The Associations between Snack Intake and Cariogenic Oral Microorganism Colonization in Young Children of a Low Socioeconomic Status
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Oral Sample Collection and Quantification
2.3. Snack Data Collection
2.4. Covariates
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Child Cohort
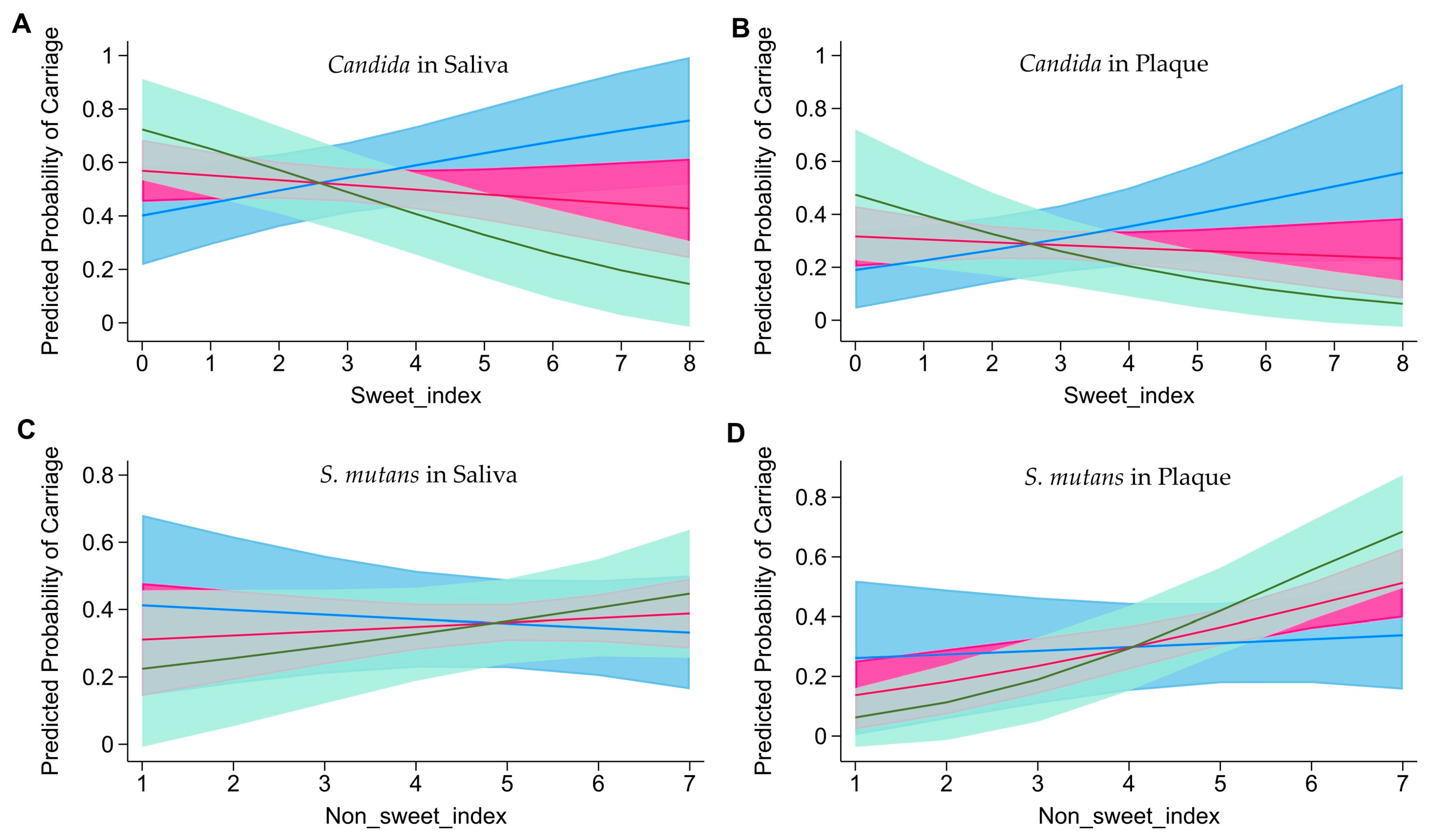
3.2. Association between Snack Intake and Cariogenic Microorganism Carriage
3.3. Rank of the Predictive Factors of Cariogenic Microorganism Carriage
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dye, B.A.; Hsu, K.L.; Afful, J. Prevalence and Measurement of Dental Caries in Young Children. Pediatr. Dent. 2015, 37, 200–216. [Google Scholar] [PubMed]
- Fleming, E.; Afful, J. Prevalence of total and untreated dental caries among youth: United States, 2015–2016. In NCHS Data Brief; no 307; National Center for Health Statistics: Hyattsville, MD, USA, 2018. [Google Scholar]
- Palmer, C.; Kent, R., Jr.; Loo, C.; Hughes, C.; Stutius, E.; Pradhan, N.; Dahlan, M.; Kanasi, E.; Arevalo Vasquez, S.; Tanner, A. Diet and caries-associated bacteria in severe early childhood caries. J. Dent. Res. 2010, 89, 1224–1229. [Google Scholar] [CrossRef] [PubMed]
- Manchanda, S.; Sardana, D.; Peng, S.; Lo, E.C.M.; Chandwani, N.; Yiu, C.K.Y. Is Mutans Streptococci count a risk predictor of Early Childhood Caries? A systematic review and meta-analysis. BMC Oral Health 2023, 23, 648. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, F.G.; Silva, D.S.; Hebling, J.; Spolidorio, L.C.; Spolidorio, D.M. Presence of mutans streptococci and Candida spp. in dental plaque/dentine of carious teeth and early childhood caries. Arch. Oral Biol. 2006, 51, 1024–1028. [Google Scholar] [CrossRef]
- Al-Ahmad, A.; Auschill, T.M.; Dakhel, R.; Wittmer, A.; Pelz, K.; Heumann, C.; Hellwig, E.; Arweiler, N.B. Prevalence of Candida albicans and Candida dubliniensis in caries-free and caries-active children in relation to the oral microbiota-a clinical study. Clin. Oral. Investig. 2016, 20, 1963–1971. [Google Scholar] [CrossRef] [PubMed]
- Samaranayake, L.P.; Hughes, A.; Weetman, D.A.; MacFarlane, T.W. Growth and acid production of Candida species in human saliva supplemented with glucose. J. Oral. Pathol. 1986, 15, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Klinke, T.; Kneist, S.; de Soet, J.J.; Kuhlisch, E.; Mauersberger, S.; Forster, A.; Klimm, W. Acid production by oral strains of Candida albicans and Lactobacilli. Caries Res. 2009, 43, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Moon, Y.; Li, L.; Rustchenko, E.; Wakabayashi, H.; Zhao, X.; Feng, C.; Gill, S.R.; McLaren, S.; Malmstrom, H.; et al. Candida albicans Carriage in Children with Severe Early Childhood Caries (S-ECC) and Maternal Relatedness. PLoS ONE 2016, 11, e0164242. [Google Scholar] [CrossRef] [PubMed]
- Gregoire, S.; Xiao, J.; Silva, B.B.; Gonzalez, I.; Agidi, P.S.; Klein, M.I.; Ambatipudi, K.S.; Rosalen, P.L.; Bauserman, R.; Waugh, R.E.; et al. Role of glucosyltransferase B in interactions of Candida albicans with Streptococcus mutans and with an experimental pellicle on hydroxyapatite surfaces. Appl. Environ. Microbiol. 2011, 77, 6357–6367. [Google Scholar] [CrossRef]
- Hwang, G.; Marsh, G.; Gao, L.; Waugh, R.; Koo, H. Binding Force Dynamics of Streptococcus mutans-glucosyltransferase B to Candida albicans. J. Dent. Res. 2015, 94, 1310–1317. [Google Scholar] [CrossRef]
- Koo, H.; Bowen, W.H. Candida albicans and Streptococcus mutans: A potential synergistic alliance to cause virulent tooth decay in children. Future Microbiol. 2014, 9, 1295–1297. [Google Scholar] [CrossRef]
- Zero, D. Recaldent™—Evidence for clinical activity. Adv. Dent. Res. 2009, 21, 30–34. [Google Scholar] [CrossRef]
- Dunford, E.; Popkin, B. 37 year snacking trends for US children 1977–2014. Pediatr. Obes. 2018, 13, 247–255. [Google Scholar] [CrossRef]
- Xue, H.; Maguire, R.L.; Liu, J.; Kollins, S.H.; Murphy, S.K.; Hoyo, C.; Fuemmeler, B.F. Snacking frequency and dietary intake in toddlers and preschool children. Appetite 2019, 142, 104369. [Google Scholar] [CrossRef]
- Evans, E.W.; Hayes, C.; Palmer, C.A.; Bermudez, O.I.; Cohen, S.A.; Must, A. Dietary intake and severe early childhood caries in low-income, young children. J. Acad. Nutr. Diet. 2013, 113, 1057–1061. [Google Scholar] [CrossRef]
- MacKeown, J.M.; Faber, M. Frequency of consumption of cariogenic food items by 4-month-old to 24-month-old children: Comparison between two rural communities in KwaZulu–Natal, South Africa. Int. J. Food Sci. Nutr. 2005, 56, 95–103. [Google Scholar] [CrossRef]
- Dotsey, R.P.; Moser, E.A.S.; Eckert, G.J.; Gregory, R.L. Effects of Cola-Flavored Beverages and Caffeine on Streptococcus mutans Biofilm Formation and Metabolic Activity. J. Clin. Pediatr. Dent. 2017, 41, 294–299. [Google Scholar] [CrossRef]
- Cornejo, C.F.; Soken, L.J.; Salgado, P.A.; Gliosca, L.A.; Squassi, A.F. Detection of Streptococcus mutans and Streptococcus sobrinus and Their Association with Oral Microbiome Stressors in 6–18-month-old Infants. Int. J. Clin. Pediatr. Dent. 2023, 16, 68–73. [Google Scholar] [CrossRef]
- Habibian, M.; Beighton, D.; Stevenson, R.; Lawson, M.; Roberts, G. Relationships between dietary behaviours, oral hygiene and mutans streptococci in dental plaque of a group of infants in southern England. Arch. Oral Biol. 2002, 47, 491–498. [Google Scholar] [CrossRef]
- Weber-Gasparoni, K.; Goebel, B.M.; Drake, D.R.; Kramer, K.W.; Warren, J.J.; Reeve, J.; Dawson, D.V. Factors associated with mutans streptococci among young WIC-enrolled children. J. Public. Health Dent. 2012, 72, 269–278. [Google Scholar] [CrossRef]
- Mohan, A.; Morse, D.E.; O’Sullivan, D.M.; Tinanoff, N. The relationship between bottle usage/content, age, and number of teeth with mutans streptococci colonization in 6–24-month-old children. Community Dent. Oral Epidemiol. 1998, 26, 12–20. [Google Scholar] [PubMed]
- Ingemansson Hultquist, A.; Lingström, P.; Bågesund, M. Risk factors for early colonization of mutans streptococci—A multiple logistic regression analysis in Swedish 1-year-olds. BMC Oral Health 2014, 14, 147. [Google Scholar] [CrossRef] [PubMed]
- Roeters, F.J.; van der Hoeven, J.S.; Burgersdijk, R.C.; Schaeken, M.J. Lactobacilli, mutants streptococci and dental caries: A longitudinal study in 2-year-old children up to the age of 5 years. Caries Res. 1995, 29, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Wan, A.K.; Seow, W.K.; Purdie, D.M.; Bird, P.S.; Walsh, L.J.; Tudehope, D.I. A longitudinal study of Streptococcus mutans colonization in infants after tooth eruption. J. Dent. Res. 2003, 82, 504–508. [Google Scholar] [CrossRef]
- Alkhars, N.; Zeng, Y.; Alomeir, N.; Al Jallad, N.; Wu, T.T.; Aboelmagd, S.; Youssef, M.; Jang, H.; Fogarty, C.; Xiao, J. Oral Candida Predicts Streptococcus mutans Emergence in Underserved US Infants. J. Dent. Res. 2022, 101, 54–62. [Google Scholar] [CrossRef]
- Xiao, J.; Fogarty, C.; Wu, T.T.; Alkhers, N.; Zeng, Y.; Thomas, M.; Youssef, M.; Wang, L.; Cowen, L.; Abdelsalam, H.; et al. Oral health and Candida carriage in socioeconomically disadvantaged US pregnant women. BMC Pregnancy Childbirth 2019, 19, 480. [Google Scholar] [CrossRef] [PubMed]
- Little, W.A.; Korts, D.C.; Thomson, L.A.; Bowen, W.H. Comparative recovery of Streptococcus mutans on ten isolation media. J. Clin. Microbiol. 1977, 5, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Chaffee, B.W.; Feldens, C.A.; Rodrigues, P.H.; Vítolo, M.R. Feeding practices in infancy associated with caries incidence in early childhood. Community Dent. Oral Epidemiol. 2015, 43, 338–348. [Google Scholar] [CrossRef]
- Peres, M.A.; Sheiham, A.; Liu, P.; Demarco, F.F.; Silva, A.E.; Assunção, M.C.; Menezes, A.M.; Barros, F.C.; Peres, K.G. Sugar Consumption and Changes in Dental Caries from Childhood to Adolescence. J. Dent. Res. 2016, 95, 388–394. [Google Scholar] [CrossRef]
- EWG. Children’s Cereals: Sugar by the Pound. 2014. Available online: https://www.ewg.org/research/childrens-cereals (accessed on 30 January 2024).
- Pizzo, G.; Giuliana, G.; Milici, M.E.; Giangreco, R. Effect of dietary carbohydrates on the in vitro epithelial adhesion of Candida albicans, Candida tropicalis, and Candida krusei. New Microbiol. 2000, 23, 63–71. [Google Scholar]
- Negrini, T.C.; Ren, Z.; Miao, Y.; Kim, D.; Simon-Soro, Á.; Liu, Y.; Koo, H.; Arthur, R.A. Dietary sugars modulate bacterial-fungal interactions in saliva and inter-kingdom biofilm formation on apatitic surface. Front. Cell. Infect. Microbiol. 2022, 12, 993640. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Percentage or Mean (SD) | ||
|---|---|---|---|
| 12 Months a (n = 123) | 18 Months a (n = 97) | 24 Months a (n = 104) | |
| Race__White | 24.4% | 23.7% | 24.0% |
| __Black | 54.5% | 53.6% | 54.8% |
| Female | 51.2% | 50.5% | 51.9% |
| Maternal education (High school or less) | 56.1% | 54.6% | 55.8% |
| Dad as care provider | 43.1% | 48.5% | 45.2% |
| Exclusively breastfeeding at 12 months | 15.5% | 15.5% | 13.5% |
| History of antibiotics use | 12.2% | 16.5% | 18.3% |
| Tooth brushing | 66.7% | 92.8% | 99.0% |
| Number of erupted teeth | 5.8 (2.7) | 13.2 (3.2) | 17.1 (1.9) |
| Plaque score | 0.24 (0.42) | 0.49 (0.63) | 0.63 (0.68) |
| ECC | 3.3% | 12.4% | 25.0% |
| Predictors | |||
| Snack__Sweet index | 2.1 (1.5) | 3.1 (1.7) | 3.6 (1.7) |
| Snack__Non-sweet index | 4.5 (1.4) | 5.2 (0.9) | 5.2 (1.1) |
| Outcomes | |||
| S. mutans carriage__saliva | 21.1% | 41.2% | 50.0% |
| S. mutans carriage__plaque | 21.7% | 36.1% | 56.7% |
| Candida carriage__saliva | 50.4% | 54.6% | 40.4% |
| Candida carriage__plaque | 25.8% | 23.7% | 29.8% |
| Sweet Index | Non-Sweet Index | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| S. mutans carriage__saliva | 1.05 | 0.85, 1.29 | 0.68 | 1.06 | 0.80, 1.42 | 0.68 |
| S. mutans carriage__plaque | 0.99 | 0.79, 1.25 | 0.95 | 1.67 | 1.14, 2.46 | 0.01 |
| Candida carriage__saliva | 0.91 | 0.75, 1.09 | 0.31 | 1.13 | 0.89, 1.45 | 0.32 |
| Candida carriage__plaque | 0.93 | 0.75, 1.16 | 0.54 | 0.81 | 0.61, 1.07 | 0.13 |
| S. mutans | Candida | |||||||
|---|---|---|---|---|---|---|---|---|
| Saliva | Plaque | Saliva | Plaque | |||||
| OR (95%CI) | p | OR (95%CI) | p | OR (95%CI) | p | OR (95%CI) | p | |
| Sweet Index | 1.70 (0.80, 3.64) | 0.17 | 1.54 (0.66, 3.56) | 0.26 | 2.69 (1.34, 5.43) | 0.006 | 2.80 (1.29, 6.09) | 0.01 |
| Time | 1.12 (0.93, 1.35) | 0.22 | 1.16 (0.94, 1.42) | 0.17 | 1.17 (0.99, 1.38) | 0.06 | 1.17 (0.98, 1.40) | 0.04 |
| Sweet Index × Time | 0.97 (0.94, 1.01) | 0.19 | 0.98 (0.94, 1.02) | 0.29 | 0.94 (0.91, 0.98) | 0.002 | 0.94 (0.90, 0.98) | 0.004 |
| Non-sweet Index | 0.64 (0.26, 1.60) | 0.34 | 0.45 (0.13, 1.56) | 0.21 | 1.37 (0.63, 2.95) | 0.43 | 1.25 (0.53, 2.95) | 0.62 |
| Time | 0.86 (0.64, 1.16) | 0.34 | 0.74 (0.50, 1.09) | 0.26 | 1.02 (0.79, 1.32) | 0.86 | 1.11 (0.83, 1.47) | 0.49 |
| Non-sweet index × Time | 1.03 (0.98, 1.08) | 0.25 | 1.08 (1.00, 1.16) | 0.04 | 0.99 (0.95, 1.03) | 0.62 | 0.97 (0.93, 1.02) | 0.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkadi, A.; Alkhars, N.; Manning, S.; Xu, H.; Sohn, M.; Xiao, J.; Meng, Y. The Associations between Snack Intake and Cariogenic Oral Microorganism Colonization in Young Children of a Low Socioeconomic Status. Nutrients 2024, 16, 1113. https://doi.org/10.3390/nu16081113
Alkadi A, Alkhars N, Manning S, Xu H, Sohn M, Xiao J, Meng Y. The Associations between Snack Intake and Cariogenic Oral Microorganism Colonization in Young Children of a Low Socioeconomic Status. Nutrients. 2024; 16(8):1113. https://doi.org/10.3390/nu16081113
Chicago/Turabian StyleAlkadi, Ahmed, Naemah Alkhars, Samantha Manning, Hongzhe Xu, Michael Sohn, Jin Xiao, and Ying Meng. 2024. "The Associations between Snack Intake and Cariogenic Oral Microorganism Colonization in Young Children of a Low Socioeconomic Status" Nutrients 16, no. 8: 1113. https://doi.org/10.3390/nu16081113
APA StyleAlkadi, A., Alkhars, N., Manning, S., Xu, H., Sohn, M., Xiao, J., & Meng, Y. (2024). The Associations between Snack Intake and Cariogenic Oral Microorganism Colonization in Young Children of a Low Socioeconomic Status. Nutrients, 16(8), 1113. https://doi.org/10.3390/nu16081113






