Time-Restricted Eating and Bone Health: A Systematic Review with Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources and Search Strategy
2.2. Eligibility Criteria
2.3. Data Collection
2.4. Quality and Certainty Assessment
2.5. Data Synthesis
2.5.1. Measure of Intervention Effect
2.5.2. Outcome Data for Evidence Synthesis
2.5.3. Sensitivity Analyses
2.6. Other Metodological Considerations
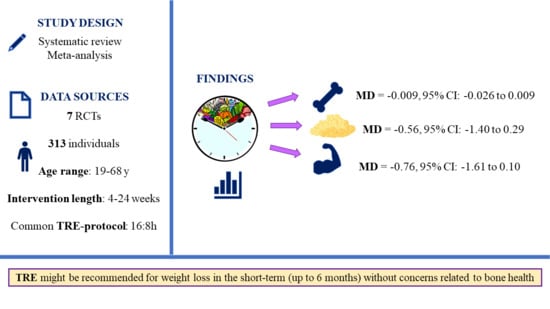
3. Results
3.1. Literature Search
3.2. Study Characteristics
3.3. Population
3.4. Time-Restricted Eating Interventions
3.5. Control Conditions
3.6. Bone Health Outcomes
3.7. Comparisons Not Included in the Meta-Analysis
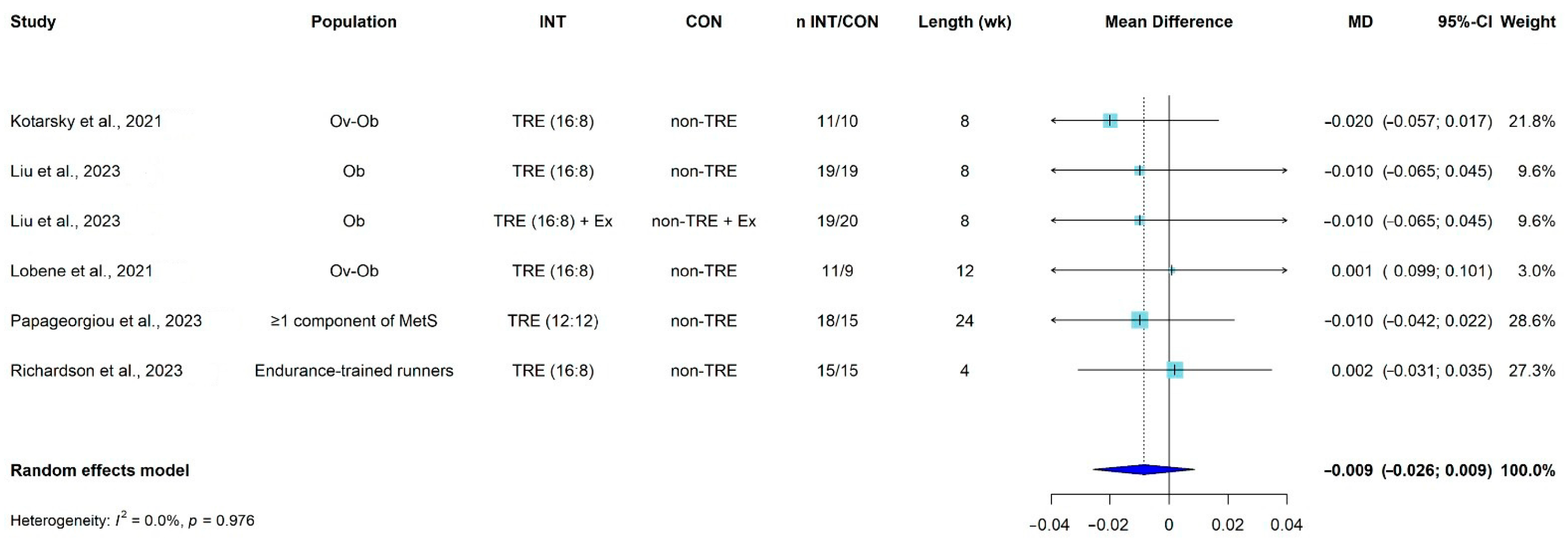
3.8. Meta-Analysis
3.8.1. Sensitivity Analyses
3.8.2. Risk of Bias and Certainty Assessment
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, N.K.; Sowa, H.; Hinoi, E.; Ferron, M.; Ahn, J.D.; Confavreux, C.; Dacquin, R.; Mee, P.J.; McKee, M.D.; Jung, D.Y.; et al. Endocrine regulation of energy metabolism by the skeleton. Cell 2007, 130, 456–469. [Google Scholar] [CrossRef]
- Smith, C.; Sim, M.; Dalla Via, J.; Levinger, I.; Duque, G. The Interconnection Between Muscle and Bone: A Common Clinical Management Pathway. Calcif. Tissue Int. 2024, 114, 24–37. [Google Scholar] [CrossRef]
- Karsenty, G. Osteocalcin: A Multifaceted Bone-Derived Hormone. Annu. Rev. Nutr. 2023, 43, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Villareal, D.T.; Shah, K.; Banks, M.R.; Sinacore, D.R.; Klein, S. Effect of weight loss and exercise therapy on bone metabolism and mass in obese older adults: A one-year randomized controlled trial. J. Clin. Endocrinol. Metab. 2008, 93, 2181–2187. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, M.; Kerschan-Schindl, K.; Sathyapalan, T.; Pietschmann, P. Is Weight Loss Harmful for Skeletal Health in Obese Older Adults? Gerontology 2020, 66, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Paccou, J.; Tsourdi, E.; Meier, C.; Palermo, A.; Pepe, J.; Body, J.J.; Zillikens, M.C. Bariatric surgery and skeletal health: A narrative review and position statement for management by the European Calcified Tissue Society (ECTS). Bone 2022, 154, 116236. [Google Scholar] [CrossRef] [PubMed]
- Rizzoli, R.; Chevalley, T. Bone health: Biology and nutrition. Curr. Opin. Clin. Nutr. Metab. Care 2024, 27, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Rizzoli, R. Nutrition: Its role in bone health. Best. Pract. Res. Clin. Endocrinol. Metab. 2008, 22, 813–829. [Google Scholar] [CrossRef]
- New, S.A. Exercise, bone and nutrition. Proc. Nutr. Soc. 2001, 60, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Mirzababaei, A.; Mirzaei, K.; Khorrami-Nezhad, L.; Maghbooli, Z.; Keshavarz, S.A. Metabolically healthy/unhealthy components may modify bone mineral density in obese people. Arch. Osteoporos. 2017, 12, 95. [Google Scholar] [CrossRef]
- Salamat, M.R.; Salamat, A.H.; Abedi, I.; Janghorbani, M. Relationship between Weight, Body Mass Index, and Bone Mineral Density in Men Referred for Dual-Energy X-Ray Absorptiometry Scan in Isfahan, Iran. J. Osteoporos. 2013, 2013, 205963. [Google Scholar] [CrossRef] [PubMed]
- Morin, S.; Leslie, W.D. High bone mineral density is associated with high body mass index. Osteoporos. Int. 2009, 20, 1267–1271. [Google Scholar] [CrossRef] [PubMed]
- Torres-Costoso, A.; Martínez-Vizcaíno, V.; Baptista, F.; Reina-Gutiérrez, S.; Núñez de Arenas-Arroyo, S.; Hernández-Castillejo, L.E.; Garrido-Miguel, M. Body composition phenotypes and bone health in young adults: A cluster analysis. Clin. Nutr. 2023, 42, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.G.; Kim, Y.; Lim, H.; Kang, M.J.; Park, K.H. Relationship Between Bone Mineral Density and Body Composition According to Obesity Status in Children. Endocr. Pract. 2021, 27, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Armamento-Villareal, R. Obesity and Skeletal Fragility. J. Clin. Endocrinol. Metab. 2024, 109, e466–e477. [Google Scholar] [CrossRef]
- Fleischer, J.G.; Das, S.K.; Bhapkar, M.; Manoogian, E.N.C.; Panda, S. Associations between the timing of eating and weight-loss in calorically restricted healthy adults: Findings from the CALERIE study. Exp. Gerontol. 2022, 165, 111837. [Google Scholar] [CrossRef]
- Clayton, D.J.; Varley, I.; Papageorgiou, M. Intermittent fasting and bone health: A bone of contention? Br. J. Nutr. 2023, 130, 1487–1499. [Google Scholar] [CrossRef]
- Zibellini, J.; Seimon, R.V.; Lee, C.M.; Gibson, A.A.; Hsu, M.S.; Shapses, S.A.; Nguyen, T.V.; Sainsbury, A. Does Diet-Induced Weight Loss Lead to Bone Loss in Overweight or Obese Adults? A Systematic Review and Meta-Analysis of Clinical Trials. J. Bone Miner. Res. 2015, 30, 2168–2178. [Google Scholar] [CrossRef]
- Harper, C.; Pattinson, A.L.; Fernando, H.A.; Zibellini, J.; Seimon, R.V.; Sainsbury, A. Effects of obesity treatments on bone mineral density, bone turnover and fracture risk in adults with overweight or obesity. Horm. Mol. Biol. Clin. Investig. 2016, 28, 133–149. [Google Scholar] [CrossRef]
- Villareal, D.T.; Fontana, L.; Weiss, E.P.; Racette, S.B.; Steger-May, K.; Schechtman, K.B.; Klein, S.; Holloszy, J.O. Bone mineral density response to caloric restriction-induced weight loss or exercise-induced weight loss: A randomized controlled trial. Arch. Intern. Med. 2006, 166, 2502–2510. [Google Scholar] [CrossRef]
- Schwartz, A.V.; Johnson, K.C.; Kahn, S.E.; Shepherd, J.A.; Nevitt, M.C.; Peters, A.L.; Walkup, M.P.; Hodges, A.; Williams, C.C.; Bray, G.A. Effect of 1 year of an intentional weight loss intervention on bone mineral density in type 2 diabetes: Results from the Look AHEAD randomized trial. J. Bone Miner. Res. 2012, 27, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Iwaniec, U.T.; Turner, R.T. Influence of body weight on bone mass, architecture and turnover. J. Endocrinol. 2016, 230, R115–R130. [Google Scholar] [CrossRef] [PubMed]
- Shapses, S.A.; Sukumar, D. Bone metabolism in obesity and weight loss. Annu. Rev. Nutr. 2012, 32, 287–309. [Google Scholar] [CrossRef] [PubMed]
- Barnosky, A.; Kroeger, C.M.; Trepanowski, J.F.; Klempel, M.C.; Bhutani, S.; Hoddy, K.K.; Gabel, K.; Shapses, S.A.; Varady, K.A. Effect of alternate day fasting on markers of bone metabolism: An exploratory analysis of a 6-month randomized controlled trial. Nutr. Healthy Aging 2017, 4, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Stekovic, S.; Hofer, S.J.; Tripolt, N.; Aon, M.A.; Royer, P.; Pein, L.; Stadler, J.T.; Pendl, T.; Prietl, B.; Url, J.; et al. Alternate Day Fasting Improves Physiological and Molecular Markers of Aging in Healthy, Non-obese Humans. Cell Metab. 2019, 30, 462–476.e6. [Google Scholar] [CrossRef]
- Liu, L.; Chen, W.; Wu, D.; Hu, F. Metabolic Efficacy of Time-Restricted Eating in Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Clin. Endocrinol. Metab. 2022, 107, 3428–3441. [Google Scholar] [CrossRef] [PubMed]
- Turner-McGrievy, G.M.; Wirth, M.D.; Bernhart, J.A.; Aydin, H. The Fasting and Shifted Timing (FAST) of Eating Study: A pilot feasibility randomized crossover intervention assessing the acceptability of three different fasting diet approaches. Appetite 2022, 176, 106135. [Google Scholar] [CrossRef]
- Jan, Y.C.; Chiang, S.W.; Hsieh, T.C. Exploring the successful experience of time-restricted eating in overweight adults: A qualitative study. Appetite 2023, 188, 106979. [Google Scholar] [CrossRef]
- Panda, S. The arrival of circadian medicine. Nat. Rev. Endocrinol. 2019, 15, 67–69. [Google Scholar] [CrossRef]
- Li, C.I.; Liu, C.S.; Lin, W.Y.; Meng, N.H.; Chen, C.C.; Yang, S.Y.; Chen, H.J.; Lin, C.C.; Li, T.C. Glycated Hemoglobin Level and Risk of Hip Fracture in Older People with Type 2 Diabetes: A Competing Risk Analysis of Taiwan Diabetes Cohort Study. J. Bone Miner. Res. 2015, 30, 1338–1346. [Google Scholar] [CrossRef]
- Hofbauer, L.C.; Busse, B.; Eastell, R.; Ferrari, S.; Frost, M.; Müller, R.; Burden, A.M.; Rivadeneira, F.; Napoli, N.; Rauner, M. Bone fragility in diabetes: Novel concepts and clinical implications. Lancet Diabetes Endocrinol. 2022, 10, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Swanson, C.M.; Kohrt, W.M.; Buxton, O.M.; Everson, C.A.; Wright, K.P., Jr.; Orwoll, E.S.; Shea, S.A. The importance of the circadian system & sleep for bone health. Metabolism 2018, 84, 28–43. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, M.; Biver, E.; Mareschal, J.; Phillips, N.E.; Hemmer, A.; Biolley, E.; Schwab, N.; Manoogian, E.N.C.; Gonzalez Rodriguez, E.; Aeberli, D.; et al. The Effects Of Time-Restricted Eating (Tre) And Weight Loss On Bone Metabolism And Health: An Exploratory Analysis In A 6-Month Randomised Controlled Trial. Clin. Nutr. ESPEN 2023, 54, 558. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.4. 2023. Available online: www.training.cochrane.org/handbook (accessed on 1 August 2023).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; DeBeer, H.; et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.; Turner, R. Power analysis for random-effects meta-analysis. Res. Synth. Methods 2017, 8, 290–302. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Balduzzi, S.; Rücker, G.; Schwarzer, G. How to perform a meta-analysis with R: A practical tutorial. Evid. Based Ment. Health 2019, 22, 153–160. [Google Scholar] [CrossRef]
- Kotarsky, C.J.; Johnson, N.R.; Mahoney, S.J.; Mitchell, S.L.; Schimek, R.L.; Stastny, S.N.; Hackney, K.J. Time-restricted eating and concurrent exercise training reduces fat mass and increases lean mass in overweight and obese adults. Physiol. Rep. 2021, 9, e14868. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, S.; Ji, H.; Dai, Z. Effects of time-restricted feeding and walking exercise on the physical health of female college students with hidden obesity: A randomized trial. Front. Public Health 2023, 11, 1020887. [Google Scholar] [CrossRef] [PubMed]
- Lobene, A.J.; Panda, S.; Mashek, D.G.; Manoogian, E.N.C.; Gallant, K.M.H.; Chow, L.S. Time-Restricted Eating for 12 Weeks Does Not Adversely Alter Bone Turnover in Overweight Adults. Nutrients 2021, 13, 1155. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, M.; Biver, E.; Mareschal, J.; Phillips, N.E.; Hemmer, A.; Biolley, E.; Schwab, N.; Manoogian, E.N.C.; Gonzalez Rodriguez, E.; Aeberli, D.; et al. The effects of time-restricted eating and weight loss on bone metabolism and health: A 6-month randomized controlled trial. Obesity 2023, 31 (Suppl. 1), 85–95. [Google Scholar] [CrossRef] [PubMed]
- Richardson, C.E.; Tovar, A.P.; Davis, B.A.; Van Loan, M.D.; Keim, N.L.; Casazza, G.A. An Intervention of Four Weeks of Time-Restricted Eating (16/8) in Male Long-Distance Runners Does Not Affect Cardiometabolic Risk Factors. Nutrients 2023, 15, 985. [Google Scholar] [CrossRef] [PubMed]
- Lowe, D.A.; Wu, N.; Rohdin-Bibby, L.; Moore, A.H.; Kelly, N.; Liu, Y.E.; Philip, E.; Vittinghoff, E.; Heymsfield, S.B.; Olgin, J.E.; et al. Effects of Time-Restricted Eating on Weight Loss and Other Metabolic Parameters in Women and Men With Overweight and Obesity: The TREAT Randomized Clinical Trial. JAMA Intern. Med. 2020, 180, 1491–1499. [Google Scholar] [CrossRef]
- Rohatgi, A. WebPlotDigitizer Version 4.6. Available online: https://automeris.io/WebPlotDigitizer.html (accessed on 2 December 2023).
- McGrath, S.; Zhao, X.; Steele, R.; Thombs, B.D.; Benedetti, A. Estimating the sample mean and standard deviation from commonly reported quantiles in meta-analysis. Stat. Methods Med. Res. 2020, 29, 2520–2537. [Google Scholar] [CrossRef]
- Martens, C.R.; Rossman, M.J.; Mazzo, M.R.; Jankowski, L.R.; Nagy, E.E.; Denman, B.A.; Richey, J.J.; Johnson, S.A.; Ziemba, B.P.; Wang, Y.; et al. Short-term time-restricted feeding is safe and feasible in non-obese healthy midlife and older adults. GeroScience 2020, 42, 667–686. [Google Scholar] [CrossRef]
- Chow, L.S.; Manoogian, E.N.C.; Alvear, A.; Fleischer, J.G.; Thor, H.; Dietsche, K.; Wang, Q.; Hodges, J.S.; Esch, N.; Malaeb, S.; et al. Time-Restricted Eating Effects on Body Composition and Metabolic Measures in Humans who are Overweight: A Feasibility Study. Obesity 2020, 28, 860–869. [Google Scholar] [CrossRef]
- Ezpeleta, M.; Cienfuegos, S.; Lin, S.; Pavlou, V.; Gabel, K.; Tussing-Humphreys, L.; Varady, K.A. Time-restricted eating: Watching the clock to treat obesity. Cell Metab. 2024, 36, 301–314. [Google Scholar] [CrossRef]
- Lin, S.; Cienfuegos, S.; Ezpeleta, M.; Gabel, K.; Pavlou, V.; Mulas, A.; Chakos, K.; McStay, M.; Wu, J.; Tussing-Humphreys, L.; et al. Time-Restricted Eating Without Calorie Counting for Weight Loss in a Racially Diverse Population: A Randomized Controlled Trial. Ann. Intern. Med. 2023, 176, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Varady, K.A.; Cienfuegos, S.; Ezpeleta, M.; Gabel, K. Clinical application of intermittent fasting for weight loss: Progress and future directions. Nat. Rev. Endocrinol. 2022, 18, 309–321. [Google Scholar] [CrossRef]
- Savvidis, C.; Tournis, S.; Dede, A.D. Obesity and bone metabolism. Hormones 2018, 17, 205–217. [Google Scholar] [CrossRef]
- Rector, R.S.; Loethen, J.; Ruebel, M.; Thomas, T.R.; Hinton, P.S. Serum markers of bone turnover are increased by modest weight loss with or without weight-bearing exercise in overweight premenopausal women. Appl. Physiol. Nutr. Metab. 2009, 34, 933–941. [Google Scholar] [CrossRef]
- Lucey, A.J.; Paschos, G.K.; Cashman, K.D.; Martínéz, J.A.; Thorsdottir, I.; Kiely, M. Influence of moderate energy restriction and seafood consumption on bone turnover in overweight young adults. Am. J. Clin. Nutr. 2008, 87, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Chao, D.; Espeland, M.A.; Farmer, D.; Register, T.C.; Lenchik, L.; Applegate, W.B.; Ettinger, W.H., Jr. Effect of voluntary weight loss on bone mineral density in older overweight women. J. Am. Geriatr. Soc. 2000, 48, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Soltani, S.; Hunter, G.R.; Kazemi, A.; Shab-Bidar, S. The effects of weight loss approaches on bone mineral density in adults: A systematic review and meta-analysis of randomized controlled trials. Osteoporos. Int. 2016, 27, 2655–2671. [Google Scholar] [CrossRef]
- Conte, C.; Epstein, S.; Napoli, N. Insulin resistance and bone: A biological partnership. Acta Diabetol. 2018, 55, 305–314. [Google Scholar] [CrossRef]
- Zeb, F.; Wu, X.; Chen, L.; Fatima, S.; Haq, I.U.; Chen, A.; Majeed, F.; Feng, Q.; Li, M. Effect of time-restricted feeding on metabolic risk and circadian rhythm associated with gut microbiome in healthy males. Br. J. Nutr. 2020, 123, 1216–1226. [Google Scholar] [CrossRef]
- Turner, L.; Charrouf, R.; Martínez-Vizcaíno, V.; Hutchison, A.; Heilbronn, L.K.; Fernández-Rodríguez, R. The effects of time-restricted eating versus habitual diet on inflammatory cytokines and adipokines in the general adult population: A systematic review with meta-analysis. Am. J. Clin. Nutr. 2024, 119, 206–220. [Google Scholar] [CrossRef]
- Allison, K.C.; Hopkins, C.M.; Ruggieri, M.; Spaeth, A.M.; Ahima, R.S.; Zhang, Z.; Taylor, D.M.; Goel, N. Prolonged, Controlled Daytime versus Delayed Eating Impacts Weight and Metabolism. Curr. Biol. 2021, 31, 650–657.e653. [Google Scholar] [CrossRef] [PubMed]
- Andriessen, C.; Fealy, C.E.; Veelen, A.; van Beek, S.M.M.; Roumans, K.H.M.; Connell, N.J.; Mevenkamp, J.; Moonen-Kornips, E.; Havekes, B.; Schrauwen-Hinderling, V.B.; et al. Three weeks of time-restricted eating improves glucose homeostasis in adults with type 2 diabetes but does not improve insulin sensitivity: A randomised crossover trial. Diabetologia 2022, 65, 1710–1720. [Google Scholar] [CrossRef] [PubMed]
- Bantle, A.E.; Lau, K.J.; Wang, Q.; Malaeb, S.; Harindhanavudhi, T.; Manoogian, E.N.C.; Panda, S.; Mashek, D.G.; Chow, L.S. Time-restricted eating did not alter insulin sensitivity or β-cell function in adults with obesity: A randomized pilot study. Obesity 2023, 31 (Suppl. 1), 108–115. [Google Scholar] [CrossRef] [PubMed]
- Bao, R.; Sun, Y.; Jiang, Y.; Ye, L.; Hong, J.; Wang, W. Effects of Time-Restricted Feeding on Energy Balance: A Cross-Over Trial in Healthy Subjects. Front. Endocrinol. 2022, 13, 870054. [Google Scholar] [CrossRef]
- Brady, A.J.; Langton, H.M.; Mulligan, M.; Egan, B. Effects of 8 wk of 16:8 Time-restricted Eating in Male Middle- and Long-Distance Runners. Med. Sci. Sports Exerc. 2021, 53, 633–642. [Google Scholar] [CrossRef]
- Cienfuegos, S.; Gabel, K.; Kalam, F.; Ezpeleta, M.; Lin, S.; Varady, K.A. Changes in body weight and metabolic risk during time restricted feeding in premenopausal versus postmenopausal women. Exp. Gerontol. 2021, 154, 111545. [Google Scholar] [CrossRef]
- Cienfuegos, S.; Gabel, K.; Kalam, F.; Ezpeleta, M.; Pavlou, V.; Lin, S.; Wiseman, E.; Varady, K.A. The effect of 4-h versus 6-h time restricted feeding on sleep quality, duration, insomnia severity and obstructive sleep apnea in adults with obesity. Nutr. Health 2022, 28, 5–11. [Google Scholar] [CrossRef]
- Cienfuegos, S.; Gabel, K.; Kalam, F.; Ezpeleta, M.; Wiseman, E.; Pavlou, V.; Lin, S.; Oliveira, M.L.; Varady, K.A. Effects of 4- and 6-h Time-Restricted Feeding on Weight and Cardiometabolic Health: A Randomized Controlled Trial in Adults with Obesity. Cell Metab. 2020, 32, 366–378.e363. [Google Scholar] [CrossRef]
- Correia, J.M.; Santos, I.; Pezarat-Correia, P.; Minderico, C.; Schoenfeld, B.J.; Mendonca, G.V. Effects of Time-Restricted Feeding on Supramaximal Exercise Performance and Body Composition: A Randomized and Counterbalanced Crossover Study in Healthy Men. Int. J. Environ. Res. Public Health 2021, 18, 7227. [Google Scholar] [CrossRef]
- Correia, J.M.; Santos, P.D.G.; Pezarat-Correia, P.; Minderico, C.S.; Infante, J.; Mendonca, G.V. Effect of Time-Restricted Eating and Resistance Training on High-Speed Strength and Body Composition. Nutrients 2023, 15, 285. [Google Scholar] [CrossRef]
- Crose, A.; Alvear, A.; Singroy, S.; Wang, Q.; Manoogian, E.; Panda, S.; Mashek, D.G.; Chow, L.S. Time-Restricted Eating Improves Quality of Life Measures in Overweight Humans. Nutrients 2021, 13, 1430. [Google Scholar] [CrossRef]
- Da Silva, B.R.; Kirkham, A.A.; Ford, K.L.; Haykowsky, M.J.; Paterson, D.I.; Joy, A.A.; Pituskin, E.; Thompson, R.; Prado, C.M. Time-Restricted Eating in Breast Cancer Survivors: Effects on Body Composition and Nutritional Status. Nutr. Cancer 2023, 75, 1309–1314. [Google Scholar] [CrossRef]
- de Oliveira Maranhão Pureza, I.R.; da Silva Junior, A.E.; Silva Praxedes, D.R.; Lessa Vasconcelos, L.G.; de Lima Macena, M.; Vieira de Melo, I.S.; de Menezes Toledo Florêncio, T.M.; Bueno, N.B. Effects of time-restricted feeding on body weight, body composition and vital signs in low-income women with obesity: A 12-month randomized clinical trial. Clin. Nutr. 2021, 40, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Domaszewski, P.; Konieczny, M.; Dybek, T.; Łukaniszyn-Domaszewska, K.; Anton, S.; Sadowska-Krępa, E.; Skorupska, E. Comparison of the effects of six-week time-restricted eating on weight loss, body composition, and visceral fat in overweight older men and women. Exp. Gerontol. 2023, 174, 112116. [Google Scholar] [CrossRef] [PubMed]
- Domaszewski, P.; Konieczny, M.; Pakosz, P.; Łukaniszyn-Domaszewska, K.; Mikuláková, W.; Sadowska-Krępa, E.; Anton, S. Effect of a six-week times restricted eating intervention on the body composition in early elderly men with overweight. Sci. Rep. 2022, 12, 9816. [Google Scholar] [CrossRef] [PubMed]
- Ferrocino, I.; Pellegrini, M.; D’Eusebio, C.; Goitre, I.; Ponzo, V.; Fadda, M.; Rosato, R.; Mengozzi, G.; Beccuti, G.; Merlo, F.D.; et al. The Effects of Time-Restricted Eating on Metabolism and Gut Microbiota: A Real-Life Study. Nutrients 2022, 14, 2569. [Google Scholar] [CrossRef] [PubMed]
- Gasmi, M.; Sellami, M.; Denham, J.; Padulo, J.; Kuvacic, G.; Selmi, W.; Khalifa, R. Time-restricted feeding influences immune responses without compromising muscle performance in older men. Nutrition 2018, 51–52, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.E.; Waldman, H.S.; Abel, M.G.; McCurdy, K.W.; McAllister, M.J. Impact of Time Restricted Feeding on Fitness Variables in Professional Resistance Trained Firefighters. J. Occup. Environ. Med. 2021, 63, 343–349. [Google Scholar] [CrossRef]
- Haganes, K.L.; Silva, C.P.; Eyjólfsdóttir, S.K.; Steen, S.; Grindberg, M.; Lydersen, S.; Hawley, J.A.; Moholdt, T. Time-restricted eating and exercise training improve HbA1c and body composition in women with overweight/obesity: A randomized controlled trial. Cell Metab. 2022, 34, 1457–1471.e1454. [Google Scholar] [CrossRef]
- He, M.; Wang, J.; Liang, Q.; Li, M.; Guo, H.; Wang, Y.; Deji, C.; Sui, J.; Wang, Y.W.; Liu, Y.; et al. Time-restricted eating with or without low-carbohydrate diet reduces visceral fat and improves metabolic syndrome: A randomized trial. Cell Rep. Med. 2022, 3, 100777. [Google Scholar] [CrossRef]
- Hutchison, A.T.; Regmi, P.; Manoogian, E.N.C.; Fleischer, J.G.; Wittert, G.A.; Panda, S.; Heilbronn, L.K. Time-Restricted Feeding Improves Glucose Tolerance in Men at Risk for Type 2 Diabetes: A Randomized Crossover Trial. Obesity 2019, 27, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Isenmann, E.; Dissemond, J.; Geisler, S. The Effects of a Macronutrient-Based Diet and Time-Restricted Feeding (16:8) on Body Composition in Physically Active Individuals-A 14-Week Randomised Controlled Trial. Nutrients 2021, 13, 3122. [Google Scholar] [CrossRef] [PubMed]
- Jamshed, H.; Steger, F.L.; Bryan, D.R.; Richman, J.S.; Warriner, A.H.; Hanick, C.J.; Martin, C.K.; Salvy, S.J.; Peterson, C.M. Effectiveness of Early Time-Restricted Eating for Weight Loss, Fat Loss, and Cardiometabolic Health in Adults With Obesity: A Randomized Clinical Trial. JAMA Intern. Med. 2022, 182, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Kesztyüs, D.; Cermak, P.; Gulich, M.; Kesztyüs, T. Adherence to Time-Restricted Feeding and Impact on Abdominal Obesity in Primary Care Patients: Results of a Pilot Study in a Pre-Post Design. Nutrients 2019, 11, 2854. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xing, C.; Zhang, J.; Zhao, H.; Shi, W.; He, B. Eight-hour time-restricted feeding improves endocrine and metabolic profiles in women with anovulatory polycystic ovary syndrome. J. Transl. Med. 2021, 19, 148. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.J.; Wang, Y.T.; Chan, L.C.; Chu, N.F. Effect of time-restricted feeding on body composition and cardio-metabolic risk in middle-aged women in Taiwan. Nutrition 2022, 93, 111504. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Huang, Y.; Huang, C.; Yang, S.; Wei, X.; Zhang, P.; Guo, D.; Lin, J.; Xu, B.; Li, C.; et al. Calorie Restriction with or without Time-Restricted Eating in Weight Loss. N. Engl. J. Med. 2022, 386, 1495–1504. [Google Scholar] [CrossRef] [PubMed]
- Lobene, A.; Panda, S.; Mashek, D.; Gallant, K.H.; Chow, L. Time Restricted Eating for 12 Weeks Does Not Adversely Alter Bone Mineral Content and Bone Metabolism in Overweight Adults. J. Bone Miner. Res. 2020, 35 (Suppl. 1), 232–233. [Google Scholar] [CrossRef]
- Manoogian, E.N.C.; Zadourian, A.; Lo, H.C.; Gutierrez, N.R.; Shoghi, A.; Rosander, A.; Pazargadi, A.; Ormiston, C.K.; Wang, X.; Sui, J.; et al. Feasibility of time-restricted eating and impacts on cardiometabolic health in 24-h shift workers: The Healthy Heroes randomized control trial. Cell Metab. 2022, 34, 1442–1456.e1447. [Google Scholar] [CrossRef]
- Mayra, S.T.; Chondropoulos, K.; De Leon, A.; Kravat, N.; Johnston, C.S. The feasibility and preliminary efficacy of early time-restricted eating on diet quality in college students: A randomized study. Obes. Res. Clin. Pract. 2022, 16, 413–420. [Google Scholar] [CrossRef]
- McAllister, M.J.; Pigg, B.L.; Renteria, L.I.; Waldman, H.S. Time-restricted feeding improves markers of cardiometabolic health in physically active college-age men: A 4-week randomized pre-post pilot study. Nutr. Res. 2020, 75, 32–43. [Google Scholar] [CrossRef]
- Moro, T.; Tinsley, G.; Bianco, A.; Marcolin, G.; Pacelli, Q.F.; Battaglia, G.; Palma, A.; Gentil, P.; Neri, M.; Paoli, A. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J. Transl. Med. 2016, 14, 290. [Google Scholar] [CrossRef] [PubMed]
- Moro, T.; Tinsley, G.; Longo, G.; Grigoletto, D.; Bianco, A.; Ferraris, C.; Guglielmetti, M.; Veneto, A.; Tagliabue, A.; Marcolin, G.; et al. Time-restricted eating effects on performance, immune function, and body composition in elite cyclists: A randomized controlled trial. J. Int. Soc. Sports Nutr. 2020, 17, 65. [Google Scholar] [CrossRef] [PubMed]
- Moro, T.; Tinsley, G.; Pacelli, F.Q.; Marcolin, G.; Bianco, A.; Paoli, A. Twelve Months of Time-restricted Eating and Resistance Training Improves Inflammatory Markers and Cardiometabolic Risk Factors. Med. Sci. Sports Exerc. 2021, 53, 2577–2585. [Google Scholar] [CrossRef]
- Parr, E.; Kouw, I.; Wheeler, M.; Radford, B.; Hall, R.; Senden, J.; Goessens, J.; Van Loon, L.; Hawley, J. Short-term time-restricted eating does not lower muscle protein synthesis rates in men with overweight/obesity. Obes. Rev. 2022, 23. [Google Scholar] [CrossRef]
- Phillips, N.E.; Mareschal, J.; Schwab, N.; Manoogian, E.N.C.; Borloz, S.; Ostinelli, G.; Gauthier-Jaques, A.; Umwali, S.; Gonzalez Rodriguez, E.; Aeberli, D.; et al. The Effects of Time-Restricted Eating versus Standard Dietary Advice on Weight, Metabolic Health and the Consumption of Processed Food: A Pragmatic Randomised Controlled Trial in Community-Based Adults. Nutrients 2021, 13, 1042. [Google Scholar] [CrossRef]
- Pureza, I.; Melo, I.S.V.; Macena, M.L.; Praxedes, D.R.S.; Vasconcelos, L.G.L.; Silva-Júnior, A.E.; Florêncio, T.; Bueno, N.B. Acute effects of time-restricted feeding in low-income women with obesity placed on hypoenergetic diets: Randomized trial. Nutrition 2020, 77, 110796. [Google Scholar] [CrossRef]
- Steger, F.L.; Jamshed, H.; Bryan, D.R.; Richman, J.S.; Warriner, A.H.; Hanick, C.J.; Martin, C.K.; Salvy, S.J.; Peterson, C.M. Early time-restricted eating affects weight, metabolic health, mood, and sleep in adherent completers: A secondary analysis. Obesity 2023, 31 (Suppl. 1), 96–107. [Google Scholar] [CrossRef]
- Steger, F.L.; Jamshed, H.; Martin, C.K.; Richman, J.S.; Bryan, D.R.; Hanick, C.J.; Salvy, S.J.; Warriner, A.H.; Peterson, C.M. Impact of early time-restricted eating on diet quality, meal frequency, appetite, and eating behaviors: A randomized trial. Obesity 2023, 31 (Suppl. 1), 127–138. [Google Scholar] [CrossRef]
- Stratton, M.T.; Tinsley, G.M.; Alesi, M.G.; Hester, G.M.; Olmos, A.A.; Serafini, P.R.; Modjeski, A.S.; Mangine, G.T.; King, K.; Savage, S.N.; et al. Four weeks of time-restricted feeding combined with resistance training does not differentially influence measures of body composition, muscle performance, resting energy expenditure, and blood biomarkers. Nutrients 2020, 12, 1126. [Google Scholar] [CrossRef]
- Sutton, E.F.; Beyl, R.; Early, K.S.; Cefalu, W.T.; Ravussin, E.; Peterson, C.M. Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes. Cell Metab. 2018, 27, 1212–1221.e1213. [Google Scholar] [CrossRef]
- Teong, X.T.; Liu, K.; Vincent, A.D.; Bensalem, J.; Liu, B.; Hattersley, K.J.; Zhao, L.; Feinle-Bisset, C.; Sargeant, T.J.; Wittert, G.A.; et al. Intermittent fasting plus early time-restricted eating versus calorie restriction and standard care in adults at risk of type 2 diabetes: A randomized controlled trial. Nat. Med. 2023, 29, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.A.; Zaman, A.; Sloggett, K.J.; Steinke, S.; Grau, L.; Catenacci, V.A.; Cornier, M.A.; Rynders, C.A. Early time-restricted eating compared with daily caloric restriction: A randomized trial in adults with obesity. Obesity 2022, 30, 1027–1038. [Google Scholar] [CrossRef]
- Tinsley, G.M.; Forsse, J.S.; Butler, N.K.; Paoli, A.; Bane, A.A.; La Bounty, P.M.; Morgan, G.B.; Grandjean, P.W. Time-restricted feeding in young men performing resistance training: A randomized controlled trial. Eur. J. Sport. Sci. 2017, 17, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Tinsley, G.M.; Moore, M.L.; Graybeal, A.J.; Paoli, A.; Kim, Y.; Gonzales, J.U.; Harry, J.R.; VanDusseldorp, T.A.; Kennedy, D.N.; Cruz, M.R. Time-restricted feeding plus resistance training in active females: A randomized trial. Am. J. Clin. Nutr. 2019, 110, 628–640. [Google Scholar] [CrossRef] [PubMed]
- Tinsley, G.M.; Paoli, A. Time-restricted eating and age-related muscle loss. Aging 2019, 11, 8741–8742. [Google Scholar] [CrossRef]
- Tovar, A.P.; Richardson, C.E.; Keim, N.L.; Van Loan, M.D.; Davis, B.A.; Casazza, G.A. Four Weeks of 16/8 Time Restrictive Feeding in Endurance Trained Male Runners Decreases Fat Mass, without Affecting Exercise Performance. Nutrients 2021, 13, 2941. [Google Scholar] [CrossRef]
- Valenzuela, P.L.; Castillo-García, A.; Lucia, A. Calorie Restriction with or without Time-Restricted Eating in Weight Loss. N. Engl. J. Med. 2022, 387, 280–281. [Google Scholar] [CrossRef] [PubMed]
- Vidmar, A.P.; Naguib, M.; Raymond, J.K.; Salvy, S.J.; Hegedus, E.; Wee, C.P.; Goran, M.I. Time-Limited Eating and Continuous Glucose Monitoring in Adolescents with Obesity: A Pilot Study. Nutrients 2021, 13, 3697. [Google Scholar] [CrossRef]
- Wei, X.; Lin, B.; Huang, Y.; Yang, S.; Huang, C.; Shi, L.; Liu, D.; Zhang, P.; Lin, J.; Xu, B.; et al. Effects of Time-Restricted Eating on Nonalcoholic Fatty Liver Disease: The TREATY-FLD Randomized Clinical Trial. JAMA Netw. Open 2023, 6, e233513. [Google Scholar] [CrossRef]
- Xie, Z.; Sun, Y.; Ye, Y.; Hu, D.; Zhang, H.; He, Z.; Zhao, H.; Yang, H.; Mao, Y. Randomized controlled trial for time-restricted eating in healthy volunteers without obesity. Nat. Commun. 2022, 13, 1003. [Google Scholar] [CrossRef] [PubMed]
- Zeb, F.; Wu, X.; Chen, L.; Fatima, S.; Ijaz Ul, H.; Chen, A.; Xu, C.; Jianglei, R.; Feng, Q.; Li, M. Time-restricted feeding is associated with changes in human gut microbiota related to nutrient intake. Nutrition 2020, 78, 110797. [Google Scholar] [CrossRef] [PubMed]
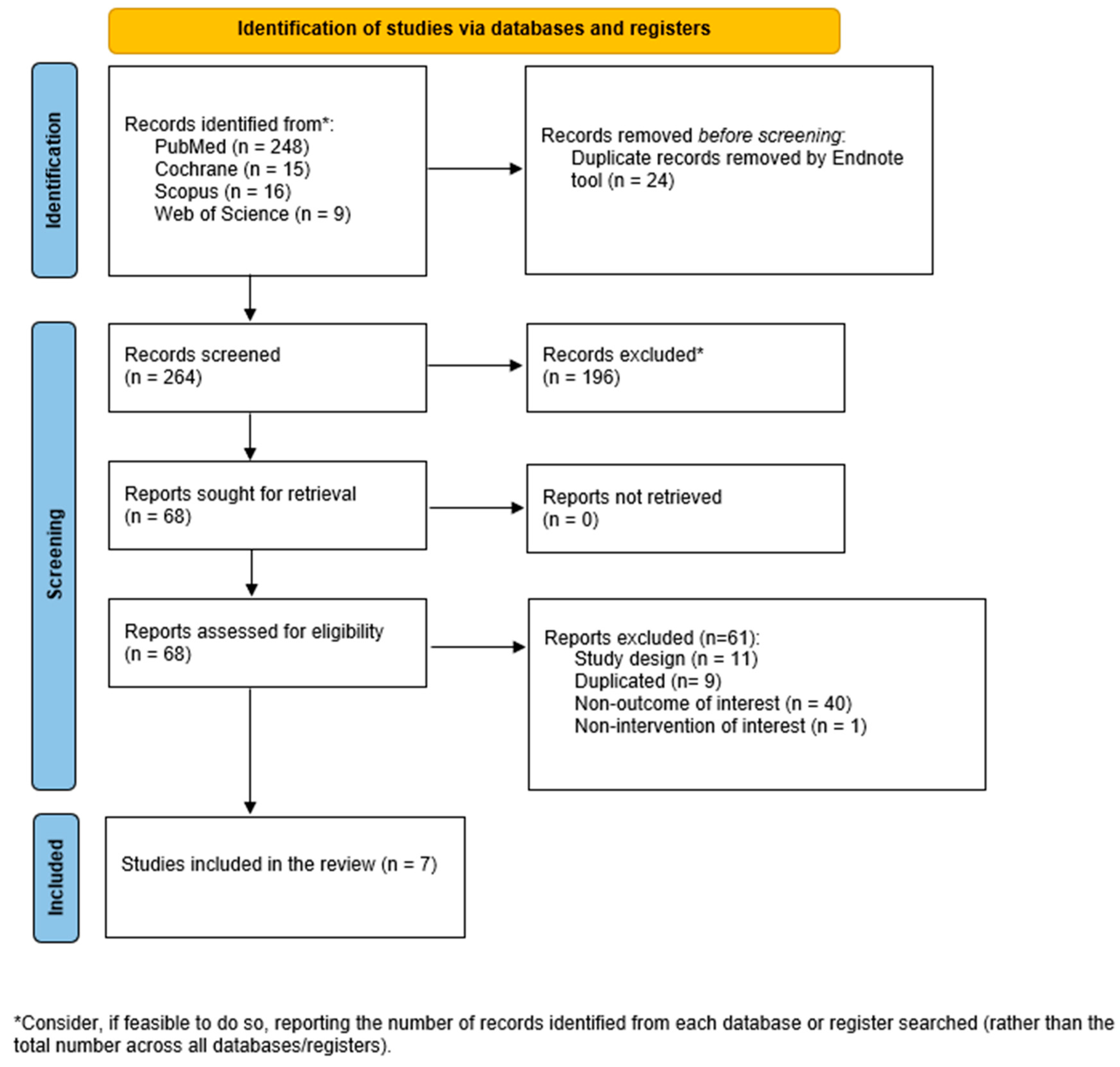
| Author Year Country | Sample Characteristics * | TRE Protocol | Length (Weeks) | Comparator | BMD/BMC Assessment | Outcomes | Results |
|---|---|---|---|---|---|---|---|
| Lowe et al., 2020 [47] USA | Overweight and obese adults aged 18–64 y N (% female):116 (40.7%) TRE: 59; CON: 57 Age (y): RE: 46.8 ± 10.8; CON: 46.1 ± 10.3 BMI(kg/m2): TRE = 32.9 ± 4.9/CON = 32.6 ± 3.4 | 16:8 (eating window from 12 pm to 8 pm) | 12 | non-TRE (CON) | DXA: Hologic Horizon/A system (Hologic Inc., Marlborough, MA, USA). | BMC total | Weight: Significant weight loss in TRE but not significant between-groups differences compared to CON. Bone: No significant increment on BMC. |
| Martens et al., 2020 [50] USA | Healthy non-obese midlife and older adults N (% female): 22 (54.5%) TRE:12; CON: 10 Age (y): TRE: 66.0 ± 2.0; CON: 68.0 ± 2.0 BMI (kg/m2): GA (Non-TRE, TRE) = 25.7 ± 0.7/GB (TRE, Non-TRE) = 23.9 ± 0.9 | 16:8 (self-selected, eating window from 10–11 am to 6–7 pm) | 6 | non-TRE (CON) | DXA: Lunar/Prodigy, GE Healthcare, Chicago IL, USA. | BMD (total and regional) | Weight: Body weight maintenance-throughout the TRE intervention (Non-TRE 69.3 ± 2.7 vs. TRF 69.4 ± 2.8 kg; p = 0.82). Bone: No change in BMD between-groups. Total and regional BMD were not different between conditions. |
| Kotarsky et al., 2021 [42] USA | Physically inactive and overweight or obese N (% female): 21 (85.7%) TRE: 11; CON: 10 Age (y): TRE: 45 ± 3; CON: 44 ± 2 BMI (kg/m2): TRE= 29.8 ± 0.8/CON = 29.4 ± 0.8 | 16:8 (eating window from 12 pm to 8 pm) | 8 | non-TRE (CON), both groups were performing concurrent training | DXA: on a Lunar Prodigy, Model #8915 (GE Healthcare). | BMD, BMC (total) | Weight: Losses of total body mass were significantly greater for TRE (3.3%) relative to NE (0.2%) pre-to post-intervention, of which TRE had significantly greater losses of fat mass (9.0%) compared to CON (3.3%). Lean mass increased during the intervention for both TRE (0.6%) and CON (1.9%), with no group differences. Bone: No significant change or differences between-groups. |
| Lobene et al., 2021 [44] USA | Overweight and obese adults aged 18–65 y N (% female): 20 (85%) TRE: 11; CON: 9 Age (y): TRE: 46.5 ± 3.7; CON: 44.2 ± 4.1 BMI (kg/m2): TRE: 33.8 ± 2.3; CON: 34.4 ± 2.6 | 16:8 (self-selected) | 12 | non-TRE (CON) | DXA: scans using the enCore software (GE Healthcare, Chicago, IL, USA, version 16.2). | BMD total and bone turnover (P1NP, NTX, PTH) | Weight: Body weight, fat mass, lean mass, and visceral fat were reduced in the TRE group compared to pre-intervention (−3.7% ± 0.5; −4.0% ± 0.9; −3.0 ± 0.8, and −11.1% ± 4.0, respectively), and changes in body weight, lean mass, and visceral fat were significant compared to the non-TRE group (all p < 0.05). Bone: No significant treatment effects on bone health outcomes |
| Liu et al., 2023 [43] China | Female college students with hidden obesity N (% female): 77 (100%); TRE: 19; CON: 19; EX: 20; TRE + EX: 19 Age (y): TRE: 20.3 ± 1.8; CON: 20.1 ± 1.8; EX: 20.1 ± 1.4; TRE + EX: 19.9 ± 0.6 BMI (kg/m2): TRE = 21.63 ± 1.24/CON = 20.32 ± 1.06 | 16:8 (eating window from 10 am to 6 pm) | 8 | Control, EX and TRE + EX | DXA: Hologic, Horizon, WI, USA. | BMD (total) | Weight: Significant weight loss, BMI, lean tissue mass on TRE. Bone: Total BMD (TRE, EX, and TRE + EX) and the CON group showed no significant differences (p > 0.05). |
| Richardson et al., 2023 [46] USA | Long-distance male runners N (% female): 15 (0%) Age (y): 28.7 ± 5.2 BMI (kg/m2): 23.3 (calculated from primary data on weight and height) | 16:8 (self-selected) | 4 | non-TRE (12 h eating window) 4 weeks intervention Wash-out: 2 to 4 weeks | DXA: Hologic Discovery QDR Series 94994; Hologic, Inc. | BMD total, BMD z-score | Weight: Significantly losses of fat mass, leg fat mass, and percent body fat in the TRE intervention, with no change in fat-free mass. Bone: No change. |
| Papageorgiou et al., 2023 [45] Switzerland | Adults with ≥1 component of metabolic syndrome N (% female): 42 (76%); TRE: 23; CON: 19 Age (y): TRE: 47 (range: 32–57); CON: 45 (range: 27–50) BMI (kg/m2): TRE = 28.51 ± 4.47/CON = 27.37 ± 5.18 | 12:12 (self-selected) | 24 | non-TRE (SDA) | DXA: GE Healthcare Lunar iDXA at Lausanne site, GE Healthcare Lunar Prodigy Advance at Bern site. | BMD/BMC (total), and bone turnover markers (P1NP, NTX, PTH, CTX, vit D, IGF-1) | Weight: Participants significantly lost weight after 6 months of TRE. Bone: No overall detrimental effects of 6 months of TRE on bone health outcomes. Those who lost weight following the CON intervention (SDA) experienced small, albeit non-significant, increases in CTX levels without parallel changes in P1NP levels and a small loss of total body BMC. Weight loss responders with TRE tended to have reduced bone resorption (CTX) whereas no change occurred in bone formation (P1NP). As opposed to the bone loss observed in weight loss responders with SDA, total body BMC/BMD remained unaltered in weight loss responders after TRE. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Rodríguez, R.; Garrido-Miguel, M.; Bizzozero-Peroni, B.; Díaz-Goñi, V.; Rodríguez-Gutiérrez, E.; Guzmán-Pavón, M.J.; Meseguer-Henarejos, A.B.; Torres-Costoso, A. Time-Restricted Eating and Bone Health: A Systematic Review with Meta-Analysis. Nutrients 2024, 16, 876. https://doi.org/10.3390/nu16060876
Fernández-Rodríguez R, Garrido-Miguel M, Bizzozero-Peroni B, Díaz-Goñi V, Rodríguez-Gutiérrez E, Guzmán-Pavón MJ, Meseguer-Henarejos AB, Torres-Costoso A. Time-Restricted Eating and Bone Health: A Systematic Review with Meta-Analysis. Nutrients. 2024; 16(6):876. https://doi.org/10.3390/nu16060876
Chicago/Turabian StyleFernández-Rodríguez, Rubén, Miriam Garrido-Miguel, Bruno Bizzozero-Peroni, Valentina Díaz-Goñi, Eva Rodríguez-Gutiérrez, María José Guzmán-Pavón, Ana Belén Meseguer-Henarejos, and Ana Torres-Costoso. 2024. "Time-Restricted Eating and Bone Health: A Systematic Review with Meta-Analysis" Nutrients 16, no. 6: 876. https://doi.org/10.3390/nu16060876
APA StyleFernández-Rodríguez, R., Garrido-Miguel, M., Bizzozero-Peroni, B., Díaz-Goñi, V., Rodríguez-Gutiérrez, E., Guzmán-Pavón, M. J., Meseguer-Henarejos, A. B., & Torres-Costoso, A. (2024). Time-Restricted Eating and Bone Health: A Systematic Review with Meta-Analysis. Nutrients, 16(6), 876. https://doi.org/10.3390/nu16060876