The Role of Nutrition in the Development and Management of Chronic Obstructive Pulmonary Disease
Abstract
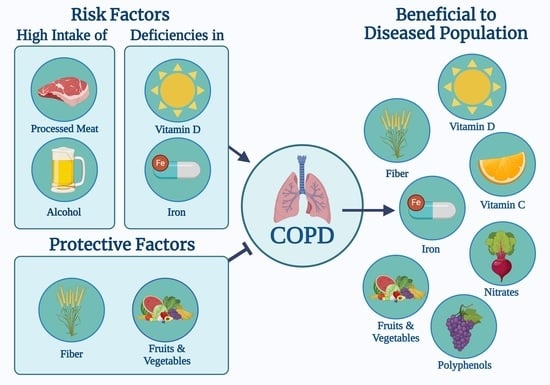
:1. Introduction
2. Meat
2.1. Health Outcomes
2.2. Mechanisms
2.3. Recommendations
3. Fruit and Vegetables
3.1. Health Outcomes
3.2. Mechanisms
3.3. Recommendations
4. Dietary Fiber
4.1. Health Outcomes
4.2. Mechanisms
4.3. Recommendations
5. Vitamin D
5.1. Health Outcomes
5.2. Mechanisms
5.3. Recommendations
6. Vitamins A, B, C, and E
6.1. Health Outcomes
6.2. Mechanisms
6.3. Recommendations
7. Iron
7.1. Health Outcomes
7.2. Mechanisms
7.3. Recommendations
8. Nitrates
8.1. Health Outcomes
8.2. Mechanisms
8.3. Recommendations
9. Other
9.1. Alcohol
9.2. Polyphenols
9.3. Dietary Patterns
9.4. Omega-3 Polyunsaturated Fatty Acids (PUFAs)
10. Practical Implications and Current Limitations
11. Conclusions
| Which Dietary and Nutritional Supplements May Be Beneficial for Patients with COPD? | |||
|---|---|---|---|
| Diet and/or Nutritional Supplement | Risk of COPD Development? | Intake Has Potential Benefit Once COPD Diagnosis is Established? | Specific Dosage |
| Meat | ↑ | <75 g/week | |
| Fruits and Vegetables | ↓ | ✔ | |
| Fiber | ↓ | ✔ | ≥26.5 g/day |
| Vitamin D | ↑ (deficiency) | ✔ | Serum 25(OH)D levels ≥ 55 nmol/L |
| Vitamin C | ? | ✔ | 400–1000 mg daily |
| Iron | ↑ (deficiency) | ✔ | Intravenous ferric carboxymaltose |
| Nitrate | ? | ✔ | BRJ with 12.9 mmol of nitrate twice weekly |
| Heavy Alcohol Consumption | ↑ | ||
| Polyphenols | ↓ | ✔ | |
| Prudent Diet | ↓ | ||
| Western Style Diet | ↑ | ||
| DASH Diet | ↓ | ||
| Mediterranean Diet | ? | ||
| n-3 PUFAs | ? | ✔ | |
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Safiri, S.; Carson-Chahhoud, K.; Noori, M.; Nejadghaderi, S.A.; Sullman, M.J.M.; Ahmadian Heris, J.; Ansarin, K.; Mansournia, M.A.; Collins, G.S.; Kolahi, A.A.; et al. Burden of chronic obstructive pulmonary disease and its attributable risk factors in 204 countries and territories, 1990–2019: Results from the Global Burden of Disease Study 2019. BMJ 2022, 378, e069679. [Google Scholar] [CrossRef] [PubMed]
- Agustí, A.; Celli, B.R.; Criner, G.J.; Halpin, D.; Anzueto, A.; Barnes, P.; Bourbeau, J.; Han, M.K.; Martinez, F.J.; Montes de Oca, M.; et al. Global Initiative for Chronic Obstructive Lung Disease 2023 Report: GOLD Executive Summary. Eur. Respir. J. 2023, 61, 2300239. [Google Scholar] [CrossRef] [PubMed]
- Kerley, C.P.; James, P.E.; McGowan, A.; Faul, J.; Cormican, L. Dietary nitrate improved exercise capacity in COPD but not blood pressure or pulmonary function: A 2 week, double-blind randomised, placebo-controlled crossover trial. Int. J. Food Sci. Nutr. 2019, 70, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Berry, M.J.; Justus, N.W.; Hauser, J.I.; Case, A.H.; Helms, C.C.; Basu, S.; Rogers, Z.; Lewis, M.T.; Miller, G.D. Dietary nitrate supplementation improves exercise performance and decreases blood pressure in COPD patients. Nitric Oxide 2015, 48, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Tramontano, A.; Palange, P. Nutritional State and COPD: Effects on Dyspnoea and Exercise Tolerance. Nutrients 2023, 15, 1786. [Google Scholar] [CrossRef] [PubMed]
- Pavitt, M.J.; Tanner, R.J.; Lewis, A.; Buttery, S.; Mehta, B.; Jefford, H.; Curtis, K.J.; Banya, W.A.S.; Husain, S.; Satkunam, K.; et al. Oral nitrate supplementation to enhance pulmonary rehabilitation in COPD: ON-EPIC a multicentre, double-blind, placebo-controlled, randomised parallel group study. Thorax 2020, 75, 547–555. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Qian, D.C.; Diao, J.A.; Cho, M.H.; Silverman, E.K.; Gusev, A.; Manrai, A.K.; Martin, A.R.; Patel, C.J. Prediction and stratification of longitudinal risk for chronic obstructive pulmonary disease across smoking behaviors. Nat. Commun. 2023, 14, 8297. [Google Scholar] [CrossRef] [PubMed]
- Van Iersel, L.E.J.; Beijers, R.J.H.C.; Gosker, H.R.; Schols, A.M.W.J. Nutrition as a modifiable factor in the onset and progression of pulmonary function impairment in COPD: A systematic review. Nutr. Rev. 2022, 80, 1434–1444. [Google Scholar] [CrossRef] [PubMed]
- Lamprecht, B.; McBurnie, M.A.; Vollmer, W.M.; Gudmundsson, G.; Welte, T.; Nizankowska-Mogilnicka, E.; Studnicka, M.; Bateman, E.; Anto, J.M.; Burney, P.; et al. COPD in never smokers: Results from the population-based burden of obstructive lung disease study. Chest 2011, 139, 752–763. [Google Scholar] [CrossRef] [PubMed]
- Yang, I.A.; Jenkins, C.R.; Salvi, S.S. Chronic obstructive pulmonary disease in never-smokers: Risk factors, pathogenesis, and implications for prevention and treatment. Lancet Respir. Med. 2022, 10, 497–511. [Google Scholar] [CrossRef] [PubMed]
- Beijers, R.J.H.C.; Steiner, M.C.; Schols, A.M.W.J. The role of diet and nutrition in the management of COPD. Eur. Respir. Rev. 2023, 32, 230003. [Google Scholar] [CrossRef] [PubMed]
- Billingsley, H.; Rodriguez-Miguelez, P.; Del Buono, M.G.; Abbate, A.; Lavie, C.J.; Carbone, S. Lifestyle Interventions with a Focus on Nutritional Strategies to Increase Cardiorespiratory Fitness in Chronic Obstructive Pulmonary Disease, Heart Failure, Obesity, Sarcopenia, and Frailty. Nutrients 2019, 11, 2849. [Google Scholar] [CrossRef] [PubMed]
- Fekete, M.; Csípő, T.; Fazekas-Pongor, V.; Fehér, Á.; Szarvas, Z.; Kaposvári, C.; Horváth, K.; Lehoczki, A.; Tarantini, S.; Varga, J.T. The Effectiveness of Supplementation with Key Vitamins, Minerals, Antioxidants and Specific Nutritional Supplements in COPD-A Review. Nutrients 2023, 15, 2741. [Google Scholar] [CrossRef] [PubMed]
- Fekete, M.; Pákó, J.; Szőllősi, G.; Tóth, K.; Szabó, M.; Horváth, D.; Varga, J.T. Significance of nutritional status in chronic obstructive pulmonary disease: A survey. Orvosi Hetil. 2020, 161, 1711–1719. [Google Scholar] [CrossRef] [PubMed]
- Lei, T.; Lu, T.; Yu, H.; Su, X.; Zhang, C.; Zhu, L.; Yang, K.; Liu, J. Efficacy of Vitamin C Supplementation on Chronic Obstructive Pulmonary Disease (COPD): A Systematic Review and Meta-Analysis. Int. J. Chronic Obstr. Pulm. Dis. 2022, 17, 2201–2216. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Lahera, J.; Romera, D.; Gómez Mendieta, A.; Martínez Verdasco, A.; Fernández-Bujarrabal, J.; Santiago, A.; Alcolea, S.; Martínez-Abad, Y.; Prados, C.; Villasante, C.; et al. Prevalence of vitamin D deficiency in patients with chronic obstructive pulmonary disease. Eur. Respir. J. 2015, 46, PA3977. [Google Scholar] [CrossRef]
- Varraso, R.; Jiang, R.; Barr, R.G.; Willett, W.C.; Camargo, C.A. Prospective study of cured meats consumption and risk of chronic obstructive pulmonary disease in men. Am. J. Epidemiol. 2007, 166, 1438–1445. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Camargo, C.A.; Varraso, R.; Paik, D.C.; Willett, W.C.; Barr, R.G. Consumption of cured meats and prospective risk of chronic obstructive pulmonary disease in women. Am. J. Clin. Nutr. 2008, 87, 1002–1008. [Google Scholar] [CrossRef] [PubMed]
- Varraso, R.; Dumas, O.; Boggs, K.M.; Willett, W.C.; Speizer, F.E.; Camargo, C.A. Processed Meat Intake and Risk of Chronic Obstructive Pulmonary Disease among Middle-aged Women. EClinicalMedicine 2019, 14, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Paik, D.C.; Hankinson, J.L.; Barr, R.G. Cured meat consumption, lung function, and chronic obstructive pulmonary disease among United States adults. Am. J. Respir. Crit. Care Med. 2007, 175, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Salari-Moghaddam, A.; Milajerdi, A.; Larijani, B.; Esmaillzadeh, A. Processed red meat intake and risk of COPD: A systematic review and dose-response meta-analysis of prospective cohort studies. Clin. Nutr. 2019, 38, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Kaluza, J.; Larsson, S.C.; Linden, A.; Wolk, A. Consumption of Unprocessed and Processed Red Meat and the Risk of Chronic Obstructive Pulmonary Disease: A Prospective Cohort Study of Men. Am. J. Epidemiol. 2016, 184, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Okubo, H.; Shaheen, S.O.; Ntani, G.; Jameson, K.A.; Syddall, H.E.; Sayer, A.A.; Dennison, E.M.; Cooper, C.; Robinson, S.M.; Group, H.C.S. Processed meat consumption and lung function: Modification by antioxidants and smoking. Eur. Respir. J. 2014, 43, 972–982. [Google Scholar] [CrossRef] [PubMed]
- De Batlle, J.; Mendez, M.; Romieu, I.; Balcells, E.; Benet, M.; Donaire-Gonzalez, D.; Ferrer, J.J.; Orozco-Levi, M.; Antó, J.M.; Garcia-Aymerich, J.; et al. Cured meat consumption increases risk of readmission in COPD patients. Eur. Respir. J. 2012, 40, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Karwowska, M.; Kononiuk, A. Nitrates/Nitrites in Food-Risk for Nitrosative Stress and Benefits. Antioxidants 2020, 9, 241. [Google Scholar] [CrossRef] [PubMed]
- Jakszyn, P.; Agudo, A.; Ibáñez, R.; García-Closas, R.; Pera, G.; Amiano, P.; González, C.A. Development of a food database of nitrosamines, heterocyclic amines, and polycyclic aromatic hydrocarbons. J. Nutr. 2004, 134, 2011–2014. [Google Scholar] [CrossRef] [PubMed]
- Ricciardolo, F.L.; Di Stefano, A.; Sabatini, F.; Folkerts, G. Reactive nitrogen species in the respiratory tract. Eur. J. Pharmacol. 2006, 533, 240–252. [Google Scholar] [CrossRef] [PubMed]
- MacNee, W. ABC of chronic obstructive pulmonary disease: Pathology, pathogenesis, and pathophysiology. BMJ 2006, 332, 1202–1204. [Google Scholar] [CrossRef]
- Shuval, H.I.; Gruener, N. Epidemiological and toxicological aspects of nitrates and nitrites in the environment. Am. J. Public Health 1972, 62, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Freeman, G.; Crane, S.C.; Stephens, R.J.; Furiosi, N.J. Pathogenesis of the nitrogen dioxide-induced lesion in the rat lung: A review and presentation of new observations. Am. Rev. Respir. Dis. 1968, 98, 429–443. [Google Scholar] [CrossRef] [PubMed]
- Uribarri, J.; Woodruff, S.; Goodman, S.; Cai, W.; Chen, X.; Pyzik, R.; Yong, A.; Striker, G.E.; Vlassara, H. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J. Am. Diet. Assoc. 2010, 110, 911–916.e12. [Google Scholar] [CrossRef] [PubMed]
- Uribarri, J.; Cai, W.; Peppa, M.; Goodman, S.; Ferrucci, L.; Striker, G.; Vlassara, H. Circulating glycotoxins and dietary advanced glycation endproducts: Two links to inflammatory response, oxidative stress, and aging. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Eagan, T.M.; Ueland, T.; Wagner, P.D.; Hardie, J.A.; Mollnes, T.E.; Damås, J.K.; Aukrust, P.; Bakke, P.S. Systemic inflammatory markers in COPD: Results from the Bergen COPD Cohort Study. Eur. Respir. J. 2010, 35, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Ley, S.H.; Sun, Q.; Willett, W.C.; Eliassen, A.H.; Wu, K.; Pan, A.; Grodstein, F.; Hu, F.B. Associations between red meat intake and biomarkers of inflammation and glucose metabolism in women. Am. J. Clin. Nutr. 2014, 99, 352–360. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, A.; Gibney, M.J.; Brennan, L. Dietary intake patterns are reflected in metabolomic profiles: Potential role in dietary assessment studies. Am. J. Clin. Nutr. 2011, 93, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, L.M.; Yde, C.C.; Van Hecke, T.; Jessen, R.; Young, J.F.; De Smet, S.; Bertram, H.C. Impact of red meat consumption on the metabolome of rats. Mol. Nutr. Food Res. 2017, 61, 1600387. [Google Scholar] [CrossRef] [PubMed]
- Ottiger, M.; Nickler, M.; Steuer, C.; Bernasconi, L.; Huber, A.; Christ-Crain, M.; Henzen, C.; Hoess, C.; Thomann, R.; Zimmerli, W.; et al. Gut, microbiota-dependent trimethylamine-N-oxide is associated with long-term all-cause mortality in patients with exacerbated chronic obstructive pulmonary disease. Nutrition 2018, 45, 135–141.e1. [Google Scholar] [CrossRef] [PubMed]
- GOLD. Global Strategy for Prevention, Diagnosis and Management of COPD: 2024 Report; 4. 2024. Available online: https://goldcopd.org/2024-gold-report/ (accessed on 3 April 2024).
- Qian, F.; Riddle, M.C.; Wylie-Rosett, J.; Hu, F.B. Red and Processed Meats and Health Risks: How Strong Is the Evidence? Diabetes Care 2020, 43, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Kaluza, J.; Harris, H.R.; Linden, A.; Wolk, A. Long-term consumption of fruits and vegetables and risk of chronic obstructive pulmonary disease: A prospective cohort study of women. Int. J. Epidemiol. 2018, 47, 1897–1909. [Google Scholar] [CrossRef] [PubMed]
- Zhai, H.; Wang, Y.; Jiang, W. Fruit and Vegetable Intake and the Risk of Chronic Obstructive Pulmonary Disease: A Dose-Response Meta-Analysis of Observational Studies. Biomed. Res. Int. 2020, 2020, 3783481. [Google Scholar] [CrossRef] [PubMed]
- Tabak, C.; Smit, H.A.; Heederik, D.; Ocké, M.C.; Kromhout, D. Diet and chronic obstructive pulmonary disease: Independent beneficial effects of fruits, whole grains, and alcohol (the MORGEN study). Clin. Exp. Allergy 2001, 31, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Kaluza, J.; Larsson, S.C.; Orsini, N.; Linden, A.; Wolk, A. Fruit and vegetable consumption and risk of COPD: A prospective cohort study of men. Thorax 2017, 72, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, S.O.; Jameson, K.A.; Syddall, H.E.; Aihie Sayer, A.; Dennison, E.M.; Cooper, C.; Robinson, S.M.; Group, H.C.S. The relationship of dietary patterns with adult lung function and COPD. Eur. Respir. J. 2010, 36, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Seyedrezazadeh, E.; Moghaddam, M.P.; Ansarin, K.; Asghari Jafarabadi, M.; Sharifi, A.; Sharma, S.; Kolahdooz, F. Dietary Factors and Risk of Chronic Obstructive Pulmonary Disease: A Systemic Review and Meta-Analysis. Tanaffos 2019, 18, 294–309. [Google Scholar] [PubMed]
- Ochs-Balcom, H.M.; Grant, B.J.; Muti, P.; Sempos, C.T.; Freudenheim, J.L.; Browne, R.W.; McCann, S.E.; Trevisan, M.; Cassano, P.A.; Iacoviello, L.; et al. Antioxidants, oxidative stress, and pulmonary function in individuals diagnosed with asthma or COPD. Eur. J. Clin. Nutr. 2006, 60, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Keranis, E.; Makris, D.; Rodopoulou, P.; Martinou, H.; Papamakarios, G.; Daniil, Z.; Zintzaras, E.; Gourgoulianis, K.I. Impact of dietary shift to higher-antioxidant foods in COPD: A randomised trial. Eur. Respir. J. 2010, 36, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, M.; Jamil, R.; Attia, F. Vitamin C (Ascorbic Acid); StatPearls: St. Petersburg, FL, USA, 2023. [Google Scholar]
- Jiang, Q. Natural forms of vitamin E: Metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic. Biol. Med. 2014, 72, 76–90. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.G.; Olmedilla-Alonso, B.; Hornero-Méndez, D.; Mercadante, A.Z.; Osorio, C.; Vargas-Murga, L.; Meléndez-Martínez, A.J. Comprehensive Database of Carotenoid Contents in Ibero-American Foods. A Valuable Tool in the Context of Functional Foods and the Establishment of Recommended Intakes of Bioactives. J. Agric. Food Chem. 2018, 66, 5055–5107. [Google Scholar] [CrossRef] [PubMed]
- Gęgotek, A.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Activity of Ascorbic Acid. Antioxidants 2022, 11, 1993. [Google Scholar] [CrossRef] [PubMed]
- Singh, U.; Devaraj, S.; Jialal, I. Vitamin E, oxidative stress, and inflammation. Annu. Rev. Nutr. 2005, 25, 151–174. [Google Scholar] [CrossRef] [PubMed]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Baldrick, F.R.; Elborn, J.S.; Woodside, J.V.; Treacy, K.; Bradley, J.M.; Patterson, C.C.; Schock, B.C.; Ennis, M.; Young, I.S.; McKinley, M.C. Effect of fruit and vegetable intake on oxidative stress and inflammation in COPD: A randomised controlled trial. Eur. Respir. J. 2012, 39, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Bulsiewicz, W.J. The Importance of Dietary Fiber for Metabolic Health. Am. J. Lifestyle Med. 2023, 17, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Soliman, G.A. Dietary Fiber, Atherosclerosis, and Cardiovascular Disease. Nutrients 2019, 11, 1155. [Google Scholar] [CrossRef] [PubMed]
- Kaluza, J.; Harris, H.; Wallin, A.; Linden, A.; Wolk, A. Dietary Fiber Intake and Risk of Chronic Obstructive Pulmonary Disease: A Prospective Cohort Study of Men. Epidemiology 2018, 29, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Varraso, R.; Willett, W.C.; Camargo, C.A. Prospective study of dietary fiber and risk of chronic obstructive pulmonary disease among US women and men. Am. J. Epidemiol. 2010, 171, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Kan, H.; Stevens, J.; Heiss, G.; Rose, K.M.; London, S.J. Dietary fiber, lung function, and chronic obstructive pulmonary disease in the atherosclerosis risk in communities study. Am. J. Epidemiol. 2008, 167, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Szmidt, M.K.; Kaluza, J.; Harris, H.R.; Linden, A.; Wolk, A. Long-term dietary fiber intake and risk of chronic obstructive pulmonary disease: A prospective cohort study of women. Eur. J. Nutr. 2020, 59, 1869–1879. [Google Scholar] [CrossRef] [PubMed]
- Valisoltani, N.; Ghoreishy, S.M.; Imani, H.; Rajabi Harsini, A.; Jowshan, M.; Travica, N.; Mohammadi, H. Fiber intake and risk of chronic obstructive pulmonary disease: A systematic review and dose response meta-analysis. Food Sci. Nutr. 2023, 11, 6775–6788. [Google Scholar] [CrossRef] [PubMed]
- Hanson, C.; Lyden, E.; Rennard, S.; Mannino, D.M.; Rutten, E.P.; Hopkins, R.; Young, R. The Relationship between Dietary Fiber Intake and Lung Function in the National Health and Nutrition Examination Surveys. Ann. Am. Thorac. Soc. 2016, 13, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.J.; Lee, S.H.; Chang, J.H.; Lee, H.S.; Kang, E.H.; Lee, S.W. The Impact of Changes in the Intake of Fiber and Antioxidants on the Development of Chronic Obstructive Pulmonary Disease. Nutrients 2021, 13, 580. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, A.; Frazer, Z.A.; Hansbro, P.M.; Yang, I.A. COPD and the gut-lung axis: The therapeutic potential of fibre. J. Thorac. Dis. 2019, 11 (Suppl. 17), S2173–S2180. [Google Scholar] [CrossRef] [PubMed]
- McNabney, S.M.; Henagan, T.M. Short Chain Fatty Acids in the Colon and Peripheral Tissues: A Focus on Butyrate, Colon Cancer, Obesity and Insulin Resistance. Nutrients 2017, 9, 1348. [Google Scholar] [CrossRef] [PubMed]
- Vinolo, M.A.; Rodrigues, H.G.; Nachbar, R.T.; Curi, R. Regulation of inflammation by short chain fatty acids. Nutrients 2011, 3, 858–876. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, C.B.; Lichatz, R.; Pich, A.; Mühlfeld, C.; Woltemate, S.; Vital, M.; Brandenberger, C. Short-chain fatty acids improve inflamm-aging and acute lung injury in old mice. Am. J. Physiol. Lung Cell Mol. Physiol. 2023, 324, L480–L492. [Google Scholar] [CrossRef] [PubMed]
- Chassaing, B.; Vijay-Kumar, M.; Gewirtz, A.T. How diet can impact gut microbiota to promote or endanger health. Curr. Opin. Gastroenterol. 2017, 33, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Satija, A.; Hu, F.B. Cardiovascular benefits of dietary fiber. Curr. Atheroscler. Rep. 2012, 14, 505–514. [Google Scholar] [CrossRef] [PubMed]
- American Association of Clinical Endocrinologists. Vitamin D Deficiency; American Association of Clinical Endocrinologists: Jacksonville, FL, USA, 2019. [Google Scholar]
- Herrick, K.A.; Storandt, R.J.; Afful, J.; Pfeiffer, C.M.; Schleicher, R.L.; Gahche, J.J.; Potischman, N. Vitamin D status in the United States, 2011–2014. Am. J. Clin. Nutr. 2019, 110, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Wan, X.; Liu, J.; Zhang, D.; Luo, P.; Du, W.; Chen, L.; Su, J.; Hang, D.; Zhou, J.; et al. Vitamin D status and chronic obstructive pulmonary disease risk: A prospective UK Biobank study. BMJ Open Respir. Res. 2023, 10, e001684. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, R.; Aleva, F.E.; Schrumpf, J.A.; Daniels, J.M.; Bet, P.M.; Boersma, W.G.; Bresser, P.; Spanbroek, M.; Lips, P.; van den Broek, T.J.; et al. Vitamin D supplementation in chronic obstructive pulmonary disease patients with low serum vitamin D: A randomized controlled trial. Am. J. Clin. Nutr. 2022, 116, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Khan, D.M.; Ullah, A.; Randhawa, F.A.; Iqtadar, S.; Butt, N.F.; Waheed, K. Role of Vitamin D in reducing number of acute exacerbations in Chronic Obstructive Pulmonary Disease (COPD) patients. Pak. J. Med. Sci. 2017, 33, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Janssens, W.; Bouillon, R.; Claes, B.; Carremans, C.; Lehouck, A.; Buysschaert, I.; Coolen, J.; Mathieu, C.; Decramer, M.; Lambrechts, D. Vitamin D deficiency is highly prevalent in COPD and correlates with variants in the vitamin D-binding gene. Thorax 2010, 65, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Jolliffe, D.A.; James, W.Y.; Hooper, R.L.; Barnes, N.C.; Greiller, C.L.; Islam, K.; Bhowmik, A.; Timms, P.M.; Rajakulasingam, R.K.; Choudhury, A.B.; et al. Prevalence, determinants and clinical correlates of vitamin D deficiency in patients with Chronic Obstructive Pulmonary Disease in London, UK. J. Steroid Biochem. Mol. Biol. 2018, 175, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Herr, C.; Greulich, T.; Koczulla, R.A.; Meyer, S.; Zakharkina, T.; Branscheidt, M.; Eschmann, R.; Bals, R. The role of vitamin D in pulmonary disease: COPD, asthma, infection, and cancer. Respir. Res. 2011, 12, 31. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, S.O.; Jameson, K.A.; Robinson, S.M.; Boucher, B.J.; Syddall, H.E.; Sayer, A.A.; Cooper, C.; Holloway, J.W.; Dennison, E.M. Relationship of vitamin D status to adult lung function and COPD. Thorax 2011, 66, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Afzal, S.; Lange, P.; Bojesen, S.E.; Freiberg, J.J.; Nordestgaard, B.G. Plasma 25-hydroxyvitamin D, lung function and risk of chronic obstructive pulmonary disease. Thorax 2014, 69, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Baneen, U.; Naseem, S. Correlation of severity of chronic obstructive pulmonary disease with serum vitamin-D level. J. Family Med. Prim. Care 2019, 8, 2268–2277. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, J.; Yu, M.; Sun, J. The efficacy of vitamin D therapy for patients with COPD: A meta-analysis of randomized controlled trials. Ann. Palliat. Med. 2020, 9, 286–297. [Google Scholar] [CrossRef]
- Zendedel, A.; Gholami, M.; Anbari, K.; Ghanadi, K.; Bachari, E.C.; Azargon, A. Effects of Vitamin D Intake on FEV1 and COPD Exacerbation: A Randomized Clinical Trial Study. Glob. J. Health Sci. 2015, 7, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Dong, T.; Wang, S.; Jing, H.; Chen, J. Vitamin D. EBioMedicine 2019, 45, 563–577. [Google Scholar] [CrossRef] [PubMed]
- Menon, B.; Nima, G.; Dogra, V.; Mittal, A.; Kaur, C.; Mittal, U. Evaluation of vitamin D in bronchial asthma and the effect of vitamin D supplementation on asthma severity and control: A randomised control trial. Eur. Respir. J. 2014, 44, P4049. [Google Scholar]
- Fu, L.; Fei, J.; Tan, Z.X.; Chen, Y.H.; Hu, B.; Xiang, H.X.; Zhao, H.; Xu, D.X. Low Vitamin D Status Is Associated with Inflammation in Patients with Chronic Obstructive Pulmonary Disease. J. Immunol. 2021, 206, 515–523. [Google Scholar] [CrossRef]
- Uh, S.T.; Koo, S.M.; Kim, Y.K.; Kim, K.U.; Park, S.W.; Jang, A.S.; Kim, D.J.; Kim, Y.H.; Park, C.S. Inhibition of vitamin d receptor translocation by cigarette smoking extracts. Tuberc. Respir. Dis. 2012, 73, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Sundar, I.K.; Hwang, J.W.; Wu, S.; Sun, J.; Rahman, I. Deletion of vitamin D receptor leads to premature emphysema/COPD by increased matrix metalloproteinases and lymphoid aggregates formation. Biochem. Biophys. Res. Commun. 2011, 406, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, J.P.; Zhou, A.; Hyppönen, E. Vitamin D Deficiency Increases Mortality Risk in the UK Biobank: A Nonlinear Mendelian Randomization Study. Ann. Intern. Med. 2022, 175, 1552–1559. [Google Scholar] [CrossRef] [PubMed]
- Kennel, K.A.; Drake, M.T.; Hurley, D.L. Vitamin D deficiency in adults: When to test and how to treat. Mayo Clin. Proc. 2010, 85, 752–757, quiz 757–758. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, F.; Lee, A.H.; Terasawa, K.; Kagawa, Y. Folate intake associated with lung function, breathlessness and the prevalence of chronic obstructive pulmonary disease. Asia Pac. J. Clin. Nutr. 2010, 19, 103–109. [Google Scholar]
- Van de Bool, C.; Mattijssen-Verdonschot, C.; van Melick, P.P.; Spruit, M.A.; Franssen, F.M.; Wouters, E.F.; Schols, A.M.; Rutten, E.P. Quality of dietary intake in relation to body composition in patients with chronic obstructive pulmonary disease eligible for pulmonary rehabilitation. Eur. J. Clin. Nutr. 2014, 68, 159–165. [Google Scholar] [CrossRef]
- Lin, Y.C.; Wu, T.C.; Chen, P.Y.; Hsieh, L.Y.; Yeh, S.L. Comparison of plasma and intake levels of antioxidant nutrients in patients with chronic obstructive pulmonary disease and healthy people in Taiwan: A case-control study. Asia Pac. J. Clin. Nutr. 2010, 19, 393–401. [Google Scholar] [PubMed]
- Hirayama, F.; Lee, A.H.; Binns, C.W.; Zhao, Y.; Hiramatsu, T.; Tanikawa, Y.; Nishimura, K.; Taniguchi, H. Do vegetables and fruits reduce the risk of chronic obstructive pulmonary disease? A case-control study in Japan. Prev. Med. 2009, 49, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Paiva, S.A.; Godoy, I.; Vannucchi, H.; Fávaro, R.M.; Geraldo, R.R.; Campana, A.O. Assessment of vitamin A status in chronic obstructive pulmonary disease patients and healthy smokers. Am. J. Clin. Nutr. 1996, 64, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Britton, J.R.; Pavord, I.D.; Richards, K.A.; Knox, A.J.; Wisniewski, A.F.; Lewis, S.A.; Tattersfield, A.E.; Weiss, S.T. Dietary antioxidant vitamin intake and lung function in the general population. Am. J. Respir. Crit. Care Med. 1995, 151, 1383–1387. [Google Scholar] [CrossRef] [PubMed]
- Kelly, Y.; Sacker, A.; Marmot, M. Nutrition and respiratory health in adults: Findings from the health survey for Scotland. Eur. Respir. J. 2003, 21, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Cassano, P.A. Antioxidant nutrients and pulmonary function: The Third National Health and Nutrition Examination Survey (NHANES III). Am. J. Epidemiol. 2000, 151, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Schünemann, H.J.; Grant, B.J.; Freudenheim, J.L.; Muti, P.; Browne, R.W.; Drake, J.A.; Klocke, R.A.; Trevisan, M. The relation of serum levels of antioxidant vitamins C and E, retinol and carotenoids with pulmonary function in the general population. Am. J. Respir. Crit. Care Med. 2001, 163, 1246–1255. [Google Scholar] [CrossRef] [PubMed]
- Timoneda, J.; Rodríguez-Fernández, L.; Zaragozá, R.; Marín, M.P.; Cabezuelo, M.T.; Torres, L.; Viña, J.R.; Barber, T. Vitamin A Deficiency and the Lung. Nutrients 2018, 10, 1132. [Google Scholar] [CrossRef] [PubMed]
- Tug, T.; Karatas, F.; Terzi, S.M. Antioxidant vitamins (A, C and E) and malondialdehyde levels in acute exacerbation and stable periods of patients with chronic obstructive pulmonary disease. Clin. Investig. Med. 2004, 27, 123–128. [Google Scholar]
- Cheng, X.; Hu, Y.; Ruan, Z.; Zang, G.; Chen, X.; Qiu, Z. Association between B-vitamins intake and frailty among patients with chronic obstructive pulmonary disease. Aging Clin. Exp. Res. 2023, 35, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Paulin, F.V.; Zagatto, A.M.; Chiappa, G.R.; Müller, P.T. Addition of vitamin B12 to exercise training improves cycle ergometer endurance in advanced COPD patients: A randomized and controlled study. Respir. Med. 2017, 122, 23–29. [Google Scholar] [CrossRef]
- Joshi, P.; Kim, W.J.; Lee, S.A. The effect of dietary antioxidant on the COPD risk: The community-based KoGES (Ansan-Anseong) cohort. Int. J. Chronic Obstr. Pulm. Dis. 2015, 10, 2159–2168. [Google Scholar] [CrossRef]
- Agler, A.H.; Kurth, T.; Gaziano, J.M.; Buring, J.E.; Cassano, P.A. Randomised vitamin E supplementation and risk of chronic lung disease in the Women’s Health Study. Thorax 2011, 66, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: A randomised placebo-controlled trial. Lancet 2002, 360, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, A.; Raj, H.G.; Chhabra, S.K. Effect of vitamin E supplementation with standard treatment on oxidant-antioxidant status in chronic obstructive pulmonary disease. Indian J. Med. Res. 2008, 128, 705–711. [Google Scholar] [PubMed]
- Al-Azzawi, M.A.; AboZaid, M.M.N.; Ibrahem, R.A.L.; Sakr, M.A. Therapeutic effects of black seed oil supplementation on chronic obstructive pulmonary disease patients: A randomized controlled double blind clinical trial. Heliyon 2020, 6, e04711. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A.; Ansari, S.M.; Zahida, M. Dose antioxidant ascorbic acid supplementation delay lung function deterioration in stable patients with chronic obstructive pulmonary disease. Rawal Med. J. 2010, 35, 133–136. [Google Scholar]
- Schwartz, J.; Weiss, S.T. Relationship between dietary vitamin C intake and pulmonary function in the First National Health and Nutrition Examination Survey (NHANES I). Am. J. Clin. Nutr. 1994, 59, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Tunstall-Pedoe, H.; Bolton-Smith, C.; Hannah, M.K.; Morrison, C. Association of dietary antioxidants and waist circumference with pulmonary function and airway obstruction. Am. J. Epidemiol. 2001, 153, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Bentley, A.R.; Kritchevsky, S.B.; Harris, T.B.; Holvoet, P.; Jensen, R.L.; Newman, A.B.; Lee, J.S.; Yende, S.; Bauer, D.; Cassano, P.A.; et al. Dietary antioxidants and forced expiratory volume in 1 s decline: The Health, Aging and Body Composition study. Eur. Respir. J. 2012, 39, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Dey, D.; Sengupta, S.; Bhattacharyya, P. Long-term use of Vitamin-C in chronic obstructive pulmonary disease: Early pilot observation. Lung India 2021, 38, 500–501. [Google Scholar] [CrossRef] [PubMed]
- Ives, S.J.; Harris, R.A.; Witman, M.A.; Fjeldstad, A.S.; Garten, R.S.; McDaniel, J.; Wray, D.W.; Richardson, R.S. Vascular dysfunction and chronic obstructive pulmonary disease: The role of redox balance. Hypertension 2014, 63, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Orozco-Levi, M.; Colmenares-Mejía, C.; Ruíz, J.; Valencia-Barón, Y.D.; Ramírez-Sarmiento, A.; Quintero-Lesmes, D.C.; Serrano, N.C. Effect of Antioxidants in the Treatment of COPD Patients: Scoping Review. J. Nutr. Metab. 2021, 2021, 7463391. [Google Scholar] [CrossRef] [PubMed]
- Neha, K.; Haider, M.R.; Pathak, A.; Yar, M.S. Medicinal prospects of antioxidants: A review. Eur. J. Med. Chem. 2019, 178, 687–704. [Google Scholar] [CrossRef] [PubMed]
- Shan, M.R.; Zhou, S.N.; Fu, C.N.; Song, J.W.; Wang, X.Q.; Bai, W.W.; Li, P.; Song, P.; Zhu, M.L.; Ma, Z.M.; et al. Vitamin B6 inhibits macrophage activation to prevent lipopolysaccharide-induced acute pneumonia in mice. J. Cell. Mol. Med. 2020, 24, 3139–3148. [Google Scholar] [CrossRef] [PubMed]
- Surman, S.L.; Penkert, R.R.; Sealy, R.E.; Jones, B.G.; Marion, T.N.; Vogel, P.; Hurwitz, J.L. Consequences of Vitamin A Deficiency: Immunoglobulin Dysregulation, Squamous Cell Metaplasia, Infectious Disease, and Death. Int. J. Mol. Sci. 2020, 21, 5570. [Google Scholar] [CrossRef] [PubMed]
- Kuzkaya, N.; Weissmann, N.; Harrison, D.G.; Dikalov, S. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: Implications for uncoupling endothelial nitric-oxide synthase. J. Biol. Chem. 2003, 278, 22546–22554. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Miguelez, P.; Gregg, J.; Seigler, N.; Bass, L.; Thomas, J.; Pollock, J.S.; Sullivan, J.C.; Dillard, T.A.; Harris, R.A. Acute Tetrahydrobiopterin Improves Endothelial Function in Patients With COPD. Chest 2018, 154, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Oxidative Stress in Chronic Obstructive Pulmonary Disease. Antioxidants 2022, 11, 965. [Google Scholar] [CrossRef] [PubMed]
- Giustina, A.D.; Danielski, L.G.; Novochadlo, M.M.; Goldim, M.P.S.; Joaquim, L.; Metzker, K.L.L.; Carli, R.J.; Denicol, T.; Cidreira, T.; Vieira, T.; et al. Vitamin B6 reduces oxidative stress in lungs and liver in experimental sepsis. An. Acad. Bras. Cienc. 2019, 91, e20190434. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Gong, J.; Li, L.; Zhi, S.; Yang, G.; Li, P.; Li, R.; Li, J. Vitamin E relieves chronic obstructive pulmonary disease by inhibiting COX2-mediated p-STAT3 nuclear translocation through the EGFR/MAPK signaling pathway. Lab. Investig. 2022, 102, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Doseděl, M.; Jirkovský, E.; Macáková, K.; Krčmová, L.K.; Javorská, L.; Pourová, J.; Mercolini, L.; Remião, F.; Nováková, L.; Mladěnka, P.; et al. Vitamin C-Sources, Physiological Role, Kinetics, Deficiency, Use, Toxicity, and Determination. Nutrients 2021, 13, 615. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Peiró, M.; Martín-Ontiyuelo, C.; Rodó-Pi, A.; Piccari, L.; Admetlló, M.; Durán, X.; Rodríguez-Chiaradía, D.A.; Barreiro, E. Iron Replacement and Redox Balance in Non-Anemic and Mildly Anemic Iron Deficiency COPD Patients: Insights from a Clinical Trial. Biomedicines 2021, 9, 1191. [Google Scholar] [CrossRef] [PubMed]
- Nickol, A.H.; Frise, M.C.; Cheng, H.Y.; McGahey, A.; McFadyen, B.M.; Harris-Wright, T.; Bart, N.K.; Curtis, M.K.; Khandwala, S.; O’Neill, D.P.; et al. A cross-sectional study of the prevalence and associations of iron deficiency in a cohort of patients with chronic obstructive pulmonary disease. BMJ Open 2015, 5, e007911. [Google Scholar] [CrossRef] [PubMed]
- Martín-Ontiyuelo, C.; Rodó-Pin, A.; Echeverría-Esnal, D.; Admetlló, M.; Duran-Jordà, X.; Alvarado, M.; Gea, J.; Barreiro, E.; Rodríguez-Chiaradía, D.A. Intravenous Iron Replacement Improves Exercise Tolerance in COPD: A Single-Blind Randomized Trial. Arch. Bronconeumol. 2022, 58, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Grasmuk-Siegl, E.; Urban, M.H.; Scherrer, S.; Funk, G.C. Effect of intravenous ferric carboxymaltose on exercise capacity and quality of life in patients with COPD: A pilot study. Wien. Klin. Wochenschr. 2023, 135, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Martín-Ontiyuelo, C.; Rodó-Pin, A.; Sancho-Muñoz, A.; Martinez-Llorens, J.M.; Admetlló, M.; Molina, L.; Gea, J.; Barreiro, E.; Chiaradía, D.A.R. Is iron deficiency modulating physical activity in COPD? Int. J. Chronic Obstr. Pulm. Dis. 2019, 14, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, F.; Du, M.; Huang, K.; Wang, C. Efficacy and safety of iron supplementation in patients with heart failure and iron deficiency: A meta-analysis. Br. J. Nutr. 2019, 121, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Sun, L.; Liu, C.; Li, L. Causal association between iron deficiency anemia and chronic obstructive pulmonary disease: A bidirectional two-sample Mendelian randomization study. Heart Lung 2023, 58, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Santer, P.; McGahey, A.; Frise, M.C.; Petousi, N.; Talbot, N.P.; Baskerville, R.; Bafadhel, M.; Nickol, A.H.; Robbins, P.A. Intravenous iron and chronic obstructive pulmonary disease: A randomised controlled trial. BMJ Open Respir. Res. 2020, 7, e000577. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Ganz, T. Hepcidin and Iron in Health and Disease. Annu. Rev. Med. 2023, 74, 261–277. [Google Scholar] [CrossRef] [PubMed]
- Hoon, M.W.; Johnson, N.A.; Chapman, P.G.; Burke, L.M. The effect of nitrate supplementation on exercise performance in healthy individuals: A systematic review and meta-analysis. Int. J. Sport Nutr. Exerc. Metab. 2013, 23, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Pavitt, M.J.; Lewis, A.; Buttery, S.C.; Fernandez, B.O.; Mikus-Lelinska, M.; Banya, W.A.S.; Feelisch, M.; Polkey, M.I.; Hopkinson, N.S. Dietary nitrate supplementation to enhance exercise capacity in hypoxic COPD: EDEN-OX, a double-blind, placebo-controlled, randomised cross-over study. Thorax 2022, 77, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Kenjale, A.A.; Ham, K.L.; Stabler, T.; Robbins, J.L.; Johnson, J.L.; Vanbruggen, M.; Privette, G.; Yim, E.; Kraus, W.E.; Allen, J.D. Dietary nitrate supplementation enhances exercise performance in peripheral arterial disease. J. Appl. Physiol. 2011, 110, 1582–1591. [Google Scholar] [CrossRef] [PubMed]
- Shaltout, H.A.; Eggebeen, J.; Marsh, A.P.; Brubaker, P.H.; Laurienti, P.J.; Burdette, J.H.; Basu, S.; Morgan, A.; Dos Santos, P.C.; Norris, J.L.; et al. Effects of supervised exercise and dietary nitrate in older adults with controlled hypertension and/or heart failure with preserved ejection fraction. Nitric Oxide 2017, 69, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Woessner, M.N.; Levinger, I.; Allen, J.D.; McIlvenna, L.C.; Neil, C. The Effect of Dietary Inorganic Nitrate Supplementation on Cardiac Function during Submaximal Exercise in Men with Heart Failure with Reduced Ejection Fraction (HFrEF): A Pilot Study. Nutrients 2020, 12, 2132. [Google Scholar] [CrossRef] [PubMed]
- Zamani, P.; Rawat, D.; Shiva-Kumar, P.; Geraci, S.; Bhuva, R.; Konda, P.; Doulias, P.T.; Ischiropoulos, H.; Townsend, R.R.; Margulies, K.B.; et al. Effect of inorganic nitrate on exercise capacity in heart failure with preserved ejection fraction. Circulation 2015, 131, 371–380, discussion 380. [Google Scholar] [CrossRef] [PubMed]
- Velmurugan, S.; Gan, J.M.; Rathod, K.S.; Khambata, R.S.; Ghosh, S.M.; Hartley, A.; Van Eijl, S.; Sagi-Kiss, V.; Chowdhury, T.A.; Curtis, M.; et al. Dietary nitrate improves vascular function in patients with hypercholesterolemia: A randomized, double-blind, placebo-controlled study. Am. J. Clin. Nutr. 2016, 103, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; He, S.; Chen, F.; Liang, L.; Pan, J. Efficacy and safety of nitrate supplementation on exercise tolerance in chronic obstructive pulmonary disease: A systematic review and meta-analysis. Medicine 2022, 101, e28578. [Google Scholar] [CrossRef] [PubMed]
- Alshafie, S.; El-Helw, G.O.; Fayoud, A.M.; Elrashedy, A.A.; Gbreel, M.I.; Alfayoumi, S.S.; Mohamed, I.M.; Abdelwadoud, G.T.; Isa, A.S.; Ragab, K.M.; et al. Efficacy of dietary nitrate-rich beetroot juice supplementation in patients with chronic obstructive pulmonary disease (COPD): A systematic review and meta-analysis. Clin. Nutr. ESPEN 2021, 42, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, A.I.; Wilkerson, D.P.; Dobson, L.; Kelly, J.; Winyard, P.G.; Jones, A.M.; Benjamin, N.; Shore, A.C.; Gilchrist, M. The effect of dietary nitrate supplementation on the oxygen cost of cycling, walking performance and resting blood pressure in individuals with chronic obstructive pulmonary disease: A double blind placebo controlled, randomised control trial. Nitric Oxide 2015, 48, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Friis, A.L.; Steenholt, C.B.; Løkke, A.; Hansen, M. Dietary beetroot juice—Effects on physical performance in COPD patients: A randomized controlled crossover trial. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 1765–1773. [Google Scholar] [CrossRef] [PubMed]
- Behnia, M.; Wheatley, C.M.; Avolio, A.; Johnson, B.D. Influence of dietary nitrate supplementation on lung function and exercise gas exchange in COPD patients. Nitric Oxide 2018, 76, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Cuenca, E.; Maté-Muñoz, J.L.; García-Fernández, P.; Serra-Paya, N.; Estevan, M.C.; Herreros, P.V.; Garnacho-Castaño, M.V. Effects of Beetroot Juice Supplementation on Cardiorespiratory Endurance in Athletes. A Systematic Review. Nutrients 2017, 9, 43. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, P.; Rengarajan, T.; Thangavel, J.; Nishigaki, Y.; Sakthisekaran, D.; Sethi, G.; Nishigaki, I. The vascular endothelium and human diseases. Int. J. Biol. Sci. 2013, 9, 1057–1069. [Google Scholar] [CrossRef] [PubMed]
- Theodorakopoulou, M.P.; Alexandrou, M.E.; Bakaloudi, D.R.; Pitsiou, G.; Stanopoulos, I.; Kontakiotis, T.; Boutou, A.K. Endothelial dysfunction in COPD: A systematic review and meta-analysis of studies using different functional assessment methods. ERJ Open Res. 2021, 7, 00983-2020. [Google Scholar] [CrossRef] [PubMed]
- Sterling, S.A.; Palzes, V.A.; Lu, Y.; Kline-Simon, A.H.; Parthasarathy, S.; Ross, T.; Elson, J.; Weisner, C.; Maxim, C.; Chi, F.W. Associations Between Medical Conditions and Alcohol Consumption Levels in an Adult Primary Care Population. JAMA Netw. Open 2020, 3, e204687. [Google Scholar] [CrossRef] [PubMed]
- Kaluza, J.; Harris, H.R.; Linden, A.; Wolk, A. Alcohol Consumption and Risk of Chronic Obstructive Pulmonary Disease: A Prospective Cohort Study of Men. Am. J. Epidemiol. 2019, 188, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Lange, P.; Groth, S.; Mortensen, J.; Appleyard, M.; Nyboe, J.; Jensen, G.; Schnohr, P. Pulmonary function is influenced by heavy alcohol consumption. Am. Rev. Respir. Dis. 1988, 137, 1119–1123. [Google Scholar] [CrossRef] [PubMed]
- Tabak, C.; Smit, H.A.; Räsänen, L.; Fidanza, F.; Menotti, A.; Nissinen, A.; Feskens, E.J.; Heederik, D.; Kromhout, D. Alcohol consumption in relation to 20-year COPD mortality and pulmonary function in middle-aged men from three European countries. Epidemiology 2001, 12, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Li, T.; Li, J.H.; Miao, S.Y.; Xiao, X.Z. The Effects of Resveratrol on Inflammation and Oxidative Stress in a Rat Model of Chronic Obstructive Pulmonary Disease. Molecules 2017, 22, 1529. [Google Scholar] [CrossRef] [PubMed]
- Boots, A.W.; Haenen, G.R.; Bast, A. Oxidant metabolism in chronic obstructive pulmonary disease. Eur. Respir. J. Suppl. 2003, 46, 14s–27s. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Xu, Y.; Li, K.; Liu, L. Heavy metal levels and flavonoid intakes are associated with chronic obstructive pulmonary disease: An NHANES analysis (2007–2010 to 2017–2018). BMC Public Health 2023, 23, 2335. [Google Scholar] [CrossRef] [PubMed]
- Bondonno, N.P.; Parmenter, B.H.; Dalgaard, F.; Murray, K.; Rasmussen, D.B.; Kyrø, C.; Cassidy, A.; Bondonno, C.P.; Lewis, J.R.; Croft, K.D.; et al. Flavonoid intakes inversely associate with COPD in smokers. Eur. Respir. J. 2022, 60. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Marchetti, N.; Ganjian, H.; Kelsen, S.G.; Criner, G.J.; Sajjan, U. Oral Treatment with Quercetin Reduces Markers of Inflammation in COPD Patients; American Thoracic Society: New York, NY, USA, 2023. [Google Scholar]
- Cobb, K.; Payne, C.; Lavender, R.; Simovic, T.; Harris, R.; Pollock, J.; Baban, B.; Mannino, D.; Nana-Sinkam, P.; Rodriguez Miguelez, P. Resveratrol Reduces Arterial Stiffness and Improves Functional Capacity in Patients with COPD. FASEB J. 2022, 36. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Beijers, R.J.; Gosker, H.R.; Sanders, K.J.; de Theije, C.; Kelders, M.; Clarke, G.; Cryan, J.F.; van den Borst, B.; Schols, A.M. Resveratrol and metabolic health in COPD: A proof-of-concept randomized controlled trial. Clin. Nutr. 2020, 39, 2989–2997. [Google Scholar] [CrossRef] [PubMed]
- Mennen, L.I.; Walker, R.; Bennetau-Pelissero, C.; Scalbert, A. Risks and safety of polyphenol consumption. Am. J. Clin. Nutr. 2005, 81 (Suppl. S1), 326S–329S. [Google Scholar] [CrossRef] [PubMed]
- Varraso, R.; Fung, T.T.; Hu, F.B.; Willett, W.; Camargo, C.A. Prospective study of dietary patterns and chronic obstructive pulmonary disease among US men. Thorax 2007, 62, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Varraso, R.; Fung, T.T.; Barr, R.G.; Hu, F.B.; Willett, W.; Camargo, C.A. Prospective study of dietary patterns and chronic obstructive pulmonary disease among US women. Am. J. Clin. Nutr. 2007, 86, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Gu, S.; Wang, X.; Qi, X. Associations of adherence to the DASH diet and the Mediterranean diet with chronic obstructive pulmonary disease among US adults. Front. Nutr. 2023, 10, 1031071. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; Johansson, I.; Blomberg, A.; Sundström, B. Adherence to a Mediterranean-like Diet as a Protective Factor Against COPD: A Nested Case-Control Study. COPD J. Chronic Obstr. Pulm. Dis. 2019, 16, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Wood, L.G. Omega-3 polyunsaturated fatty acids and chronic obstructive pulmonary disease. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Broekhuizen, R.; Wouters, E.F.; Creutzberg, E.C.; Weling-Scheepers, C.A.; Schols, A.M. Polyunsaturated fatty acids improve exercise capacity in chronic obstructive pulmonary disease. Thorax 2005, 60, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Laviano, A.; Lonnqvist, F.; Muscaritoli, M.; Öhlander, M.; Schols, A. Targeted medical nutrition for cachexia in chronic obstructive pulmonary disease: A randomized, controlled trial. J. Cachexia Sarcopenia Muscle 2018, 9, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, K.; Takahashi, H.; Kasai, C.; Kiyokawa, N.; Watanabe, T.; Fujii, S.; Kashiwagura, T.; Honma, M.; Satake, M.; Shioya, T. Effects of nutritional supplementation combined with low-intensity exercise in malnourished patients with COPD. Respir. Med. 2010, 104, 1883–1889. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, K.; Takahashi, H.; Kashiwagura, T.; Yamada, K.; Yanagida, S.; Homma, M.; Dairiki, K.; Sasaki, H.; Kawagoshi, A.; Satake, M.; et al. Effect of anti-inflammatory supplementation with whey peptide and exercise therapy in patients with COPD. Respir. Med. 2012, 106, 1526–1534. [Google Scholar] [CrossRef] [PubMed]
- Fekete, M.; Szarvas, Z.; Fazekas-Pongor, V.; Lehoczki, A.; Tarantini, S.; Varga, J.T. Effects of omega-3 supplementation on quality of life, nutritional status, inflammatory parameters, lipid profile, exercise tolerance and inhaled medications in chronic obstructive pulmonary disease. Ann. Palliat. Med. 2022, 11, 2819–2829. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes: From molecules to man. Biochem. Soc. Trans. 2017, 45, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. FDA Announces New Qualified Health Claims for EPA and DHA Omega-3 Consumption and the Risk of Hypertension and Coronary Heart Disease. Available online: https://www.fda.gov/food/cfsan-constituent-updates/fda-announces-new-qualified-health-claims-epa-and-dha-omega-3-consumption-and-risk-hypertension-and (accessed on 3 April 2014).
- Rimm, E.B.; Appel, L.J.; Chiuve, S.E.; Djoussé, L.; Engler, M.B.; Kris-Etherton, P.M.; Mozaffarian, D.; Siscovick, D.S.; Lichtenstein, A.H.; American Heart Association Nutrition Committee of the Council on Lifestyle and Cardiometabolic Health; et al. Seafood Long-Chain n-3 Polyunsaturated Fatty Acids and Cardiovascular Disease: A Science Advisory From the American Heart Association. Circulation 2018, 138, e35–e47. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.M.; Innis, S.; Ammerican Dietetic Assocition; Dietitians of Canada. Position of the American Dietetic Association and Dietitians of Canada: Dietary fatty acids. J. Am. Diet. Assoc. 2007, 107, 1599–1611. [Google Scholar] [PubMed]
- Krupa, K.; Fritz, K.; Parmar, M. Omega-3 Fatty Acids; StatPearls Publishing: St. Petersburg, FL, USA, 2023. [Google Scholar]
- American Lung Association. Nutrition and COPD. 2023. Available online: https://www.lung.org/lung-health-diseases/lung-disease-lookup/copd/living-with-copd/nutrition (accessed on 3 April 2024).
- Schols, A.M.; Ferreira, I.M.; Franssen, F.M.; Gosker, H.R.; Janssens, W.; Muscaritoli, M.; Pison, C.; Rutten-van Mölken, M.; Slinde, F.; Steiner, M.C.; et al. Nutritional assessment and therapy in COPD: A European Respiratory Society statement. Eur. Respir. J. 2014, 44, 1504–1520. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, A.H.; Appel, L.J.; Vadiveloo, M.; Hu, F.B.; Kris-Etherton, P.M.; Rebholz, C.M.; Sacks, F.M.; Thorndike, A.N.; Van Horn, L.; Wylie-Rosett, J. 2021 Dietary Guidance to Improve Cardiovascular Health: A Scientific Statement From the American Heart Association. Circulation 2021, 144, e472–e487. [Google Scholar] [CrossRef] [PubMed]
- Ikizler, T.A.; Burrowes, J.D.; Byham-Gray, L.D.; Campbell, K.L.; Carrero, J.J.; Chan, W.; Fouque, D.; Friedman, A.N.; Ghaddar, S.; Goldstein-Fuchs, D.J.; et al. KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 Update. Am. J. Kidney Dis. 2020, 76 (Suppl. 1), S1–S107. [Google Scholar] [CrossRef] [PubMed]
- American Heart Association. Managing Blood Pressure with a Heart-Healthy Diet. Available online: https://www.heart.org/en/health-topics/high-blood-pressure/changes-you-can-make-to-manage-high-blood-pressure/managing-blood-pressure-with-a-heart-healthy-diet (accessed on 1 April 2024).
- Discharge Packet for Patients Diagnosed with Heart Failure. 2019. Available online: https://www.heart.org/-/media/files/health-topics/heart-failure/hf-discharge-packet.pdf?la=en&hash=90463681A07EE6230276BC27A08F5D337D1D6D8C (accessed on 1 April 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heefner, A.; Simovic, T.; Mize, K.; Rodriguez-Miguelez, P. The Role of Nutrition in the Development and Management of Chronic Obstructive Pulmonary Disease. Nutrients 2024, 16, 1136. https://doi.org/10.3390/nu16081136
Heefner A, Simovic T, Mize K, Rodriguez-Miguelez P. The Role of Nutrition in the Development and Management of Chronic Obstructive Pulmonary Disease. Nutrients. 2024; 16(8):1136. https://doi.org/10.3390/nu16081136
Chicago/Turabian StyleHeefner, Allison, Tijana Simovic, Kasey Mize, and Paula Rodriguez-Miguelez. 2024. "The Role of Nutrition in the Development and Management of Chronic Obstructive Pulmonary Disease" Nutrients 16, no. 8: 1136. https://doi.org/10.3390/nu16081136