Impact of Melatonin Supplementation on Sports Performance and Circulating Biomarkers in Highly Trained Athletes: A Systematic Review of Randomized Controlled Trials
Abstract
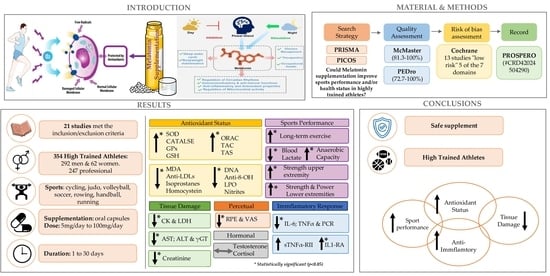
:1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Literature Search Strategy
2.3. Eligibility Criteria
2.4. Study Selection
2.5. Data Extraction
- Study: name of the first author, year of publication, country in which the study was conducted;
- Sample: The number of participants and the main characteristics related to their sports practice: trained, professional, or competitive athletes, initial sample size, age, sex, anthropometric and physical characteristics, withdrawals from the study, and final group sample size;
- Study design: types of randomized clinical trial;
- Intervention: description of each intervention, dose, pharmaceutical form, composition, timing and hour of supplementation, duration, and washout periods;
- Outcomes: all the outcomes assessed related to sports performance and/or health status (through clinical laboratory analytical biomarkers);
- Results: studies that specify the results where statistical differences were found between the experimental conditions (melatonin supplementation group and the placebo group).
2.6. Methodological Quality Assessment
2.7. Risk of Bias Assessment
3. Results
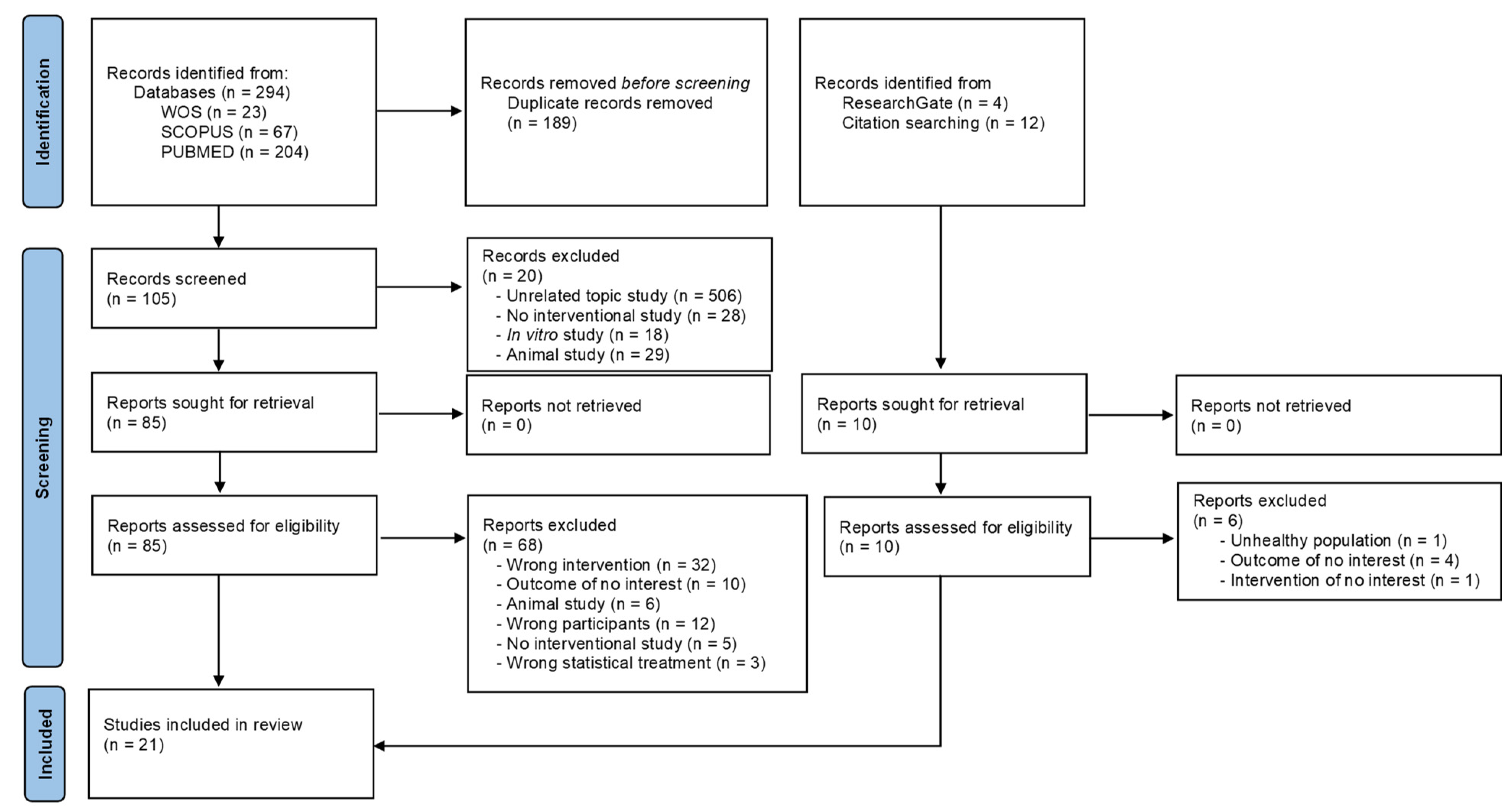
3.1. Literature Search and Study Selection
3.2. Assessment of Methodological Quality
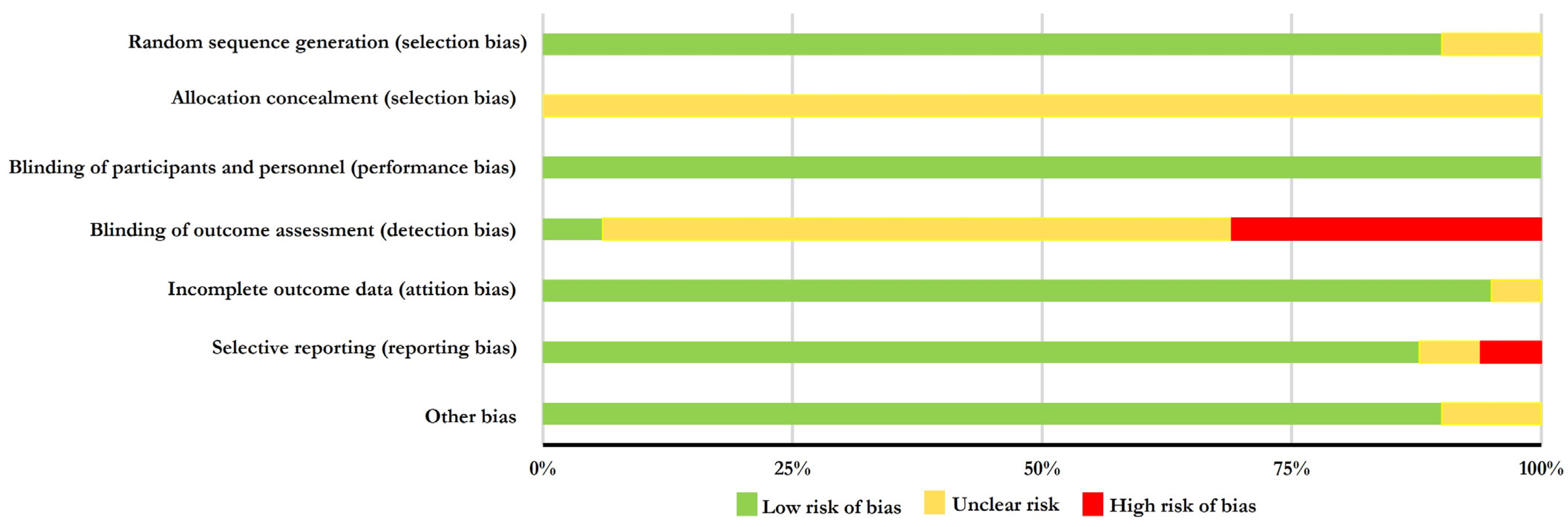
3.3. Risk of Bias Assessment
3.4. Characteristics of the Studies Included in the Systematic Review
3.5. Results Summary of the Studies Included in This Systematic Review Considering Health Circulating Biomarkers
3.5.1. Hematological Biomarkers
3.5.2. Biochemical Parameters
- Blood Glucose
- Lipid Metabolism
- Kidney Function
- Liver Function
3.5.3. Hormone Response
3.5.4. Inflammatory Response
3.5.5. Muscle Damage
3.5.6. Antioxidant Status
- Antioxidant Enzymes
- Oxidative Stress Biomarkers
- Antioxidant Status
- Glutathione Homeostasis
3.5.7. Perceptual and Cognitive Response
3.5.8. Physiological Parameters
3.6. Summarized Results of the Studies Included in the Systematic Review Based on Sports Performance
3.7. Summarized Results of the Studies Included in the Systematic Review Based on Melatonin Parameters
3.7.1. Melatonin Bioavailability
3.7.2. Adverse Effects
4. Discussion
4.1. Melatonin Supplementation
4.2. Antioxidant Status
4.3. Inflammatory Response
4.4. Tissue Damage
4.5. Immune System
4.6. Hormonal Response
4.7. Physical Performance
4.8. Limitations and Strengths
5. Practical Applications
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- do Amaral, F.G.; Cipolla-Neto, J. A brief review about melatonin, a pineal hormone. Arch. Endocrinol. Metab. 2018, 62, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.C.; Hardeland, R.; Lopez-Burillo, S.; Mayo, J.C.; Sainz, R.M.; Reiter, R.J. Melatonin: A hormone, a tissue factor, an autocoid, a paracoid, and an antioxidant vitamin. J. Pineal. Res. 2003, 34, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Acuña-Castroviejo, D.; Escames, G.; Venegas, C.; Díaz-Casado, M.E.; Lima-Cabello, E.; Lopez, L.C.; Rosales-Corral, S.; Tan, D.-X.; Reiter, R.J. Extrapineal melatonin: Sources, regulation, and potential functions. Cell. Mol. Life Sci. 2014, 71, 2997–3025. [Google Scholar] [CrossRef] [PubMed]
- Majidinia, M.; Reiter, R.J.; Shakouri, S.K.; Mohebbi, I.; Rastegar, M.; Kaviani, M.; Darband, S.G.; Jahanban-Esfahlan, R.; Nabavi, S.M.; Yousefi, B. The multiple functions of melatonin in regenerative medicine. Ageing Res. Rev. 2018, 45, 33–52. [Google Scholar] [CrossRef] [PubMed]
- Pivonello, C.; Negri, M.; Patalano, R.; Amatrudo, F.; Montò, T.; Liccardi, A.; Graziadio, C.; Muscogiuri, G.; Pivonello, R.; Colao, A. The role of melatonin in the molecular mechanisms underlying metaflammation and infections in obesity: A narrative review. Obes. Rev. 2022, 23, e13390. [Google Scholar] [CrossRef] [PubMed]
- Holloszy, J.O.; Coyle, E.F.; Gifford, J.R.; Trinity, J.D.; Kwon, O.S.; Layec, G.; Garten, R.S.; Park, S.Y.; Nelson, A.D.; Richardson, R.S.; et al. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1984, 56, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Zunner, B.E.M.; Wachsmuth, N.B.; Eckstein, M.L.; Scherl, L.; Schierbauer, J.R.; Haupt, S.; Stumpf, C.; Reusch, L.; Moser, O. Myokines and Resistance Training: A Narrative Review. Int. J. Mol. Sci. 2022, 23, 3501. [Google Scholar] [CrossRef]
- Fernández-Lázaro, D.; Mielgo-Ayuso, J.; del Valle Soto, M.; Adams, D.P.; Gutiérrez-Abejón, E.; Seco-Calvo, J. Impact of Optimal Timing of Intake of Multi-Ingredient Performance Supplements on Sports Performance, Muscular Damage, and Hormonal Behavior across a Ten-Week Training Camp in Elite Cyclists: A Randomized Clinical Trial. Nutrients 2021, 13, 3746. [Google Scholar] [CrossRef]
- Gabriel, B.M.; Zierath, J.R. The Limits of Exercise Physiology: From Performance to Health. Cell Metab. 2017, 25, 1000–1011. [Google Scholar] [CrossRef]
- Fernández-Lázaro, D.; Seco-Calvo, J. Nutrition, Nutritional Status and Functionality. Nutrients 2023, 15, 1944. [Google Scholar] [CrossRef]
- Powers, S.K.; Jackson, M.J. Exercise-Induced Oxidative Stress: Cellular Mechanisms and Impact on Muscle Force Production. Physiol. Rev. 2008, 88, 1243–1276. [Google Scholar] [CrossRef] [PubMed]
- Stožer, A.; Vodopivc, P.; Bombek, L.K. Pathophysiology of exercise-induced muscle damage and its structural, functional, metabolic, and clinical consequences. Physiol. Res. 2020, 69, 565–598. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Lázaro, D.; Sánchez-Serrano, N.; Rabail, R.; Aadil, R.M.; Mielgo-Ayuso, J.; Radesca Fabiano, K.; Garrosa, E. Is Probiotics Supplementation an Appropriate Strategy to Modulate Inflammation in Physically Active Healthy Adults or Athletes? A Systematic Review. Appl. Sci. 2023, 13, 3448. [Google Scholar] [CrossRef]
- Fernández-Lázaro, D.; Fernandez-Lazaro, C.I.; Mielgo-Ayuso, J.; Adams, D.P.; García Hernández, J.L.; González-Bernal, J.; González-Gross, M. Glycophosphopeptical AM3 Food Supplement: A Potential Adjuvant in the Treatment and Vaccination of SARS-CoV-2. Front. Immunol. 2021, 12, 6988672. [Google Scholar] [CrossRef] [PubMed]
- Mata-Ordoñez, F.; Bastos, P.C.; Domínguez, R.; Sánchez-Oliver, A.J. Importance of sleep in the performance and health of the athlete. E-Motion 2018, 1, 70–82. [Google Scholar] [CrossRef]
- Mochón-Benguigui, S.; Carneiro-Barrera, A.; Dote-Montero, M.; Castillo, M.J.; Amaro-Gahete, F.J. Sleep and Anabolic/Catabolic Hormonal Profile in Sedentary Middle-Aged Adults: The FIT-AGEING Study. Int. J. Mol. Sci. 2022, 23, 14709. [Google Scholar] [CrossRef] [PubMed]
- Morrison, M.; Halson, S.L.; Weakley, J.; Hawley, J.A. Sleep, circadian biology and skeletal muscle interactions: Implications for metabolic health. Sleep Med. Rev. 2022, 66, 101700. [Google Scholar] [CrossRef] [PubMed]
- Reis, V.M. Effects of Exercise on Biomarkers in Health and Disease: Some New Insights with Special Focus on Extreme Exercise and Healthy Ageing. Int. J. Environ. Res. Public Health 2020, 17, 1986. [Google Scholar] [CrossRef] [PubMed]
- Rojano-Ortega, D.; Peña Amaro, J.; Berral-Aguilar, A.J.; Berral-de la Rosa, F.J. Effects of Beetroot Supplementation on Recovery After Exercise-Induced Muscle Damage: A Systematic Review. Sports Health 2022, 14, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Lázaro, D.; Mielgo-Ayuso, J.; Calvo, J.S.; Martínez, A.C.; García, A.C.; Fernandez-Lazaro, C.I. Modulation of Exercise-Induced Muscle Damage, Inflammation, and Oxidative Markers by Curcumin Supplementation in a Physically Active Population: A Systematic Review. Nutrients 2020, 12, 501. [Google Scholar] [CrossRef]
- Fernández-Lázaro, D.; Domínguez-Ortega, C.; Busto, N.; Santamaría-Peláez, M.; Roche, E.; Gutiérez-Abejón, E.; Mielgo-Ayuso, J. Influence of N-Acetylcysteine Supplementation on Physical Performance and Laboratory Biomarkers in Adult Males: A Systematic Review of Controlled Trials. Nutrients 2023, 15, 2463. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Lázaro, D.; Fernandez-Lazaro, C.I.; Mielgo-Ayuso, J.; Navascués, L.J.; Martínez, A.C.; Seco-Calvo, J. The Role of Selenium Mineral Trace Element in Exercise: Antioxidant Defense System, Muscle Performance, Hormone Response, and Athletic Performance. A Systematic Review. Nutrients 2020, 12, 1790. [Google Scholar] [CrossRef] [PubMed]
- Cipolla-Neto, J.; Do Amaral, F.G. Melatonin as a Hormone: New Physiological and Clinical Insights. Endocr. Rev. 2018, 39, 990–1028. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, É.; Marinho, D.A.; Neiva, H.P.; Lourenço, O. Inflammatory Effects of High and Moderate Intensity Exercise—A Systematic Review. Front. Physiol. 2020, 10, 1550. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Aging, Melatonin, and the Pro- and Anti-Inflammatory Networks. Int. J. Mol. Sci. 2019, 20, 1223. [Google Scholar] [CrossRef] [PubMed]
- Carrascal, L.; Nunez-Abades, P.; Ayala, A.; Cano, M. Role of Melatonin in the Inflammatory Process and its Therapeutic Potential. Curr. Pharm. Des. 2018, 24, 1563–1588. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.-X.; Manchester, L.C.; Esteban-Zubero, E.; Zhou, Z.; Reiter, R.J. Melatonin as a Potent and Inducible Endogenous Antioxidant: Synthesis and Metabolism. Molecules 2015, 20, 18886–18906. [Google Scholar] [CrossRef] [PubMed]
- Tordjman, S.; Chokron, S.; Delorme, R.; Charrier, A.; Bellissant, E.; Jaafari, N.; Fougerou, C. Melatonin: Pharmacology, Functions and Therapeutic Benefits. Curr. Neuropharmacol. 2017, 15, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Chitimus, D.M.; Popescu, M.R.; Voiculescu, S.E.; Panaitescu, A.M.; Pavel, B.; Zagrean, L.; Zagrean, A.-M. Melatonin’s Impact on Antioxidative and Anti-Inflammatory Reprogramming in Homeostasis and Disease. Biomolecules 2020, 10, 1211. [Google Scholar] [CrossRef]
- Tan, D.-X.; Manchester, L.C.; Reiter, R.J.; Qi, W.-B.; Karbownik, M.; Calvo, J.R. Significance of Melatonin in Antioxidative Defense System: Reactions and Products. Neurosignals 2000, 9, 137–159. [Google Scholar] [CrossRef]
- Faria, V.S.; Messias, L.H.D.; Pejon, T.M.M.; Beck, W.R. Influence of Acute Melatonin Administration on Human Physical Performance: A Systematic Review. Sports Health 2024, 16, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.; Brunnhuber, K.; Chalkidou, K.; Chalmers, I.; Clarke, M.; Fenton, M.; Forbes, C.; Glanville, J.; Hicks, N.J.; Moody, J.; et al. How to formulate research recommendations. BMJ 2006, 333, 804–806. [Google Scholar] [CrossRef] [PubMed]
- Moseley, A.M.; Elkins, M.R.; Van der Wees, P.J.; Pinheiro, M.B. Using research to guide practice: The Physiotherapy Evidence Database (PEDro). Braz. J. Phys. Ther. 2020, 24, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Law, M.; Stewart, D.; Letts, L.; Pollock, N.; Bosch, J.W.M. Guidelines for Critical Review of Qualitative Studies; McMaster University Occupational Therapy Evidence-Based Practice Research Group: Hamilton, ON, Canada, 1998; pp. 1–9. [Google Scholar]
- Higgins, J.P.T.; Eldridge, S.; Li, T. Cochrane Handbook for Systematic Reviews of Interventions Version 6.2; updated February 2021. Cochran: Pittsburg, PA, USA, 2021. Available online: https://training.cochrane.org/handbook (accessed on 26 February 2024).
- Sterne, J.A.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: Arevised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Beck, W.R.; Messias, L.H.D.; da Silva, F.C.; Manchado-Gobatto, F.B.; Gobatto, C.A. Acute melatonin administration enhances aerobic tolerance: An analysis of biochemical and hematological parameters. Mot. Rev. Educ. Física 2018, 24, e1018169. [Google Scholar] [CrossRef]
- Brandenberger, K.J.; Ingalls, C.P.; Rupp, J.C.; Doyle, J.A. Consumption of a 5-mg Melatonin Supplement Does Not Affect 32.2-km Cycling Time Trial Performance. J. Strength Cond. Res. 2018, 32, 2872–2877. [Google Scholar] [CrossRef] [PubMed]
- Cheikh, M.; Hammouda, O.; Gaamouri, N.; Driss, T.; Chamari, K.; Ben Cheikh, R.; Dogui, M.; Souissi, N. Melatonin ingestion after exhaustive late-evening exercise improves sleep quality and quantity, and short-term performances in teenage athletes. Chronobiol. Int. 2018, 35, 1281–1293. [Google Scholar] [CrossRef] [PubMed]
- Cheikh, M.; Makhlouf, K.; Ghattassi, K.; Graja, A.; Ferchichi, S.; Kallel, C.; Houda, M.; Souissi, N.; Hammouda, O. Melatonin ingestion after exhaustive late-evening exercise attenuate muscle damage, oxidative stress, and inflammation during intense short term effort in the following day in teenage athletes. Chronobiol. Int. 2020, 37, 236–247. [Google Scholar] [CrossRef]
- Czuczejko, J.; Sielski, Ł.; Woźniak, B.; Woźniak, A.; Szewczyk-Golec, K. Melatonin supplementation improves oxidative and inflammatory state in the blood of professional athletes during the preparatory period for competitions. Free Radic. Res. 2019, 53, 198–209. [Google Scholar] [CrossRef]
- Farjallah, M.; Hammouda, O.; Zouch, M.; Ghattassi, K.; Graja, A.; Driss, T.; Chamari, K.; Souissi, N. Effect of melatonin ingestion on physical performance, metabolic responses, and recovery after an intermittent training session. Physiol. Int. 2018, 105, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Farjallah, M.A.; Ghattassi, K.; Kamoun, A.; Graja, A.; Ben Mahmoud, L.; Driss, T.; Jamoussi, K.; Sahnoun, Z.; Souissi, N.; Zmijewski, P.; et al. Melatonin supplementation alleviates cellular damage and physical performance decline induced by an intensive training period in professional soccer players. PLoS ONE 2022, 17, e0273719. [Google Scholar] [CrossRef] [PubMed]
- Farjallah, M.A.; Graja, A.; Ghattassi, K.; Ben Mahmoud, L.; Elleuch, H.; Ayadi, F.; Driss, T.; Jammoussi, K.; Sahnoun, Z.; Souissi, N.; et al. Melatonin Ingestion Prevents Liver Damage and Improves Biomarkers of Renal Function Following a Maximal Exercise. Res. Q. Exerc. Sport 2023, 94, 869–879. [Google Scholar] [CrossRef] [PubMed]
- Farjallah, M.; Graja, A.; Mahmoud, L.; Ghattassi, K.; Boudaya, M.; Driss, T.; Jamoussi, K.; Sahnoun, Z.; Souissi, N.; Hammouda, O. Effects of melatonin ingestion on physical performance and biochemical responses following exhaustive running exercise in soccer players. Biol. Sport 2022, 39, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Farjallah, M.A.; Hammouda, O.; Ben Mahmoud, L.; Graja, A.; Ghattassi, K.; Boudaya, M.; Jammoussi, K.; Sahnoun, Z.; Souissi, N. Melatonin supplementation ameliorates oxidative stress, antioxidant status and physical performances recovery during a soccer training camp. Biol. Rhythm. Res. 2020, 51, 441–452. [Google Scholar] [CrossRef]
- Ghattassi, K.; Hammouda, O.; Graja, A.; Boudhina, N.; Chtourou, H.; Hadhri, S.; Driss, T.; Souissi, N. Morning melatonin ingestion and diurnal variation of short-term maximal performances in soccer players. Physiol. Int. 2016, 103, 94–104. [Google Scholar] [CrossRef]
- Ghattassi, K.; Farjallah, M.A.; Graja, A.; Romdhani, M.; Boudhina, N.; Guimard, A.; Driss, T.; Souissi, N.; Chtourou, H.; Hammouda, O. Nocturnal Melatonin Ingestion Improves Soccer Players’ Short-Term Maximal Performances on the Following Day. Res. Q. Exerc. Sport, 2024; 1–8, Online ahead of print. [Google Scholar] [CrossRef]
- Ghattassi, K.; Graja, A.; Hammouda, O.; Chtourou, H.; Boudhina, N.; Chaouachi, A.; Souissi, N. Effect of nocturnal melatonin ingestion on short-term anaerobic performance in soccer players. Biol. Rhythm. Res. 2014, 45, 885–893. [Google Scholar] [CrossRef]
- Khaleghi-Mamaghani, E.; Rahmani-Nia, F.; Arazi, H. Evaluation of the Effects of Melatonin Supplementation on the Physical and Physiological Performance Following Total Night Sleep Deprivation in Trained Young Males. J. Turk. Sleep Med. 2021, 8, 151–158. [Google Scholar] [CrossRef]
- Leonardo-Mendonça, R.C.; Ocaña-Wilhelmi, J.; de Haro, T.; de Teresa-Galván, C.; Guerra-Hernández, E.; Rusanova, I.; Fernández-Ortiz, M.; Sayed, R.K.; Escames, G.; Acuña-Castroviejo, D. The benefit of a supplement with the antioxidant melatonin on redox status and muscle damage in resistance-trained athletes. Appl. Physiol. Nutr. Metab. 2017, 42, 700–707. [Google Scholar] [CrossRef]
- Maldonado; Manfredi, M.; Ribas-Serna, J.; Garcia-Moreno, H.; Calvo, J. Melatonin administrated immediately before an intense exercise reverses oxidative stress, improves immunological defenses and lipid metabolism in football players. Physiol. Behav. 2012, 105, 1099–1103. [Google Scholar] [CrossRef]
- Mero, A.A.; Vähälummukka, M.; Hulmi, J.J.; Kallio, P.; von Wright, A. Effects of resistance exercise session after oral ingestion of melatonin on physiological and performance responses of adult men. Eur. J. Appl. Physiol. 2006, 96, 729–739. [Google Scholar] [CrossRef]
- Ochoa, J.J.; Díaz-Castro, J.; Kajarabille, N.; García, C.; Guisado, I.M.; De Teresa, C.; Guisado, R. Melatonin supplementation ameliorates oxidative stress and inflammatory signaling induced by strenuous exercise in adult human males. J. Pineal Res. 2011, 51, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Franco, M.; Planells, E.; Quintero, B.; Acuña-Castroviejo, D.; Rusanova, I.; Escames, G.; Molina-López, J. Effect of Melatonin Supplementation on Antioxidant Status and DNA Damage in High Intensity Trained Athletes. Int. J. Sports Med. 2017, 38, 1117–1125. [Google Scholar] [CrossRef]
- Paryab, N.; Taheri, M.; H’mida, C.; Irandoust, K.; Mirmoezzi, M.; Trabelsi, K.; Ammar, A.; Chtourou, H. Melatonin supplementation improves psychomotor and physical performance in collegiate student-athletes following a sleep deprivation night. Chronobiol. Int. 2021, 38, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Souissi, A.; Souissi, N.; Dabboubi, R.; Souissi, N. Effect of melatonin on inflammatory response to prolonged exercise. Biol. Rhythm Res. 2020, 51, 560–565. [Google Scholar] [CrossRef]
- Seabra, M.D.L.V.; Bignotto, M.; Pinto, L.R., Jr.; Tufik, S. Randomized, double-blind clinical trial, controlled with placebo, of the toxicology of chronic melatonin treatment. J. Pineal Res. 2000, 29, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Poza, J.J.; Pujol, M.; Ortega-Albás, J.J.; Romero, O. Melatonin in sleep disorders. Neurología 2022, 37, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Jafari-Koulaee, A.; Bagheri-Nesami, M. The effect of melatonin on sleep quality and insomnia in patients with cancer: A systematic review study. Sleep Med. 2021, 82, 96–103. [Google Scholar] [CrossRef]
- Mantle, D.; Smits, M.; Boss, M.; Miedema, I.; van Geijlswijk, I. Efficacy and safety of supplemental melatonin for delayed sleep–wake phase disorder in children: An overview. Sleep Med. X 2020, 2, 100022. [Google Scholar] [CrossRef]
- Lissoni, P.; Barni, S.; Tancini, G.; Crispino, S.; Paolorossi, F.; Lucini, V.; Mariani, M.; Cattaneo, G.; Esposti, D.; Esposti, G.; et al. Clinical Study of Melatonin in Untreatable Advanced Cancer Patients. Tumori J. 1987, 73, 475–480. [Google Scholar] [CrossRef]
- Lissoni, P. Is there a role for melatonin in supportive care? Support. Care Cancer 2002, 10, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Lissoni, P.; Barni, S.; Mandalà, M.; Ardizzoia, A.; Paolorossi, F.; Vaghi, M.; Longarini, R.; Malugani, F.; Tancini, G. Decreased toxicity and increased efficacy of cancer chemotherapy using the pineal hormone melatonin in metastatic solid tumour patients with poor clinical status. Eur. J. Cancer 1999, 35, 1688–1692. [Google Scholar] [CrossRef] [PubMed]
- Lissoni, P.; Chilelli, M.; Villa, S.; Cerizza, L.; Tancini, G. Five years survival in metastatic non-small cell lung cancer patients treated with chemotherapy alone or chemotherapy and melatonin: A randomized trial. J. Pineal Res. 2003, 35, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Moroni, I.; Garcia-Bennett, A.; Chapman, J.; Grunstein, R.R.; Gordon, C.J.; Comas, M. Pharmacokinetics of exogenous melatonin in relation to formulation, and effects on sleep: A systematic review. Sleep Med. Rev. 2021, 57, 101431. [Google Scholar] [CrossRef] [PubMed]
- Kopustinskiene, D.M.; Bernatoniene, J. Molecular Mechanisms of Melatonin-Mediated Cell Protection and Signaling in Health and Disease. Pharmaceutics 2021, 13, 129. [Google Scholar] [CrossRef]
- European Food Safety Authority. Scientific Opinion on the Supplement 1. European Food Safety Authority. Scientific Opinion on the Substantiation of a Health Claim Related to Melatonin and Reduction of Sleep Onset Latency (ID 1698, 1780, 4080) Pursuant to Article 13(1) of Regulation (EC) No 1924/. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/2241 (accessed on 26 February 2024).
- Almendros-Ruiz, A.; Lopez-Moro, A.; Conde-Pipò, J.; Santalla, A.; Requena, B.; Mariscal-Arcas, M. The Effects of Melatonin Supplementation on Professional Football Player Performance: A Systematic Review. Nutrients 2023, 15, 4467. [Google Scholar] [CrossRef] [PubMed]
- Anisimov, V. Effects of Exogenous Melatonin—A Review. Toxicol. Pathol. 2003, 31, 589–603. [Google Scholar] [CrossRef]
- Peuhkuri, K.; Sihvola, N.; Korpela, R. Dietary factors and fluctuating levels of melatonin. Food Nutr. Res. 2012, 56, 17252. [Google Scholar] [CrossRef]
- Meng, X.; Li, Y.; Li, S.; Zhou, Y.; Gan, R.-Y.; Xu, D.-P.; Li, H.-B. Dietary Sources and Bioactivities of Melatonin. Nutrients 2017, 9, 367. [Google Scholar] [CrossRef]
- World Anti-Doping Agency (W.A.D.A.). Prohibited List. Available online: https://www.wada-ama.org/sites/default/files/resources/files/2022list_final_en.pdf (accessed on 3 February 2024).
- Afonso, A.G.; Fernandez-Lazaro, D.; Adams, D.P.; Monserdà-Vilaró, A.; Fernandez-Lazaro, C.I. Effects of Withania somnifera (Ashwagandha) on Hematological and Biochemical Markers, Hormonal Behavior, and Oxidant Response in Healthy Adults: A Systematic Review. Curr. Nutr. Rep. 2023, 12, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Concepcion-Huertas, M.; Chirosa, L.J.; De Haro, T.; Chirosa, I.J.; Romero, V.; Aguilar-Martinez, D.; Leonardo-Mendonça, R.C.; Doerrier, C.; Escames, G.; Acuña-Castroviejo, D. Changes in the redox status and inflammatory response in handball players during one-year of competition and training. J. Sports Sci. 2013, 31, 1197–1207. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.B.; Ali, A.; Bilal, M.; Rashid, S.M.; Wani, A.B.; Bhat, R.R.; Rehman, M.U. Melatonin and Health: Insights of Melatonin Action, Biological Functions, and Associated Disorders. Cell Mol. Neurobiol. 2023, 43, 2437–2458. [Google Scholar] [CrossRef]
- Kruidenier, L.; Kuiper, I.; Lamers, C.B.H.W.; Verspaget, H.W. Intestinal oxidative damage in inflammatory bowel disease: Semi-quantification, localization, and association with mucosal antioxidants. J. Pathol. 2003, 201, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Lou, A.; Wang, L.; Lai, W.; Zhu, D.; Wu, W.; Wang, Z.; Cai, Z.; Yang, M. Advanced oxidation protein products induce inflammatory responses and invasive behaviour in fibroblast-like synoviocytes via the RAGE-NF-κB pathway. Bone Jt. Res. 2021, 10, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Sofic, E.; Rimpapa, Z.; Kundurovic, Z.; Sapcanin, A.; Tahirovic, I.; Rustembegovic, A.; Cao, G. Antioxidant capacity of the neurohormone melatonin. J. Neural Transm. 2005, 112, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Pieri, C.; Marra, M.; Moroni, F.; Recchioni, R.; Marcheselli, F. Melatonin: A peroxyl radical scavenger more effective than vitamin E. Life Sci. 1994, 55, PL271–PL276. [Google Scholar] [CrossRef]
- Fernández-Lázaro, D. Ergogenic Strategies for Optimizing Performance and Health in Regular Physical Activity Participants: Evaluation of the Efficacy of Compressive Cryotherapy, Exposure to Intermittent Hypoxia at Rest and Sectoralized Lung Training. Ph.D. Thesis, University of León, León, Spain, 2020. Available online: https://dialnet.unirioja.es/servlet/dctes?codigo=286163 (accessed on 16 February 2024).
- Fernández-Lázaro, D.; González-Bernal, J.J.; Sánchez-Serrano, N.; Navascués, L.J.; Del Río, A.A.; Mielgo-Ayuso, J. Physical exercise as a multimodal tool for COVID-19: Could it be used as a preventive strategy? Int. J. Environ. Res. Public Health 2020, 17, 8496. [Google Scholar] [CrossRef]
- Favero, G.; Franceschetti, L.; Bonomini, F.; Rodella, L.F.; Rezzani, R. Melatonin as an Anti-Inflammatory Agent Modulating Inflammasome Activation. Int. J. Endocrinol. 2017, 2017, 1835195. [Google Scholar] [CrossRef]
- Markus, R.P.; Cecon, E.; Pires-Lapa, M.A. Immune-Pineal Axis: Nuclear Factor κB (NF-κB) Mediates the Shift in the Melatonin Source from Pinealocytes to Immune Competent Cells. Int. J. Mol. Sci. 2013, 14, 10979–10997. [Google Scholar] [CrossRef]
- Tan, P.H.; Sagoo, P.; Chan, C.; Yates, J.B.; Campbell, J.; Beutelspacher, S.C.; Foxwell, B.M.; Lombardi, G.; George, A.J. Inhibition of NF-kappa B and oxidative pathways in human dendritic cells by antioxidative vitamins generates regulatory T cells. J. Immunol. 2005, 174, 7633–7644. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Lázaro, D.; Seco-Calvo, J.; Pascual-Fernández, J.; Domínguez-Ortega, C.; Del Valle Soto, M.; Mielgo-Ayuso, J. 6-Week Supplementation with Tribulus terrestris L. to Trained Male CrossFit® Athletes on Muscle, Inflammation, and Antioxidant Biomarkers: A Randomized, Single-Blind, Placebo-Controlled Trial. Int. J. Environ. Res. Public Health 2022, 19, 16158. [Google Scholar] [CrossRef]
- Yao, C.; Liu, X.; Zhou, Z.; Xiang, Y.; Yuan, S.; Xie, W.; Zhou, M.; Hu, Z.; Li, Y.; Ji, A.; et al. Melatonin attenuates expression of cyclooxygenase-2 (COX-2) in activated microglia induced by lipopolysaccharide (LPS). J. Toxicol. Environ. Health Part A 2019, 82, 437–446. [Google Scholar] [CrossRef]
- Mortezaee, K.; Potes, Y.; Mirtavoos-Mahyari, H.; Motevaseli, E.; Shabeeb, D.; Musa, A.E.; Najafi, M.; Farhood, B. Boosting immune system against cancer by melatonin: A mechanistic viewpoint. Life Sci. 2019, 238, 116960. [Google Scholar] [CrossRef]
- Vinther, A.G.; Clässeon, M.H. The influence of melatonin on immune system and cancer. Ugeskr. Laeger 2015, 177, V10140568. [Google Scholar] [CrossRef] [PubMed]
- Talib, W.H. Melatonin and Cancer Hallmarks. Molecules 2018, 23, 518. [Google Scholar] [CrossRef]
- Fernández-Lázaro, D.; Santamaría, G.; Sánchez-Serrano, N.; Caeiro, E.L.; Seco-Calvo, J. Efficacy of Therapeutic Exercise in Reversing Decreased Strength, Impaired Respiratory Function, Decreased Physical Fitness, and Decreased Quality of Life Caused by the Post-COVID-19 Syndrome. Viruses 2022, 14, 2797. [Google Scholar] [CrossRef] [PubMed]
- Cicchella, A.; Stefanelli, C.; Massaro, M. Upper Respiratory Tract Infections in Sport and the Immune System Response. A Review. Biology 2021, 10, 362. [Google Scholar] [CrossRef]
- Simpson, R.J.; Kunz, H.; Agha, N.; Graff, R. Exercise and the regulation of immune functions. Prog. Mol. Biol. Transl. Sci. 2015, 135, 355–380. [Google Scholar]
- Mubashshir, M.; Ahmad, N.; Negi, T.; Rawal, R.; Singhvi, N.; Khatoon, H.; Laxmi, V.; Dubey, O.; Sharma, R.B.; Negi, G.; et al. Therapeutic Benefits of Melatonin against COVID-19. Neuroimmunomodulation 2023, 30, 196–205. [Google Scholar] [CrossRef]
- Fernández-Lázaro, D.; Mielgo-Ayuso, J.; Del Valle Soto, M.; Adams, D.P.; González-Bernal, J.J.; Seco-Calvo, J. The Effects of 6 Weeks of Tribulus terrestris L. Supplementation on Body Composition, Hormonal Response, Perceived Exertion, and CrossFit® Performance: A Randomized, Single-Blind, Placebo-Controlled Study. Nutrients 2021, 7, 3969. [Google Scholar] [CrossRef] [PubMed]
- Minich, D.M.; Henning, M.; Darley, C.; Fahoum, M.; Schuler, C.B.; Frame, J. Is Melatonin the “Next Vitamin D”?: A Review of Emerging Science, Clinical Uses, Safety, and Dietary Supplements. Nutrients 2022, 14, 3934. [Google Scholar] [CrossRef] [PubMed]
| Criteria | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Population “P” | The sample must be composed of men and/or women trained athletes. | Participants receiving any type of medication or treatment; unhealthy individuals with gastrointestinal problems, with inflammatory and/or immunological pathologies, with musculoskeletal pathology. In general, individuals with chronic diseases. |
| Individuals within the sample must be explicitly referred to as “trained athletes” in the manuscript or meet the criteria to be classified as “physically active” for exceeding the exercise recommendations of the American College of Sports Medicine (ACSM). | ||
| Intervention “I” | Include a supplementation intervention that should involve the use of melatonin in monotherapy, with clear information on dosage and duration of melatonin supplementation. | Administration together with other nutritional supplements. |
| Comparison “C” | Include either a placebo or control group (parallel group studies design) or experimental condition (cross-over studies design). | With other doses of melatonin, or with other nutritional supplements. |
| Outcomes “O” | Any parameter related to sports performance, and/or biomarkers related to health status, that reports on the bioavailability and adverse effects of melatonin. | None. |
| Study design “S” | Human intervention studies, randomized controlled trials, and/or randomized controlled crossover trials. | Observational studies and studies that used a targeted analytical approach. |
| Study, Year | Item | Total | % | Quality Score | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | ||||
| Beck et al., 2018 [38] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 14 | 87.5 | VG |
| Brandeberger et al., 2018 [39] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 14 | 87.5 | VG |
| Cheikh et al., 2018 [40] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 15 | 93.8 | E |
| Cheikh et al., 2020 [41] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 15 | 93.8 | E |
| Czucejko et al., 2019 [42] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 14 | 87.5 | VG |
| Farjallah et al., 2018 [43] | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 14 | 87.5 | VG |
| Farjallah et al., 2022 [44] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 16 | 100 | E |
| Farjallah et al., 2023 [45] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 15 | 93.8 | E |
| Farjallah et al., 2022 [46] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 16 | 100 | E |
| Farjallah et al., 2020 [47] | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 14 | 87.5 | VG |
| Ghatassi et al., 2016 [48] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 15 | 93.8 | E |
| Ghatassi et al., 2024 [49] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 15 | 93.8 | E |
| Ghatassi et al., 2014 [50] | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 13 | 81.3 | VG |
| Khaleghi-Mamaghani et al., 2021 [51] | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 13 | 81.3 | VG |
| Leonardo-Mendonça et al., 2017 [52] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 14 | 87.5 | VG |
| Maldonado et al., 2012 [53] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 14 | 87.5 | VG |
| Mero et al., 2006 [54] | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 13 | 81.3 | VG |
| Ochoa et al., 2011 [55] | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 13 | 81.3 | VG |
| Ortiz-Franco et al. 2017, [56] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 14 | 87.5 | VG |
| Paryab et al., 2021 [57] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 15 | 93.8 | VG |
| Souissi et al., 2018 [58] | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 14 | 87.5 | VG |
| Study, Year | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Total | % | Quality Score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beck et al., 2018 [38] | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 9 | 81.8 | G |
| Brandeberger et al., 2018 [39] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 8 | 72.7 | G |
| Cheikh et al., 2018 [40] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 10 | 90.9 | E |
| Cheikh et al., 2020 [41] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 10 | 90.9 | E |
| Czucejko et al., 2019 [42] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 10 | 90.9 | E |
| Farjallah et al., 2018 [43] | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 9 | 81.8 | G |
| Farjallah et al., 2022 [44] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 10 | 90.9 | E |
| Farjallah et al., 2023 [45] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 10 | 90.9 | E |
| Farjallah et al., 2022 [46] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 11 | 100 | E |
| Farjallah et al., 2020 [47] | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 9 | 81.8 | G |
| Ghatassi et al., 2016 [48] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 10 | 90.9 | E |
| Ghatassi et al., 2024 [49] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 10 | 90.9 | E |
| Ghatassi et al., 2014 [50] | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 9 | 81.8 | G |
| Khaleghi-Mamaghani et al., 2021 [51] | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 9 | 81.8 | G |
| Leonardo-Mendonça et al., 2017 [52] | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 9 | 81.8 | G |
| Maldonado et al., 2012 [53] | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 9 | 81.8 | G |
| Mero et al., 2006 [54] | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 9 | 81.8 | G |
| Ochoa et al., 2011 [55] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 10 | 90.9 | E |
| Ortiz-Franco et al., 2017 [56] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 10 | 90.9 | E |
| Paryab et al., 2021 [57] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 10 | 90.9 | E |
| Souissi et al., 2018 [58] | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 9 | 81.8 | G |
| Study | Random Sequence Generation (Selection Bias) | Allocation Concealment (Selection Bias) | Blinding of Participants and Personnel (Performance Bias) | Blinding of Outcome Assessment (Detection Bias) | Incomplete Outcome Data (Attrition Bias) | Selective Reporting (Reporting Bias) | Other Bias | Overall Risk Rating | Sample Size Was Justified |
|---|---|---|---|---|---|---|---|---|---|
| Beck et al., 2018 [38] |  |  |  |  |  |  |  |  | N |
| Brandeberger et al., 2018 [39] |  |  |  |  |  |  |  |  | N |
| Cheikh et al., 2018 [40] |  |  |  |  |  |  |  |  | N |
| Cheikh et al., 2020 [41] |  |  |  |  |  |  |  |  | N |
| Czucejko et al., 2019 [42] |  |  |  |  |  |  |  |  | N |
| Farjallah et al., 2018 [43] |  |  |  |  |  |  |  |  | N |
| Farjallah et al., 2022 [44] |  |  |  |  |  |  |  |  | Y |
| Farjallah et al., 2023 [45] |  |  |  |  |  |  |  |  | N |
| Farjallah et al., 2022 [46] |  |  |  |  |  |  |  |  | N |
| Farjallah et al., 2020 [47] |  |  |  |  |  |  |  |  | N |
| Ghatassi et al., 2016 [48] |  |  |  |  |  |  |  |  | N |
| Ghatassi et al., 2024 [49] |  |  |  |  |  |  |  |  | N |
| Ghatassi et al., 2014 [50] |  |  |  |  |  |  |  |  | N |
| Khaleghi-Mamaghani et al., 2021 [51] |  |  |  |  |  |  |  |  | N |
| Leonardo-Mendonça et al., 2017 [52] |  |  |  |  |  |  |  |  | N |
| Maldonado et al., 2012 [53] |  |  |  |  |  |  |  |  | N |
| Mero et al., 2006, Finland [54] |  |  |  |  |  |  |  |  | N |
| Ochoa et al., 2011 [55] |  |  |  |  |  |  |  |  | N |
| Ortiz-Franco et al., 2017 [56] |  |  |  |  |  |  |  |  | N |
| Paryab et al., 2021 [57] |  |  |  |  |  |  |  |  | N |
| Souissi et al., 2018 [58] |  |  |  |  |  |  |  |  | N |
| First Author, Year of Publication and Country (Reference) | Study Design | Characteristics of Participants: Baseline Sample (n, Sex, and Sport Discipline), Age (Mean ± SD), Anthropometric Parameters (Height, Weight, BMI, Body Fat and Muscle Mass) (Mean ± SD), Maximal Oxygen Consumption/Maximal Aerobic Speed (Mean ± SD) and Withdrawals | Intervention | Outcomes | Results IG vs. CG |
|---|---|---|---|---|---|
| Beck et al., 2018, Brazil [38] | Randomized, double-blind, placebo-controlled crossover trial | 11 ♂ trained athletes in multiple sports (soccer, handball, basketball, cycling) Age: 24.18 ± 3.92 years Height: 182 ± 5 cm Weight: 87.07 ± 12.48 kg BMI: 26.18 ± 3.63 kg/m2 Body Fat: 16.28 ± 5.77% Study withdrawals: 0 | Melatonin: 6 mg tablets (2 mg calcium, 55 mg phosphorus) (Optimum Nutrition, Inc., Downers Grove, IL, USA) Placebo: same conditions with no melatonin 30 min before exercise 1 day 06:00–09.00 p.m. Washout period: 48 to 72 h | RCB Hemoglobin Hematocrit WBC Neutrophils Lymphocytes Monocytes Uric Acid Urea Creatinine Total Proteins Glucose Total cholesterol Triglycerides CK LDH Time to exhaustion test | ↔ RCB ↔ Hemoglobin ↔ Hematocrit ↔ WBC ↔ Neutrophils ↔ Lymphocytes ↔ Monocytes ↔ Uric Acid ↔Urea ↔ Creatinine ↔ Total Proteins ↔ Glucose ↓ * Total cholesterol ↔ Triglycerides ↔ CK ↔ LDH ↑ * Time to exhaustion test |
| Brandeberger et al., 2018, USA [39] | Randomized, double-blind, placebo-controlled, crossover trial | 10 ♂ endurance-trained cyclists Age: 25.1 ± 4.0 years Height: 176 ± 7.1 cm Body Fat: 9.2 ± 13.2% VO2max: 62.7 ± 6.3 mL/kg/min Study withdrawals: 0 | Melatonin: 5 mg Tablets (NR) Placebo: (multivitamin) similar size and shape to the melatonin 15 min before exercise 1 day 02:00–06.00 p.m. Washout period: ≥7 days | Rectal temperature RPE Medium Power Average Cycling Cadence 32.2 km cycling time trial | ↔ Rectal temperature ↔ RPE ↔ Medium Power ↔ Average Cycling Cadence ↔ 32.2 km cycling time trial |
| Cheikh et al., 2018, France [40] | Randomized, double-blind, placebo-controlled crossover trial | 10 ♂ national-level judo competitors (currently training ~8 h/week) Age: 15.4 ± 0.3 years Weight: 60.6 ± 5.7 kg Height: 167.9 ± 6.9 cm BMI: 21.21 ± 2.5 kg/m2 Study withdrawals: 0 | Melatonin: 10 mg Tablets MEL-10 mg (Jamieson lab Toronto, Montreal, Vancouver, Canada N8W5B5) Placebo: starch and cellulose 2 h after exercise (15 min before bedtime) 1 day 10:00 p.m. Washout period: 7 days | RPE Hooper index (Fatigue) YYIRT-1 Manual pressure force 5-Jump Test Vertical jump Horizontal jump | ↓ * RPE ↓ Hooper Index ↑ * YYIRT-1 ↔ Manual pressure force ↑ 5-Jump Test ↔ Vertical jump ↔ Horizontal jump |
| Cheikh et al., 2020, France [41] | Randomized, double-blind, placebo-controlled crossover trial | 14 ♂ volleyball players from Tunisian league 1 (training 4 days per week for an average of 1.5–2 h) Age: 14.5 ± 0.52 years Weight: 65.68 ± 7.72 kg Height: 181.57 ± 7.38 cm BMI: 21.21 ± 2.5 kg/m2 Study withdrawals: 0 | Melatonin: 10 mg Tablets MEL-10 mg (Jamieson lab Toronto, Montreal, Vancouver, Canada N8W5B5) Placebo: starch and cellulose 2 h after exercise (15 min before bedtime) 1 day 10:00 p.m. Washout period: 7 days | WBC Neutrophils Lymphocytes CK LDH AST CRP MDA Homocysteine Fatigue Inex Medium Power Peak Power Time trial | ↓ * WBC ↓ * Neutrophils ↓ * Lymphocytes ↓ * CK ↓ * LDH ↓ * AST ↓ * CRP ↓ * MDA ↓ *Homocysteine ↓ * Fatigue Inex ↑ * Medium Power ↑ * Peak Power ↓ * Time trial |
| Czucejko et al., 2019, Poland [42] | Randomized, double-blind, placebo-controlled, parallel group trial | 81 ♂ and ♀ IG: 47 ♀ second and third league soccer players (Zawisza Bydgoszcz Sports Club, Bydgoszcz, Poland), 19 ♂ rowers (Bydgoszcz Rowing Club, Poland); CG 15 ♂ non-training young adults Melatonin Group: Age: 20.95 ± 2.5 years Weight: 89.7 ± 8.5 kg Height: 1.85 ± 0.2 m BMI: 26.2 ± 0.2 kg/m2 Control Group: Age: 20.50 ± 2.0 years Weight: 82.1 ± 6.5 kg Height: 1.82 ± 0.11 m BMI: 24.8 ± 0.1 kg/m2 Study withdrawals: 0 66 participants in IG 15 participants in CG | Melatonin: Orally in a single dose 5 mg melatonin (NR) daily Placebo: NR 1 h before bedtime 30 days No adverse effects were reported | Glucose IL-6 CRP MDA 8-iso-PGF2α Isoprostane Ox-LDLs SOD GRd GSH GPx Melatonin | ↓ * Glucose ↓ * IL-6 ↓ * CRP ↓ * MDA ↓ * 8-iso-PGF2α Isoprostane ↓ * Ox-LDLs ↔ SOD ↓ * GRd ↑ * GSH ↔ GPx ↑ Melatonin |
| Farjallah et al., 2018, Tunisia [43] | Randomized, double-blind, placebo-controlled crossover trial | 15 ♀ elite athletes (Tunisian handball national team) Age: 17.4 ± 0.4 years Weight: 76.4 ± 5.6 kg Height: 176.0 ± 4.2 cm Study withdrawals: 0 | Melatonin: 6 mg Tablets quick release (Jamieson Laboratories Toronto, Montreal, Canada) Placebo: lactose, starch, and cellulose 30 min before exercise 4:00 and 4:30 p.m. 1 day Washout period: 2 weeks | Glucose RPE Blood Lactate Modified agility T-test Squat jump Counter movement jump Maximum standing ball throw velocity test Maximum jump ball throw velocity test 20-m sprint | ↓ * Glucose ↔ RPE ↓ * Blood Lactate ↔ Modified agility T-test ↔ Squat jump ↔ Counter movement jump ↔ Maximum standing ball throw velocity test ↔ Maximum jump ball throw velocity test ↔ 20-m sprint |
| Farjallah et al., 2022, Tunisia [44] | Randomized, double-blind, placebo-controlled, parallel group trial | 24 ♂ professional soccer players from Tunisian first league Age: 18.8 ± 1.3 years Weight: 70.0 ± 10.6 kg Height: 181 ± 8 cm BMI: 21.27 ± 1.87 kg/m2 Study withdrawals: 4 10 participants in IG 10 participants in CG | Melatonin: 5 mg Capsules (Jamieson Laboratories Toronto, Montreal, Canada) Placebo: lactose, starch, and cellulose After exercise 6 days 7.00 p.m. | Creatinine Urea Glucose Total Cholesterol HDL LDL Triglycerides Total Proteins γ-glutamyl transferase Alkaline Phosphatase CK AOPP SOD Squat jump Countermovement jump 5-jump test Modified agility T-test 20-m sprint | ↓ Creatinine ↓ Urea ↔ Glucose ↔ Total Cholesterol ↔ HDL ↔ LDL ↔ Triglycerides ↔ Total Proteins ↓ γ-glutamyl transferase ↓ Alkaline Phosphatase ↓ CK ↓ AOPP ↑ * SOD ↑ Squat jump ↑ Countermovement jump ↑ 5-jump test ↓ Modified agility T-test ↓ 20-m sprint |
| Farjallah et al., 2023, Tunisia [45] | Randomized, double-blind, placebo-controlled, parallel group trial | 12 ♂ professional soccer players from Tunisian first league (soccer experience from 5–7 years) Age: 17.54 ± 0.78 years, Weight: 70.31 ± 3.86 kg Height: 1.80 ± 0.08 m Maximal Aerobic Speed: 16.85 ± 0.63 km/h Study withdrawals: 0 | Melatonin: 6 mg Tablets quick release (Jamieson Laboratories Toronto, Montreal, Canada) Placebo: lactose, starch, and cellulose 30 min before exercise 5:00 ± 0:30 p.m. 1 day Washout period: 48 h | WBC Neutrophils Lymphocytes Monocytes Glucose Total Cholesterol HDL LDL Triglycerides Creatinine Urea Total Proteins AST ALT γ-glutamyl transferase Alkaline Phosphatase Blood Lactate Heart Rate Time to exhaustion Distance covered | ↔ WBC ↔ Neutrophils ↔ Lymphocytes ↔ Monocytes ↔ Glucose ↔ Total Cholesterol ↔ HDL ↔ LDL ↔ Triglycerides ↓ * Creatinine ↔ Urea ↔ Total Proteins ↓ * AST ↓ * ALT ↓ * γ-glutamyl transferase ↓ Alkaline Phosphatase ↔ Blood Lactate ↓ Heart Rate ↑ Time to exhaustion ↑ Distance covered |
| Farjallah et al., 2022, Tunisia [46] | Randomized, double-blind, placebo-controlled crossover trial | 13 ♂ professional soccer players from Tunisian first league Age: 17.5 ± 0.8 years Weight: 70.0 ± 3.9 kg Height: 180 ± 8 cm Maximal Aerobic Speed: 16.85 ± 0.63 km/h Study withdrawals: 0 | Melatonin: 6 mg Capsules (Jamieson Laboratories Toronto, Montreal, Canada) Placebo: lactose, starch, and cellulose 10 min after exercise 1 day 05.00 p.m.–00.30 a.m. Washout period: 2 days | Uric Acid Total Bilirubin CK LDH MDA AOPP SOD GPx GRd RPE Running exercise test | ↓ Uric Acid ↓ Total Bilirubin ↓ CK ↓ LDH ↓ MDA ↓ AOPP ↑ SOD ↑ GPx ↔ GRd ↔ RPE ↔ Running exercise test |
| Farjallah et al., 2020, Tunisia [47] | Randomized, double-blind, placebo-controlled, parallel group trial | 20 ♂ professional soccer players from Tunisian first league Age: 18.9 ±1.3 years Weight: 70.1 ± 10.6 kg Height: 180 ± 1.0 cm Study withdrawals: 0 10 participants in IG 10 participants in CG | Melatonin: 5 mg Capsules (Jamieson Laboratories Toronto, Montreal, Canada) Placebo: lactose, starch, and cellulose After exercise 6 days 7.00 p.m. | CK LDH MAD SOD VAS Repeated sprint ability test | ↓ * CK ↓ * LDH ↓ * MAD ↑ * SOD ↓ * VAS ↓ Repeated sprint ability test |
| Ghatassi et al., 2016, Tunisia [48] | Randomized, double-blind, placebo-controlled crossover trial | 12 ♂ soccer players (Tunisian League 3) Age: 17.9 ± 1.3 years Weight: 62.0 ± 8.8 kg Height: 174 ± 6 cm | Melatonin: 5 mg Capsules (Jamieson Laboratories Toronto, Montreal, Canada) Placebo: lactose, starch, and cellulose 30 min before exercise 1 day 07.30 a.m. Washout period: 36 h | Medicine-ball throw Manual pressure force 5-jump test Agility T-test Reaction time Vigilance tests | ↑ * Medicine-ball throw ↑ * Manual pressure force ↔ 5-jump test ↔ Agility T-test ↓ * Reaction time ↓ * Vigilance tests |
| Ghatassi et al., 2024, Tunisia [49] | Randomized, double-blind, placebo-controlled crossover trial | 12 ♂ soccer players (Tunisian League) Age: 22.9 ± 1.3 years Weight: 72.0 ± 8.8 kg Height: 1.80 ± 0.05 m | Melatonin: 5 mg Capsules (Jamieson Laboratories Toronto, Montreal, Canada) Placebo: lactose, starch, and cellulose 30 min before exercise 1 day 07.30 a.m. Washout period: 48 h | Glucose Hooper’s index Manual pressure force Squat jump RPE Reaction time Vigilance Test Blood lactate Peak Power Average Power Modified agility T-test | ↔ Glucose ↓ Hooper’s index ↑ Manual pressure force ↑ Squat jump ↓ RPE ↓ Reaction time ↔ Vigilance Test ↔ Blood lactate ↑ Peak Power ↑ Average Power ↔ Modified agility T-test |
| Ghatassi et al., 2014, Tunisia [50] | Randomized, double-blind, placebo-controlled crossover trial | 12 ♂ soccer players (Tunisian League 3) Age: 17.9 ± 1.3 years Weight: 62.0 ± 8.8 kg Height: 174 ± 6 cm | Melatonin: 5 mg or 8 mg Capsules (Jamieson Laboratories Toronto, Montreal, Canada) Placebo: lactose, starch, and cellulose 30 min before exercise 07.30 a.m. 1 day Washout period: NR 3 test sessions at 9:00 p.m. on different days | Medicine-ball throw Manual pressure force 5-jump test Squat jump Counter movement jump Agility T-test Reaction time Vigilance tests | Melatonin: 5 mg ↔ Medicine-ball throw ↑ Manual pressure force ↔ 5-jump test ↔ Squat jump ↔ Counter movement jump ↔ Agility T-test Melatonin: 8 mg ↓ Medicine-ball throw ↓ * Manual pressure force ↓ 5-jump test ↓ * Squat jump ↓ * Counter movement jump ↔ Modified agility T-test |
| Khaleghi-Mamaghani et al., 2021, Turkey [51] | Randomized, double-blind, placebo-controlled crossover trial | 10 ♂ highly trained (3–4 days per week for an average of 2-h training in a day) Age: 23.4 ± 1.71 years Weight: 74.28 ± 6.69 kg Height: 176 ± 6.42 cm BMI: 23.96 ± 1.63 kg/m2 Body Fat: 13.4 ± 2.75% | Melatonin: 10 mg (NR) Placebo: (NR) 30 to 45 min after exercise 1 day 11.00 a.m. Washout period: 1 week | Heart Rate DBP SBP Blood Lactate Reaction time Dynamic balance Static balance Jump strength Manual pressure force Squat Bench press Anaerobic peak power Anaerobic minimum power Average power Fatigue index | ↓ Heart Rate ↓ DBP ↓ SBP ↓ Blood Lactate ↑ Reaction time ↑ Dynamic balance ↑ Static balance ↔ Jump strength ↔ Manual pressure force ↔ Squat ↔ Bench press ↓ Anaerobic peak power ↓ Anaerobic minimum power ↓ Average power ↓ Fatigue index |
| Leonardo-Mendonça et al., 2017, Spain [52] | Randomized, double-blind, placebo-controlled, parallel group trial | 24 ♂ resistance-trained athletes Age: 20.3 ± 0.71 years Weight: 74.7 ± 3.22 kg Height: 176.7 ± 1.83 cm Study withdrawals: 0 12 participants in IG 12 participants in CG | Melatonin: 100 mg per day gelatinous capsules (NR) Placebo: capsules excipients (lactose, colloidal silica) 30 min before bedtime 4 weeks | RCB Hemoglobin Hematocrit Leukocyte Glucose Total Cholesterol Triglycerides Creatinine Urea Uric Acid CK LDH AST ALT AOPP Lipid peroxidation ORAC Nitrites GSH GSSG GSH:GSSG GPx GRd GPx:GRd | ↔ RBC ↔ Hemoglobin ↔ Hematocrit ↔ Leukocyte ↔ Glucose ↓ * Total Cholesterol ↔ Triglycerides ↓ Creatinine ↔ Urea ↔ Uric Acid ↓ * CK ↓ * LDH ↔ AST ↔ ALT ↓ * AOPP ↓ * Lipid peroxidation ↑ * ORAC ↓ * Nitrites ↔ GSH ↓ * GSSG ↓ * GSH:GSSG ↓ * GPx ↓ * GRd ↓ * GPx:GRd |
| Maldonado et al., 2012, Spain [53] | Randomized, single-blind, placebo-controlled, parallel group trial | 16 ♂ professional active soccer players (from the Sevilla football club of the second division of Spain, belonging to the Spanish Professional Football League) Age (Range): 18–20 years Weight: 68.2 ± 1.5 kg Height: 177.2 ± 6.9 cm Study withdrawals: 0 8 participants in IG 8 participants in CG | Melatonin: 6 mg (NR) Placebo: (NR) 30 min before exercise 1 day | RCB Hemoglobin Hematocrit WBC Neutrophils Lymphocytes Natural Killer Ig M Ig G Ig A Cortisol Testosterone Glucose Total Cholesterol Triglycerides Total Proteins Creatinine Urea Uric Acid CK LDH AST ALT MDA TAS | ↔ RCB ↔ Hemoglobin ↔ Hemoglobin ↔ WBC ↔ Neutrophils ↔ Lymphocytes ↔ Natural Killer ↔ Ig M ↔ Ig G ↑ * Ig A ↔ Cortisol ↔ Testosterone ↔ Glucose ↔ Total Cholesterol ↓ * Triglycerides ↔ Total Proteins ↔ Creatinine ↔ Urea ↔ Uric Acid ↔ CK ↔ LDH ↔ AST ↔ ALT ↓ * MDA ↑ * TAS |
| Mero et al., 2006, Finland [54] | Randomized, double-blind, placebo-controlled crossover trial | 10 ♂ high strength and resistance-trained (regular exercise four times a week with 4.8 ± 2.0 years’ experience in strength and resistance training) Age: 24.0 ± 3.0 years Weight: 74.7 ± 5.4 kg Height: 178.0 ± 5.0 cm Body Fat: 14.3 ± 3.4% Study withdrawals: 0 | Melatonin: 6 mg Tablets (University of Pharmacy, Finland), Placebo: 6 mg Tablets 60 min before exercise 1 day Washout period: 14 days | Glucose Cortisol Testosterone Grow Hormone Lactate Counter movement jump Squat Bench press Resistance Exercise Serum melatonin | ↔ Glucose ↔ Cortisol ↔ Testosterone ↔ Grow Hormone ↔ Lactate ↔ Counter movement jump ↔ Squat ↔ Bench press ↔ Resistance Exercise ↑ * Serum melatonin |
| Ochoa et al., 2011, Spain [55] | Randomized, double-blind, placebo-controlled, parallel group trial | 20 ♂ highly trained athletes daily (running) Characteristics of participants NR Study withdrawals: 0 8 participants in IG 8 participants in CG | Melatonin: 5 capsules 3 mg (Natrol, Chatsworth, CA, USA) Total dose 15 mg 1 capsule 2 days before the test with dinner, 3 capsules on the previous day (breakfast, lunch, and dinner), 1 capsule the same day of the run, 1 h before physical exercise test Placebo: beer yeast, cellulose, acacia, silica stearic acid, magnesium stearate, cellulose gum, maltodextrin 3 days | RBC Hemoglobin Reduction plasma viscosity Cholesterol Phospholipids Total Bilirubin Total Proteins Creatinine TNF-α IL-6 sTNF-α-RII IL-1ra TAS 15-F2t-Isoprostane 8-OHdG CAT GPx Serum melatonin | ↔ RBC ↔ Hemoglobin ↔ Reduction plasma viscosity ↓ Cholesterol ↓ Phospholipids ↓ Total Bilirubin ↔ Total Proteins ↓ Creatinine ↓ * TNF-α ↓ * IL-6 ↑ sTNF-α-RII ↑ * IL-1ra ↑ * TAS ↓ 15-F2t-Isoprostane ↓ 8-OHdG ↑ * CAT ↑ * GPx ↑ * Serum melatonin |
| Ortiz-Franco et al., 2017, Spain [56] | Randomized, double-blind, placebo-controlled, parallel group trial | 14 ♂ highly trained athletes Age: CG: 28.43 ± 4.39 years IG: 26.00 ± 6.03 years Weight: CG: 78.39 ± 6.68 kg IG: 79.96 ± 7.29 kg Height: CG: 176.9 ± 3.89 cm IG: 179.9 ± 6.04 cm BMI: CG: 25.06 ± 2.20 kg/m2 IG: 24.70 ± 19.8 kg/m2 Fat Mass: CG: 13.21 ± 3.91 kg IG: 14.79 ± 3.60 kg Muscle Mass: CG: 61.96 ± 4.13 kg IG: 61.94 ± 4.21 kg Study withdrawals: 0 7 participants in IG 7 participants in CG | Melatonin: 1 daily capsule containing 20 mg of melatonin (Acofarma®, Barcelona, Spain) Placebo: 1 daily capsule containing lactose Before exercise NR 14 days | RBC Hemoglobin Hematocrit Transferrin Ferritin Serum Iron Glucose Urea Creatinine Uric Acid Total Cholesterol HDL LDL Triglycerides Total Bilirubin Albumin Prealbumin TAC SOD GPx DNA damage Serum melatonin | ↔ RBC ↔ Hemoglobin ↔ Hematocrit ↔Transferrin ↔ Ferritin ↔ Serum Iron ↔ Glucose ↔ Urea ↔ Creatinine ↔ Uric Acid ↔ Total Cholesterol ↔ HDL ↔ LDL ↔ Triglycerides ↔ Total Bilirubin ↔ Albumin ↔ Prealbumin ↑ * TAC ↔ SOD ↑ * GPx ↓ * DNA damage ↑ * Serum melatonin |
| Paryab et al., 2021, Tunisia [57] | Randomized, double-blind, placebo-controlled, repeated-measures crossover trial | 33 ♂ university championship professional athletes (running) Age: 20.0 ± 2.0 years Weight: Body Mass: 83.4 ± 14.4 kg Height: 180.0 ± 1.0 cm Study withdrawals: 23 10 participants in IG/CG | Melatonin: 6 mg Tablets (NR) Placebo: 6 mg Tablets (NR) 30 min before training 1 day 08:00 a.m. Washout period: 3 days | Blood Lactate Reaction time Static balance Dynamic balance Anaerobic power | ↓ * Blood Lactate ↓ * Reaction time ↑ * Static balance ↑ * Dynamic balance ↑ * Anaerobic power |
| Souissi et al., 2020 Tunisia [58] | Randomized, double-blind, placebo-controlled crossover trial | 8 ♂ highly trained athletes (students of Institute of Sports and Physical Education) Age: 21.8 ± 0.9 years Weight: NR BMI: 21.0 ± 0.8 kg/m2 Study withdrawals: 0 | Melatonin: 6 mg Capsules (NR) Placebo: 6 mg Capsules (NR) 50 min before exercise 1 day 09:00 a.m. Washout period: NR 2 test sessions at 8:00 a.m. on different days | CRP LDH AST ALT | ↔ CRP ↔ LDH ↔ AST ↔ ALT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Celorrio San Miguel, A.M.; Roche, E.; Herranz-López, M.; Celorrio San Miguel, M.; Mielgo-Ayuso, J.; Fernández-Lázaro, D. Impact of Melatonin Supplementation on Sports Performance and Circulating Biomarkers in Highly Trained Athletes: A Systematic Review of Randomized Controlled Trials. Nutrients 2024, 16, 1011. https://doi.org/10.3390/nu16071011
Celorrio San Miguel AM, Roche E, Herranz-López M, Celorrio San Miguel M, Mielgo-Ayuso J, Fernández-Lázaro D. Impact of Melatonin Supplementation on Sports Performance and Circulating Biomarkers in Highly Trained Athletes: A Systematic Review of Randomized Controlled Trials. Nutrients. 2024; 16(7):1011. https://doi.org/10.3390/nu16071011
Chicago/Turabian StyleCelorrio San Miguel, Ana M., Enrique Roche, María Herranz-López, Marta Celorrio San Miguel, Juan Mielgo-Ayuso, and Diego Fernández-Lázaro. 2024. "Impact of Melatonin Supplementation on Sports Performance and Circulating Biomarkers in Highly Trained Athletes: A Systematic Review of Randomized Controlled Trials" Nutrients 16, no. 7: 1011. https://doi.org/10.3390/nu16071011
APA StyleCelorrio San Miguel, A. M., Roche, E., Herranz-López, M., Celorrio San Miguel, M., Mielgo-Ayuso, J., & Fernández-Lázaro, D. (2024). Impact of Melatonin Supplementation on Sports Performance and Circulating Biomarkers in Highly Trained Athletes: A Systematic Review of Randomized Controlled Trials. Nutrients, 16(7), 1011. https://doi.org/10.3390/nu16071011