A Comparative Analysis between Whole Chinese Yam and Peeled Chinese Yam: Their Hypolipidemic Effects via Modulation of Gut Microbiome in High-Fat Diet-Fed Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Whole Chinese Yam and Peeled Chinese Yam
2.2. Animal Experimental Design and Diets
2.3. Sample Collection
2.4. Serum Biochemical Analysis
2.5. Histopathological Analysis
2.6. Gut Microbiota Analysis
2.7. Statistical Analysis
3. Results
3.1. The Effects of WY and PY on Body Weight, Food Intake, and White Adipose Tissues in Mice
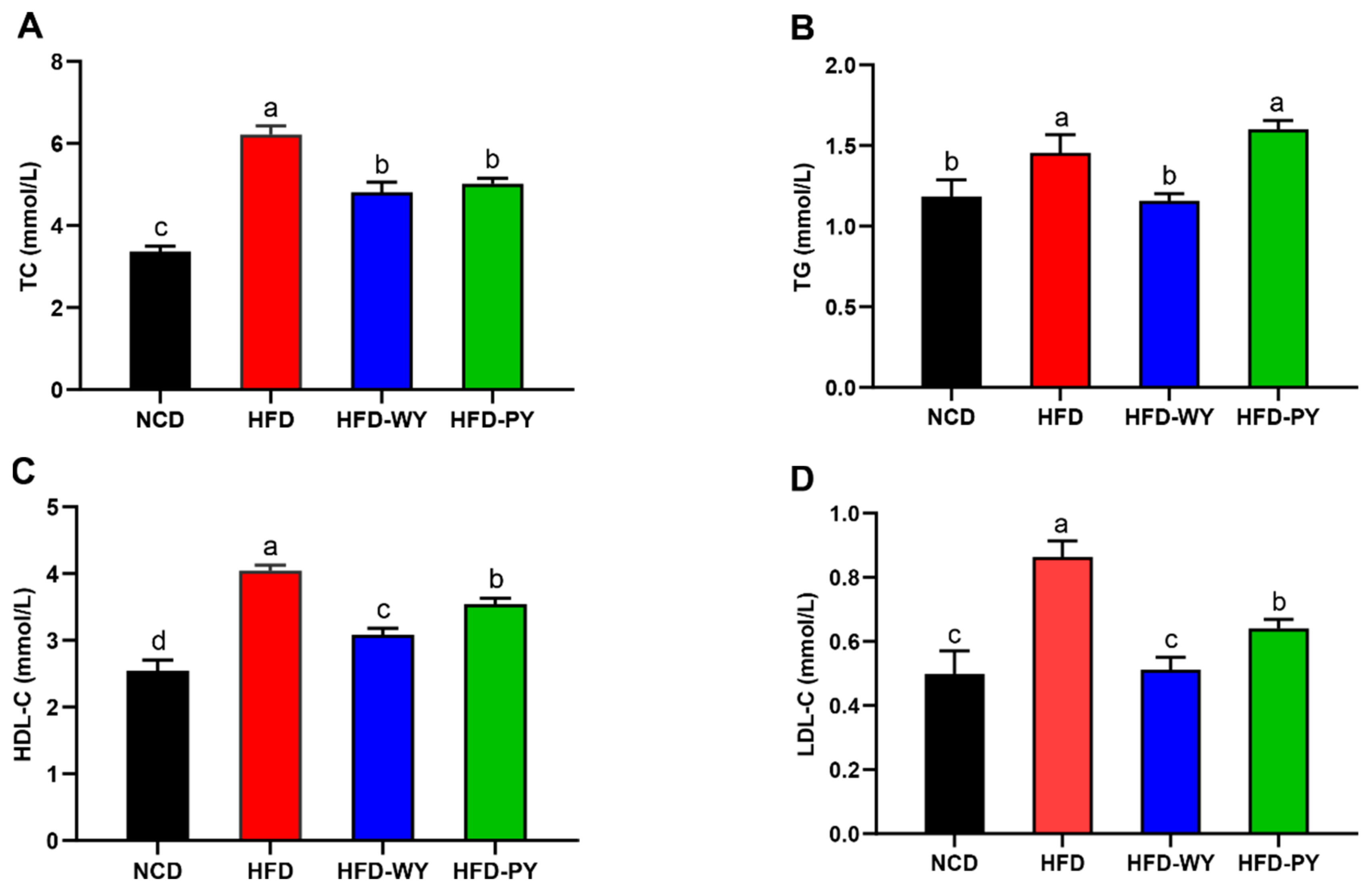
3.2. The Effects of WY and PY on Serum Lipid Profiles
3.3. The Effects of WY and PY on the WAT Hypertrophy and Hepatic Steatosis
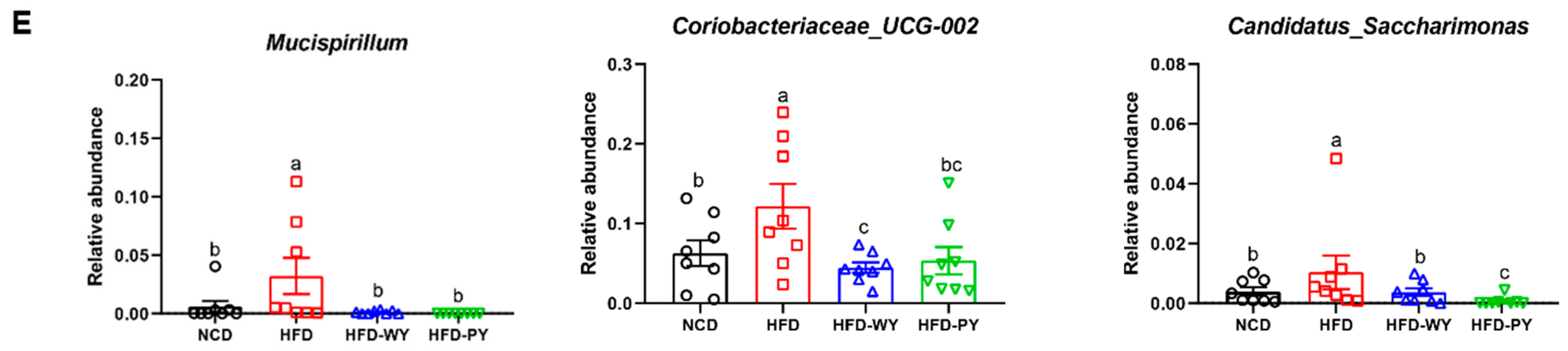
3.4. The Effects of WY and PY on the Gut Microbiota Composition
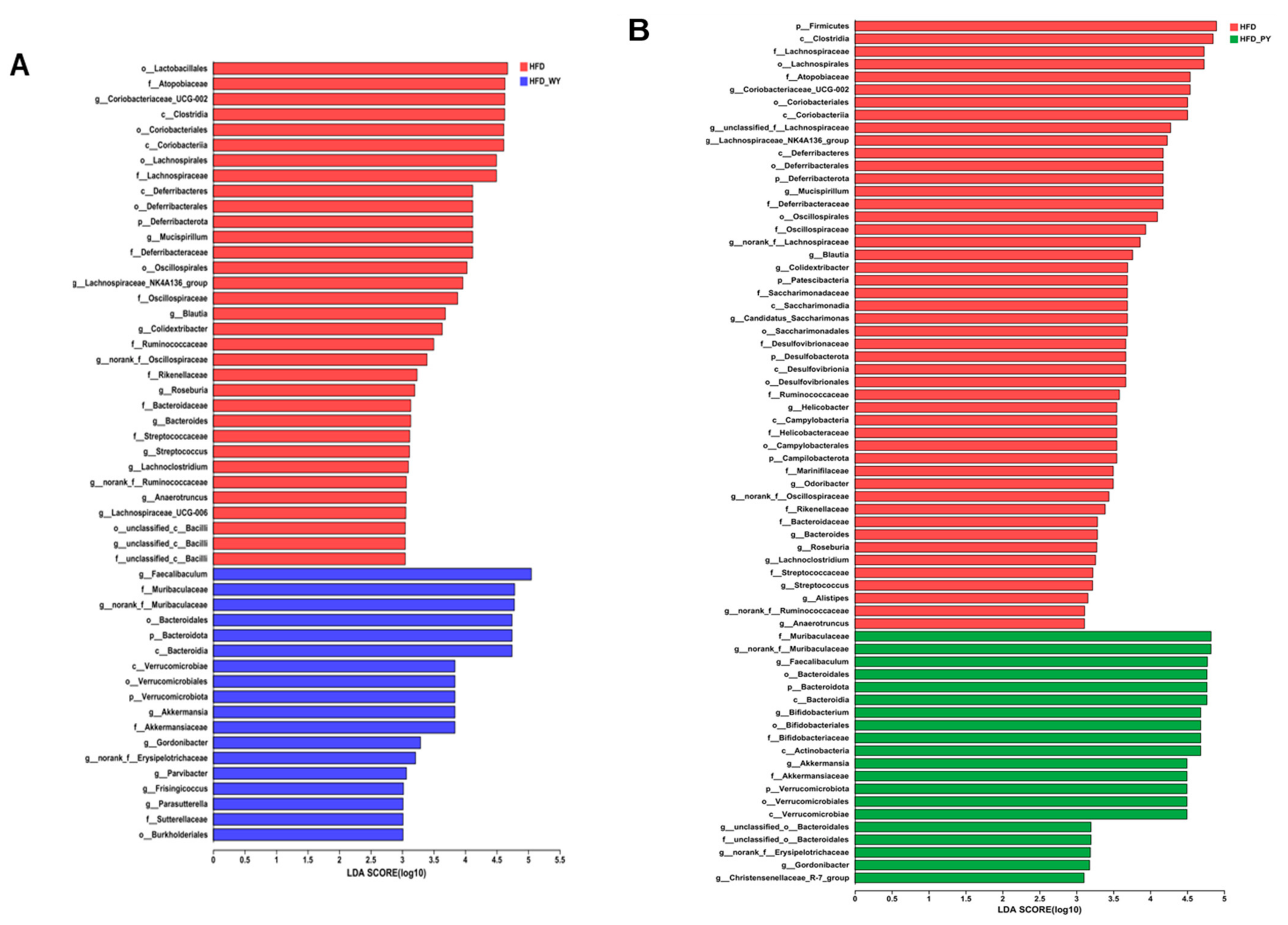
3.5. Key Phylotypes of Gut Microbiota Responding to WY and PY Supplementation
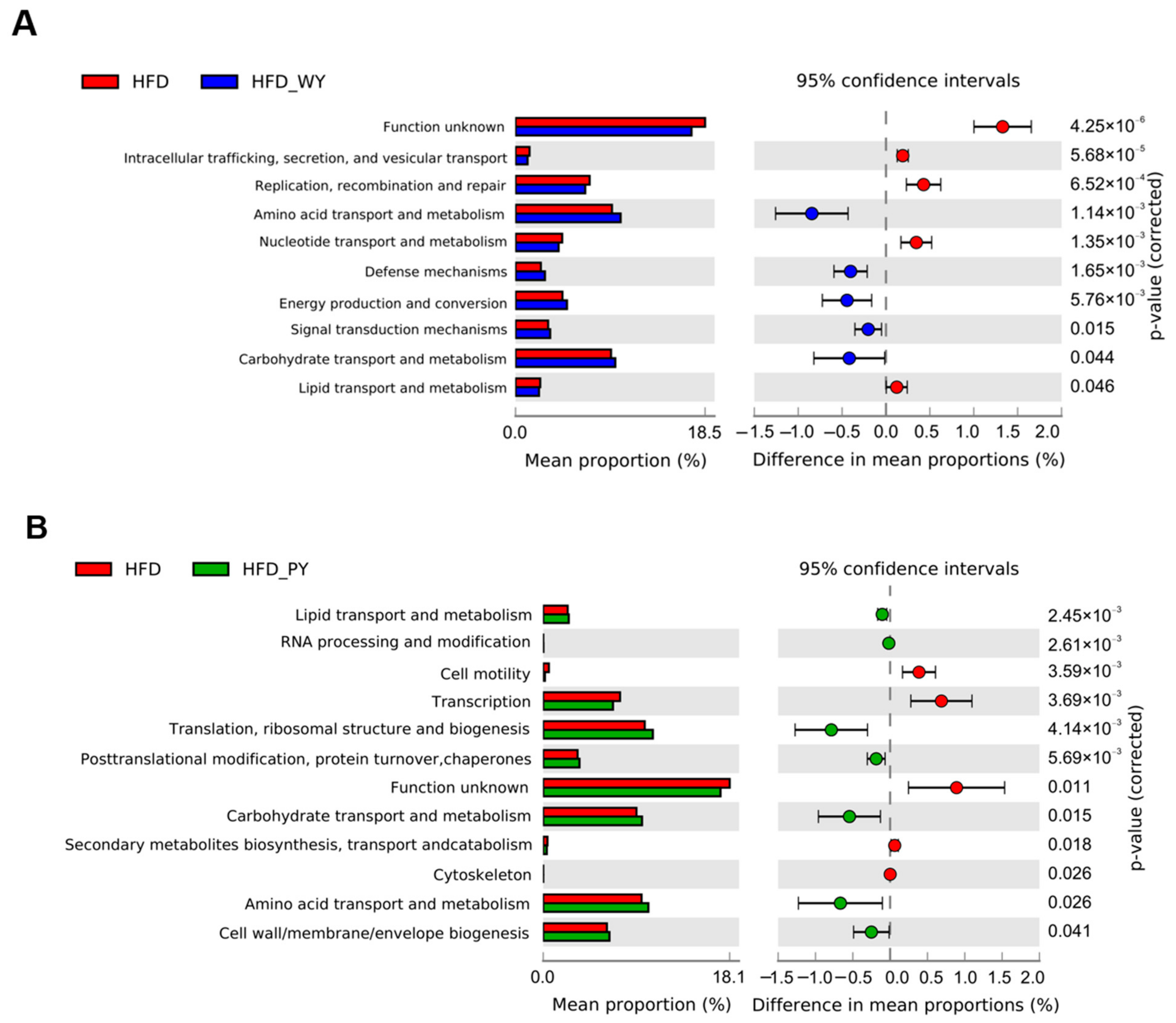
3.6. Metabolic Pathways Altered by WY and PY Supplementation in the Gut Microbiome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chew, N.W.; Ng, C.H.; Tan, D.J.H.; Kong, G.; Lin, C.; Chin, Y.H.; Lim, W.H.; Huang, D.Q.; Quek, J.; Fu, C.E. The global burden of metabolic disease: Data from 2000 to 2019. Cell Metab. 2023, 35, 414–428.e3. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Shi, B.; Li, C.; Cheng, Y. Diacylglycerol-enriched oil from hydrolysis of soybean oil with rhizopus oryzae lipase against high-fat diet-induced obesity in mice. Grain Oil Sci. Technol. 2018, 1, 53–58. [Google Scholar] [CrossRef]
- Nan, G.; Liu, L.; Wu, H.; Yin, S.; Li, C.; Zhao, H.; Chen, H.; Wu, Q. Transcriptomic and metabonomic profiling reveals the antihyperlipidemic effects of tartary buckwheat sprouts in high-fat-diet-fed mice. J. Agric. Food Chem. 2022, 70, 13302–13312. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.-F.; Yue, Z.-L.; Wang, Y.-N.; Li, Y.-D.; Li, C.; Liu, X.-T.; Shi, R.-H.; Huo, N.-N.; Li, D.-D.; Gao, S. Synergistic effect of polysaccharides and flavonoids on lipid and gut microbiota in hyperlipidemic rats. Food Funct. 2023, 14, 921–933. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Song, X.; Zhou, H.; Zhou, X.; Xia, Y.; Dong, X.; Zhong, W.; Tang, S.; Wang, L.; Wen, S.; et al. Gut microbiome associates with lipid-lowering effect of rosuvastatin in vivo. Front. Microbiol. 2018, 9, 530. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The firmicutes/bacteroidetes ratio: A relevant marker of gut dysbiosis in obese patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef]
- Grigor’eva, I.N. Gallstone disease, obesity and the firmicutes/bacteroidetes ratio as a possible biomarker of gut dysbiosis. J. Pers. Med. 2021, 11, 13. [Google Scholar] [CrossRef]
- Hou, D.; Zhao, Q.; Yousaf, L.; Khan, J.; Xue, Y.; Shen, Q. Consumption of mung bean (Vigna radiata L.) attenuates obesity, ameliorates lipid metabolic disorders and modifies the gut microbiota composition in mice fed a high-fat diet. J. Funct. Foods 2020, 64, 103687. [Google Scholar] [CrossRef]
- Zhou, M.; Johnston, L.J.; Wu, C.; Ma, X. Gut microbiota and its metabolites: Bridge of dietary nutrients and obesity-related diseases. Crit. Rev. Food Sci. Nutr. 2023, 63, 3236–3253. [Google Scholar] [CrossRef]
- Hou, D.; Tang, J.; Feng, Q.; Niu, Z.; Shen, Q.; Wang, L.; Zhou, S. Gamma-aminobutyric acid (gaba): A comprehensive review of dietary sources, enrichment technologies, processing effects, health benefits, and its applications. Crit. Rev. Food Sci. Nutr. 2023, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ji, S.; Xu, T.; Zhong, Y.; Xu, M.; Liu, Y.; Li, M.; Fan, B.; Wang, F.; Xiao, J.; et al. Chinese yam (Dioscorea): Nutritional value, beneficial effects, and food and pharmaceutical applications. Trends Food Sci. Technol. 2023, 134, 29–40. [Google Scholar] [CrossRef]
- Darkwa, K.; Olasanmi, B.; Asiedu, R.; Asfaw, A. Review of empirical and emerging breeding methods and tools for yam (Dioscorea spp.) improvement: Status and prospects. Plant Breed. 2020, 139, 474–497. [Google Scholar] [CrossRef]
- Zhou, S.; Huang, G.; Chen, G. Extraction, structural analysis, derivatization and antioxidant activity of polysaccharide from chinese yam. Food Chem. 2021, 361, 130089. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Zhou, Y.; Zhang, R.; Chen, Y.; Wang, F.; Zhang, M. Gut microbial fermentation promotes the intestinal anti-inflammatory activity of chinese yam polysaccharides. Food Chem. 2023, 402, 134003. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Y.; Zhang, Y.; Huo, Z.; Wang, G.; He, Y.; Man, S.; Gao, W. Effects of the polysaccharides extracted from chinese yam (Dioscorea opposita thunb.) on cancer-related fatigue in mice. Food Funct. 2021, 12, 10602–10614. [Google Scholar] [CrossRef] [PubMed]
- Zong, A.; Guan, Q.; Ma, W.; Liu, L.; Jia, M.; Du, F.; Xu, T. Antidiabetic effect of chinese yam (Dioscorea opposita thunb.) in high-fat diet/streptozotocin-induced diabetic rats. Starch Stärke 2023, 75, 2200281. [Google Scholar] [CrossRef]
- Li, T.; Teng, H.; An, F.; Huang, Q.; Chen, L.; Song, H. The beneficial effects of purple yam (Dioscorea alata L.) resistant starch on hyperlipidemia in high-fat-fed hamsters. Food Funct. 2019, 10, 2642–2650. [Google Scholar] [CrossRef]
- Cheng, Z.; Hu, M.; Tao, J.; Yang, H.; Yan, P.; An, G.; Wang, H. The protective effects of chinese yam polysaccharide against obesity-induced insulin resistance. J. Funct. Foods 2019, 55, 238–247. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, J.; Jiao, J.; Su, J.; Sun, W.-T.; Guo, Y.; Ma, S.-X.; Zhang, T.; Meng, D.-X. Effect of nano yam polysaccharide on the blood glucose and blood lipid in rats. Pak. J. Pharm. Sci. 2020, 33, 481–487. [Google Scholar]
- Liu, Y.; Li, H.; Fan, Y.; Man, S.; Liu, Z.; Gao, W.; Wang, T. Antioxidant and antitumor activities of the extracts from chinese yam (Dioscorea opposite thunb.) flesh and peel and the effective compounds. J. Food Sci. 2016, 81, H1553–H1564. [Google Scholar] [CrossRef] [PubMed]
- Pessôa, L.; Rêgo, T.; Asht, L.; Rêgo-Monteiro, I.; Fortunato, R.; Feijó, M.; Correia-Santos, A.M.; Costa, C.; Boaventura, G. Serum and liver lipids distributions in streptozotocin induced diabetic rat treated with diet containing yam (Dioscorea bulbifera) flour. Nutr. Hosp. Organo Of. Soc. Esp. Nutr. Parenter. Enter. 2015, 31, 1647–1653. [Google Scholar]
- Hou, D.; Zhao, Q.; Yousaf, L.; Xue, Y.; Shen, Q. Whole mung bean (Vigna radiata L.) supplementation prevents high-fat diet-induced obesity and disorders in a lipid profile and modulates gut microbiota in mice. Eur. J. Nutr. 2020, 59, 3617–3634. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.; Tang, J.; Huan, M.; Liu, F.; Zhou, S.; Shen, Q. Alteration of fecal microbiome and metabolome by mung bean coat improves diet-induced non-alcoholic fatty liver disease in mice. Food Sci. Hum. Wellness 2022, 11, 1259–1272. [Google Scholar] [CrossRef]
- Langille, M.G.I.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Vega Thurber, R.L.; Knight, R.; et al. Predictive functional profiling of microbial communities using 16s rrna marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef]
- Li, C.; Li, Y.; Ma, Y.; Wang, D.; Zheng, Y.; Wang, X. Effect of black and white sesame on lowering blood lipids of rats with hyperlipidemia induced by high-fat diet. Grain Oil Sci. Technol. 2020, 3, 57–63. [Google Scholar] [CrossRef]
- Li, Q.; Li, W.; Gao, Q.; Zou, Y. Hypoglycemic effect of chinese yam (Dioscorea opposita rhizoma) polysaccharide in different structure and molecular weight. J. Food Sci. 2017, 82, 2487–2494. [Google Scholar] [CrossRef]
- Nie, Y.; Luo, F. Dietary fiber: An opportunity for a global control of hyperlipidemia. Oxidative Med. Cell. Longev. 2021, 2021, 5542342. [Google Scholar] [CrossRef] [PubMed]
- Kusano, Y.; Tsujihara, N.; Masui, H.; Kozai, H.; Takeuchi, W. Consumption of japanese yam improves lipid metabolism in high-cholesterol diet-fed rats. J. Nutr. Sci. Vitaminol. 2016, 62, 350–360. [Google Scholar] [CrossRef]
- Yap, P.-G.; Choi, S.-B.; Liong, M.-T. Allantoin, a potential metabolite that promotes ampk phosphorylation and suppresses cholesterol biosynthesis via the mevalonate pathway and bloch pathway. Appl. Biochem. Biotechnol. 2020, 191, 226–244. [Google Scholar] [CrossRef]
- Yang, M.H.; Chin, Y.W.; Yoon, K.D.; Kim, J. Phenolic compounds with pancreatic lipase inhibitory activity from korean yam (Dioscorea opposita). J. Enzym. Inhib. Med. Chem. 2014, 29, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, C.; Kou, X.; Wang, Y.; Yu, Y.; Zhen, N.; Jiang, J.; Zhaxi, P.; Xue, Z. Synergistic hypolipidemic effects and mechanisms of phytochemicals: A review. Foods 2022, 11, 2774. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.; Feng, Q.; Tang, J.; Shen, Q.; Zhou, S. An update on nutritional profile, phytochemical compounds, health benefits, and potential applications in the food industry of pulses seed coats: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2023, 63, 1960–1982. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Yu, J.; Yang, Y.; Li, Z.; Zhang, Y.; Zhang, F.; Wang, Q.; Xie, Y.; Zhao, B.; Wu, C. Strain-level screening of human gut microbes identifies blautia producta as a new anti-hyperlipidemic probiotic. Gut Microbes 2023, 15, 2228045. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, T.; Si, Y.; Cao, B.; Zhang, Y.; Zheng, X.; Feng, W. Integrated metabolomics and 16s rrna sequencing to investigate the regulation of chinese yam on antibiotic-induced intestinal dysbiosis in rats. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3382–3390. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, H.; Liu, F.; Chen, W. Effects of chinese yam (Dioscorea oppositifolia L.) dietary supplementation on intestinal microflora, digestive enzyme activity and immunity in rainbow trout (Oncorhynchus mykiss). Aquac. Res. 2020, 51, 4698–4712. [Google Scholar] [CrossRef]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The influence of probiotics on the firmicutes/bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ren, Y.; Li, S.; Guo, C.; Gao, Z. Development of a polyphenol-enriched whole kiwifruit dietary supplement and its potential in ameliorating hyperlipidemia. J. Sci. Food Agric. 2023, 104, 2140–2155. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Cantabrana, C.; Delgado, S.; Ruiz, L.; Ruas-Madiedo, P.; Sánchez, B.; Margolles, A. Bifidobacteria and their health-promoting effects. In Bugs as Drugs; ASM Press: Washington, DC, USA, 2018; pp. 73–98. [Google Scholar]
- Ottman, N.; Geerlings, S.Y.; Aalvink, S.; de Vos, W.M.; Belzer, C. Action and function of Akkermansia muciniphila in microbiome ecology, health and disease. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 637–642. [Google Scholar] [CrossRef]
- Yu, Y.; Lu, J.; Sun, L.; Lyu, X.; Chang, X.-Y.; Mi, X.; Hu, M.-G.; Wu, C.; Chen, X. Akkermansia muciniphila: A potential novel mechanism of nuciferine to improve hyperlipidemia. Biomed. Pharmacother. 2021, 133, 111014. [Google Scholar] [CrossRef]
- Katiraei, S.; de Vries, M.R.; Costain, A.H.; Thiem, K.; Hoving, L.R.; van Diepen, J.A.; Smits, H.H.; Bouter, K.E.; Rensen, P.C.N.; Quax, P.H.A.; et al. Akkermansia muciniphila exerts lipid-lowering and immunomodulatory effects without affecting neointima formation in hyperlipidemic apoe*3-leiden.Cetp mice. Mol. Nutr. Food Res. 2020, 64, 1900732. [Google Scholar] [CrossRef]
- Aoki, R.; Kamikado, K.; Suda, W.; Takii, H.; Mikami, Y.; Suganuma, N.; Hattori, M.; Koga, Y. A proliferative probiotic bifidobacterium strain in the gut ameliorates progression of metabolic disorders via microbiota modulation and acetate elevation. Sci. Rep. 2017, 7, 43522. [Google Scholar] [CrossRef]
- Feng, Q.; Niu, Z.; Zhang, S.; Wang, L.; Dong, L.; Hou, D.; Zhou, S. Protective effects of white kidney bean (Phaseolus vulgaris L.) against diet-induced hepatic steatosis in mice are linked to modification of gut microbiota and its metabolites. Nutrients 2023, 15, 3033. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Du, Y.; Zhang, C.; Wang, L. Black rice regulates lipid metabolism, liver injury, oxidative stress and adipose accumulation in high-fat/cholesterol diet mice based on gut microbiota and untargeted metabonomics. J. Nutr. Biochem. 2023, 117, 109320. [Google Scholar] [CrossRef]
- Li, X.; Suo, J.; Huang, X.; Dai, H.; Bian, H.; Zhu, M.; Lin, W.; Han, N. Whole grain qingke attenuates high-fat diet-induced obesity in mice with alterations in gut microbiota and metabolite profile. Front. Nutr. 2021, 8, 761727. [Google Scholar] [CrossRef]
- Ma, Y.; Zhu, L.; Ke, H.; Jiang, S.; Zeng, M. Oyster (Crassostrea gigas) polysaccharide ameliorates obesity in association with modulation of lipid metabolism and gut microbiota in high-fat diet fed mice. Int. J. Biol. Macromol. 2022, 216, 916–926. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.; Zheng, H.; Liu, W.-H.; Cheng, R.; Lan, H.; Sun, T.; Zhao, W.; Li, J.; Shen, X.; Li, H.; et al. Lacticaseibacillus paracasei k56 attenuates high-fat diet-induced obesity by modulating the gut microbiota in mice. Probiotics Antimicrob. Proteins 2023, 15, 844–855. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Tan, L.; Kong, L. Multiple levels of health benefits from resistant starch. J. Agric. Food Res. 2022, 10, 100380. [Google Scholar] [CrossRef]
- Huang, H.; Jiang, Q.; Chen, Y.; Li, X.; Mao, X.; Chen, X.; Huang, L.; Gao, W. Preparation, physico–chemical characterization and biological activities of two modified starches from yam (Dioscorea opposita thunb.). Food Hydrocoll. 2016, 55, 244–253. [Google Scholar] [CrossRef]
- Yuan, H.C.; Meng, Y.; Bai, H.; Shen, D.Q.; Wan, B.C.; Chen, L.Y. Meta-analysis indicates that resistant starch lowers serum total cholesterol and low-density cholesterol. Nutr. Res. 2018, 54, 1–11. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Q.; Lin, J.; Niu, Z.; Wu, T.; Shen, Q.; Hou, D.; Zhou, S. A Comparative Analysis between Whole Chinese Yam and Peeled Chinese Yam: Their Hypolipidemic Effects via Modulation of Gut Microbiome in High-Fat Diet-Fed Mice. Nutrients 2024, 16, 977. https://doi.org/10.3390/nu16070977
Feng Q, Lin J, Niu Z, Wu T, Shen Q, Hou D, Zhou S. A Comparative Analysis between Whole Chinese Yam and Peeled Chinese Yam: Their Hypolipidemic Effects via Modulation of Gut Microbiome in High-Fat Diet-Fed Mice. Nutrients. 2024; 16(7):977. https://doi.org/10.3390/nu16070977
Chicago/Turabian StyleFeng, Qiqian, Jinquan Lin, Zhitao Niu, Tong Wu, Qun Shen, Dianzhi Hou, and Sumei Zhou. 2024. "A Comparative Analysis between Whole Chinese Yam and Peeled Chinese Yam: Their Hypolipidemic Effects via Modulation of Gut Microbiome in High-Fat Diet-Fed Mice" Nutrients 16, no. 7: 977. https://doi.org/10.3390/nu16070977
APA StyleFeng, Q., Lin, J., Niu, Z., Wu, T., Shen, Q., Hou, D., & Zhou, S. (2024). A Comparative Analysis between Whole Chinese Yam and Peeled Chinese Yam: Their Hypolipidemic Effects via Modulation of Gut Microbiome in High-Fat Diet-Fed Mice. Nutrients, 16(7), 977. https://doi.org/10.3390/nu16070977





