The Influence of High-Dose Parenteral Vitamin C on the Incidence and Severity of Postoperative Pulmonary Complications in Cardiac Surgery with Extracorporeal Circulation: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Participants
2.3. Interventions
- Intervention group (B): 50 mg/kg/6 h as a 30 min i.v. infusion of VitC in 50 mL of normal saline every 6 h, under UV protection.
- Control group (A): an equal volume of normal saline every 6 h as a 30 min i.v. infusion, under UV protection.
2.4. Outcome
2.5. Sample Size
2.6. Randomization and Masking
2.7. Statistical Methods
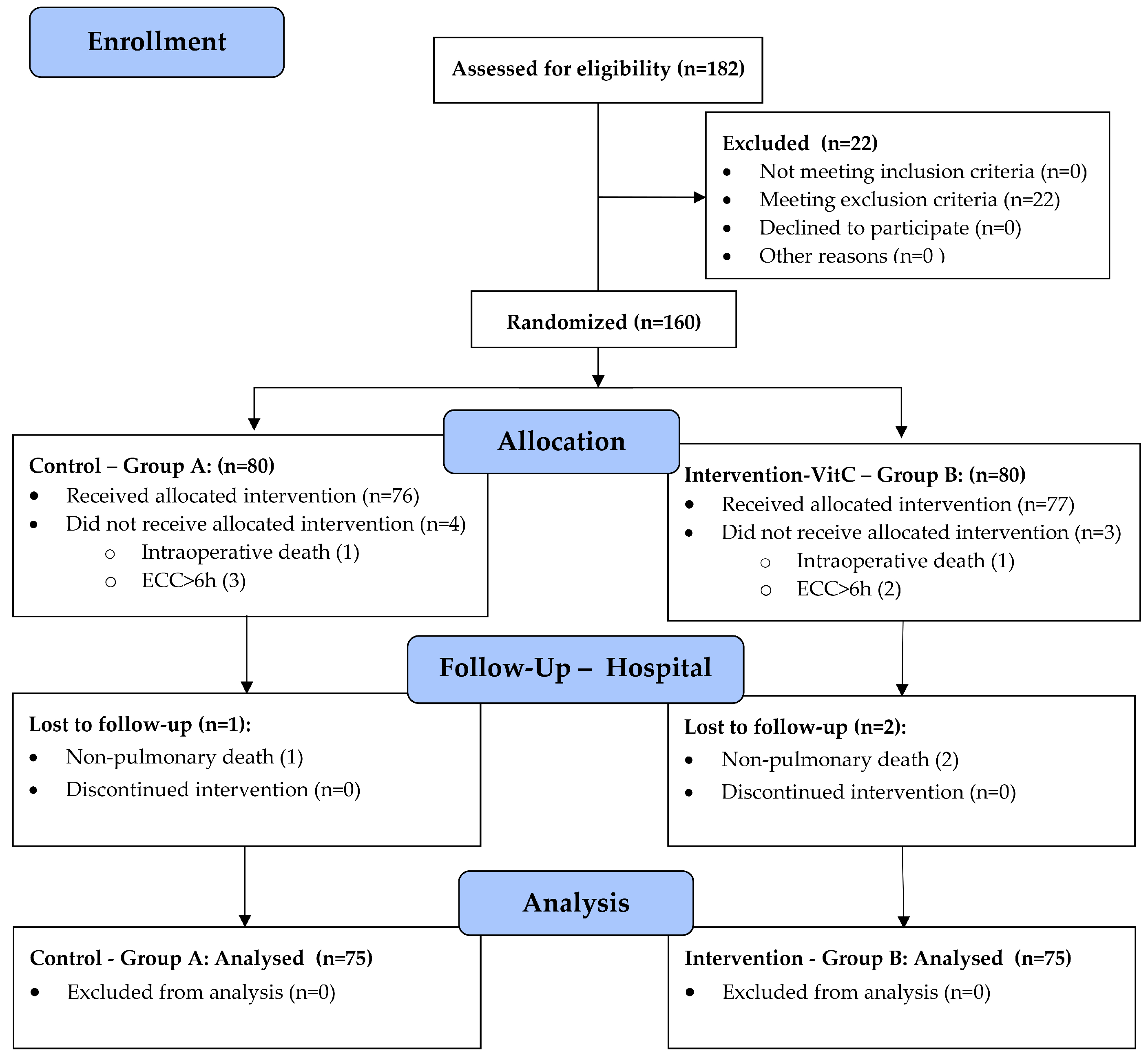
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Squiccimarro, E.; Labriola, C.; Malvindi, P.G.; Margari, V.; Guida, P.; Visicchio, G.; Kounakis, G.; Favale, A.; Dambruoso, P.; Mastrototaro, G.; et al. Prevalence and Clinical Impact of Systemic Inflammatory Reaction After Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2019, 33, 1682–1690. [Google Scholar] [CrossRef]
- Churpek, M.M.; Zadravecz, F.J.; Winslow, C.; Howell, M.D.; Edelson, D.P. Incidence and Prognostic Value of the Systemic Inflammatory Response Syndrome and Organ Dysfunctions in Ward Patients. Am. J. Respir. Crit. Care Med. 2015, 192, 958–964. [Google Scholar] [CrossRef]
- McGuinness, J.; Bouchier-Hayes, D.; Redmond, J.M. Understanding the inflammatory response to cardiac surgery. Surgeon 2008, 6, 162–171. [Google Scholar] [CrossRef]
- Semler, M.W.; Wheeler, A.P. Systemic inflammatory response syndrome after cardiac surgery: Time for a change. Chest 2014, 145, 1181–1182. [Google Scholar] [CrossRef] [PubMed]
- Warltier, D.C.; Laffey, J.G.; Boylan, J.F.; Cheng, D.C. The Systemic Inflammatory Response to Cardiac Surgery: Implications for the Anesthesiologist. Anesthesiology 2002, 97, 215–252. [Google Scholar] [CrossRef] [PubMed]
- MacCallum, N.S.; Finney, S.J.; Gordon, S.E.; Quinlan, G.J.; Evans, T.W. Modified criteria for the systemic inflammatory response syndrome improves their utility following cardiac surgery. Chest 2014, 145, 1197–1203. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, H.; Wang, H.; Zheng, Z.; Ooi, O.C. Validation of prognostic accuracy of the SOFA score, SIRS criteria, and qSOFA score for in-hospital mortality among cardiac-, thoracic-, and vascular-surgery patients admitted to a cardiothoracic intensive care unit. J. Card. Surg. 2020, 35, 118–127. [Google Scholar] [CrossRef]
- Biglioli, P.; Cannata, A.; Alamanni, F.; Naliato, M.; Porqueddu, M.; Zanobini, M.; Tremoli, E.; Parolari, A. Biological effects of off-pump vs. on-pump coronary artery surgery: Focus on inflammation, hemostasis and oxidative stress. Eur. J. Cardio-Thorac. Surg. 2003, 24, 260–269. [Google Scholar] [CrossRef]
- Landis, R.C.; Brown, J.R.; Fitzgerald, D.; Likosky, D.S.; Shore-Lesserson, L.; Baker, R.A.; Hammon, J.W. Attenuating the Systemic Inflammatory Response to Adult Cardiopulmonary Bypass: A Critical Review of the Evidence Base. J. Extra-Corpor. Technol. 2014, 46, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Larmann, J.; Theilmeier, G. Inflammatory response to cardiac surgery: Cardiopulmonary bypass versus non-cardiopulmonary bypass surgery. Best. Pract. Res. Clin. Anaesthesiol. 2004, 18, 425–438. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Y. Mass intraoperative endothelial glycocalyx shedding affects postoperative systemic inflammation response. BMC Anesthesiol. 2024, 24, 76. [Google Scholar] [CrossRef]
- Joseph, D.; Puttaswamy, R.K.; Krovvidi, H. Non-respiratory functions of the lung. Contin. Educ. Anaesth. Crit. Care Pain 2013, 13, 98–102. [Google Scholar] [CrossRef]
- Miskovic, A.; Lumb, A.B. Postoperative pulmonary complications. Br. J. Anaesth. 2017, 118, 317–334. [Google Scholar] [CrossRef]
- Fischer, M.-O.; Brotons, F.; Briant, A.R.; Suehiro, K.; Gozdzik, W.; Sponholz, C.; Kirkeby-Garstad, I.; Joosten, A.; Nigro Neto, C.; Kunstyr, J.; et al. Postoperative Pulmonary Complications After Cardiac Surgery: The VENICE International Cohort Study. J. Cardiothorac. Vasc. Anesth. 2022, 36, 2344–2351. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.P.; Kugelmass, A.D.; Cohen, D.J.; Reynolds, M.R.; Culler, S.D.; Dee, A.D.; Simon, A.W. The frequency and cost of complications associated with coronary artery bypass grafting surgery: Results from the United States Medicare program. Ann. Thorac. Surg. 2008, 85, 1980–1986. [Google Scholar] [CrossRef]
- Abbott, T.E.F.; Fowler, A.J.; Pelosi, P.; Gama de Abreu, M.; Møller, A.M.; Canet, J.; Creagh-Brown, B.; Mythen, M.; Gin, T.; Lalu, M.M.; et al. A systematic review and consensus definitions for standardised end-points in perioperative medicine: Pulmonary complications. Br. J. Anaesth. 2018, 120, 1066–1079. [Google Scholar] [CrossRef] [PubMed]
- Mali, S.; Haghaninejad, H. Pulmonary complications following cardiac surgery. Arch. Med. Sci. Atheroscler. Dis. 2019, 4, e280–e285. [Google Scholar] [CrossRef] [PubMed]
- O’Donohue, W.J., Jr. Postoperative pulmonary complications. When are preventive and therapeutic measures necessary? Postgrad. Med. 1992, 91, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Jammer, I.; Wickboldt, N.; Sander, M.; Smith, A.; Schultz, M.J.; Pelosi, P.; Leva, B.; Rhodes, A.; Hoeft, A.; Walder, B.; et al. Standards for definitions and use of outcome measures for clinical effectiveness research in perioperative medicine: European Perioperative Clinical Outcome (EPCO) definitions: A statement from the ESA-ESICM joint taskforce on perioperative outcome measures. Eur. J. Anaesthesiol. 2015, 32, 88–105. [Google Scholar] [CrossRef]
- Costa Leme, A.; Hajjar, L.A.; Volpe, M.S.; Fukushima, J.T.; De Santis Santiago, R.R.; Osawa, E.A.; Pinheiro de Almeida, J.; Gerent, A.M.; Franco, R.A.; Zanetti Feltrim, M.I.; et al. Effect of Intensive vs Moderate Alveolar Recruitment Strategies Added to Lung-Protective Ventilation on Postoperative Pulmonary Complications: A Randomized Clinical Trial. JAMA 2017, 317, 1422–1432. [Google Scholar] [CrossRef] [PubMed]
- Futier, E.; Constantin, J.M.; Paugam-Burtz, C.; Pascal, J.; Eurin, M.; Neuschwander, A.; Marret, E.; Beaussier, M.; Gutton, C.; Lefrant, J.Y.; et al. A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. N. Engl. J. Med. 2013, 369, 428–437. [Google Scholar] [CrossRef]
- Hulzebos, E.H.; Helders, P.J.; Favié, N.J.; De Bie, R.A.; Brutel de la Riviere, A.; Van Meeteren, N.L. Preoperative intensive inspiratory muscle training to prevent postoperative pulmonary complications in high-risk patients undergoing CABG surgery: A randomized clinical trial. JAMA 2006, 296, 1851–1857. [Google Scholar] [CrossRef]
- Kroenke, K.; Lawrence, V.A.; Theroux, J.F.; Tuley, M.R. Operative risk in patients with severe obstructive pulmonary disease. Arch. Intern. Med. 1992, 152, 967–971. [Google Scholar] [CrossRef]
- Oudemans-van Straaten, H.M.; Spoelstra-de Man, A.M.; de Waard, M.C. Vitamin C revisited. Crit. Care 2014, 18, 460. [Google Scholar] [CrossRef]
- Ballmer, P.E.; Reinhart, W.H.; Jordan, P.; Bühler, E.; Moser, U.K.; Gey, K.F. Depletion of plasma vitamin C but not of vitamin E in response to cardiac operations. J. Thorac. Cardiovasc. Surg. 1994, 108, 311–320. [Google Scholar] [CrossRef]
- Bowie, A.G.; O’Neill, L.A. Vitamin C inhibits NF-kappa B activation by TNF via the activation of p38 mitogen-activated protein kinase. J. Immunol. 2000, 165, 7180–7188. [Google Scholar] [CrossRef]
- Padayatty, S.J.; Sun, H.; Wang, Y.; Riordan, H.D.; Hewitt, S.M.; Katz, A.; Wesley, R.A.; Levine, M. Vitamin C pharmacokinetics: Implications for oral and intravenous use. Ann. Intern. Med. 2004, 140, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Schellhorn, H.E. New developments and novel therapeutic perspectives for vitamin C. J. Nutr. 2007, 137, 2171–2184. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, R.; Yamazaki, E. Vitamin C requirement in surgical patients. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Lykkesfeldt, J.; Tveden-Nyborg, P. The Pharmacokinetics of Vitamin C. Nutrients 2019, 11, 2412. [Google Scholar] [CrossRef] [PubMed]
- Long, M.T.; Hess, A.S.; McCarthy, D.P.; DeCamp, M.M. Power for the Sickest: Vitamin C for Vasoplegia after Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2020, 34, 1123. [Google Scholar] [CrossRef]
- Lykkesfeldt, J. On the effect of vitamin C intake on human health: How to (mis)interprete the clinical evidence. Redox Biol. 2020, 34, 101532. [Google Scholar] [CrossRef]
- Doseděl, M.; Jirkovský, E.; Macáková, K.; Krčmová, L.K.; Javorská, L.; Pourová, J.; Mercolini, L.; Remião, F.; Nováková, L.; Mladěnka, P.; et al. Vitamin C-Sources, Physiological Role, Kinetics, Deficiency, Use, Toxicity, and Determination. Nutrients 2021, 13, 615. [Google Scholar] [CrossRef]
- Hill, A.; Clasen, K.C.; Wendt, S.; Majoros, Á.G.; Stoppe, C.; Adhikari, N.K.; Heyland, D.K.; Benstoem, C. Effects of Vitamin C on Organ Function in Cardiac Surgery Patients: A Systematic Review and Meta-Analysis. Nutrients 2019, 11, 2103. [Google Scholar] [CrossRef]
- Hill, A.; Borgs, C.; Fitzner, C.; Stoppe, C. Perioperative Vitamin C and E levels in Cardiac Surgery Patients and Their Clinical Significance. Nutrients 2019, 11, 2157. [Google Scholar] [CrossRef]
- Hill, A.; Wendt, S.; Benstoem, C.; Neubauer, C.; Meybohm, P.; Langlois, P.; Adhikari, N.K.; Heyland, D.K.; Stoppe, C. Vitamin C to Improve Organ Dysfunction in Cardiac Surgery Patients-Review and Pragmatic Approach. Nutrients 2018, 10, 974. [Google Scholar] [CrossRef]
- Carr, A.C.; Rosengrave, P.C.; Bayer, S.; Chambers, S.; Mehrtens, J.; Shaw, G.M. Hypovitaminosis C and vitamin C deficiency in critically ill patients despite recommended enteral and parenteral intakes. Crit. Care 2017, 21, 300. [Google Scholar] [CrossRef]
- Creagan, E.T.; Moertel, C.G.; O’Fallon, J.R.; Schutt, A.J.; O’Connell, M.J.; Rubin, J.; Frytak, S. Failure of high-dose vitamin C (ascorbic acid) therapy to benefit patients with advanced cancer. A controlled trial. N. Engl. J. Med. 1979, 301, 687–690. [Google Scholar] [CrossRef] [PubMed]
- Pauling, L. Ascorbic acid and the common cold. Am. J. Clin. Nutr. 1971, 24, 1294–1299. [Google Scholar] [CrossRef]
- Coppock, D.; Violet, P.C.; Vasquez, G.; Belden, K.; Foster, M.; Mullin, B.; Magee, D.; Mikell, I.; Shah, L.; Powers, V.; et al. Pharmacologic Ascorbic Acid as Early Therapy for Hospitalized Patients with COVID-19: A Randomized Clinical Trial. Life 2022, 12, 453. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Rao, X.; Li, Y.; Zhu, Y.; Liu, F.; Guo, G.; Luo, G.; Meng, Z.; De Backer, D.; Xiang, H.; et al. Pilot trial of high-dose vitamin C in critically ill COVID-19 patients. Ann. Intensive Care 2021, 11, 5. [Google Scholar] [CrossRef]
- Cheng, R.Z. Can early and high intravenous dose of vitamin C prevent and treat coronavirus disease 2019 (COVID-19)? Med. Drug Discov. 2020, 5, 100028. [Google Scholar] [CrossRef]
- PDQ® Integrative. Alternative, and Complementary Therapies Editorial Board; PDQ Intravenous Vitamin C; National Cancer Institute: Bethesda, MD, USA, 2022. Available online: https://www.cancer.gov/about-cancer/treatment/cam/hp/vitamin-c-pdq (accessed on 12 October 2023). [PubMed]
- Fowler, A.A., 3rd; Syed, A.A.; Knowlson, S.; Sculthorpe, R.; Farthing, D.; DeWilde, C.; Farthing, C.A.; Larus, T.L.; Martin, E.; Brophy, D.F.; et al. Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J. Transl. Med. 2014, 12, 32. [Google Scholar] [CrossRef]
- Dingchao, H.; Zhiduan, Q.; Liye, H.; Xiaodong, F. The protective effects of high-dose ascorbic acid on myocardium against reperfusion injury during and after cardiopulmonary bypass. Thorac. Cardiovasc. Surg. 1994, 42, 276–278. [Google Scholar] [CrossRef]
- Chen, P.; Reed, G.; Jiang, J.; Wang, Y.; Sunega, J.; Dong, R.; Ma, Y.; Esparham, A.; Ferrell, R.; Levine, M.; et al. Pharmacokinetic Evaluation of Intravenous Vitamin C: A Classic Pharmacokinetic Study. Clin. Pharmacokinet. 2022, 61, 1237–1249. [Google Scholar] [CrossRef]
- Yanase, F.; Fujii, T.; Naorungroj, T.; Belletti, A.; Luethi, N.; Carr, A.C.; Young, P.J.; Bellomo, R. Harm of IV High-Dose Vitamin C Therapy in Adult Patients: A Scoping Review. Crit. Care Med. 2020, 48, e620–e628. [Google Scholar] [CrossRef]
- Takahashi, T.; Lord, B.; Schulze, P.C.; Fryer, R.M.; Sarang, S.S.; Gullans, S.R.; Lee, R.T. Ascorbic acid enhances differentiation of embryonic stem cells into cardiac myocytes. Circulation 2003, 107, 1912–1916. [Google Scholar] [CrossRef]
- Spoelstra-de Man, A.M.E.; Elbers, P.W.G.; Oudemans-van Straaten, H.M. Making sense of early high-dose intravenous vitamin C in ischemia/reperfusion injury. Crit. Care 2018, 22, 70. [Google Scholar] [CrossRef]
- May, J.M.; Harrison, F.E. Role of vitamin C in the function of the vascular endothelium. Antioxid. Redox Signal. 2013, 19, 2068–2083. [Google Scholar] [CrossRef]
- Ashor, A.W.; Lara, J.; Mathers, J.C.; Siervo, M. Effect of vitamin C on endothelial function in health and disease: A systematic review and meta-analysis of randomised controlled trials. Atherosclerosis 2014, 235, 9–20. [Google Scholar] [CrossRef]
- Hemilä, H.; Chalker, E.; de Man, A.M.E. Vitamin C May Improve Left Ventricular Ejection Fraction: A Meta-Analysis. Front. Cardiovasc. Med. 2022, 9, 789729. [Google Scholar] [CrossRef]
- Hemilä, H.; Chalker, E. Vitamin C may reduce the duration of mechanical ventilation in critically ill patients: A meta-regression analysis. J. Intensive Care 2020, 8, 15. [Google Scholar] [CrossRef]
- Mangoush, O.; Nakamura, K.; Al-Ruzzeh, S.; Athanasiou, T.; Chester, A.; Amrani, M. Effect of ascorbic acid on endothelium-dependent vasodilatation of human arterial conduits for coronary artery bypass grafting. Eur. J. Cardiothorac. Surg. 2003, 24, 541–546. [Google Scholar] [CrossRef][Green Version]
- Wang, D.; Wang, M.; Zhang, H.; Zhu, H.; Zhang, N.; Liu, J. Effect of Intravenous Injection of Vitamin C on Postoperative Pulmonary Complications in Patients Undergoing Cardiac Surgery: A Double-Blind, Randomized Trial. Drug Des. Devel. Ther. 2020, 14, 3263–3270. [Google Scholar] [CrossRef]
- Li, C.C. Changes of creatine phosphokinase and malondialdehyde in the serum and clinical use of large doses of vitamin C following open heart surgery. Zhonghua Wai Ke Za Zhi 1990, 28, 16–17. [Google Scholar]
- Knuf, K.M.; Maani, C.V.; Cummings, A.K. Clinical agreement in the American Society of Anesthesiologists physical status classification. Perioper. Med. 2018, 7, 14. [Google Scholar] [CrossRef]
- Lambden, S.; Laterre, P.F.; Levy, M.M.; Francois, B. The SOFA score-development, utility and challenges of accurate assessment in clinical trials. Crit. Care 2019, 23, 374. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, Z.; Huang, W.; Zhang, X.; Guo, Y.; Yu, P. Comparison of Tools for Postoperative Pulmonary Complications After Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2023, 37, 1442–1448. [Google Scholar] [CrossRef]
- Cvetković, V.M.; Nikolić, N.; Radovanović Nenadić, U.; Öcal, A.; Noji, E.K.; Zečević, M. Preparedness and Preventive Behaviors for a Pandemic Disaster Caused by COVID-19 in Serbia. Int. J. Environ. Res. Public Health 2020, 17, 4124. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Cheng, C.; Wei, X. Incidence of postoperative pulmonary complications in patients undergoing minimally invasive versus median sternotomy valve surgery: Propensity score matching. J. Cardiothorac. Surg. 2021, 16, 287. [Google Scholar] [CrossRef]
- Tanner, T.G.; Colvin, M.O. Pulmonary Complications of Cardiac Surgery. Lung 2020, 198, 889–896. [Google Scholar] [CrossRef]
- Naveed, A.; Azam, H.; Murtaza, H.G.; Ahmad, R.A.; Baig, M.A.R. Incidence and risk factors of Pulmonary Complications after Cardiopulmonary bypass. Pak. J. Med. Sci. 2017, 33, 993–996. [Google Scholar] [CrossRef]
- Canet, J.; Hardman, J.; Sabaté, S.; Langeron, O.; Abreu, M.G.; Gallart, L.; Belda, J.; Markstaller, K.; Pelosi, P.; Mazo, V. PERISCOPE study: Predicting post-operative pulmonary complications in Europe. Eur. J. Anaesthesiol. 2011, 28, 459–461. [Google Scholar] [CrossRef]
- Canet, J.; Gallart, L.; Gomar, C.; Paluzie, G.; Vallès, J.; Castillo, J.; Sabaté, S.; Mazo, V.; Briones, Z.; Sanchis, J. Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology 2010, 113, 1338–1350. [Google Scholar] [CrossRef]
- Weissman, C. Pulmonary complications after cardiac surgery. Semin. Cardiothorac. Vasc. Anesth. 2004, 8, 185–211. [Google Scholar] [CrossRef]
- Gologorsky, E.; Gologorsky, A.; Salerno, T.A. Lung-Centered Open Heart Surgery: A Call for a Paradigm Change. Front. Cardiovasc. Med. 2016, 3, 12. [Google Scholar] [CrossRef][Green Version]
- Khera, R.; Vaughan-Sarrazin, M.; Rosenthal, G.E.; Girotra, S. Racial disparities in outcomes after cardiac surgery: The role of hospital quality. Curr. Cardiol. Rep. 2015, 17, 29. [Google Scholar] [CrossRef]
- Mathis, M.R.; Duggal, N.M.; Likosky, D.S.; Haft, J.W.; Douville, N.J.; Vaughn, M.T.; Maile, M.D.; Blank, R.S.; Colquhoun, D.A.; Strobel, R.J.; et al. Intraoperative Mechanical Ventilation and Postoperative Pulmonary Complications after Cardiac Surgery. Anesthesiology 2019, 131, 1046–1062. [Google Scholar] [CrossRef]
- Hickey, S.; Roberts, H. Evolution and Deficiency. In Ascorbate: The Science of Vitamin C; Hickey, S., Roberts, H., Eds.; Lulu Press: Morrisville, NC, USA, 2004; pp. 66–72. [Google Scholar]
- Rowe, S.; Carr, A.C. Global Vitamin C Status and Prevalence of Deficiency: A Cause for Concern? Nutrients 2020, 12, 2008. [Google Scholar] [CrossRef]
- Carpenter, K.J. The discovery of vitamin C. Ann. Nutr. Metab. 2012, 61, 259–264. [Google Scholar] [CrossRef]
- Richards, E. Introduction. In Vitamin C and Cancer: Medicine or Politics? Palgrave Macmillan UK: London, UK, 1991; pp. 1–14. [Google Scholar]
- Iizuka, Y.; Yoshinaga, K.; Takahashi, K.; Oki, S.; Chiba, Y.; Sanui, M.; Kimura, N.; Yamaguchi, A. Association between Plasma Ascorbic Acid Levels and Postoperative Delirium in Older Patients Undergoing Cardiovascular Surgery: A Prospective Observational Study. J. Cardiovasc. Dev. Dis. 2023, 10, 293. [Google Scholar] [CrossRef] [PubMed]
- Nabzdyk, C.S.; Bittner, E.A. Vitamin C in the critically ill—indications and controversies. World J. Crit. Care Med. 2018, 7, 52–61. [Google Scholar] [CrossRef]
| Perioperative Parameters | Group A (n = 75) | Group B (n = 75) | p-Value (Test) * |
|---|---|---|---|
| 1. Demographic and Anthropometric | |||
| Age (years) | 66.9 ± 8.7 | 66.3 ± 8.6 | 0.672 (t) |
| Male gender | 59 (78.7%) | 55 (73.3%) | 0.444 (chi) |
| BMI (kg/m2) | 28.4 ± 3.9 | 26.7 ± 3.3 | 0.005 (t) |
| 2. CVD Risk | |||
| HTA | 75 (100%) | 73 (97.3%) | 0.497 (fet) |
| DM | 36 (48%) | 36 (48%) | 1.000 (fet) |
| HLP | 72 (96%) | 68 (90.7%) | 0.190 (chi) |
| Smoking | 52 (69.3%) | 52 (69.3%) | 1.000 (chi) |
| 3. CV Status and Comorbidities | |||
| Recent MI | 41 (54.7%) | 34 (45.3%) | 0.253 (chi) |
| AP | 61 (81.3%) | 58 (77.3%) | 0.545 (chi) |
| TAs (mmHg) | 145.8 ± 26.3 | 139.8 ± 16.9 | 0.100 (t) |
| TAd (mmHg) | 81.9 ± 13.1 | 77.3 ± 11.6 | 0.022 (t) |
| EF-LV (%) | 46.5 ± 9.1 | 48.4 ± 8.0 | 0.187 (t) |
| HR (beats/min) | 70.4 ± 9.3 | 69.5 ± 9.7 | 0.565 (t) |
| Sinus | 68 (90.7%) | 66 (88%) | 0.597 (chi) |
| AF | 8 (10.7%) | 9 (12%) | 0.979 (chi) |
| CVD | 11 (14.7%) | 5 (6.7%) | 0.113 (chi) |
| CRF | 23 (30.7%) | 12 (16%) | 0.034 (chi) |
| COVID-19 | 22 (29.3%) | 17 (22.7%) | 0.352 (chi) |
| 4. Pulmonary status (PPC Score) | |||
| 0 | 20 (26.7%) | 25 (33.3%) | 0.373 (chi) |
| 1 | 55 (73.3%) | 50 (66.7%) | |
| 5. ASA Score | |||
| 3 | 65 (86.7%) | 66 (88.0%) | 0.806 (chi) |
| 4 | 10 (13.3%) | 9 (12.0%) | |
| 6. Surgery | |||
| CABG | 53 (70.7%) | 49 (65.3%) | 0.484 (chi) |
| Aortic valve | 9 (12%) | 13 (17.3%) | 0.356 (chi) |
| Mitral valve | 2 (2.7%) | 4 (5.3%) | 0.681 (fet) |
| Combined | 11 (14.7%) | 9 (12%) | 0.631 (chi) |
| Duration of surgery (min) | 245.7 ± 40.2 | 219.9 ± 45.0 | <0.001 (t) |
| ECC time (min) | 86.8 ± 27.3 | 80.7 ± 19.1 | 0.114 (t) |
| ACC time (min) | 55.6 ± 20.7 | 56.3 ± 15.4 | 0.799 (t) |
| Primary Outcome Measures | Group A (n = 75) | Group B (n = 75) | p-Value (Test) * |
|---|---|---|---|
| 1. PPCs Incidence | |||
| PPC ≥ 3 (n, %) | 45 (60.0%) | 10 (13.3%) | <0.001 (chi) |
| 2. PPCs Severity | |||
| PPC severity score | 3 (2) | 1 (1) | <0.001 (mw) |
| Grade 0 (n, %) | 5 (6.7%) | 14 (18.7%) | <0.001 (mw) |
| Grade 1 (n, %) | 3 (4.0%) | 31 (41.3%) | |
| Grade 2 (n, %) | 22 (29.3%) | 20 (26.7%) | |
| Grade 3 (n, %) | 23 (30.7%) | 7 (9.3%) | |
| Grade 4 (n, %) | 18 (24.0%) | 3 (4.0%) | |
| Grade 5 (n, %) | 4 (5.3%) | 0 | |
| 3. PPCs Types | |||
| Pneumonia (n, %) | 32 (42.7%) | 13 (17.3%) | <0.001 (chi) |
| Pneumothorax (n, %) | 10 (13.3%) | 3 (4%) | 0.042 (chi) |
| Pleural effusion (n, %) | 51 (68%) | 38 (50.7%) | 0.031 (chi) |
| Re-intubation (n, %) | 16 (21.3%) | 2 (2.7%) | <0.001 (chi) |
| Secondary Outcome Measures | Group A (n = 75) | Group B (n = 75) | p-Value (Test) * |
|---|---|---|---|
| 1. Pulmonary oxygenation and ventilation | |||
| Horowitz index (PaO2/FiO2) 48 h | 268.9 ± 112.6 | 312.6 ± 107.4 | 0.008 (t) |
| Alveolar–arterial gradient (A-aDO2) 48 h | 17.3 ± 5.4 | 17.9 ± 4.7 | 0.432 (t) |
| Total MV time (h) | 5.2 ± 1.6 | 5.4 ± 1.2 | 0.493 (t) |
| 2. Inflammatory markers (48 h) ** | |||
| Procalcitonin | 0.3 (0.6) | 0.5 (0.8) | 0.032 (mw) |
| C-reactive protein | 167.4 (82.9) | 95 (56.2) | <0.001 (mw) |
| Leucocytes | 13.3 ± 3.3 | 12.4 ± 3.4 | 0.102 (t) |
| Neutrophils | 80.6 ± 5.5 | 80.5 ± 5.1 | 0.881 (t) |
| Lymphocytes | 12 ± 4.6 | 12.6 ± 4 | 0.418 (t) |
| Sedimentation rate | 22 (18) | 20 (6) | 0.023 (mw) |
| Fibrinogen | 5.1 ± 1.4 | 5.2 ± 1 | 0.775 (t) |
| Albumin | 31.8 ± 3.5 | 31.4 ± 3.2 | 0.479 (t) |
| D-dimer | 0.5 (0.5) | 0.6 (0.4) | 0.877 (mw) |
| Ferritin | 202 (277) | 200 (104) | 0.287 (mw) |
| 3. Postoperative complications (non-pulmonary) | |||
| PONV | 20 (26.7%) | 30 (40.0%) | 0.083 (chi) |
| Delirium | 22 (29.3%) | 16 (21.3%) | 0.260 (chi) |
| Transfusion | 32 (42.7%) | 36 (48%) | 0.512 (chi) |
| Acute renal failure | 8 (10.7%) | 1 (1.3%) | 0.034 (fet) |
| Wound infection | 15 (20%) | 5 (6.7%) | 0.016 (chi) |
| CPR | 4 (5.3%) | 0 (0%) | 0.120 (fet) |
| 4. Renal function (48 h) ** | |||
| GFR < 60 mL/min | 24 (32%) | 10 (13.3%) | 0.006 (chi) |
| Creatinine | 99.6 ± 44.6 | 87.8 ± 27.5 | 0.054 (t) |
| Urea | 7.1 ± 3 | 6.3 ± 1.9 | 0.041(t) |
| 5. Postoperative organ dysfunction | |||
| SOFA score | 4 (2) | 4 (1) | 0.132 (mw) |
| ASA/SOFA ratio | 1.0 (0.4) | 0.8 (0.25) | 0.190 (mw) |
| 6. ICU outcome measures | |||
| ICU re-admission | 15 (20%) | 4 (5.3%) | 0.007 (chi) |
| ICU stay | 48 (24) | 32 (24) | <0.001 (mw) |
| 7. Hospital outcome measures | |||
| Hospital stay | 8 (2) | 8 (2) | 0.092 (mw) |
| Hospital mortality | 8 (10.7%) | 1 (1.3%) | 0.034 (fet) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karadžić Kočica, M.; Ristić, A.; Soldatović, I.; Lazović, D.; Čumić, J.; Grujić, M.; Karan, R.; Terzić, D.; Palibrk, I.; Kočica, M.; et al. The Influence of High-Dose Parenteral Vitamin C on the Incidence and Severity of Postoperative Pulmonary Complications in Cardiac Surgery with Extracorporeal Circulation: A Randomized Controlled Trial. Nutrients 2024, 16, 761. https://doi.org/10.3390/nu16060761
Karadžić Kočica M, Ristić A, Soldatović I, Lazović D, Čumić J, Grujić M, Karan R, Terzić D, Palibrk I, Kočica M, et al. The Influence of High-Dose Parenteral Vitamin C on the Incidence and Severity of Postoperative Pulmonary Complications in Cardiac Surgery with Extracorporeal Circulation: A Randomized Controlled Trial. Nutrients. 2024; 16(6):761. https://doi.org/10.3390/nu16060761
Chicago/Turabian StyleKaradžić Kočica, Milica, Arsen Ristić, Ivan Soldatović, Dejan Lazović, Jelena Čumić, Miloš Grujić, Radmila Karan, Duško Terzić, Ivan Palibrk, Mladen Kočica, and et al. 2024. "The Influence of High-Dose Parenteral Vitamin C on the Incidence and Severity of Postoperative Pulmonary Complications in Cardiac Surgery with Extracorporeal Circulation: A Randomized Controlled Trial" Nutrients 16, no. 6: 761. https://doi.org/10.3390/nu16060761
APA StyleKaradžić Kočica, M., Ristić, A., Soldatović, I., Lazović, D., Čumić, J., Grujić, M., Karan, R., Terzić, D., Palibrk, I., Kočica, M., & Marković, D. (2024). The Influence of High-Dose Parenteral Vitamin C on the Incidence and Severity of Postoperative Pulmonary Complications in Cardiac Surgery with Extracorporeal Circulation: A Randomized Controlled Trial. Nutrients, 16(6), 761. https://doi.org/10.3390/nu16060761








