA [50]. However, in the review by Bullock et al. (2022), which contrasts several tools, the authors did not identify a single tool suitable for detecting the risk of malnutrition in adult oncological patients [48].
This review stands out for its ability to gather information on an understudied topic, thus opening doors to future research on this subject and highlighting the need for more scientific literature on this pathological entity which is prevalent among oncology patients. Similarly, this review helps to determine the best available evidence on currently validated screening tools. It also provides guidance for health professionals involved in the patient care process. However, there are some methodological limitations that need to be addressed. The time available to carry out this review was limited, which affected the length of the literature search; it ran from May to December 2023, including articles in English, Spanish, and German, thus incurring a potential selection bias that could have had an impact on the results of this research.
Finally, this area has not been sufficiently studied, so the literature does not yield solid conclusions and will need to be supported by further studies. Future nutritional screening tools and studies should consider in their scoring systems other diseases that involve nutritional risk or nutritional complications, such as cachexia or sarcopenia, and patients with chronic disease (diabetes mellitus, hypertension), weight-control medication use, or inflammatory digestive diseases. These conditions and their influence on malnutrition in outpatients could not be assessed in this review because the data from the studies were insufficient or associated comorbidities were not assessed as part of the nutritional screening tools.
5. Conclusions
Malnutrition in these patients is influenced by various parameters such as tumour type and location, cancer stage, and age, as well as dietetic and dietary aspects such as recent weight loss, dietary intake, and digestive disorders. The gender factor does not seem to play a role in patient malnutrition.
Therefore, the choice of nutritional screening tool for oncological outpatients should consider these factors. It is essential to adjust the choice to the particularities of the disease and the patient in order to obtain accurate results. Among the tools evaluated, Nutriscore is effective in identifying nutritional risk in patients with head and neck tumours, but not in cases of colorectal cancer. Although it addresses essential aspects, it does not consider other important aspects for assessing nutritional status. The same applies to MUST and NRS-2002, which are useful, although their efficacy may vary according to the type of tumour. Furthermore, MNA shows good results in elderly cancer patients, while SNAQ is not reliable for predicting malnutrition and is discarded as a baseline.
More research is needed on nutritional screening tools for ambulatory cancer patients, highlighting the urgent need to carry out more studies to evaluate and compare tools, as well as to develop new ones that are better adapted to this pathology, using the factors indicated in this study.
Author Contributions
D.G.-A. and L.C.-A. developed the concept. D.G.-A. wrote the manuscript and L.C.-A. critically appraised the document at all stages. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2020.
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLO-BOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- de Ulíbarri Pérez, J.I. Cribado Nutricional; control de la desnutrición clínica. Nutr. Hosp. 2014, 4, 797–811. [Google Scholar] [CrossRef]
- Jensen, G.L.; Mirtallo, J.; Compher, C.; Dhaliwal, R.; Forbes, A.; Grijalba, R.F.; Hardy, G.; Kondrup, J.; Labadarios, D.; Nyulasi, I.; et al. Adult Starvation and Disease-Related Malnutrition: A Proposal for Etiology-Based Diagnosis in the Clinical Practice Setting from the International Consensus Guideline Committee. J. Parenter. Enter. Nutr. 2010, 34, 156–159. [Google Scholar] [CrossRef]
- Virizuela, J.A.; Camblor-Álvarez, M.; Luengo-Pérez, L.M.; Grande, E.; Álvarez-Hernández, J.; Sendrós-Madroño, M.J.; Ji-ménez-Fonseca, P.; Cervera-Peris, M.; Ocón-Bretón, M.J. Nutritional Support and Parenteral Nutrition in Cancer Patients: An Expert Consensus Report. Clin. Transl. Oncol. 2018, 20, 619–629. [Google Scholar] [CrossRef]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and Classification of Cancer Cachexia: An International Consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Meyer, F.; Valentini, L. Disease-Related Malnutrition and Sarcopenia as Determinants of Clinical Outcome. Visc. Med. 2019, 35, 282–291. [Google Scholar] [CrossRef] [PubMed]
- García de Lorenzo, A.; Álvarez Hernández, J.; Planas, M.; Burgos, R.; Araujo, K. Multidisciplinary consensus work-team on the approach to hospital malnutrition in Spain. Multidisciplinary consensus on the approach to hospital malnutrition in Spain. Nutr Hosp. 2011, 26, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.; Wells, L.; Nwulu, U.; Currow, D.; Johnson, M.J.; Skipworth, R.J.E. Validated Screening Tools for the Assessment of Cachexia, Sarcopenia, and Malnutrition: A Systematic Review. Am. J. Clin. Nutr. 2018, 108, 1196–1208. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM Criteria for the Diagnosis of Malnutrition—A Consensus Report from the Global Clinical Nutrition Community. Clin. Nutr. 2019, 38, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN Guidelines on Nutrition in Cancer Patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef]
- Arribas, L.; Hurtós, L.; Sendrós, M.J.; Peiró, I.; Salleras, N.; Fort, E.; Sánchez-Migallón, J.M. NUTRISCORE: A New Nutritional Screening Tool for Oncological Outpatients. Nutrition 2017, 33, 297–303. [Google Scholar] [CrossRef] [PubMed]
- De Van Der Schueren, M.A.E.; Keller, H.; Cederholm, T.; Barazzoni, R.; Compher, C.; Correia, M.I.T.D.; Gonzalez, M.C.; Jager-Wittenaar, H.; Pirlich, M.; Steiber, A.; et al. Global Leadership Initiative on Malnutrition (GLIM): Guidance on Validation of the Operational Criteria for the Diagnosis of Protein-Energy Malnutrition in Adults. Clin. Nutr. 2020, 39, 2872–2880. [Google Scholar] [CrossRef]
- Gabrielson, D.K.; Scaffidi, D.; Leung, E.; Stoyanoff, L.; Robinson, J.; Nisenbaum, R.; Brezden-Masley, C.; Darling, P.B. Use of an Abridged Scored Patient-Generated Subjective Global Assessment (abPG-SGA) as a Nutritional Screening Tool for Cancer Patients in an Outpatient Setting. Nutr. Cancer 2013, 65, 234–239. [Google Scholar] [CrossRef]
- Ottery, F.D. Definition of Standardized Nutritional Assessment and Interventional Pathways in Oncology. Nutrition 1996, 12, S15–S19. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.L.; Elliott, L.; Fuchs-Tarlovsky, V.; Levin, R.M.; Voss, A.C.; Piemonte, T. Oncology Evidence-Based Nutrition Practice Guideline for Adults. J. Acad. Nutr. Diet. 2017, 117, 297–310.e47. [Google Scholar] [CrossRef]
- Gascón-Ruiz, M.; Casas-Deza, D.; Torres-Ramón, I.; Zapata-García, M.; Alonso, N.; Sesma, A.; Lambea, J.; Álvarez-Alejandro, M.; Quílez, E.; Isla, D.; et al. Comparation of Different Malnutrition Screening Tools According to GLIM Criteria in Cancer Outpatients. Eur. J. Clin. Nutr. 2022, 76, 698–702. [Google Scholar] [CrossRef] [PubMed]
- Gascón-Ruiz, M.; Casas-Deza, D.; Marti-Pi, M.; Torres-Ramón, I.; Zapata-García, M.; Sesma, A.; Lambea, J.; Álvarez-Alejandro, M.; Quilez, E.; Isla, D.; et al. Diagnosis of Malnutrition According to GLIM Criteria Predicts Complications and 6-Month Survival in Cancer Outpatients. Biomedicines 2022, 10, 2201. [Google Scholar] [CrossRef]
- Jendretzki, J.; Henniger, D.; Schiffmann, L.; Wolz, C.; Kollikowski, A.; Meining, A.; Einsele, H.; Winkler, M.; Löffler, C. Every Fifth Patient Suffered a High Nutritional Risk—Results of a Prospective Patient Survey in an Oncological Outpatient Center. Front. Nutr. 2022, 9, 1033265. [Google Scholar] [CrossRef]
- Opanga, Y.; Kaduka, L.; Bukania, Z.; Mutisya, R.; Korir, A.; Thuita, V.; Mwangi, M.; Muniu, E.; Mbakaya, C. Nutritional Status of Cancer Outpatients Using Scored Patient Generated Subjective Global Assessment in Two Cancer Treatment Centers, Nairobi, Kenya. BMC Nutr. 2017, 3, 63. [Google Scholar] [CrossRef]
- Helfenstein, S.F.; Uster, A.; Rühlin, M.; Pless, M.; Ballmer, P.E.; Imoberdorf, R. Are Four Simple Questions Able to Predict Weight Loss in Outpatients with Metastatic Cancer? A Prospective Cohort Study Assessing the Simplified Nutritional Appe-tite Questionnaire. Nutr. Cancer 2016, 68, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Hettiarachchi, J.; Madubhashini, P.; Miller, M. Agreement between the Malnutrition Universal Screening Tool and the Patient-Generated Subjective Global Assessment for Cancer Outpatients Receiving Chemotherapy: A Cross-Sectional Study. Nutr. Cancer 2018, 70, 1275–1282. [Google Scholar] [CrossRef]
- Auma, C.M.N.; Mweu, M.M.; Opiyo, R.O. Performance of Malnutrition Universal Screening Tool and Patient-Generated Global Subjective Assessment in Screening for Cancer-Related Malnutrition in Nairobi, Kenya. F1000Research 2022, 11, 755. [Google Scholar] [CrossRef]
- Bozzetti, F. Forcing the Vicious Circle: Sarcopenia Increases Toxicity, Decreases Response to Chemotherapy and Worsens with Chemotherapy. Ann. Oncol. 2017, 28, 2107–2118. [Google Scholar] [CrossRef]
- Bozzetti, F.; Mariani, L.; Lo Vullo, S.; Amerio, M.L.; Biffi, R.; Caccialanza, R.; Capuano, G.; Correja, I.; Cozzaglio, L.; Di Leo, A.; et al. The Nutritional Risk in Oncology: A Study of 1453 Cancer Outpatients. Support. Care Cancer 2012, 20, 1919–1928. [Google Scholar] [CrossRef]
- Hauner, H.; Kocsis, A.; Jaeckel, B.; Martignoni, M.; Hauner, D.; Holzapfel, C. Häufigkeit eines Risikos für Mangelernährung bei Patienten in onkologischen Schwerpunktpraxen—Eine Querschnittserhebung. DMW Dtsch. Med. Wochenschr. 2020, 145, e1–e9. [Google Scholar] [CrossRef]
- Kaduka, L.U.; Bukania, Z.N.; Opanga, Y.; Mutisya, R.; Korir, A.; Thuita, V.; Nyongesa, C.; Mwangi, M.; Mbakaya, C.F.L.; Muniu, E. Malnutrition and Cachexia among Cancer Out-Patients in Nairobi, Kenya. J. Nutr. Sci. 2017, 6, e63. [Google Scholar] [CrossRef] [PubMed]
- Sobrini, P.; Sánchez-Castellano, C.; Cruz-Jentoft, A.J. MNA-SF as a Screening Tool for Malnutrition Diagnosed with the Glim Criteria in Older Persons with Cancer. Eur. Geriatr. Med. 2021, 12, 653–656. [Google Scholar] [CrossRef]
- De Groot, L.M.; Lee, G.; Ackerie, A.; Van Der Meij, B.S. Malnutrition Screening and Assessment in the Cancer Care Ambula-tory Setting: Mortality Predictability and Validity of the Patient-Generated Subjective Global Assessment Short Form (PG-SGA SF) and the GLIM Criteria. Nutrients 2020, 12, 2287. [Google Scholar] [CrossRef]
- Carretero, I.V.; Ravasco, P. PP078-MON: Must in Cancer Patients Undergoing Chemotherapy: Valid? Clin. Nutr. 2014, 33, S159. [Google Scholar] [CrossRef]
- Tobberup, R.; Thoresen, L. Accuracy of Screening Tools in Predicting Malnutrition in Cancer Outpatients According to the Glim Criteria. Clin. Nutr. ESPEN 2020, 40, 565–566. [Google Scholar] [CrossRef]
- Anzévui, A.; Frateur, L.; Bertrand, B.; Gihousse, D.; Joly, E.; Hamoir, M.; Méan, A. Sun applicability of the Malnutrition Screening Tool for Cancer patients (MSTC) in adult outpatients, suffering from any tumour types, in Belgium. Clin. Nutr. Suppl. 2012, 7, 107–108. [Google Scholar] [CrossRef]
- Kuzma, S.; Mahon, E.; Ramage, J.; Camilleri, B.; Todd, A. Malnutrition prevalence in an outpatient cancer care setting. Asia Pac. J. Clin. Oncol. 2014, 10, 126–209. [Google Scholar] [CrossRef]
- Segura, A.; Pardo, J.; Jara, C.; Zugazabeitia, L.; Carulla, J.; De Las Peñas, R.; García-Cabrera, E.; Luz Azuara, M.; Casadó, J.; Gómez-Candela, C. An Epidemiological Evaluation of the Prevalence of Malnutrition in Spanish Patients with Locally Ad-vanced or Metastatic Cancer. Clin. Nutr. 2005, 24, 801–814. [Google Scholar] [CrossRef] [PubMed]
- Bossi, P.; Delrio, P.; Mascheroni, A.; Zanetti, M. The Spectrum of Malnutrition/Cachexia/Sarcopenia in Oncology According to Different Cancer Types and Settings: A Narrative Review. Nutrients 2021, 13, 1980. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Martínez, L.; Castro-Eguiluz, D.; Copca-Mendoza, E.T.; Pérez-Camargo, D.A.; Reyes-Torres, C.A.; Ávila, E.A.; López-Córdova, G.; Fuentes-Hernández, M.R.; Cetina-Pérez, L.; Milke-García, M.D.P. Nutritional Assessment Tools for the Identification of Malnutrition and Nutritional Risk Associated with Cancer Treatment. Rev. Investig. Clin. 2018, 70, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.C.; Cespedes Feliciano, E.M.; Caan, B.J. The evolution of body composition in oncology-epidemiology, clinical trials, and the future of patient care: Facts and numbers. J. Cachexia Sarcopenia Muscle 2018, 9, 1200–1208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tang, M.; Zhang, Q.; Zhang, K.P.; Guo, Z.Q.; Xu, H.X.; Yuan, K.T.; Yu, M.; Braga, M.; Cederholm, T.; et al. The GLIM criteria as an effective tool for nutrition assessment and survival prediction in older adult cancer patients. Clin. Nutr. 2021, 40, 1224–1232. [Google Scholar] [CrossRef]
- Martinovic, D.; Tokic, D.; Puizina Mladinic, E.; Usljebrka, M.; Kadic, S.; Lesin, A.; Vilovic, M.; Lupi-Ferandin, S.; Ercegovic, S.; Kumric, M.; et al. Nutritional Management of Patients with Head and Neck Cancer—A Comprehensive Review. Nutrients 2023, 15, 1864. [Google Scholar] [CrossRef]
- Sonneborn-Papakostopoulos, M.; Dubois, C.; Mathies, V.; Heß, M.; Erickson, N.; Ernst, T.; Huebner, J. Quality of life, symptoms and dietary habits in oncology outpatients with malnutrition: A cross-sectional study. Med. Oncol. 2021, 38, 20. [Google Scholar] [CrossRef]
- Argiles, J.M. Cancer-associated malnutrition. Eur. J. Oncol. Nurs. 2005, 9, S39–S50. [Google Scholar] [CrossRef]
- Peixoto da Silva, S.; Santos, J.M.O.; Costa ESilva, M.P.; Gil da Costa, R.M.; Medeiros, R. Cancer cachexia and its pathophysiology: Links with sarcopenia, anorexia and asthenia. J. Cachexia Sarcopenia Muscle 2020, 11, 619–635. [Google Scholar] [CrossRef]
- Zhang, X.; Edwards, B.J. Malnutrition in Older Adults with Cancer. Curr. Oncol. Rep. 2019, 21, 80. [Google Scholar] [CrossRef]
- Nicolini, A.; Ferrari, P.; Masoni, M.C.; Fini, M.; Pagani, S.; Giampietro, O.; Carpi, A. Malnutrition, anorexia and cachexia in cancer patients: A mini-review on pathogenesis and treatment. Biomed. Pharmacother. 2013, 67, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Dewys, W.D.; Begg, C.; Lavin, P.T.; Band, P.R.; Bennett, J.M.; Bertino, J.R.; Cohen, M.H.; Douglass, H.O.; Engstrom, P.F.; Ezdinli, E.Z.; et al. Prognostic Effect of Weight Loss Prior Tochemotherapy in Cancer Patients. Am. J. Med. 1980, 69, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Tchekmedyan, N. Cost and benefits of nutrition support in cancer. Oncology 1995, 9, 79–84. [Google Scholar]
- Bullock, A.F.; Greenley, S.L.; McKenzie, G.A.G.; Paton, L.W.; Johnson, M.J. Relationship between Markers of Malnutrition and Clinical Outcomes in Older Adults with Cancer: Systematic Review, Narrative Synthesis and Meta-Analysis. Eur. J. Clin. Nutr. 2020, 74, 1519–1535. [Google Scholar] [CrossRef] [PubMed]
- Serón-Arbeloa, C.; Labarta-Monzón, L.; Puzo-Foncillas, J.; Mallor-Bonet, T.; Lafita-López, A.; Bueno-Vidales, N.; Montoro-Huguet, M. Malnutrition Screening and Assessment. Nutrients 2022, 14, 2392. [Google Scholar] [CrossRef]
- Zhang, Z.; Pereira, S.; Luo, M.; Matheson, E. Evaluation of Blood Biomarkers Associated with Risk of Malnutrition in Older Adults: A Systematic Review and Meta-Analysis. Nutrients 2017, 9, 829. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).