The Power of Psychobiotics in Depression: A Modern Approach through the Microbiota–Gut–Brain Axis: A Literature Review
Abstract
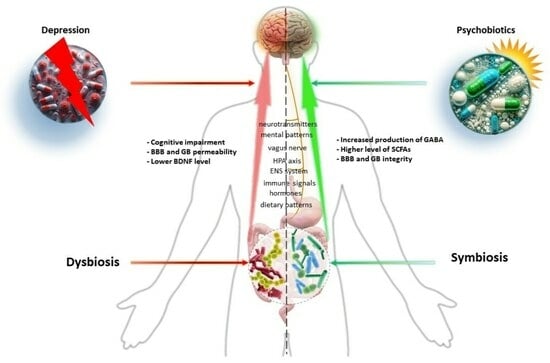
:1. Introduction
2. The Microbiota–Gut–Brain (MGB) Axis and Depression: Understanding the Essential Connections
2.1. Gut Microbiota Metabolites in Antidepressant Mechanisms
2.1.1. Short-Chain Fatty Acids (SCFAs)
2.1.2. Kynurenine Pathway Metabolites and Neurotransmitters
2.2. Gut Microbiota and CNS Functioning and BBB Permeability
2.3. Dysregulated MGB Axis in Depression: Chronic Stress Response
2.4. Gut Microbiota and Inflammation
3. Impact of Psychobiotics and Healthy Dietary Patterns on Depressive Disorders
3.1. Preclinical Studies on Animal Models
3.2. Clinical Human Studies on Psychobiotics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- World Health Organization (WHO). Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 25 March 2023).
- Institute of Health Metrics and Evaluation. Global Health Data Exchange (GHDx). Available online: http://ghdx.healthdata.org/gbd-results-tool?params=gbd-api-2019-permalink/d780dffbe8a381b25e1416884959e88b (accessed on 1 May 2021).
- Gałecki, P.; Bliźniewska-Kowalska, K.; Maes, M.; Su, K.P. Neuroimmunology and (Epi)Genetics in Depressive Disorders. J. Pers. Med. 2021, 11, 670. [Google Scholar] [CrossRef] [PubMed]
- Halaris, A.; Sohl, E.; Whitham, E.A. Treatment-Resistant Depression Revisited: A Glimmer of Hope. J. Pers. Med. 2021, 11, 155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, B.; Zhang, J.; Dong, J.; Ma, J.; Zhang, Y.; Jin, K.; Lu, J. Effect of prebiotics, probiotics, synbiotics on depression: Results from a meta-analysis. BMC Psychiatry 2023, 23, 477. [Google Scholar] [CrossRef] [PubMed]
- Firth, J.; Marx, W.; Dash, S.; Carney, R.; Teasdale, S.B.; Solmi, M.; Stubbs, B.; Schuch, F.B.; Carvalho, A.F.; Jacka, F.; et al. The Effects of Dietary Improvement on Symptoms of Depression and Anxiety: A Meta-Analysis of Randomized Controlled Trials. Psychosom. Med. 2019, 81, 265–280. [Google Scholar] [CrossRef] [PubMed]
- Ceolin, G.; Breda, V.; Koning, E.; Meyyappan, A.C.; Gomes, F.A.; Moreira, J.D.; Gerchman, F.; Brietzke, E. A Possible Antidepressive Effect of Dietary Interventions: Emergent Findings and Research Challenges. Curr. Treat. Options Psychiatry 2022, 9, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Wallace, C.J.K.; Milev, R.V. The Efficacy, Safety, and Tolerability of Probiotics on Depression: Clinical Results from an Open-Label Pilot Study. Front. Psychiatry 2021, 12, 618279. [Google Scholar] [CrossRef] [PubMed]
- Radjabzadeh, D.; Bosch, J.A.; Uitterlinden, A.G.; Zwinderman, A.H.; Ikram, M.A.; van Meurs, J.B.J.; Luik, A.I.; Nieuwdorp, M.; Lok, A.; van Duijn, C.M.; et al. Gut microbiome-wide association study of depressive symptoms. Nat. Commun. 2022, 13, 7128. [Google Scholar] [CrossRef]
- Barros-Santos, T.; Silva, K.S.O.; Libarino-Santos, M.; Cata-Preta, E.G.; Reis, H.S.; Tamura, E.K.; de Oliveira-Lima, A.J.; Berro, L.F.; Uetanabaro, A.P.T.; Marinho, E.A.V. Effects of chronic treatment with new strains of Lactobacillus plantarum on cognitive, anxiety- and depressive-like behaviors in male mice. PLoS ONE 2020, 15, e0234037. [Google Scholar] [CrossRef] [PubMed]
- Colombo, A.V.; Sadler, R.K.; Llovera, G.; Singh, V.; Roth, S.; Heindl, S.; Sebastian Monasor, L.; Verhoeven, A.; Peters, F.; Parhizkar, S.; et al. Microbiota-derived short chain fatty acids modulate microglia and promote Aβ plaque deposition. Elife 2021, 10, e59826. [Google Scholar] [CrossRef]
- Mirzaei, R.; Bouzari, B.; Hosseini-Fard, S.R.; Mazaheri, M.; Ahmadyousefi, Y.; Abdi, M.; Jalalifar, S.; Karimitabar, Z.; Teimoori, A.; Keyvani, H.; et al. Role of microbiota-derived short-chain fatty acids in nervous system disorders. Biomed. Pharmacother. 2021, 139, 111661. [Google Scholar] [CrossRef]
- Mack, I.; Schwille-Kiuntke, J.; Mazurak, N.; Niesler, B.; Zimmermann, K.; Mönnikes, H.; Enck, P. A Nonviable Probiotic in Irritable Bowel Syndrome: A Randomized, Double-Blind, Placebo-Controlled, Multicenter Study. Clin. Gastroenterol. Hepatol. 2022, 20, 1039–1047.e9. [Google Scholar] [CrossRef] [PubMed]
- Puricelli, C.; Rolla, R.; Gigliotti, L.; Boggio, E.; Beltrami, E.; Dianzani, U.; Keller, R. The Gut-Brain-Immune Axis in Autism Spectrum Disorders: A State-of-Art Report. Front. Psychiatry 2022, 12, 755171. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Dinan, T.G. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef]
- Bi, C.; Guo, S.; Hu, S.; Chen, J.; Ye, M.; Liu, Z. The microbiota–gut–brain axis and its modulation in the therapy of depression: Comparison of efficacy of conventional drugs and traditional Chinese medicine approaches. Pharmacol. Res. 2022, 183, 106372. [Google Scholar] [CrossRef] [PubMed]
- Sonali, S.; Ray, B.; Ahmed Tousif, H.; Rathipriya, A.G.; Sunanda, T.; Mahalakshmi, A.M.; Rungratanawanich, W.; Essa, M.M.; Qoronfleh, M.W.; Chidambaram, S.B.; et al. Mechanistic Insights into the Link between Gut Dysbiosis and Major Depression: An Extensive Review. Cells 2022, 11, 1362. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Tu, H.; Chen, T. The Microbiota–Gut–Brain Axis in Depression: The Potential Pathophysiological Mechanisms and Microbiota Combined Antidepression Effect. Nutrients 2022, 14, 2081. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, G.K.; Ramadan, H.K.-A.; Elbeh, K.; Haridy, N.A. Bridging the gap: Associations between gut microbiota and psychiatric disorders. Middle East. Curr. Psychiatry 2024, 31, 2. [Google Scholar] [CrossRef]
- Rusch, J.A.; Layden, B.T.; Dugas, L.R. Signalling cognition: The gut microbiota and hypothalamic-pituitary-adrenal axis. Front. Endocrinol. 2023, 14, 1130689. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Anand, N.; Gorantla, V.R.; Chidambaram, S.B. The Role of Gut Dysbiosis in the Pathophysiology of Neuropsychiatric Disorders. Cells 2022, 12, 54. [Google Scholar] [CrossRef]
- Andrioaie, I.-M.; Duhaniuc, A.; Nastase, E.V.; Iancu, L.S.; Luncă, C.; Trofin, F.; Anton-Păduraru, D.-T.; Dorneanu, O.-S. The Role of the Gut Microbiome in Psychiatric Disorders. Microorganisms 2022, 10, 2436. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Liu, Z.; Dong, X.; Hu, T.; Wang, L.; Li, J.; Liu, X.; Sun, J. Fecal Microbiota Transplantation from Healthy Donors Reduced Alcohol-induced Anxiety and Depression in an Animal Model of Chronic Alcohol Exposure. Chin. J. Physiol. 2018, 61, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.; Xie, R.; Lin, L.; Jiang, J.; Du, L.; Zeng, X.; Li, G.; Wang, C.; Qiao, Y. Fecal microbiota transplantation ameliorates gut microbiota imbalance and intestinal barrier damage in rats with stress-induced depressive-like behavior. Eur. J. Neurosci. 2021, 53, 3598–3611. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.; Tan, Y.; Qu, Y.; Chang, L.; Wang, S.; Wei, Y.; Wang, X.; Hashimoto, K. A role of the subdiaphragmatic vagus nerve in depression-like phenotypes in mice after fecal microbiota transplantation from Chrna7 knock-out mice with depression-like phenotypes. Brain Behav. Immun. 2021, 94, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Bures, J.; Cyrany, J.; Kohoutova, D.; Förstl, M.; Rejchrt, S.; Kvetina, J.; Vorisek, V.; Kopacova, M. Small intestinal bacterial overgrowth syndrome. World J. Gastroenterol. 2010, 16, 2978–2990. [Google Scholar] [CrossRef] [PubMed]
- Kossewska, J.; Bierlit, K.; Trajkovski, V. Personality, Anxiety, and Stress in Patients with Small Intestine Bacterial Overgrowth Syndrome. The Polish Preliminary Study. Int. J. Environ. Res. Public Health 2022, 20, 93. [Google Scholar] [CrossRef] [PubMed]
- Chojnacki, C.; Popławski, T.; Konrad, P.; Fila, M.; Błasiak, J.; Chojnacki, J. Antimicrobial treatment improves tryptophan metabolism and mood of patients with small intestinal bacterial overgrowth. Nutr. Metab. 2022, 19, 66. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ma, L.; Chang, L.; Pu, Y.; Qu, Y.; Hashimoto, K. A key role of the subdiaphragmatic vagus nerve in the depression-like phenotype and abnormal composition of gut microbiota in mice after lipopolysaccharide administration. Transl. Psychiatry 2020, 10, 186. [Google Scholar] [CrossRef] [PubMed]
- Aaronson, S.T.; Sears, P.; Ruvuna, F.; Bunker, M.; Conway, C.R.; Dougherty, D.D.; Reimherr, F.W.; Schwartz, T.L.; Zajecka, J.M. A 5-Year Observational Study of Patients With Treatment-Resistant Depression Treated With Vagus Nerve Stimulation or Treatment as Usual: Comparison of Response, Remission, and Suicidality. Am. J. Psychiatry 2017, 174, 640–648. [Google Scholar] [CrossRef]
- Gold, S.M.; Köhler-Forsberg, O.; Moss-Morris, R.; Mehnert, A.; Miranda, J.J.; Bullinger, M.; Steptoe, A.; Whooley, M.A.; Otte, C. Comorbid depression in medical diseases. Nat. Rev. Dis. Primers 2020, 6, 69. [Google Scholar] [CrossRef]
- Gilbert, J.A.; Blaser, M.J.; Caporaso, J.G.; Jansson, J.K.; Lynch, S.V.; Knight, R. Current understanding of the human microbiome. Nat. Med. 2018, 24, 392–400. [Google Scholar] [CrossRef]
- Góralczyk-Bińkowska, A.; Szmajda-Krygier, D.; Kozłowska, E. The Microbiota–Gut–Brain Axis in Psychiatric Disorders. Int. J. Mol. Sci. 2022, 23, 11245. [Google Scholar] [CrossRef] [PubMed]
- Bastiaanssen, T.F.S.; Cowan, C.S.M.; Claesson, M.J.; Dinan, T.G.; Cryan, J.F. Making Sense of … the Microbiome in Psychiatry. Int. J. Neuropsychopharmacol. 2019, 22, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef]
- Berer, K.; Krishnamoorthy, G. Microbial view of central nervous system autoimmunity. FEBS Lett. 2014, 588, 4207–4213. [Google Scholar] [CrossRef]
- Sandoval-Motta, S.; Aldana, M.; Martínez-Romero, E.; Frank, A. The Human Microbiome and the Missing Heritability Problem. Front. Genet. 2017, 8, 80. [Google Scholar] [CrossRef]
- Leviatan, S.; Shoer, S.; Rothschild, D.; Gorodetski, M.; Segal, E. An expanded reference map of the human gut microbiome reveals hundreds of previously unknown species. Nat. Commun. 2022, 13, 3863. [Google Scholar] [CrossRef] [PubMed]
- Forster, S.C.; Kumar, N.; Anonye, B.O.; Almeida, A.; Viciani, E.; Stares, M.D.; Dunn, M.; Mkandawire, T.T.; Zhu, A.; Shao, Y.; et al. A human gut bacterial genome and culture collection for improved metagenomic analyses. Nat. Biotechnol. 2019, 37, 186–192. [Google Scholar] [CrossRef]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the human intestinal microbial flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef]
- Margolis, K.G.; Cryan, J.F.; Mayer, E.A. The Microbiota-Gut-Brain Axis: From Motility to Mood. Gastroenterology 2021, 160, 1486–1501. [Google Scholar] [CrossRef]
- Flint, H.J.; Scott, K.P.; Duncan, S.H.; Louis, P.; Forano, E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes 2012, 3, 289–306. [Google Scholar] [CrossRef] [PubMed]
- Dicks, L.M.T. Gut Bacteria and Neurotransmitters. Microorganisms 2022, 10, 1838. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, J.; Chen, Y. Regulation of Neurotransmitters by the Gut Microbiota and Effects on Cognition in Neurological Disorders. Nutrients 2021, 13, 2099. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H. Immune regulation by microbiome metabolites. Immunology 2018, 154, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Suárez, V.J.; Redondo-Flórez, L.; Rubio-Zarapuz, A.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. Microbiota Implications in Endocrine-Related Diseases: From Development to Novel Therapeutic Approaches. Biomedicines 2024, 12, 221. [Google Scholar] [CrossRef] [PubMed]
- Koppel, N.; Maini Rekdal, V.; Balskus, E.P. Chemical transformation of xenobiotics by the human gut microbiota. Science 2017, 356, 6344. [Google Scholar] [CrossRef]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Nageshwar Reddy, D. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef]
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Tóth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Kundu, P.; et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014, 6, 263ra158. [Google Scholar] [CrossRef]
- Baxter, N.T.; Schmidt, A.W.; Venkataraman, A.; Kim, K.S.; Waldron, C.; Schmidt, T.M. Dynamics of Human Gut Microbiota and Short-Chain Fatty Acids in Response to Dietary Interventions with Three Fermentable Fibers. mBio 2019, 10, e02566-18. [Google Scholar] [CrossRef]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.-H.; Sperandio, M.; Di Ciaula, A. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int. J. Mol. Sci. 2022, 23, 1105. [Google Scholar] [CrossRef]
- Singh, V.; Lee, G.; Son, H.; Koh, H.; Kim, E.S.; Unno, T.; Shin, J.-H. Butyrate producers, “The Sentinel of Gut”: Their intestinal significance with and beyond butyrate, and prospective use as microbial therapeutics. Front. Microbiol. 2023, 13, 1103836. [Google Scholar] [CrossRef]
- Sleeth, M.L.; Thompson, E.L.; Ford, H.E.; Zac-Varghese, S.E.K.; Frost, G. Free fatty acid receptor 2 and nutrient sensing: A proposed role for fibre, fermentable carbohydrates and short-chain fatty acids in appetite regulation. Nutr. Res. Rev. 2010, 23, 135–145. [Google Scholar] [CrossRef]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Reigstad, C.S.; Salmonson, C.E.; Rainey, J.F., 3rd; Szurszewski, J.H.; Linden, D.R.; Sonnenburg, J.L.; Farrugia, G.; Kashyap, P.C. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015, 29, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Church, J.S.; Bannish, J.A.M.; Adrian, L.A.; Rojas Martinez, K.; Henshaw, A.; Schwartzer, J.J. Serum short chain fatty acids mediate hippocampal BDNF and correlate with decreasing neuroinflammation following high pectin fiber diet in mice. Front. Neurosci. 2023, 17, 1134080. [Google Scholar] [CrossRef] [PubMed]
- Skonieczna-Żydecka, K.; Grochans, E.; Maciejewska, D.; Szkup, M.; Schneider-Matyka, D.; Jurczak, A.; Łoniewski, I.; Kaczmarczyk, M.; Marlicz, W.; Czerwińska-Rogowska, M.; et al. Faecal Short Chain Fatty Acids Profile is Changed in Polish Depressive Women. Nutrients 2018, 10, 1939. [Google Scholar] [CrossRef] [PubMed]
- Opeyemi, O.M.; Rogers, M.B.; Firek, B.A.; Janesko-Feldman, K.; Vagni, V.; Mullett, S.J.; Wendell, S.G.; Nelson, B.P.; New, L.A.; Mariño, E.; et al. Sustained Dysbiosis and Decreased Fecal Short-Chain Fatty Acids after Traumatic Brain Injury and Impact on Neurologic Outcome. J. Neurotrauma 2021, 38, 2610–2621. [Google Scholar] [CrossRef]
- Doll, J.P.K.; Vázquez-Castellanos, J.F.; Schaub, A.C.; Schweinfurth, N.; Kettelhack, C.; Schneider, E.; Yamanbaeva, G.; Mählmann, L.; Brand, S.; Beglinger, C.; et al. Fecal Microbiota Transplantation (FMT) as an Adjunctive Therapy for Depression—Case Report. Front. Psychiatry 2022, 13, 815422. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Zheng, S.-P.; Shi, X.; Yuan, L.-Z.; Hu, H.; Zhou, B.; Xiao, S.-L.; Wang, F. Therapeutic effect of fecal microbiota transplantation on chronic unpredictable mild stress-induced depression. Front. Cell. Infect. Microbiol. 2022, 12, 900652. [Google Scholar] [CrossRef]
- Gheorghe, C.E.; Ritz, N.L.; Martin, J.A.; Wardill, H.R.; Cryan, J.F.; Clarke, G. Investigating causality with fecal microbiota transplantation in rodents: Applications, recommendations and pitfalls. Gut Microbes 2021, 13, 1941711. [Google Scholar] [CrossRef]
- Müller, B.; Rasmusson, A.J.; Just, D.; Jayarathna, S.; Moazzami, A.; Novicic, Z.K.; Cunningham, J.L. Fecal Short-Chain Fatty Acid Ratios as Related to Gastrointestinal and Depressive Symptoms in Young Adults. Psychosom. Med. 2021, 83, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tan, Y.; Cheng, H.; Zhang, D.; Feng, W.; Peng, C. Functions of Gut Microbiota Metabolites, Current Status and Future Perspectives. Aging Dis. 2022, 13, 1106–1126. [Google Scholar] [CrossRef] [PubMed]
- Nøhr, M.K.; Egerod, K.L.; Christiansen, S.H.; Gille, A.; Offermanns, S.; Schwartz, T.W.; Møller, M. Expression of the short chain fatty acid receptor GPR41/FFAR3 in autonomic and somatic sensory ganglia. Neuroscience 2015, 290, 126–137. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhang, T.; Zeng, Y.; Pei, P.; Liu, Y.; Jia, W.; Zhao, H.; Bi, M.; Wang, S. Sodium butyrate mediates histone crotonylation and alleviated neonatal rats hypoxic–ischemic brain injury through gut–brain axis. Front. Microbiol. 2022, 13, 993146. [Google Scholar] [CrossRef]
- Kratsman, N.; Getselter, D.; Elliott, E. Sodium butyrate attenuates social behavior deficits and modifies the transcription of inhibitory/excitatory genes in the frontal cortex of an autism model. Neuropharmacology 2016, 102, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, C.; Zhu, J.; Lin, Q.; Yu, M.; Wen, J.; Feng, J.; Hu, C. Sodium Butyrate Ameliorates Oxidative Stress-Induced Intestinal Epithelium Barrier Injury and Mitochondrial Damage through AMPK-Mitophagy Pathway. Oxidative Med. Cell. Longev. 2022, 2022, 3745135. [Google Scholar] [CrossRef] [PubMed]
- Patnala, R.; Arumugam, T.V.; Gupta, N.; Dheen, S.T. HDAC Inhibitor Sodium Butyrate-Mediated Epigenetic Regulation Enhances Neuroprotective Function of Microglia during Ischemic Stroke. Mol. Neurobiol. 2017, 54, 6391–6411. [Google Scholar] [CrossRef] [PubMed]
- Erny, D.; Hrabě de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Mu, C.-L.; Farzi, A.; Zhu, W.-Y. Tryptophan Metabolism: A Link Between the Gut Microbiota and Brain. Adv. Nutr. 2020, 11, 709–723. [Google Scholar] [CrossRef]
- Gasaly, N.; de Vos, P.; Hermoso, M.A. Impact of Bacterial Metabolites on Gut Barrier Function and Host Immunity: A Focus on Bacterial Metabolism and Its Relevance for Intestinal Inflammation. Front. Immunol. 2021, 12, 658354. [Google Scholar] [CrossRef]
- Yaghoubfar, R.; Behrouzi, A.; Ashrafian, F.; Shahryari, A.; Moradi, H.R.; Choopani, S.; Hadifar, S.; Vaziri, F.; Nojoumi, S.A.; Fateh, A.; et al. Modulation of serotonin signaling/metabolism by Akkermansia muciniphila and its extracellular vesicles through the gut-brain axis in mice. Sci. Rep. 2020, 10, 22119. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Xu, W.; Wang, J.; Tang, Z.; Zhang, M. The outer membrane protein Amuc_1100 of Akkermansia muciniphila alleviates the depression-like behavior of depressed mice induced by chronic stress. Biochem. Biophys. Res. Commun. 2021, 566, 170–176. [Google Scholar] [CrossRef]
- Guo, H.; Liu, X.; Chen, T.; Wang, X.; Zhang, X. Akkermansia muciniphila Improves Depressive-Like Symptoms by Modulating the Level of 5-HT Neurotransmitters in the Gut and Brain of Mice. Mol. Neurobiol. 2024, 61, 821–834. [Google Scholar] [CrossRef] [PubMed]
- Yaghoubfar, R.; Zare BanadKoki, E.; Ashrafian, F.; Shahryari, A.; Kariman, A.; Davari, M.; Fateh, A.; Khatami, S.; Siadat, S.D. The impact of Akkermansia muciniphila and its extracellular vesicles in the regulation of serotonergic gene expression in a small intestine of mice. Anaerobe 2023, 83, 102786. [Google Scholar] [CrossRef]
- Wichers, M.C.; Maes, M. The role of indoleamine 2,3-dioxygenase (IDO) in the pathophysiology of interferon-alpha-induced depression. J. Psychiatry Neurosci. 2004, 29, 11–17. [Google Scholar] [PubMed]
- Jones, B.D.M.; Daskalakis, Z.J.; Carvalho, A.F.; Strawbridge, R.; Young, A.H.; Mulsant, B.H.; Husain, M.I. Inflammation as a treatment target in mood disorders: Review. BJPsych. Open 2020, 6, e60. [Google Scholar] [CrossRef]
- Sikander, A.; Rana, S.V.; Prasad, K.K. Role of serotonin in gastrointestinal motility and irritable bowel syndrome. Clin. Chim. Acta 2009, 403, 47–55. [Google Scholar] [CrossRef]
- Buey, B.; Forcén, A.; Grasa, L.; Layunta, E.; Mesonero, J.E.; Latorre, E. Gut Microbiota-Derived Short-Chain Fatty Acids: Novel Regulators of Intestinal Serotonin Transporter. Life 2023, 13, 1085. [Google Scholar] [CrossRef] [PubMed]
- Clarke, G.; Grenham, S.; Scully, P.; Fitzgerald, P.; Moloney, R.D.; Shanahan, F.; Dinan, T.G.; Cryan, J.F. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol. Psychiatry 2013, 18, 666–673. [Google Scholar] [CrossRef]
- Strandwitz, P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018, 1693, 128–133. [Google Scholar] [CrossRef]
- Maini Rekdal, V.; Nol Bernadino, P.; Luescher, M.U.; Kiamehr, S.; Le, C.; Bisanz, J.E.; Turnbaugh, P.J.; Bess, E.N.; Balskus, E.P. A widely distributed metalloenzyme class enables gut microbial metabolism of host- and diet-derived catechols. Elife 2020, 9, e50845. [Google Scholar] [CrossRef]
- van Kessel, S.P.; Frye, A.K.; El-Gendy, A.O.; Castejon, M.; Keshavarzian, A.; van Dijk, G.; El Aidy, S. Gut bacterial tyrosine decarboxylases restrict levels of levodopa in the treatment of Parkinson’s disease. Nat. Commun. 2019, 10, 310. [Google Scholar] [CrossRef] [PubMed]
- Otaru, N.; Ye, K.; Mujezinovic, D.; Berchtold, L.; Constancias, F.; Cornejo, F.A.; Krzystek, A.; de Wouters, T.; Braegger, C.; Lacroix, C.; et al. GABA Production by Human Intestinal Bacteroides spp.: Prevalence, Regulation, and Role in Acid Stress Tolerance. Front. Microbiol. 2021, 12, 656895. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; Ahuja, V.; Paul, J. Dysregulation of GABAergic Signalling Contributes in the Pathogenesis of Diarrhea-predominant Irritable Bowel Syndrome. J. Neurogastroenterol. Motil. 2018, 24, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, M.; Rowatt, E. The production of acetylcholine by a strain of Lactobacillus plantarum. J. Gen. Microbiol. 1947, 1, 279–298. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, K.; Misawa, H.; Moriwaki, Y.; Fujii, Y.X.; Fujii, T.; Horiuchi, Y.; Yamada, T.; Imanaka, T.; Kamekura, M. Ubiquitous expression of acetylcholine and its biological functions in life forms without nervous systems. Life Sci. 2007, 80, 2206–2209. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Eguchi, A.; Fujita, Y.; Wan, X.; Chang, L.; Yang, Y.; Shan, J.; Qu, Y.; Ma, L.; Shirayama, Y.; et al. Abnormal compositions of gut microbiota and metabolites are associated with susceptibility versus resilience in rats to inescapable electric stress. J. Affect. Disord. 2023, 331, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Knox, E.G.; Lynch, C.M.K.; Lee, Y.S.; O’Driscoll, C.M.; Clarke, G.; Cryan, J.F.; Aburto, M.R. The gut microbiota is important for the maintenance of blood-cerebrospinal fluid barrier integrity. Eur. J. Neurosci. 2023, 57, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wang, B.; Gao, H.; He, C.; Hua, R.; Liang, C.; Zhang, S.; Wang, Y.; Xin, S.; Xu, J. Vagus Nerve and Underlying Impact on the Gut Microbiota-Brain Axis in Behavior and Neurodegenerative Diseases. J. Inflamm. Res. 2022, 15, 6213–6230. [Google Scholar] [CrossRef] [PubMed]
- Egerod, K.L.; Petersen, N.; Timshel, P.N.; Rekling, J.C.; Wang, Y.; Liu, Q.; Schwartz, T.W.; Gautron, L. Profiling of G protein-coupled receptors in vagal afferents reveals novel gut-to-brain sensing mechanisms. Mol. Metab. 2018, 12, 62–75. [Google Scholar] [CrossRef]
- Madison, A.; Kiecolt-Glaser, J.K. Stress, depression, diet, and the gut microbiota: Human–bacteria interactions at the core of psychoneuroimmunology and nutrition. Curr. Opin. Behav. Sci. 2019, 28, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Jašarević, E.; Howard, C.D.; Misic, A.M.; Beiting, D.P.; Bale, T.L. Stress during pregnancy alters temporal and spatial dynamics of the maternal and offspring microbiome in a sex-specific manner. Sci. Rep. 2017, 7, 44182. [Google Scholar] [CrossRef] [PubMed]
- Bailey, M.T.; Dowd, S.E.; Galley, J.D.; Hufnagle, A.R.; Allen, R.G.; Lyte, M. Exposure to a social stressor alters the structure of the intestinal microbiota: Implications for stressor-induced immunomodulation. Brain Behav. Immun. 2011, 25, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Tsilimigras, M.C.B.; Gharaibeh, R.Z.; Sioda, M.; Gray, L.; Fodor, A.A.; Lyte, M. Interactions between Stress and Sex in Microbial Responses Within the Microbiota-Gut-Brain Axis in a Mouse Model. Psychosom. Med. 2018, 80, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Atrooz, F.; Alkadhi, K.A.; Salim, S. Understanding stress: Insights from rodent models. Curr. Res. Neurobiol. 2021, 2, 100013. [Google Scholar] [CrossRef] [PubMed]
- Tran, I.; Gellner, A.-K. Long-term effects of chronic stress models in adult mice. J. Neural Transm. 2023, 130, 1133–1151. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Fujita, Y.; Ren, Q.; Ma, M.; Dong, C.; Hashimoto, K. Bifidobacterium in the gut microbiota confer resilience to chronic social defeat stress in mice. Sci. Rep. 2017, 7, 45942. [Google Scholar] [CrossRef]
- He, H.; He, H.; Mo, L.; Yuan, Q.; Xiao, C.; Ma, Q.; Yi, S.; Zhou, T.; You, Z.; Zhang, J. Gut microbiota regulate stress resistance by influencing microglia-neuron interactions in the hippocampus. Brain Behav. Immun. Health 2024, 36, 100729. [Google Scholar] [CrossRef] [PubMed]
- Marcondes Ávila, P.R.; Fiorot, M.; Michels, M.; Dominguini, D.; Abatti, M.; Vieira, A.; de Moura, A.B.; Behenck, J.P.; Borba, L.A.; Botelho, M.E.M.; et al. Effects of microbiota transplantation and the role of the vagus nerve in gut–brain axis in animals subjected to chronic mild stress. J. Affect. Disord. 2020, 277, 410–416. [Google Scholar] [CrossRef]
- Pham, H.T.; Bendezú, J.J.; Wadsworth, M.E. HPA–SAM co-activation among racially diverse, economically disadvantaged early adolescents: Secondary analysis with a preliminary test of a multisystem, person-centered approach. Biol. Psychol. 2023, 179, 108546. [Google Scholar] [CrossRef]
- Wang, S.; Ishima, T.; Zhang, J.; Qu, Y.; Chang, L.; Pu, Y.; Fujita, Y.; Tan, Y.; Wang, X.; Hashimoto, K. Ingestion of Lactobacillus intestinalis and Lactobacillus reuteri causes depression- and anhedonia-like phenotypes in antibiotic-treated mice via the vagus nerve. J. Neuroinflamm. 2020, 17, 241. [Google Scholar] [CrossRef]
- Bharwani, A.; Mian, M.F.; Surette, M.G.; Bienenstock, J.; Forsythe, P. Oral treatment with Lactobacillus rhamnosus attenuates behavioural deficits and immune changes in chronic social stress. BMC Med. 2017, 15, 7. [Google Scholar] [CrossRef]
- Wang, S.; Ishima, T.; Qu, Y.; Shan, J.; Chang, L.; Wei, Y.; Zhang, J.; Pu, Y.; Fujita, Y.; Tan, Y.; et al. Ingestion of Faecalibaculum rodentium causes depression-like phenotypes in resilient Ephx2 knock-out mice: A role of brain–gut–microbiota axis via the subdiaphragmatic vagus nerve. J. Affect. Disord. 2021, 292, 565–573. [Google Scholar] [CrossRef]
- Kiu, R.; Hall, L.J. An update on the human and animal enteric pathogen Clostridium perfringens. Emerg. Microbes Infect. 2018, 7, 141. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Nikolova, V.L.; Smith, M.R.B.; Hall, L.J.; Cleare, A.J.; Stone, J.M.; Young, A.H. Perturbations in Gut Microbiota Composition in Psychiatric Disorders: A Review and Meta-analysis. JAMA Psychiatry 2021, 78, 1343–1354. [Google Scholar] [CrossRef]
- Donoso, F.; Cryan, J.F.; Olavarría-Ramírez, L.; Nolan, Y.M.; Clarke, G. Inflammation, Lifestyle Factors, and the Microbiome-Gut-Brain Axis: Relevance to Depression and Antidepressant Action. Clin. Pharmacol. Ther. 2023, 113, 246–259. [Google Scholar] [CrossRef]
- Kinashi, Y.; Hase, K. Partners in Leaky Gut Syndrome: Intestinal Dysbiosis and Autoimmunity. Front. Immunol. 2021, 12, 673708. [Google Scholar] [CrossRef]
- Camilleri, M. Leaky gut: Mechanisms, measurement and clinical implications in humans. Gut 2019, 68, 1516–1526. [Google Scholar] [CrossRef]
- Horn, J.; Mayer, D.E.; Chen, S.; Mayer, E.A. Role of diet and its effects on the gut microbiome in the pathophysiology of mental disorders. Transl. Psychiatry 2022, 12, 164. [Google Scholar] [CrossRef]
- Frost, R.A.; Nystrom, G.J.; Lang, C.H. Lipopolysaccharide regulates proinflammatory cytokine expression in mouse myoblasts and skeletal muscle. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2002, 283, R698–R709. [Google Scholar] [CrossRef] [PubMed]
- Barton, S.M.; Janve, V.A.; McClure, R.; Anderson, A.; Matsubara, J.A.; Gore, J.C.; Pham, W. Lipopolysaccharide Induced Opening of the Blood Brain Barrier on Aging 5XFAD Mouse Model. J. Alzheimers Dis. 2019, 67, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Farhadi, A.; Fields, J.Z.; Keshavarzian, A. Mucosal mast cells are pivotal elements in inflammatory bowel disease that connect the dots: Stress, intestinal hyperpermeability and inflammation. World J. Gastroenterol. 2007, 13, 3027–3030. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.-Q.; Yu, J. Inflammation: A mechanism of depression? Neurosci. Bull. 2014, 30, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Zádor, F.; Joca, S.; Nagy-Grócz, G.; Dvorácskó, S.; Szűcs, E.; Tömböly, C.; Benyhe, S.; Vécsei, L. Pro-Inflammatory Cytokines: Potential Links between the Endocannabinoid System and the Kynurenine Pathway in Depression. Int. J. Mol. Sci. 2021, 22, 5903. [Google Scholar] [CrossRef] [PubMed]
- Amarante-Mendes, G.P.; Adjemian, S.; Branco, L.M.; Zanetti, L.C.; Weinlich, R.; Bortoluci, K.R. Pattern Recognition Receptors and the Host Cell Death Molecular Machinery. Front. Immunol. 2018, 9, 2379. [Google Scholar] [CrossRef]
- Fukata, M.; Arditi, M. The role of pattern recognition receptors in intestinal inflammation. Mucosal Immunol. 2013, 6, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Mathur, A.; Hayward, J.A.; Man, S.M. Molecular mechanisms of inflammasome signaling. J. Leukoc. Biol. 2018, 103, 233–257. [Google Scholar] [CrossRef] [PubMed]
- Maciak, K.; Dziedzic, A.; Saluk, J. Possible role of the NLRP3 inflammasome and the gut–brain axis in multiple sclerosis-related depression. FASEB J. 2023, 37, e22687. [Google Scholar] [CrossRef]
- Man, S.M. Inflammasomes in the gastrointestinal tract: Infection, cancer and gut microbiota homeostasis. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 721–737. [Google Scholar] [CrossRef]
- Zhu, G.; Zhao, J.; Zhang, H.; Chen, W.; Wang, G. Administration of Bifidobacterium breve Improves the Brain Function of Aβ(1-42)-Treated Mice via the Modulation of the Gut Microbiome. Nutrients 2021, 13, 1602. [Google Scholar] [CrossRef]
- Zhu, G.; Guo, M.; Zhao, J.; Zhang, H.; Wang, G.; Chen, W. Integrative Metabolomic Characterization Reveals the Mediating Effect of Bifidobacterium breve on Amino Acid Metabolism in a Mouse Model of Alzheimer’s Disease. Nutrients 2022, 14, 735. [Google Scholar] [CrossRef]
- Jung, J.Y.; Han, S.-S.; Kim, Z.-H.; Kim, M.H.; Kang, H.K.; Jin, H.M.; Lee, M.H. In-Vitro Characterization of Growth Inhibition against the Gut Pathogen of Potentially Probiotic Lactic Acid Bacteria Strains Isolated from Fermented Products. Microorganisms 2021, 9, 2141. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Kim, T.-Y.; Kim, Y.; Lee, S.-H.; Kim, S.; Kang, S.W.; Yang, J.-Y.; Baek, I.-J.; Sung, Y.H.; Park, Y.-Y.; et al. Microbiota-Derived Lactate Accelerates Intestinal Stem-Cell-Mediated Epithelial Development. Cell Host Microbe 2018, 24, 833–846.e6. [Google Scholar] [CrossRef]
- Watanabe, T.; Nishio, H.; Tanigawa, T.; Yamagami, H.; Okazaki, H.; Watanabe, K.; Tominaga, K.; Fujiwara, Y.; Oshitani, N.; Asahara, T.; et al. Probiotic Lactobacillus casei strain Shirota prevents indomethacin-induced small intestinal injury: Involvement of lactic acid. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 297, G506–G513. [Google Scholar] [CrossRef]
- Kaur, S.; Sharma, P.; Mayer, M.J.; Neuert, S.; Narbad, A.; Kaur, S. Beneficial effects of GABA-producing potential probiotic Limosilactobacillus fermentum L18 of human origin on intestinal permeability and human gut microbiota. Microb. Cell Factories 2023, 22, 256. [Google Scholar] [CrossRef]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef]
- Mao, J.-H.; Kim, Y.-M.; Zhou, Y.-X.; Hu, D.; Zhong, C.; Chang, H.; Brislawn, C.J.; Fansler, S.; Langley, S.; Wang, Y.; et al. Genetic and metabolic links between the murine microbiome and memory. Microbiome 2020, 8, 53. [Google Scholar] [CrossRef]
- Magryś, A.; Pawlik, M. Postbiotic Fractions of Probiotics Lactobacillus plantarum 299v and Lactobacillus rhamnosus GG Show Immune-Modulating Effects. Cells 2023, 12, 2538. [Google Scholar] [CrossRef]
- Rudzki, L.; Ostrowska, L.; Pawlak, D.; Małus, A.; Pawlak, K.; Waszkiewicz, N.; Szulc, A. Probiotic Lactobacillus Plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: A double-blind, randomized, placebo controlled study. Psychoneuroendocrinology 2019, 100, 213–222. [Google Scholar] [CrossRef]
- Park, K.; Park, S.; Nagappan, A.; Ray, N.; Kim, J.; Yoon, S.; Moon, Y. Probiotic Escherichia coli Ameliorates Antibiotic-Associated Anxiety Responses in Mice. Nutrients 2021, 13, 811. [Google Scholar] [CrossRef]
- Wu, H.; Wei, J.; Zhao, X.; Liu, Y.; Chen, Z.; Wei, K.; Lu, J.; Chen, W.; Jiang, M.; Li, S.; et al. Neuroprotective effects of an engineered Escherichia coli Nissle 1917 on Parkinson’s disease in mice by delivering GLP-1 and modulating gut microbiota. Bioeng. Transl. Med. 2022, 8, e10351. [Google Scholar] [CrossRef]
- Secher, T.; Kassem, S.; Benamar, M.; Bernard, I.; Boury, M.; Barreau, F.; Oswald, E.; Saoudi, A. Oral Administration of the Probiotic Strain Escherichia coli Nissle 1917 Reduces Susceptibility to Neuroinflammation and Repairs Experimental Autoimmune Encephalomyelitis-Induced Intestinal Barrier Dysfunction. Front. Immunol. 2017, 8, 1096. [Google Scholar] [CrossRef]
- Kim, S.; Shin, Y.-C.; Kim, T.-Y.; Kim, Y.; Lee, Y.-S.; Lee, S.-H.; Kim, M.-N.; Eunju, O.; Kim, K.S.; Kweon, M.-N. Mucin degrader Akkermansia muciniphila accelerates intestinal stem cell-mediated epithelial development. Gut Microbes 2021, 13, 1–20. [Google Scholar] [CrossRef]
- Martin-Gallausiaux, C.; Garcia-Weber, D.; Lashermes, A.; Larraufie, P.; Marinelli, L.; Teixeira, V.; Rolland, A.; Béguet-Crespel, F.; Brochard, V.; Quatremare, T.; et al. Akkermansia muciniphila upregulates genes involved in maintaining the intestinal barrier function via ADP-heptose-dependent activation of the ALPK1/TIFA pathway. Gut Microbes 2022, 14, 2110639. [Google Scholar] [CrossRef]
- Qu, S.; Fan, L.; Qi, Y.; Xu, C.; Hu, Y.; Chen, S.; Liu, W.; Liu, W.; Si, J. Akkermansia muciniphila Alleviates Dextran Sulfate Sodium (DSS)-Induced Acute Colitis by NLRP3 Activation. Microbiol. Spectr. 2021, 9, e0073021. [Google Scholar] [CrossRef]
- Zou, R.; Shen, G.; Wu, Y.; Guo, M.; Chen, J.; Yang, S.; Zhao, H.; Zheng, H. Akkermansia muciniphila plays a neuroprotective role in HMC3 cells through the ‘gut-brain’ axis. Future Microbiol. 2023, 18, 255–266. [Google Scholar] [CrossRef]
- Patterson, A.M.; Mulder, I.E.; Travis, A.J.; Lan, A.; Cerf-Bensussan, N.; Gaboriau-Routhiau, V.; Garden, K.; Logan, E.; Delday, M.I.; Coutts, A.G.P.; et al. Human Gut Symbiont Roseburia hominis Promotes and Regulates Innate Immunity. Front. Immunol. 2017, 8, 1166. [Google Scholar] [CrossRef]
- Song, L.; Sun, Q.; Zheng, H.; Zhang, Y.; Wang, Y.; Liu, S.; Duan, L. Roseburia hominis Alleviates Neuroinflammation via Short-Chain Fatty Acids through Histone Deacetylase Inhibition. Mol. Nutr. Food Res. 2022, 66, e2200164. [Google Scholar] [CrossRef]
- Li, H.; Sun, J.; Du, J.; Wang, F.; Fang, R.; Yu, C.; Xiong, J.; Chen, W.; Lu, Z.; Liu, J. Clostridium butyricum exerts a neuroprotective effect in a mouse model of traumatic brain injury via the gut-brain axis. Neurogastroenterol. Motil. 2018, 30, e13260. [Google Scholar] [CrossRef]
- Fagundes, R.R.; Bourgonje, A.R.; Saeed, A.; Vich Vila, A.; Plomp, N.; Blokzijl, T.; Sadaghian Sadabad, M.; von Martels, J.Z.H.; van Leeuwen, S.S.; Weersma, R.K.; et al. Inulin-grown Faecalibacterium prausnitzii cross-feeds fructose to the human intestinal epithelium. Gut Microbes 2021, 13, 1993582. [Google Scholar] [CrossRef] [PubMed]
- Moosavi, S.M.; Akhavan Sepahi, A.; Mousavi, S.F.; Vaziri, F.; Siadat, S.D. The effect of Faecalibacterium prausnitzii and its extracellular vesicles on the permeability of intestinal epithelial cells and expression of PPARs and ANGPTL4 in the Caco-2 cell culture model. J. Diabetes Metab. Disord. 2020, 19, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liang, R.; Zhang, W.; Tian, K.; Li, J.; Chen, X.; Yu, T.; Chen, Q. Faecalibacterium prausnitzii-derived microbial anti-inflammatory molecule regulates intestinal integrity in diabetes mellitus mice via modulating tight junction protein expression. J. Diabetes 2020, 12, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Ueda, A.; Shinkai, S.; Shiroma, H.; Taniguchi, Y.; Tsuchida, S.; Kariya, T.; Kawahara, T.; Kobayashi, Y.; Kohda, N.; Ushida, K.; et al. Identification of Faecalibacterium prausnitzii strains for gut microbiome-based intervention in Alzheimer’s-type dementia. Cell Rep. Med. 2021, 2, 100398. [Google Scholar] [CrossRef]
- Dinan, T.G.; Stanton, C.; Cryan, J.F. Psychobiotics: A novel class of psychotropic. Biol. Psychiatry 2013, 74, 720–726. [Google Scholar] [CrossRef]
- Sarkar, A.; Lehto, S.M.; Harty, S.; Dinan, T.G.; Cryan, J.F.; Burnet, P.W.J. Psychobiotics and the Manipulation of Bacteria–Gut–Brain Signals. Trends Neurosci. 2016, 39, 763–781. [Google Scholar] [CrossRef] [PubMed]
- Zareie, M.; Johnson-Henry, K.; Jury, J.; Yang, P.-C.; Ngan, B.-Y.; McKay, D.M.; Soderholm, J.D.; Perdue, M.H.; Sherman, P.M. Probiotics prevent bacterial translocation and improve intestinal barrier function in rats following chronic psychological stress. Gut 2006, 55, 1553–1560. [Google Scholar] [CrossRef]
- Gareau, M.G.; Jury, J.; MacQueen, G.; Sherman, P.M.; Perdue, M.H. Probiotic treatment of rat pups normalises corticosterone release and ameliorates colonic dysfunction induced by maternal separation. Gut 2007, 56, 1522–1528. [Google Scholar] [CrossRef]
- Messaoudi, M.; Lalonde, R.; Violle, N.; Javelot, H.; Desor, D.; Nejdi, A.; Bisson, J.F.; Rougeot, C.; Pichelin, M.; Cazaubiel, M.; et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br. J. Nutr. 2011, 105, 755–764. [Google Scholar] [CrossRef]
- Gareau, M.G.; Wine, E.; Reardon, C.; Sherman, P.M. Probiotics prevent death caused by Citrobacter rodentium infection in neonatal mice. J. Infect. Dis. 2010, 201, 81–91. [Google Scholar] [CrossRef]
- Trzeciak, P.; Herbet, M. Role of the Intestinal Microbiome, Intestinal Barrier and Psychobiotics in Depression. Nutrients 2021, 13, 927. [Google Scholar] [CrossRef]
- Arseneault-Bréard, J.; Rondeau, I.; Gilbert, K.; Girard, S.A.; Tompkins, T.A.; Godbout, R.; Rousseau, G. Combination of Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 reduces post-myocardial infarction depression symptoms and restores intestinal permeability in a rat model. Br. J. Nutr. 2012, 107, 1793–1799. [Google Scholar] [CrossRef]
- Girard, S.-A.; Bah, T.M.; Kaloustian, S.; Lada-Moldovan, L.; Rondeau, I.; Tompkins, T.A.; Godbout, R.; Rousseau, G. Lactobacillus helveticus and Bifidobacterium longum taken in combination reduce the apoptosis propensity in the limbic system after myocardial infarction in a rat model. Br. J. Nutr. 2009, 102, 1420–1425. [Google Scholar] [CrossRef]
- Gilbert, K.; Arseneault-Bréard, J.; Flores Monaco, F.; Beaudoin, A.; Bah, T.M.; Tompkins, T.A.; Godbout, R.; Rousseau, G. Attenuation of post-myocardial infarction depression in rats by n-3 fatty acids or probiotics starting after the onset of reperfusion. Br. J. Nutr. 2013, 109, 50–56. [Google Scholar] [CrossRef]
- Callaghan, B.L.; Cowan, C.S.M.; Richardson, R. Treating Generational Stress: Effect of Paternal Stress on Development of Memory and Extinction in Offspring Is Reversed by Probiotic Treatment. Psychol. Sci. 2016, 27, 1171–1180. [Google Scholar] [CrossRef]
- Ait-Belgnaoui, A.; Colom, A.; Braniste, V.; Ramalho, L.; Marrot, A.; Cartier, C.; Houdeau, E.; Theodorou, V.; Tompkins, T. Probiotic gut effect prevents the chronic psychological stress-induced brain activity abnormality in mice. Neurogastroenterol. Motil. 2014, 26, 510–520. [Google Scholar] [CrossRef]
- Liu, Y.-W.; Liu, W.-H.; Wu, C.-C.; Juan, Y.-C.; Wu, Y.-C.; Tsai, H.-P.; Wang, S.; Tsai, Y.-C. Bifidobacterium longum and Lactobacillus helveticus Synergistically Suppress Stress-related Visceral Hypersensitivity through Hypothalamic-Pituitary-Adrenal Axis Modulation. J. Neurogastroenterol. Motil. 2018, 24, 138–146. [Google Scholar] [CrossRef]
- Liu, Y.W.; Liu, W.H.; Wu, C.C.; Juan, Y.C.; Wu, Y.C.; Tsai, H.P.; Wang, S.; Tsai, Y.C. Psychotropic effects of Lactobacillus plantarum PS128 in early life-stressed and naïve adult mice. Brain Res. 2016, 1631, 1–12. [Google Scholar] [CrossRef]
- Tian, P.; Chen, Y.; Zhu, H.; Wang, L.; Qian, X.; Zou, R.; Zhao, J.; Zhang, H.; Qian, L.; Wang, Q.; et al. Bifidobacterium breve CCFM1025 attenuates major depression disorder via regulating gut microbiome and tryptophan metabolism: A randomized clinical trial. Brain Behav. Immun. 2022, 100, 233–241. [Google Scholar] [CrossRef]
- Zhu, R.; Fang, Y.; Li, H.; Liu, Y.; Wei, J.; Zhang, S.; Wang, L.; Fan, R.; Wang, L.; Li, S.; et al. Psychobiotic Lactobacillus plantarum JYLP-326 relieves anxiety, depression, and insomnia symptoms in test anxious college via modulating the gut microbiota and its metabolism. Front. Immunol. 2023, 14, 1158137. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, S.; Zhang, M.; Ren, F.; Ren, Y.; Li, Y.; Liu, N.; Zhang, Y.; Zhang, Q.; Wang, R. Effects of Fermented Milk Containing Lacticaseibacillus paracasei Strain Shirota on Constipation in Patients with Depression: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2021, 13, 2238. [Google Scholar] [CrossRef]
- Akkasheh, G.; Kashani-Poor, Z.; Tajabadi-Ebrahimi, M.; Jafari, P.; Akbari, H.; Taghizadeh, M.; Memarzadeh, M.R.; Asemi, Z.; Esmaillzadeh, A. Clinical and metabolic response to probiotic administration in patients with major depressive disorder: A randomized, double-blind, placebo-controlled trial. Nutrition 2016, 32, 315–320. [Google Scholar] [CrossRef]
- Majeed, M.; Nagabhushanam, K.; Arumugam, S.; Majeed, S.; Ali, F. Bacillus coagulans MTCC 5856 for the management of major depression with irritable bowel syndrome: A randomised, double-blind, placebo controlled, multi-centre, pilot clinical study. Food Nutr. Res. 2018, 62. [Google Scholar] [CrossRef]
- Allen, A.P.; Hutch, W.; Borre, Y.E.; Kennedy, P.J.; Temko, A.; Boylan, G.; Murphy, E.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Bifidobacterium longum 1714 as a translational psychobiotic: Modulation of stress, electrophysiology and neurocognition in healthy volunteers. Transl. Psychiatry 2016, 6, e939. [Google Scholar] [CrossRef]
- Pinto-Sanchez, M.I.; Hall, G.B.; Ghajar, K.; Nardelli, A.; Bolino, C.; Lau, J.T.; Martin, F.-P.; Cominetti, O.; Welsh, C.; Rieder, A.; et al. Probiotic Bifidobacterium longum NCC3001 Reduces Depression Scores and Alters Brain Activity: A Pilot Study in Patients With Irritable Bowel Syndrome. Gastroenterology 2017, 153, 448–459.e8. [Google Scholar] [CrossRef]
- Miyaoka, T.; Kanayama, M.; Wake, R.; Hashioka, S.; Hayashida, M.; Nagahama, M.; Okazaki, S.; Yamashita, S.; Miura, S.; Miki, H.; et al. Clostridium butyricum MIYAIRI 588 as Adjunctive Therapy for Treatment-Resistant Major Depressive Disorder: A Prospective Open-Label Trial. Clin. Neuropharmacol. 2018, 41, 151–155. [Google Scholar] [CrossRef]
- Adikari, A.; Appukutty, M.; Kuan, G. Effects of Daily Probiotics Supplementation on Anxiety Induced Physiological Parameters among Competitive Football Players. Nutrients 2020, 12, 1920. [Google Scholar] [CrossRef]
- Nishida, K.; Sawada, D.; Kawai, T.; Kuwano, Y.; Fujiwara, S.; Rokutan, K. Para-psychobiotic Lactobacillus gasseri CP2305 ameliorates stress-related symptoms and sleep quality. J. Appl. Microbiol. 2017, 123, 1561–1570. [Google Scholar] [CrossRef]
- Steenbergen, L.; Sellaro, R.; van Hemert, S.; Bosch, J.A.; Colzato, L.S. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain Behav. Immun. 2015, 48, 258–264. [Google Scholar] [CrossRef]
- Kouchaki, E.; Tamtaji, O.R.; Salami, M.; Bahmani, F.; Daneshvar Kakhaki, R.; Akbari, E.; Tajabadi-Ebrahimi, M.; Jafari, P.; Asemi, Z. Clinical and metabolic response to probiotic supplementation in patients with multiple sclerosis: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2017, 36, 1245–1249. [Google Scholar] [CrossRef]
- Kato-Kataoka, A.; Nishida, K.; Takada, M.; Suda, K.; Kawai, M.; Shimizu, K.; Kushiro, A.; Hoshi, R.; Watanabe, O.; Igarashi, T.; et al. Fermented milk containing Lactobacillus casei strain Shirota prevents the onset of physical symptoms in medical students under academic examination stress. Benef. Microbes 2016, 7, 153–156. [Google Scholar] [CrossRef]
- Akbari, E.; Asemi, Z.; Daneshvar Kakhaki, R.; Bahmani, F.; Kouchaki, E.; Tamtaji, O.R.; Hamidi, G.A.; Salami, M. Effect of Probiotic Supplementation on Cognitive Function and Metabolic Status in Alzheimer’s Disease: A Randomized, Double-Blind and Controlled Trial. Front. Aging Neurosci. 2016, 8, 256. [Google Scholar] [CrossRef]
- Hwang, Y.-H.; Park, S.; Paik, J.-W.; Chae, S.-W.; Kim, D.-H.; Jeong, D.-G.; Ha, E.; Kim, M.; Hong, G.; Park, S.-H.; et al. Efficacy and Safety of Lactobacillus Plantarum C29-Fermented Soybean (DW2009) in Individuals with Mild Cognitive Impairment: A 12-Week, Multi-Center, Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2019, 11, 305. [Google Scholar] [CrossRef]
- Butler, M.I.; Bastiaanssen, T.F.S.; Long-Smith, C.; Berding, K.; Morkl, S.; Cusack, A.M.; Strain, C.; Busca, K.; Porteous-Allen, P.; Claesson, M.J.; et al. Recipe for a Healthy Gut: Intake of Unpasteurised Milk Is Associated with Increased Lactobacillus Abundance in the Human Gut Microbiome. Nutrients 2020, 12, 1468. [Google Scholar] [CrossRef]
- Mohammadi, A.A.; Jazayeri, S.; Khosravi-Darani, K.; Solati, Z.; Mohammadpour, N.; Asemi, Z.; Adab, Z.; Djalali, M.; Tehrani-Doost, M.; Hosseini, M.; et al. The effects of probiotics on mental health and hypothalamic–pituitary–adrenal axis: A randomized, double-blind, placebo-controlled trial in petrochemical workers. Nutr. Neurosci. 2016, 19, 387–395. [Google Scholar] [CrossRef]
- Nishihira, J.; Kagami-Katsuyama, H.; Tanaka, A.; Nishimura, M.; Kobayashi, T.; Kawasaki, Y. Elevation of natural killer cell activity and alleviation of mental stress by the consumption of yogurt containing Lactobacillus gasseri SBT2055 and Bifidobacterium longum SBT2928 in a double-blind, placebo-controlled clinical trial. J. Funct. Foods 2014, 11, 261–268. [Google Scholar] [CrossRef]
- Kazemi, A.; Noorbala, A.A.; Azam, K.; Eskandari, M.H.; Djafarian, K. Effect of probiotic and prebiotic vs placebo on psychological outcomes in patients with major depressive disorder: A randomized clinical trial. Clin. Nutr. 2019, 38, 522–528. [Google Scholar] [CrossRef]
- Moludi, J.; Khedmatgozar, H.; Nachvak, S.M.; Abdollahzad, H.; Moradinazar, M.; Sadeghpour Tabaei, A. The effects of co-administration of probiotics and prebiotics on chronic inflammation, and depression symptoms in patients with coronary artery diseases: A randomized clinical trial. Nutr. Neurosci. 2022, 25, 1659–1668. [Google Scholar] [CrossRef]
- Mysonhimer, A.R.; Cannavale, C.N.; Bailey, M.A.; Khan, N.A.; Holscher, H.D. Prebiotic Consumption Alters Microbiota but Not Biological Markers of Stress and Inflammation or Mental Health Symptoms in Healthy Adults: A Randomized, Controlled, Crossover Trial. J. Nutr. 2023, 153, 1283–1296. [Google Scholar] [CrossRef]
- Gangwisch, J.E.; Hale, L.; Garcia, L.; Malaspina, D.; Opler, M.G.; Payne, M.E.; Rossom, R.C.; Lane, D. High glycemic index diet as a risk factor for depression: Analyses from the Women’s Health Initiative. Am. J. Clin. Nutr. 2015, 102, 454–463. [Google Scholar] [CrossRef]
- Xia, G.; Han, Y.; Meng, F.; He, Y.; Srisai, D.; Farias, M.; Dang, M.; Palmiter, R.D.; Xu, Y.; Wu, Q. Reciprocal control of obesity and anxiety–depressive disorder via a GABA and serotonin neural circuit. Mol. Psychiatry 2021, 26, 2837–2853. [Google Scholar] [CrossRef]
- Macfarlane, S.; Macfarlane, G.T. Regulation of short-chain fatty acid production. Proc. Nutr. Soc. 2003, 62, 67–72. [Google Scholar] [CrossRef]
- Vijay, A.; Astbury, S.; Le Roy, C.; Spector, T.D.; Valdes, A.M. The prebiotic effects of omega-3 fatty acid supplementation: A six-week randomised intervention trial. Gut Microbes 2021, 13, 1863133. [Google Scholar] [CrossRef]
- Robertson, R.C.; Seira Oriach, C.; Murphy, K.; Moloney, G.M.; Cryan, J.F.; Dinan, T.G.; Paul Ross, R.; Stanton, C. Omega-3 polyunsaturated fatty acids critically regulate behaviour and gut microbiota development in adolescence and adulthood. Brain Behav. Immun. 2017, 59, 21–37. [Google Scholar] [CrossRef]
- Hakkarainen, R.; Partonen, T.; Haukka, J.; Virtamo, J.; Albanes, D.; Lönnqvist, J. Is low dietary intake of omega-3 fatty acids associated with depression? Am. J. Psychiatry 2004, 161, 567–569. [Google Scholar] [CrossRef]
- Richardson, A.J.; Puri, B.K. A randomized double-blind, placebo-controlled study of the effects of supplementation with highly unsaturated fatty acids on ADHD-related symptoms in children with specific learning difficulties. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2002, 26, 233–239. [Google Scholar] [CrossRef]
- Chrysohoou, C.; Tsitsinakis, G.; Siassos, G.; Psaltopoulou, T.; Galiatsatos, N.; Metaxa, V.; Lazaros, G.; Miliou, A.; Giakoumi, E.; Mylonakis, C.; et al. Fish Consumption Moderates Depressive Symptomatology in Elderly Men and Women from the IKARIA Study. Cardiol. Res. Pract. 2010, 2011, 219578. [Google Scholar] [CrossRef]
- Hoffmire, C.A.; Block, R.C.; Thevenet-Morrison, K.; van Wijngaarden, E. Associations between omega-3 poly-unsaturated fatty acids from fish consumption and severity of depressive symptoms: An analysis of the 2005–2008 National Health and Nutrition Examination Survey. Prostaglandins Leukot Essent Fat. Acids 2012, 86, 155–160. [Google Scholar] [CrossRef]
- Taram, F.; Winter, A.N.; Linseman, D.A. Neuroprotection comparison of chlorogenic acid and its metabolites against mechanistically distinct cell death-inducing agents in cultured cerebellar granule neurons. Brain Res. 2016, 1648, 69–80. [Google Scholar] [CrossRef]
- Sowndhararajan, K.; Deepa, P.; Kim, M.; Park, S.J.; Kim, S. Neuroprotective and Cognitive Enhancement Potentials of Baicalin: A Review. Brain Sci. 2018, 8, 104. [Google Scholar] [CrossRef]
- Ramaholimihaso, T.; Bouazzaoui, F.; Kaladjian, A. Curcumin in Depression: Potential Mechanisms of Action and Current Evidence—A Narrative Review. Front. Psychiatry 2020, 11, 572533. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Zhang, Y.; Zhu, L.; Ying, Y.; Hao, W.; Wang, L.; He, L.; Zhao, D.; Chen, J.-X.; Gao, Y.; et al. Liquiritin apioside alleviates colonic inflammation and accompanying depression-like symptoms in colitis by gut metabolites and the balance of Th17/Treg. Phytomedicine 2023, 120, 155039. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-Y.; Yin, Y.; Li, D.-N.; Zhao, D.-Y.; Huang, J.-Q. Biological Activities of p-Hydroxycinnamic Acids in Maintaining Gut Barrier Integrity and Function. Foods 2023, 12, 2636. [Google Scholar] [CrossRef] [PubMed]
- Pirbaglou, M.; Katz, J.; de Souza, R.J.; Stearns, J.C.; Motamed, M.; Ritvo, P. Probiotic supplementation can positively affect anxiety and depressive symptoms: A systematic review of randomized controlled trials. Nutr. Res. 2016, 36, 889–898. [Google Scholar] [CrossRef]
- Burokas, A.; Moloney, R.D.; Dinan, T.G.; Cryan, J.F. Microbiota regulation of the Mammalian gut-brain axis. Adv. Appl. Microbiol. 2015, 91, 1–62. [Google Scholar] [CrossRef]
- Sharma, R.; Gupta, D.; Mehrotra, R.; Mago, P. Psychobiotics: The Next-Generation Probiotics for the Brain. Curr. Microbiol. 2021, 78, 449–463. [Google Scholar] [CrossRef]


| Bacteria | Effects on Intestines | Effects on the CNS |
|---|---|---|
| Bifidobacterium breve | Regulation of the gut microbiota composition [123]. | Positive impacts on cognitive function, neuroprotective effect, and improvement of synaptic plasticity [123,124]. |
| Lactic acid bacteria (LAB) | Inhibition of gut pathogenic bacteria, maintenance of gut barrier integrity, and homeostasis [125]. Regeneration of epithelial cells [126]. | Reduction in neuroinflammation [127]. |
| Lactobacillaceae and Bifidobacterium | Enhancement of the integrity of the intestinal epithelial barrier by increasing the levels of junction proteins and beneficially influencing the gut microbiota [128]. | Improvement in memory and regulation of emotional behaviors by increasing the level of GABA in the hippocampus and regulation of central GABA receptor expression [129,130]. |
| Lactobacillus | Lactobacillus plantarum 299v and Lactobacillus rhamnosus GG suppress inflammation and protect against intestinal barrier damage through regulatory effects on LPS-mediated cytotoxic activity in intestinal epithelial cells [131]. | Lactobacillus plantarum 299v supports selective serotonin reuptake inhibitors (SSRIs) treatment, resulting in improved cognitive function and decreased kynurenine levels [132]. |
| Escherichia coli Nissle 1917 | Regulation of gut microbiota composition and amelioration of colonic barrier function [133,134]. | Reduction in the severity of experimental autoimmune encephalomyelitis (EAE) connected with modulation of the inflammatory response of CD4+ T cells, their migration to the CNS, and restoration of the intestinal barrier integrity [135]. Neuroprotection, improvement in motor deficits, and reduction in brain inflammation [134]. Reductive impact on anxiety-like behaviors [133]. |
| Akkermansia muciniphila | Acceleration of intestinal stem cell proliferation and cell differentiation in the gut [136]. Activation of NF-κB in intestinal cells, enhancement of barrier function and immune responses [137]. Reduction in the severity of acute colitis symptoms and inflammation [138]. | Inhibition of inflammatory cytokines in microglial cells [139]. Reduction in depressive-like behaviors and modulation of gut serotonin dynamics in mice [75]. |
| Roseburia hominis | Immunomodulation [140]. | Reduction in neuroinflammation via histone deacetylase inhibition [141]. |
| Clostridium butyricum | Protection of the gut barrier integrity, a decrease in the levels of D-lactate in plasma and IL-6 in the colon, and up-regulation of occludin expression [142]. | Neuroprotective effects against neurological dysfunction, brain edema, neurodegeneration, and BBB disruption [142]. |
| Faecalibacterium prausnitzii | Enhancement in intestinal cell health and reduction in inflammation [143]. Improvement in gut barrier integrity [144,145]. | Improvement in cognitive impairment in a mouse model of Alzheimer’s disease [146]. |
| Form, Bacterial Strain | Dose | Effects |
|---|---|---|
| Postbiotic, Bacillus coagulans MTCC 5856 | 2 trillion spores | Effective in treating patients with IBS symptoms who had been diagnosed with depression [165]. |
| Probiotic, Bifidobacterium longum 1714 | 1 × 109 CFU/d | Reduced stress symptoms and improved memory [166]. |
| Probiotic, Bifidobacterium longum NCC3001 | 1 × 1010 CFU/g | Reduced symptoms of depression and reduced reactions to negative emotional stimuli [167]. |
| Probiotic, Clostridium butyricum MIYAIRI 588 | 60 mg/d | When combined with antidepressants, this strain was effective in treating drug-resistant depression [168]. |
| Probiotic, Lactobacillus casei Shirota | 1 × 109 for 8 weeks | Reduced perceived stress [169]. |
| Probiotic, Lactobacillus gasseri CP2305 | 1 × 1010 CFU | Reduced reactions to stressful situations and improved sleep quality [170]. |
| Multi-strain probiotic supplement, Bifidobacterium bifidum W23, Bifidobacterium lactis W52, Lactobacillus acidophilus W37, Lactobacillus brevis W63, Lactobacillus casei W56, Lactobacillus salivarius W24, Lactococcus lactis (W19 and W58) | 2.5 × 109 CFU/g | Reduced susceptibility to lowered mood states [171]. |
| Multi-strain probiotic supplement, Lactobacillus acidophilus, Lactobacillus casei, Bifidobacterium bifidum, Lactobacillus fermentum | 1 capsule contained 2 × 109 CFU/g per day, administered for 12 weeks | Improved overall health; reduced anxiety symptoms and depressive conditions. Reduction in inflammation in the body [172]. |
| A fermented beverage made from black soybeans, Lactobacillus gasseri CP2305 | 190 g serving once a day for 5 weeks | Improved sleep quality and reduced stress-related symptoms in healthy adults [170]. |
| A fermented beverage made from black soybeans, Lactobacillus casei Shirota | 100 mL serving once a day for 8 weeks | Increased serotonin levels in the body. Reduced stress symptoms in people exposed to stressful situations [173]. |
| Probiotic milk drink, Lactobacillus acidophilus, Lactobacillus casei, Bifidobacterium bifidum, Lactobacillus fermentum | 200 mL serving once a day for 12 weeks | Positive effects on cognitive function in patients with Alzheimer’s disease (aged 60–95 years) [174]. |
| Fermented soybean seed paste, Lactobacillus plantarum C29 | 800 mg per day for 12 weeks | Improved cognitive function in people with mild cognitive impairment [175]. |
| Unpasteurized milk and dairy products, Lactobacilli | Unlimited consumption for 12 weeks | Reduced stress reactions and anxiety in adults [176]. |
| Probiotic yogurt, Lactobacillus acidophilus LA5 and Bifidobacterium lactis BB12 | 100 g serving once a day for 6 weeks | Positive impact on depression treatment; improvement in symptoms of depression, anxiety, and stress among adults [177]. |
| Yogurt, Lactobacillus gasseri SBT2055 and Bifidobacterium longum SBT2928 | 100 g once a day for 12 weeks | Decreased levels of stress-induced hormone adrenocorticotrophic hormone [178]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dziedzic, A.; Maciak, K.; Bliźniewska-Kowalska, K.; Gałecka, M.; Kobierecka, W.; Saluk, J. The Power of Psychobiotics in Depression: A Modern Approach through the Microbiota–Gut–Brain Axis: A Literature Review. Nutrients 2024, 16, 1054. https://doi.org/10.3390/nu16071054
Dziedzic A, Maciak K, Bliźniewska-Kowalska K, Gałecka M, Kobierecka W, Saluk J. The Power of Psychobiotics in Depression: A Modern Approach through the Microbiota–Gut–Brain Axis: A Literature Review. Nutrients. 2024; 16(7):1054. https://doi.org/10.3390/nu16071054
Chicago/Turabian StyleDziedzic, Angela, Karina Maciak, Katarzyna Bliźniewska-Kowalska, Małgorzata Gałecka, Weronika Kobierecka, and Joanna Saluk. 2024. "The Power of Psychobiotics in Depression: A Modern Approach through the Microbiota–Gut–Brain Axis: A Literature Review" Nutrients 16, no. 7: 1054. https://doi.org/10.3390/nu16071054
APA StyleDziedzic, A., Maciak, K., Bliźniewska-Kowalska, K., Gałecka, M., Kobierecka, W., & Saluk, J. (2024). The Power of Psychobiotics in Depression: A Modern Approach through the Microbiota–Gut–Brain Axis: A Literature Review. Nutrients, 16(7), 1054. https://doi.org/10.3390/nu16071054