The Role of Oxidative Stress in Alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis of Preclinical Studies
Abstract
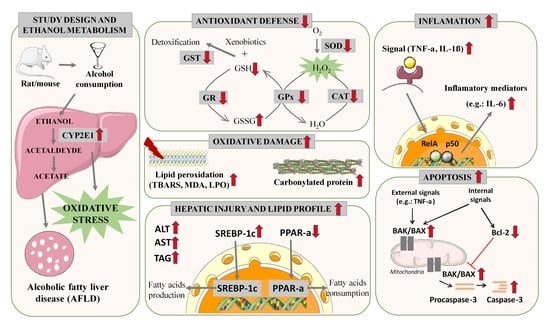
:1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria
2.2. Exclusion Criteria
2.3. Search Strategy
2.4. Study Selection
2.5. Data Extraction
2.6. Risk of Bias in Individual Studies
- (1)
- Selection bias: Was the allocation sequence properly generated and applied? Were the groups similar at baseline or were they adjusted for confounders in the analysis? Was the allocation to the different groups properly concealed?
- (2)
- Performance bias: Were the animals randomly housed during the experiment? Were the caregivers and/or investigators blinded from knowledge of which intervention each animal received during the experiment?
- (3)
- Detection bias: Were animals selected at random for outcome assessment? Was the outcome advisor blinded?
- (4)
- Attrition bias: Were incomplete outcome data adequately addressed?
- (5)
- Reporting bias: Are reports of the study free of selective outcome reporting?
- (6)
- Other biases: Was the study apparently free of other problems that could result in a high risk of bias?
2.7. Statistical Analysis
3. Results
3.1. Literature Search
3.2. Characteristics of the Included Studies
3.3. Parameters Analyzed in the Systematic Review and Meta-Analysis
3.3.1. Liver Damage
3.3.2. Lipid Profile
Triacylglycerol (TAG)
Sterol Regulatory Element-Binding Transcription Factor 1c (SREBP-1c)
Peroxisome Proliferator-Activated Receptor Alpha (PPAR-α)
Histological Analysis of the Liver
3.3.3. Ethanol Metabolism through Cytochrome P450 2E (CYP2E1)
3.3.4. Oxidative Stress Biomarkers
Antioxidant Profile in AFLD
- Superoxide Dismutase (SOD)
- Catalase (CAT)
- Glutathione Peroxidase (GPx)
- Glutathione Reductase (GR)
- Glutathione Transferase (GST)
- Reduced Glutathione (GSH)
- Reduced Glutathione (GSH)/Oxidized Glutathione (GSSG) Ratio
- Factor 2 Related to Erythroid Nuclear Factor 2 (Nrf2)
Oxidative Damage in AFLD
- Lipid Peroxidation
- Carbonylated Protein
3.3.5. Inflammation in AFLD
Tumor Necrosis Factor-α (TNF-α)
Interleukin 1 beta (IL-1β)
Interleukin-6 (IL-6)
Interleukin-10 (IL-10)
Histological Analysis of the Liver
3.3.6. Apoptosis in AFLD
Caspase-3
BCL-2-Associated Protein X (BAX)/B-Cell CLL/Lymphoma 2 (BCL-2) Ratio
3.3.7. Risk of Bias in Individual Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization—WHO. No Level of Alcohol Consumption Is Safe for Our Health. Available online: https://www.who.int/europe/news/item/04-01-2023-no-level-of-alcohol-consumption-is-safe-for-our-health (accessed on 10 February 2023).
- Salete-Granado, D.; Carbonell, C.; Puertas-Miranda, D.; Vega-Rodríguez, V.J.; García-Macia, M.; Herrero, A.B.; Marcos, M. Autophagy, Oxidative Stress, and Alcoholic Liver Disease: A Systematic Review and Potential Clinical Applications. Antioxidants 2023, 12, 1425. [Google Scholar] [CrossRef] [PubMed]
- Zima, T.; Fialová, L.; Mestek, O.; Janebová, M.; Crkovská, J.; Malbohan, I.; Stípek, S.; Mikulíková, L.; Popov, P. Oxidative stress, metabolism of ethanol and alcohol-related diseases. J. Biomed. Sci. 2001, 8, 59–70. [Google Scholar] [CrossRef] [PubMed]
- You, M.; Fischer, M.; Deeg, M.A.; Crabb, D.W. Ethanol Induces Fatty Acid Synthesis Pathways by Activation of Sterol Regulatory Element-Binding Protein (SREBP). J. Biol. Chem. 2002, 277, 29342–29347. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.K.; Yates, E.; Lilly, K.; Dhanda, A.D. Oxidative Stress in Alcohol-Related Liver Disease. World J. Hepatol. 2020, 12, 332–349. [Google Scholar] [CrossRef] [PubMed]
- Lívero, F.A.R.; Acco, A. Molecular Basis of Alcoholic Fatty Liver Disease: From Incidence to Treatment. Hepatol. Res. 2016, 46, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Diesinger, T.; Buko, V.; Lautwein, A.; Dvorsky, R.; Belonovskaya, E.; Lukivskaya, O.; Naruta, E.; Kirko, S.; Andreev, V.; Buckert, D. Drug Targeting CYP2E1 for the Treatment of Early-Stage Alcoholic Steatohepatitis. PLoS ONE 2020, 15, e0235990. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. J. Clin. Epidemiol. 2021, 134, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Hooijmans, C.R.; Rovers, M.M.; De Vries, R.B.M.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s Risk of Bias Tool for Animal Studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]
- Abdelhamid, A.M.; Elsheakh, A.R.; Abdelaziz, R.R.; Suddek, G.M. Empagliflozin Ameliorates Ethanol-Induced Liver Injury by Modulating NF-ΚB/Nrf-2/PPAR-γ Interplay in Mice. Life Sci. 2020, 256, 117908. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, A.M.; Elsheakh, A.R.; Suddek, G.M.; Abdelaziz, R.R. Telmisartan Alleviates Alcohol-Induced Liver Injury by Activation of PPAR-γ/ Nrf-2 Crosstalk in Mice. Int. Immunopharmacol. 2021, 99, 107963. [Google Scholar] [CrossRef] [PubMed]
- Al-Rejaie, S.S. Effect of Oleo-Gum-Resin on Ethanol-Induced Hepatotoxicity in Rats. J. Med. Sci. 2012, 12, 1–9. [Google Scholar] [CrossRef]
- Atef, M.M.; Hafez, Y.M.; Alshenawy, H.A.; Emam, M.N. Ameliorative Effects of Autophagy Inducer, Simvastatin on Alcohol-Induced Liver Disease in a Rat Model. J. Cell Biochem. 2019, 120, 7679–7688. [Google Scholar] [CrossRef] [PubMed]
- Bae, D.; Kim, J.; Lee, S.Y.; Choi, E.J.; Jung, M.A.; Jeong, C.S.; Na, J.R.; Kim, J.J.; Kim, S. Hepatoprotective Effects of Aqueous Extracts from Leaves of Dendropanax Morbifera Leveille against Alcohol-Induced Hepatotoxicity in Rats and in Vitro Anti-Oxidant Effects. Food Sci. Biotechnol. 2015, 24, 1495–1503. [Google Scholar] [CrossRef]
- Balasubramaniyan, V.; Sailaja, J.K.; Nalini, N. Role of Leptin on Alcohol-Induced Oxidative Stress in Swiss Mice. Pharmacol. Res. 2003, 47, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Baranisrinivasan, P.; Elumalai, E.K.; Sivakumar, C.; Therasa, S.V.; David, E. Hepatoprotective Effect of Enicostemma Littorale Blume and Eclipta Alba during Ethanol Induced Oxidative Stress in Albino Rats. Int. J. Pharmacol. 2009, 5, 268–272. [Google Scholar] [CrossRef]
- Bardag-Gorce, F.; Oliva, J.; Lin, A.; Li, J.; French, B.A.; French, S.W. Proteasome Inhibitor up Regulates Liver Antioxidative Enzymes in Rat Model of Alcoholic Liver Disease. Exp. Mol. Pathol. 2011, 90, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Bedi, O.; Bariwal, J.; Kumar, P.; Bhakuni, G.S. Hepatoprotective Activity of Morin and its Semi-Synthetic Derivatives Against Alcohol Induced Hepatotoxicity in Rats. Indian J. Physiol. Pharmacol. 2017, 61, 175–190. [Google Scholar]
- Bharrhan, S.; Koul, A.; Chopra, K.; Rishi, P. Catechin Suppresses an Array of Signalling Molecules and Modulates Alcohol-Induced Endotoxin Mediated Liver Injury in a Rat Model. PLoS ONE 2011, 6, e20635. [Google Scholar] [CrossRef] [PubMed]
- Bisht, P.; Chandrashekhara, S.; Das, K.; Tribedi, S. Effect of Cultural Condition on Evaluation of Hepatoprotective Activity of Methanolic Bark Extract of Anogeissus Latifolia on Ethanol-Induced Hepatotoxicity. Asian J. Pharm. Clin. Res. 2018, 11, 247–252. [Google Scholar] [CrossRef]
- Bispo, V.S.; Dantas, L.S.; Chaves Filho, A.B.; Pinto, I.F.D.; da Silva, R.P.; Otsuka, F.A.M.; Santos, R.B.; Santos, A.C.; Trindade, D.J.; Matos, H.R. Reduction of the DNA Damages, Hepatoprotective Effect and Antioxidant Potential of the Coconut Water, Ascorbic and Caffeic Acids in Oxidative Stress Mediated by Ethanol. An. Acad. Bras. Cienc. 2017, 89, 1095–1109. [Google Scholar] [CrossRef]
- Buko, V.; Kuzmitskaya, I.; Kirko, S.; Belonovskaya, E.; Naruta, E.; Lukivskaya, O.; Shlyahtun, A.; Ilyich, T.; Zakreska, A.; Zavodnik, I. Betulin Attenuated Liver Damage by Prevention of Hepatic Mitochondrial Dysfunction in Rats with Alcoholic Steatohepatitis. Physiol. Int. 2019, 106, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Bulle, S.; Reddyvari, H.; Reddy Vaddi, D.; Pannuru, P.; Nch, V. Therapeutic potential of P. santalinus against alcohol-induced histo-pathological changes and oxidative damage in heart and lungs. Int. J. Res. Pharm. Sci. 2015, 6, 30–311. [Google Scholar]
- Cao, Y.W.; Jiang, Y.; Zhang, D.Y.; Wang, M.; Chen, W.S.; Su, H.; Wang, Y.T.; Wan, J.B. Protective Effects of Penthorum Chinense Pursh against Chronic Ethanol-Induced Liver Injury in Mice. J. Ethnopharmacol. 2015, 161, 92–98. [Google Scholar] [CrossRef]
- Chandra, R.; Aneja, R.; Rewal, C.; Konduri, R.; Dass, S.K.; Agarwal, S. An opium alkaloid-papaverine ameliorates ethanol-induced hepatotoxicity: Diminution of oxidative stress. Indian J. Clin. Biochem. 2000, 15, 155–160. [Google Scholar] [CrossRef]
- Chang, Y.Y.; Liu, Y.C.; Kuo, Y.H.; Lin, Y.L.; Wu, Y.H.S.; Chen, J.W.; Chen, Y.C. Effects of Antrosterol from Antrodia Camphorata Submerged Whole Broth on Lipid Homeostasis, Antioxidation, Alcohol Clearance, and Anti-Inflammation in Livers of Chronic-Alcohol Fed Mice. J. Ethnopharmacol. 2017, 202, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.Y.; Kim, H.J.; Kim, T.Y.; Kim, S.Y. Enzyme-Treated Zizania Latifolia Extract Protects against Alcohol-Induced Liver Injury by Regulating the Nrf2 Pathway. Antioxidants 2021, 10, 960. [Google Scholar] [CrossRef]
- Chaturvedi, P.; George, S.; John, A. Preventive and Protective Effects of Wild Basil in Ethanol-Induced Liver Toxicity in Rats. Br. J. Biomed. Sci. 2007, 64, 10–12. [Google Scholar] [CrossRef]
- Chavan, T.; Ghadge, A.; Karandikar, M.; Pandit, V.; Ranjekar, P.; Kulkarni, O.; Kuvalekar, A.; Mantri, N. Activity of Satwa against Alcohol Injury in rats. Altern. Ther. Health Med. 2017, 23, 34–40. [Google Scholar]
- Chen, Y.L.; Peng, H.C.; Tan, S.W.; Tsai, C.Y.; Huang, Y.H.; Wu, H.Y.; Yang, S.C. Amelioration of Ethanol-Induced Liver Injury in Rats by Nanogold Flakes. Alcohol 2013, 47, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Singh, S.; Matsumoto, A.; Manna, S.K.; Abdelmegeed, M.A.; Golla, S.; Murphy, R.C.; Dong, H.; Song, B.J.; Gonzalez, F.J.; et al. Chronic Glutathione Depletion Confers Protection against Alcohol-Induced Steatosis: Implication for Redox Activation of AMP-Activated Protein Kinase Pathway. Sci. Rep. 2016, 6, 29743. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Kong, H. The Effect of Lycium Barbarum Polysaccharide on Alcohol-Induced Oxidative Stress in Rats. Molecules 2011, 16, 2542–2550. [Google Scholar] [CrossRef] [PubMed]
- Chiu, P.Y.; Lam, P.Y.; Leung, H.Y.; Leong, P.K.; Ma, C.W.; Tang, Q.T.; Ko, K.M. Co-Treatment with Shengmai San-Derived Herbal Product Ameliorates Chronic Ethanol-Induced Liver Damage in Rats. Rejuvenation Res. 2011, 14, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.; Yan, R.; Wang, S.; Li, G.; Kang, X.; Hu, Y.; Lin, M.; Shan, W.; Zhao, Y.; Wang, Z.; et al. Sinapic Acid Reduces Oxidative Stress and Pyroptosis via Inhibition of BRD4 in Alcoholic Liver Disease. Front. Pharmacol. 2021, 12, 668708. [Google Scholar] [CrossRef] [PubMed]
- Colantoni, A.; Paglia, N.L.; De Maria, N.; Emanuele, M.A.; Emanuele, N.V.; Idilman, R.; Harig, J.; Van Thiel, D.H. Influence of Sex Hormonal Status on Alcohol-Induced Oxidative Injury in Male and Female Rat Liver. Alcohol. Clin. Exp. Res. 2000, 24, 1467–1473. [Google Scholar] [PubMed]
- Cui, Y.; Ye, Q.; Wang, H.; Li, Y.; Xia, X.; Yao, W.; Qian, H. Aloin Protects against Chronic Alcoholic Liver Injury via Attenuating Lipid Accumulation, Oxidative Stress and Inflammation in Mice. Arch. Pharmacal Res. 2014, 37, 1624–1633. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Ye, Q.; Wang, H.; Li, Y.; Yao, W.; Qian, H. Hepatoprotective Potential of Aloe Vera Polysaccharides against Chronic Alcohol-Induced Hepatotoxicity in Mice. J. Sci. Food Agric. 2014, 94, 1764–1771. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Vasudevan, D.M. Effect of Lecithin in the Treatment of Ethanol Mediated Free Radical Induced Hepatotoxicity. Indian J. Clin. Biochem. 2006, 21, 62–69. [Google Scholar] [CrossRef]
- Das, S.K.; Mukherjee, S.; Vasudevan, D.M. Effects of Long Term Ethanol Consumption Mediated Oxidative Stress on Neovessel Generation in Liver. Toxicol. Mech. Methods 2012, 22, 375–382. [Google Scholar] [CrossRef] [PubMed]
- De Souza, C.E.A.; Stolf, A.M.; Dreifuss, A.A.; Lívero, F.R.; Gomes, L.O.; Petiz, L.; Beltrame, O.; Dittrich, R.L.; Telles, J.E.Q.; Cadena, S.M. Characterization of an Alcoholic Hepatic Steatosis Model Induced by Ethanol and High-Fat Diet in Rats. Braz. Arch. Biol. Technol. 2015, 58, 367–378. [Google Scholar] [CrossRef]
- Develi, S.; Evran, B.; Kalaz, E.B.; Koçak-Toker, N.; Erata, G.Ö. Protective Effect of Nigella Sativa Oil against Binge Ethanol-Induced Oxidative Stress and Liver Injury in Rats. Chin. J. Nat. Med. 2014, 12, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Dou, X.; Shen, C.; Wang, Z.; Li, S.; Zhang, X.; Song, Z. Protection of Nicotinic Acid against Oxidative Stress-Induced Cell Death in Hepatocytes Contributes to Its Beneficial Effect on Alcohol-Induced Liver Injury in Mice. J. Nutr. Biochem. 2013, 24, 1520–1528. [Google Scholar] [CrossRef] [PubMed]
- Du, S.-Y.; Zhang, Y.-L.; Bai, R.-X.; Ai, Z.-L.; Xie, B.-S.; Yang, H.-Y. Lutein Prevents Alcohol-Induced Liver Disease in Rats by Modulating Oxidative Stress and Inflammation. Int. J. Clin. Exp. Med. 2015, 8, 8785–8793. [Google Scholar] [PubMed]
- Duryee, M.J.; Dusad, A.; Hunter, C.D.; Kharbanda, K.K.; Bruenjes, J.D.; Easterling, K.C.; Siebler, J.C.; Thiele, G.M.; Chakkalakal, D.A. N-Acetyl Cysteine Treatment Restores Early Phase Fracture Healing in Ethanol-Fed Rats. Alcohol Clin. Exp. Res. 2018, 42, 1206–1216. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Chen, J.H.; Liu, C.H.; Xia, F.B.; Xiao, Z.; Zhang, X.; Wan, J.B. A Combination of Pueraria Lobata and Silybum Marianum Protects against Alcoholic Liver Disease in Mice. Phytomedicine 2019, 58, 152824. [Google Scholar] [CrossRef] [PubMed]
- Galligan, J.J.; Smathers, R.L.; Shearn, C.T.; Fritz, K.S.; Backos, D.S.; Jiang, H.; Franklin, C.C.; Orlicky, D.J.; MacLean, K.N.; Petersen, D.R. Oxidative Stress and the ER Stress Response in a Murine Model for Early-Stage Alcoholic Liver Disease. J. Toxicol. 2012, 2012, 207594. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Yuan, J.; Cheng, Y.; Chen, M.; Zhang, G.; Wu, J. Selenomethionine-Dominated Selenium-Enriched Peanut Protein Ameliorates Alcohol-Induced Liver Disease in Mice by Suppressing Oxidative Stress. Foods 2021, 10, 2979. [Google Scholar] [CrossRef] [PubMed]
- George, S.; Chaturvedi, P. A comparative study of the antioxidant properties of two different species of Ocimum of southern Africa on alcohol-induced oxidative stress. J. Med. Food. 2009, 12, 1154–1158. [Google Scholar] [CrossRef] [PubMed]
- Gustot, T.; Lemmers, A.; Moreno, C.; Nagy, N.; Quertinmont, E.; Nicaise, C.; Franchimont, D.; Louis, H.; Devière, J.; Le Moine, O. Differential Liver Sensitization to Toll-like Receptor Pathways in Mice with Alcoholic Fatty Liver. Hepatology 2006, 43, 989–1000. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Liu, J.; Bai, Y.; Hang, A.; Lu, T.; Mao, C. An Iridoid Glycoside from Cornus Officinalis Balances Intestinal Microbiome Disorder and Alleviates Alcohol-Induced Liver Injury. J. Funct. Foods 2021, 82, 104488. [Google Scholar] [CrossRef]
- Hao, L.; Sun, Q.; Zhong, W.; Zhang, W.; Sun, X.; Zhou, Z. Mitochondria-Targeted Ubiquinone (MitoQ) Enhances Acetaldehyde Clearance by Reversing Alcohol-Induced Posttranslational Modification of Aldehyde Dehydrogenase 2: A Molecular Mechanism of Protection against Alcoholic Liver Disease. Redox Biol. 2018, 14, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Zhong, W.; Sun, X.; Zhou, Z. TLR9 Signaling Protects Alcohol-Induced Hepatic Oxidative Stress but Worsens Liver Inflammation in Mice. Front. Pharmacol. 2021, 12, 709002. [Google Scholar] [CrossRef] [PubMed]
- Hasanein, P.; Seifi, R. Beneficial effects of rosmarinic acid against alcohol-induced hepatotoxicity in rats. Can. J. Physiol. Pharmacol. 2018, 96, 32–37. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Xia, F.; Nan, M.; Li, L.; Wang, X.; Zhang, Y. Regulation of Bcl-2 and the NF-KB Signaling Pathway by Succinyl Rotundic Acid in Livers of Rats with Alcoholic Hepatitis. Int. J. Agric. Biol. 2021, 25, 730–734. [Google Scholar] [CrossRef]
- Hsu, J.Y.; Lin, H.H.; Hsu, C.C.; Chen, B.C.; Chen, J.H. Aqueous Extract of Pepino (Solanum Muriactum Ait) Leaves Ameliorate Lipid Accumulation and Oxidative Stress in Alcoholic Fatty Liver Disease. Nutrients 2018, 10, 931. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Jiang, W.; Yang, Y.; Xu, W.; Liu, C.; Zhang, S.; Qian, H.; Zhang, W. Gut-Liver Axis Reveals the Protective Effect of Exopolysaccharides Isolated from Sporidiobolus Pararoseus on Alcohol-Induced Liver Injury. J. Funct. Foods 2021, 87, 104737. [Google Scholar] [CrossRef]
- Huang, Q.H.; Xu, L.Q.; Liu, Y.H.; Wu, J.Z.; Wu, X.; Lai, X.P.; Li, Y.C.; Su, Z.R.; Chen, J.N.; Xie, Y.L. Polydatin Protects Rat Liver against Ethanol-Induced Injury: Involvement of CYP2E1/ROS/Nrf2 and TLR4/NF- B P65 Pathway. Evid. Based Complement. Alternat. Med. 2017, 2017, 7953850. [Google Scholar] [CrossRef] [PubMed]
- Ilaiyaraja, N.; Khanum, F. Amelioration of Alcohol-Induced Hepatotoxicity and Oxidative Stress in Rats by Acorus Calamus. J. Diet Suppl. 2011, 8, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, J.; Veerappan, M.; Namasivayam, N. Potential Beneficial Effect of Naringenin on Lipid Peroxidation and Antioxidant Status in Rats with Ethanol-Induced Hepatotoxicity. J. Pharm. Pharmacol. 2009, 61, 1383–1390. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Chen, C.; Wang, J.; Xie, W.; Wang, M.; Li, X.; Zhang, X. Purple Potato (Solanum tuberosum L.) Anthocyanins Attenuate Alcohol-Induced Hepatic Injury by Enhancing Antioxidant Defense. J. Nat. Med. 2016, 70, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Lin, D.; Shao, H.; Yang, X. Antioxidant Properties of Komagataeibacter Hansenii CGMCC 3917 and Its Ameliorative Effects on Alcohol-Induced Liver Injury in Mice. CYTA J. Food 2019, 17, 355–364. [Google Scholar] [CrossRef]
- Jin, D.C.; Jeong, S.W.; Park, P.S. Effects of Green Tea Extract on Acute Ethanol-Induced Hepatotoxicity in Rats. J. Korean Soc. Food Sci. Nutr. 2010, 39, 343–349. [Google Scholar] [CrossRef]
- Jose, S.P.; Mohanan, R.; Sandya, S.; Asha, S.; Krishnakumar, I.M. A Novel Powder Formulation of Coconut Inflorescence Sap Inhibits Alcoholic Liver Damage by Modulating Inflammatory Markers, Extracellular Matrix Metalloproteinase, and Oxidative Stress. J. Food Biochem. 2018, 42, e12543. [Google Scholar] [CrossRef]
- Kanbak, G.; Inal, M.; Bayçu, C. Ethanol-Induced Hepatotoxicity and Protective Effect of Betaine. Cell Biochem. Funct. 2001, 19, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Kanchana, G.; Jayapriya, K. Antioxidant Effect of Livomap, a Polyherbal Formulation on Ethanol Induced Hepatotoxicity in Albino Wistar Rats. J. Appl. Pharm. Sci. 2013, 3, 52–56. [Google Scholar] [CrossRef]
- Kang, X.; Zhong, W.; Liu, J.; Song, Z.; McClain, C.J.; Kang, Y.J.; Zhou, Z. Zinc Supplementation Reverses Alcohol-Induced Steatosis in Mice through Reactivating Hepatocyte Nuclear Factor-4α and Peroxisome Proliferator-Activated Receptor-α. Hepatology 2009, 50, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Kim, M.B.; Park, Y.K.; Lee, J.Y. A Mouse Model of the Regression of Alcoholic Hepatitis: Monitoring the Regression of Hepatic Steatosis, Inflammation, Oxidative Stress, and NAD+ Metabolism upon Alcohol Withdrawal. J. Nutr. Biochem. 2022, 99, 108852. [Google Scholar] [CrossRef] [PubMed]
- Kaviarasan, S.; Sundarapandiyan, R.; Anuradha, C.V. Epigallocatechin Gallate, a Green Tea Phytochemical, Attenuates Alcohol-Induced Hepatic Protein and Lipid Damage. Toxicol. Mech. Methods 2008, 18, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Khanal, T.; Choi, J.H.; Hwang, Y.P.; Chung, Y.C.; Jeong, H.G. Saponins Isolated from the Root of Platycodon Grandiflorum Protect against Acute Ethanol-Induced Hepatotoxicity in Mice. Food Chem.Toxicol. 2009, 47, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Park, J.G.; Lee, S.M. Protective Effect of Heme Oxygenase-1 Induction against Hepatic Injury in Alcoholic Steatotic Liver Exposed to Cold Ischemia/Reperfusion. Life Sci. 2012, 90, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, G.W.; Lee, S.H.; Han, G.D. Ligularia Fischeri Extract Attenuates Liver Damage Induced by Chronic Alcohol Intake. Pharm. Biol. 2016, 54, 1465–1473. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Dwivedi, D.K.; Lahkar, M.; Jangra, A. Hepatoprotective Potential of 7,8-Dihydroxyflavone against Alcohol and High-Fat Diet Induced Liver Toxicity via Attenuation of Oxido-Nitrosative Stress and NF-ΚB Activation. Pharmacol. Rep. 2019, 71, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.R.; Ke, B.J.; Hsu, Y.W.; Lee, C.L. Dimerumic Acid and Deferricoprogen Produced by Monascus purpureus Attenuate Liquid Ethanol Diet-Induced Alcoholic Hepatitis via Suppressing NF-ΚB Inflammation Signalling Pathways and Stimulation of AMPK-Mediated Lipid Metabolism. J. Funct. Foods 2019, 60, 103393. [Google Scholar] [CrossRef]
- Lee, M.; Kim, Y.; Yoon, H.G.; You, Y.; Park, J.; Lee, Y.H.; Kim, S.; Hwang, K.; Lee, J.; Jun, W. Prevention of Ethanol-Induced Hepatotoxicity by Fermented Curcuma Longa L. in C57BL/6 Mice. Food Sci. Biotechnol. 2014, 23, 925–930. [Google Scholar] [CrossRef]
- Lee, J.Y.; An, Y.J.; Kim, J.W.; Choi, H.K.; Lee, Y.H. Effect of Angelica Keiskei Koidzumi Extract on Alcohol-Induced Hepatotoxicity in Vitro and in Vivo. J. Korean Soc. Food Sci. Nutr. 2016, 45, 1391–1397. [Google Scholar] [CrossRef]
- Lee, Y.J.; Hsu, J.D.; Lin, W.L.; Kao, S.H.; Wang, C.J. Upregulation of Caveolin-1 by Mulberry Leaf Extract and Its Major Components, Chlorogenic Acid Derivatives, Attenuates Alcoholic Steatohepatitis: Via Inhibition of Oxidative Stress. Food Funct. 2017, 8, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Nam, Y.; Choi, W.S.; Kim, T.W.; Lee, J.; Sohn, U.D. The Hepato-Protective Effect of Eupatilin on an Alcoholic Liver Disease Model of Rats. Korean J. Physiol. Pharmacol. 2020, 24, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Lee, J.S.; Lee, I.H.; Hong, J.T. Therapeutic Potency of Fermented Field Water in Ethanol-Induced Liver Injury. RSC Adv. 2020, 10, 1544–1551. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Tsai, M.C.; Lin, H.T.; Wang, C.J.; Kao, S.H. Aqueous Mulberry Leaf Extract Ameliorates Alcoholic Liver Injury Associating with Upregulation of Ethanol Metabolism and Suppression of Hepatic Lipogenesis. Evid. Comp. Alt. Med. 2021, 2021, 6658422. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gao, C.; Shi, Y.; Tang, Y.; Liu, L.; Xiong, T.; Du, M.; Xing, M.; Yao, P. Carbon Monoxide Alleviates Ethanol-Induced Oxidative Damage and Inflammatory Stress through Activating P38 MAPK Pathway. Toxicol. Appl Pharmacol. 2013, 273, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhu, L.; Wu, T.; Zhang, J.; Jiao, X.; Liu, X.; Wang, Y.; Meng, X. Effects of Triterpenoid from Schisandra Chinensis on Oxidative Stress in Alcohol-Induced Liver Injury in Rats. Cell Biochem. Biophys. 2015, 71, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, M.; Xu, Y.; Yu, X.; Xiong, T.; Du, M.; Sun, J.; Liu, L.; Tang, Y.; Yao, P. Iron-Mediated Lysosomal Membrane Permeabilization in Ethanol-Induced Hepatic Oxidative Damage and Apoptosis: Protective Effects of Quercetin. Oxid. Med. Cell Longev. 2016, 2016, 4147610. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wu, Y.; Yin, F.; Feng, Q.; Dong, X.; Zhang, R.; Yin, Z.; Luo, L. Fructose 1, 6-Diphosphate Prevents Alcohol-Induced Liver Injury through Inhibiting Oxidative Stress and Promoting Alcohol Metabolism in Mice. Eur. J. Pharmacol. 2017, 815, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Sun, L.; Yang, Y.; Wang, Z.; Yang, X.; Guo, Y. Preventive and Therapeutic Effects of Pigment and Polysaccharides in Lycium Barbarum on Alcohol-Induced Fatty Liver Disease in Mice. CYTA J. Food 2018, 16, 938–949. [Google Scholar] [CrossRef]
- Li, B.; Mao, Q.; Zhou, D.; Luo, M.; Gan, R.; Li, H.; Huang, S.; Saimaiti, A.; Shang, A.; Li, H. Effects of Tea against Alcoholic Fatty Liver Disease by Modulating Gut Microbiota in Chronic Alcohol-Exposed Mice. Foods 2021, 10, 1232. [Google Scholar] [CrossRef] [PubMed]
- Li, B.Y.; Li, H.Y.; Zhou, D.D.; Huang, S.Y.; Luo, M.; Gan, R.Y.; Mao, Q.Q.; Saimaiti, A.; Shang, A.; Li, H. Bin Effects of Different Green Tea Extracts on Chronic Alcohol Induced-Fatty Liver Disease by Ameliorating Oxidative Stress and Inflammation in Mice. Oxid. Med. Cell Longev. 2021, 2021, 5188205. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shi, J.; Zhao, L.; Guan, J.; Liu, F.; Huo, G.; Li, B. Lactobacillus Plantarum KLDS1.0344 and Lactobacillus Acidophilus KLDS1.0901 Mixture Prevents Chronic Alcoholic Liver Injury in Mice by Protecting the Intestinal Barrier and Regulating Gut Microbiota and Liver-Related Pathways. J. Agric. Food Chem. 2021, 69, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Li, B.Y.; Mao, Q.Q.; Gan, R.Y.; Cao, S.Y.; Xu, X.Y.; Luo, M.; Li, H.Y.; Li, H. Bin Protective Effects of Tea Extracts against Alcoholic Fatty Liver Disease in Mice via Modulating Cytochrome P450 2E1 Expression and Ameliorating Oxidative Damage. Food Sci. Nutr. 2021, 9, 5626–5640. [Google Scholar] [CrossRef] [PubMed]
- Lian, L.H.; Wu, Y.L.; Song, S.Z.; Wan, Y.; Xie, W.X.; Li, X.; Bai, T.; Ouyang, B.Q.; Nan, J.X. Gentiana Manshurica Kitagawa Reverses Acute Alcohol-Induced Liver Steatosis through Blocking Sterol Regulatory Element-Binding Protein-1 Maturation. J. Agric. Food Chem. 2010, 58, 13013–13019. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.P.; Chuang, W.C.; Lu, F.J.; Chen, C.Y. Anti-Oxidant and Anti-Inflammatory Effects of Hydrogen-Rich Water Alleviate Ethanol-Induced Fatty Liver in Mice. World J. Gastroenterol. 2017, 23, 4920–4934. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.A.; Ke, B.J.; Cheng, S.C.; Lee, C.L. Red Quinoa Bran Extract Prevented Alcoholic Fatty Liver Disease via Increasing Antioxidative System and Repressing Fatty Acid Synthesis Factors in Mice Fed Alcohol Liquid Diet. Molecules 2021, 26, 6973. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, X.; Liu, R.; Liu, Y.; Zhang, T.; Fu, H.; Hai, C. Oleanolic Acid Co-Administration Alleviates Ethanol-Induced Hepatic Injury via Nrf-2 and Ethanol-Metabolizing Modulating in Rats. Chem. Biol. Interact. 2014, 221, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, X.; Peng, Z.; Zhang, T.; Wu, H.; Yu, W.; Kong, D.; Liu, Y.; Bai, H.; Liu, R.; et al. The Effects of Insulin Pre-Administration in Mice Exposed to Ethanol: Alleviating Hepatic Oxidative Injury through Anti-Oxidative, Anti-Apoptotic Activities and Deteriorating Hepatic Steatosis through SRBEP-1c Activation. Int. J. Biol. Sci. 2015, 11, 569–586. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; He, H.; Wang, J.; Guo, X.; Lin, H.; Chen, H.; Jiang, C.; Chen, L.; Yao, P.; Tang, Y. Oxidative Stress-Dependent Frataxin Inhibition Mediated Alcoholic Hepatocytotoxicity through Ferroptosis. Toxicology 2020, 445, 152584. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.X.; Liu, H.; Wang, S.; Zhang, C.L.; Guo, F.F.; Zeng, T. Diallyl Disulfide Ameliorates Ethanol-Induced Liver Steatosis and Inflammation by Maintaining the Fatty Acid Catabolism and Regulating the Gut-Liver Axis. Food Chem. Toxicol. 2022, 164, 113108. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Kong, D.; Ai, D.; Xu, A.; Yu, W.; Peng, Z.; Peng, J.; Wang, Z.; Liu, R.; Li, W.; et al. Insulin Resistance Enhances Binge Ethanol-Induced Liver Injury through Promoting Oxidative Stress and up-Regulation CYP2E1. Life Sci. 2022, 303, 120681. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.H.; Tseng, H.C.; Liu, C.T.; Huang, C.J.; Chyuan, J.H.; Sheen, L.Y. Wild Bitter Gourd Protects against Alcoholic Fatty Liver in Mice by Attenuating Oxidative Stress and Inflammatory Responses. Food Funct. 2014, 5, 1027–1037. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Xu, W.; Zhang, F.; Jin, H.; Chen, Q.; Chen, L.; Shao, J.; Wu, L.; Lu, Y.; Zheng, S. Ligustrazine Prevents Alcohol-Induced Liver Injury by Attenuating Hepatic Steatosis and Oxidative Stress. Int. Immunopharmacol. 2015, 29, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.S.; Chiu, W.C.; Chen, Y.L.; Peng, H.C.; Shirakawa, H.; Yang, S.C. Fish Oil Up-Regulates Hepatic Autophagy in Rats with Chronic Ethanol Consumption. J. Nutr. Biochem. 2020, 77, 108314. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Liu, X.Y.; Noh, K.H.; Kim, M.J.; Song, Y.S. Protective Effects of Persimmon Leaf and Fruit Extracts against Acute Ethanol-Induced Hepatotoxicity. J. Food Sci. Nutr. 2007, 12, 202–208. [Google Scholar] [CrossRef]
- Madushani Herath, K.H.I.N.; Bing, S.J.; Cho, J.; Kim, A.; Kim, G.; Kim, J.S.; Kim, J.B.; Doh, Y.H.; Jee, Y. Sasa Quelpaertensis Leaves Ameliorate Alcohol-Induced Liver Injury by Attenuating Oxidative Stress in HepG2 Cells and Mice. Acta Histochem. 2018, 120, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Mai, B.; Han, L.; Zhong, J.; Shu, J.; Cao, Z.; Fang, J.; Zhang, X.; Gao, Z.; Xiao, F. Rhoifolin Alleviates Alcoholic Liver Disease In Vivo and In Vitro via Inhibition of the TLR4/NF-ΚB Signaling Pathway. Front. Pharmacol. 2022, 13, 878898. [Google Scholar] [CrossRef] [PubMed]
- Maimaitimin, K.; Jiang, Z.; Aierken, A.; Shayibuzhati, M.; Zhang, X. Hepatoprotective Effect of Alhagi sparsifolia against Alcoholic Liver Injury in Mice. Braz. J. Pharm. Sci. 2018, 54, e17732. [Google Scholar] [CrossRef]
- Mallikarjuna, K.; Sahitya Chetan, P.; Sathyavelu Reddy, K.; Rajendra, W. Ethanol Toxicity: Rehabilitation of Hepatic Antioxidant Defense System with Dietary Ginger. Fitoterapia 2008, 79, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Nelson, V.K.; Mukhopadhyay, S.; Bandhopadhyay, S.; Maganti, L.; Ghoshal, N.; Sen, G.; Biswas, T. 14-Deoxyandrographolide Targets Adenylate Cyclase and Prevents Ethanol-Induced Liver Injury through Constitutive NOS Dependent Reduced Redox Signaling in Rats. Food Chem. Toxicol. 2013, 59, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Mehanna, E.T.; Ali, A.S.A.; El-Shaarawy, F.; Mesbah, N.M.; Abo-Elmatty, D.M.; Aborehab, N.M. Anti-Oxidant and Anti-Inflammatory Effects of Lipopolysaccharide from Rhodobacter sphaeroides against Ethanol-Induced Liver and Kidney Toxicity in Experimental Rats. Molecules 2021, 26, 7437. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Tang, G.Y.; Zhao, C.N.; Liu, Q.; Xu, X.Y.; Cao, S.Y. Hepatoprotective Effects of Hovenia Dulcis Seeds against Alcoholic Liver Injury and Related Mechanisms Investigated via Network Pharmacology. World J. Gastroenterol. 2020, 26, 3432–3446. [Google Scholar] [CrossRef] [PubMed]
- Miana, J.B.; Gómez-Cambronero, L.; Lloret, A.; Pallardó, F.V.; Del Olmo, J.; Escudero, A.; Rodrigo, J.M.; Pellíin, A.; Via, J.R.; Viña, J.; et al. Mitochondrial Oxidative Stress and CD95 Ligand: A Dual Mechanism for Hepatocyte Apoptosis in Chronic Alcoholism. Hepatology 2002, 35, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Ming, L.; Qi, B.; Hao, S.; Ji, R. Camel Milk Ameliorates Inflammatory Mechanisms in an Alcohol-Induced Liver Injury Mouse Model. Sci. Rep. 2021, 11, 22811. [Google Scholar] [CrossRef] [PubMed]
- Mohan, R.; Jose, S.; Sukumaran, S.; Asha, S.; Sheethal, S.; John, G.; Krishnakumar, I.M. Curcumin-Galactomannosides Mitigate Alcohol-Induced Liver Damage by Inhibiting Oxidative Stress, Hepatic Inflammation, and Enhance Bioavailability on TLR4/MMP Events Compared to Curcumin. J. Biochem. Mol. Toxicol. 2019, 33, e22315. [Google Scholar] [CrossRef] [PubMed]
- Nagappan, A.; Jung, D.Y.; Kim, J.H.; Lee, H.; Jung, M.H. Gomisin N Alleviates Ethanol-Induced Liver Injury through Ameliorating Lipid Metabolism and Oxidative Stress. Int. J. Mol. Sci. 2018, 19, 2601. [Google Scholar] [CrossRef]
- Nie, W.; Du, Y.Y.; Xu, F.R.; Zhou, K.; Wang, Z.M.; Al-Dalali, S.; Wang, Y.; Li, X.M.; Ma, Y.H.; Xie, Y. Oligopeptides from Jinhua Ham Prevent Alcohol-Induced Liver Damage by Regulating Intestinal Homeostasis and Oxidative Stress in Mice. Food Funct. 2021, 12, 10053–10070. [Google Scholar] [CrossRef]
- Nie, W.; Xu, F.; Zhou, K.; Yang, X.; Zhou, H.; Xu, B. Stearic Acid Prevent Alcohol-Induced Liver Damage by Regulating the Gut Microbiota. Food Res. Int. 2022, 155, 111095. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.I.; Lee, M.S.; Kim, C.I.; Song, K.Y.; Park, S.C. Aspartate Modulates the Ethanol-Induced Oxidative Stress and Glutathione Utilizing Enzymes in Rat Testes. Exp. Mol. Med. 2002, 34, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Osaki, K.; Arakawa, T.; Kim, B.; Lee, M.; Jeong, C.; Kang, N. Hepatoprotcetive Effects of Oyster (Crassostrea Gigas) Extract in a Rat Model of Alcohol-Induced Oxidative Stress. J. Korean Soc. Food Sci. Nutr. 2016, 45, 805–811. [Google Scholar] [CrossRef]
- Panda, V.; Ashar, H.; Srinath, S. Antioxidant and Hepatoprotective Effect of Garcinia Indica Fruit Rind in Ethanolinduced Hepatic Damage in Rodents. Interdiscip. Toxicol. 2012, 5, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Panda, V.; Kharat, P.; Sudhamani, S. Hepatoprotective Effect of the Macrotyloma Uniflorum Seed (Horse Gram) in Ethanol-Induced Hepatic Damage in Rats. J. Biol. Act. Prod. Nat. 2015, 5, 178–191. [Google Scholar]
- Pari, L.; Suresh, A. Effect of Grape (Vitis vinifera L.) Leaf Extract on Alcohol Induced Oxidative Stress in Rats. Food Chem. Toxicol. 2008, 46, 1627–1634. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Ha, S.K.; Eom, H.; Choi, I. Narirutin Fraction from Citrus Peels Attenuates Alcoholic Liver Disease in Mice. Food Chem. Toxicol. 2013, 55, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Ahn, G.; Um, J.H.; Han, E.J.; Ahn, C.B.; Yoon, N.Y.; Je, J.Y. Hepatoprotective Effect of Chitosan-Caffeic Acid Conjugate against Ethanol-Treated Mice. Exp. Toxicol. Pathol. 2017, 69, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Fernando, I.P.S.; Han, E.J.; Kim, M.J.; Jung, K.; Kang, D.S.; Ahn, C.B.; Ahn, G. In Vivo Hepatoprotective Effects of a Peptide Fraction from Krill Protein Hydrolysates against Alcohol-Induced Oxidative Damage. Mar. Drugs 2019, 17, 690. [Google Scholar] [CrossRef] [PubMed]
- Patere, S.N.; Majumdar, A.S.; Saraf, M.N. Exacerbation of Alcohol-Induced Oxidative Stress in Rats by Polyunsaturated Fatty Acids and Iron Load. Indian J. Pharm. Sci. 2011, 73, 152–158. [Google Scholar] [PubMed]
- Peng, H.C.; Chen, Y.L.; Chen, J.R.; Yang, S.S.; Huang, K.H.; Wu, Y.C.; Lin, Y.H.; Yang, S.C. Effects of Glutamine Administration on Inflammatory Responses in Chronic Ethanol-Fed Rats. J. Nutr. Biochem. 2011, 22, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.C.; Chen, Y.L.; Yang, S.Y.; Ho, P.Y.; Yang, S.S.; Hu, J.T.; Yang, S.C. The Antiapoptotic Effects of Different Doses of β-Carotene in Chronic Ethanol-Fed Rats. Hepatobiliary Surg. Nutr. 2013, 2, 132–141. [Google Scholar] [PubMed]
- Pi, A.; Jiang, K.; Ding, Q.; Lai, S.; Yang, W.; Zhu, J.; Guo, R.; Fan, Y.; Chi, L.; Li, S. Alcohol Abstinence Rescues Hepatic Steatosis and Liver Injury via Improving Metabolic Reprogramming in Chronic Alcohol-Fed Mice. Front. Pharmacol. 2021, 12, 752148. [Google Scholar] [CrossRef] [PubMed]
- Prathibha, P.; Rejitha, S.; Harikrishnan, R.; Das, S.S.; Abhilash, P.A.; Indira, M. Additive Effect of Alpha-Tocopherol and Ascorbic Acid in Combating Ethanol-Induced Hepatic Fibrosis. Redox Rep. 2013, 18, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Qi, N.; Liu, C.; Yang, H.; Shi, W.; Wang, S.; Zhou, Y.; Wei, C.; Gu, F.; Qin, Y. Therapeutic Hexapeptide (PGPIPN) Prevents and Cures Alcoholic Fatty Liver Disease by Affecting the Expressions of Genes Related with Lipid Metabolism and Oxidative Stress. Oncotarget 2017, 8, 88079–88093. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Zhu, Y.; Liu, Y.; Yang, H.; Zhu, C.; Ma, P.; Deng, J.; Fan, D. Protective Effects of Ginsenoside Rk3 against Chronic Alcohol-Induced Liver Injury in Mice through Inhibition of Inflammation, Oxidative Stress, and Apoptosis. Food Chem. Toxicol. 2019, 126, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Rabelo, A.C.S.; de Pádua Lúcio, K.; Araújo, C.M.; de Araújo, G.R.; de Amorim Miranda, P.H.; Carneiro, A.C.A.; de Castro Ribeiro, É.M.; de Melo Silva, B.; de Lima, W.G.; Costa, D.C. Baccharis Trimera Protects against Ethanol Induced Hepatotoxicity in Vitro and in Vivo. J. Ethnopharmacol. 2018, 215, 1–13. [Google Scholar] [CrossRef]
- Rejitha, S.; Prathibha, P.; Indira, M. Amelioration of Alcohol-Induced Hepatotoxicity by the Administration of Ethanolic Extract of Sida Cordifolia Linn. Brit. J. Nutr. 2012, 108, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- Roede, J.R.; Stewart, B.J.; Petersen, D.R. Decreased Expression of Peroxiredoxin 6 in a Mouse Model of Ethanol Consumption. Free Radic. Biol. Med. 2008, 45, 1551–1558. [Google Scholar] [CrossRef] [PubMed]
- Roede, J.R.; Orlicky, D.J.; Fisher, A.B.; Petersen, D.R. Overexpression of Peroxiredoxin 6 Does Not Prevent Ethanol-Mediated Oxidative Stress and May Play a Role in Hepatic Lipid Accumulation. J. Pharmacol. Exp. Ther. 2009, 330, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Rong, S.; Zhao, Y.; Bao, W.; Xiao, X.; Wang, D.; Nussler, A.K.; Yan, H.; Yao, P.; Liu, L. Curcumin Prevents Chronic Alcohol-Induced Liver Disease Involving Decreasing ROS Generation and Enhancing Antioxidative Capacity. Phytomedicine 2012, 19, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Ronis, M.J.J.; Butura, A.; Sampey, B.P.; Shankar, K.; Prior, R.L.; Korourian, S.; Albano, E.; Ingelman-Sundberg, M.; Petersen, D.R.; Badger, T.M. Effects of N-Acetylcysteine on Ethanol-Induced Hepatotoxicity in Rats Fed via Total Enteral Nutrition. Free Radic. Biol. Med. 2005, 39, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Ronis, M.J.; Korourian, S.; Blackburn, M.L.; Badeaux, J.; Badger, T.M. The Role of Ethanol Metabolism in Development of Alcoholic Steatohepatitis in the Rat. Alcohol 2010, 44, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Samuhasaneeto, S.; Thong-Ngam, D.; Kulaputana, O.; Suyasunanont, D.; Klaikeaw, N. Curcumin Decreased Oxidative Stress, Inhibited Nf-k b Activation, and Improved Liver Pathology in Ethanol-Induced Liver Injury in Rats. J. Biomed. Biotechnol. 2009, 2009, 981963. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, N.; Rajasankar, S.; Nalini, N. Antioxidant Effect of 2-Hydroxy-4-Methoxy Benzoic Acid on Ethanol-Induced Hepatotoxicity in Rats. J. Pharm. Pharmacol. 2010, 59, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, N.; Nalini, N. Antioxidant Effect of Hemidesmus Indicus on Ethanol-Induced Hepatotoxicity in Rats. J. Med. Food 2007, 10, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Sathiavelu, J.; Senapathy, G.J.; Devaraj, R.; Namasivayam, N. Hepatoprotective Effect of Chrysin on Prooxidant-Antioxidant Status during Ethanol-Induced Toxicity in Female Albino Rats. J. Pharm. Pharmacol. 2009, 61, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, R.; Sengottuvelan, M.; Nalini, N. Protective Effect of Glycine Supplementation on the Levels of Lipid Peroxidation and Antioxidant Enzymes in the Erythrocyte of Rats with Alcohol-Induced Liver Injury. Cell Biochem. Funct. 2004, 22, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Shankari, S.G.; Karthikesan, K.; Jalaludeen, A.M.; Ashokkumar, N.; Ashokkumar, N.; Patill, S.; Brid, S. Hepatoprotective effect of morin on ethanol-induced hepatotoxicity in rats. J. Basic Clin. Physiol. Pharmacol. 2010, 21, 277–294. [Google Scholar] [CrossRef] [PubMed]
- Shearn, C.T.; Backos, D.S.; Orlicky, D.J.; Smathers-McCullough, R.L.; Petersen, D.R. Identification of 5′ AMP-Activated Kinase as a Target of Reactive Aldehydes during Chronic Ingestion of High Concentrations of Ethanol. J. Biol. Chem. 2014, 289, 15449–15462. [Google Scholar] [CrossRef] [PubMed]
- Shenbagam, M.; Nalini, N. Dose Response Effect of Rutin a Dietary Antioxidant on Alcohol-Induced Prooxidant and Antioxidant Imbalance—A Histopathologic Study. Fundam. Clin. Pharmacol. 2011, 25, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Zhao, Y.; Ding, C.; Wang, Z.; Ji, A.; Li, Z.; Feng, D.; Li, Y.; Gao, D.; Zhou, J. Salvianolic Acid A Alleviates Chronic Ethanol-Induced Liver Injury via Promotion of β-Catenin Nuclear Accumulation by Restoring SIRT1 in Rats. Toxicol. Appl. Pharmacol. 2018, 350, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Smathers, R.L.; Galligan, J.J.; Shearn, C.T.; Fritz, K.S.; Mercer, K.; Ronis, M.; Orlicky, D.J.; Davidson, N.O.; Petersen, D.R. Susceptibility of L-FABP -/- Mice to Oxidative Stress in Early-Stage Alcoholic Liver. J. Lipid. Res. 2013, 54, 1335–1345. [Google Scholar] [CrossRef] [PubMed]
- Sönmez, M.F.; Narin, F.; Akkuş, D.; Türkmen, A.B. Melatonin and Vitamin C Ameliorate Alcohol-Induced Oxidative Stress and ENOS Expression in Rat Kidney. Ren. Fail 2012, 34, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Deaciuc, I.; Song, M.; Lee, D.Y.W.; Liu, Y.; Ji, X.; McClain, C. Silymarin Protects against Acute Ethanol-Induced Hepatotoxicity in Mice. Alcohol Clin. Exp. Res. 2006, 30, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Liu, Z.; Zhang, J.; Zhang, C.; Dong, Y.; Ren, Z.; Gao, Z.; Liu, M.; Zhao, H.; Jia, L. Antioxidative and Hepatoprotective Effects of Enzymatic and Acidic-Hydrolysis of Pleurotus geesteranus Mycelium Polysaccharides on Alcoholic Liver Diseases. Carbohydr. Polym. 2018, 201, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wu, X.; Yang, D.; Fang, F.; Meng, L.; Liu, Y.; Cui, W. Protective Effect of Andrographolide on Alleviating Chronic Alcoholic Liver Disease in Mice by Inhibiting Nuclear Factor Kappa B and Tumor Necrosis Factor Alpha Activation. J. Med. Food 2020, 23, 409–415. [Google Scholar] [CrossRef]
- Song, X.; Sun, W.; Cui, W.; Jia, L.; Zhang, J. A Polysaccharide of PFP-1 from: Pleurotus Geesteranus Attenuates Alcoholic Liver Diseases via Nrf2 and NF-ΚB Signaling Pathways. Food Funct. 2021, 12, 4591–4605. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, S.; Srinivasan, P.; Manikandaselvi, S.; Thinagarbabu, R. Protective effect and antioxidant role of Achyranthus aspera, L. against ethanol-induced oxidative stress in rats. Int. J. Pharm. Pharm. Sci. 2012, 4 (Suppl. 3), 280–284. [Google Scholar]
- Sun, Q.; Zhong, W.; Zhang, W.; Zhou, Z. Defect of Mitochondrial Respiratory Chain Is a Mechanism of ROS Overproduction in a Rat Model of Alcoholic Liver Disease: Role of Zinc Deficiency. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 310, 205–214. [Google Scholar] [CrossRef]
- Tahir, M.; Rehman, M.U.; Lateef, A.; Khan, R.; Khan, A.Q.; Qamar, W.; Ali, F.; O’Hamiza, O.; Sultana, S. Diosmin Protects against Ethanol-Induced Hepatic Injury via Alleviation of Inflammation and Regulation of TNF-α and NF-ΚB Activation. Alcohol 2013, 47, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.; Liang, H.; Nie, J.; Diao, Y.; He, Q.; Hou, B.; Zhao, T.; Huang, H.; Li, Y.; Gao, X.; et al. Establishment of an Alcoholic Fatty Liver Disease Model in Mice. Am. J. Drug Alcohol Abuse 2017, 43, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Gao, C.; Xing, M.; Li, Y.; Zhu, L.; Wang, D.; Yang, X.; Liu, L.; Yao, P. Quercetin Prevents Ethanol-Induced Dyslipidemia and Mitochondrial Oxidative Damage. Food Chem. Toxicol. 2012, 50, 1194–1200. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.C.; Lin, W.L.; Lee, Y.J.; Tang, Y.C.; Wang, C.J. Polyphenol-Rich Extract of Nelumbo Nucifera Leaves Inhibits Alcohol-Induced Steatohepatitis via Reducing Hepatic Lipid Accumulation and Anti-Inflammation in C57BL/6J Mice. Food Funct. 2014, 5, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Li, Y.; Yu, H.; Gao, C.; Liu, L.; Xing, M.; Yao, P. Quercetin Attenuates Chronic Ethanol Hepatotoxicity: Implication of “Free” Iron Uptake and Release. Food Chem. Toxicol. 2014, 67, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Wei, R.; Deng, A.; Lei, T. Protective Effects of Ethanolic Extracts from Artichoke, an Edible Herbal Medicine, against Acute Alcohol-Induced Liver Injury in Mice. Nutrients 2017, 9, 1000. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Zhang, L.; Wu, T.; Fang, X.; Zhao, L. Echinacoside Ameliorates Alcohol-Induced Oxidative Stress and Hepatic Steatosis by Affecting SREBP1c/FASN Pathway via PPARα. Food Chem. Toxicol. 2021, 148, 111956. [Google Scholar] [CrossRef] [PubMed]
- Valansa, A.; Tietcheu Galani, B.R.; Djamen Chuisseu, P.D.; Tontsa Tsamo, A.; Ayissi Owona, V.B.; Yanou Njintang, N. Natural Limonoids Protect Mice from Alcohol-Induced Liver Injury. J. Basic Clin. Physiol. Pharmacol. 2020, 31, 20190271. [Google Scholar] [CrossRef] [PubMed]
- Varghese, J.; James, J.V.; Sagi, S.; Chakraborty, S.; Sukumaran, A.; Ramakrishna, B.; Jacob, M. Decreased Hepatic Iron in Response to Alcohol May Contribute to Alcohol-Induced Suppression of Hepcidin. Brit. J. Nutr. 2016, 115, 1978–1986. [Google Scholar] [CrossRef] [PubMed]
- Velvizhi, S.; Nagalashmi, T.; Essa, M.M.; Dakshayani, K.B.; Subramanian, P. Effects of alpha-ketoglutarate on lipid peroxidation and antioxidant status during chronic ethanol administration in Wistar rats. Pol. J. Pharmacol. 2002, 54, 231–236. [Google Scholar] [PubMed]
- Wang, C.; Li, X.; Wang, H.; Xie, Q.; Xu, Y. Notch1-Nuclear Factor ΚB Involves in Oxidative Stress-Induced Alcoholic Steatohepatitis. Alcohol Alcohol. 2014, 49, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Su, B.; Fan, S.; Fei, H.; Zhao, W. Protective Effect of Oligomeric Proanthocyanidins against Alcohol-Induced Liver Steatosis and Injury in Mice. Biochem. Biophys. Res. Commun. 2015, 458, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, Y.; Bai, R.; Wang, M.; Du, S. Baicalin Attenuates Alcoholic Liver Injury through Modulation of Hepatic Oxidative Stress, Inflammation and Sonic Hedgehog Pathway in Rats. Cell. Physiol. Biochem. 2016, 39, 1129–1140. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, M.; Zhang, C.; Li, S.; Yang, Q.; Zhang, J.; Gong, Z.; Han, J.; Jia, L. Antioxidant Activity and Protective Effects of Enzyme-Extracted Oudemansiella Radiata Polysaccharides on Alcohol-Induced Liver Injury. Molecules 2018, 23, 481. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Fu, Y.; Li, J.; Li, Y.; Zhao, Q.; Hu, A.; Xu, C.; Shao, D.; Chen, W. Aqueous Extract of Polygonatum sibiricum Ameliorates Ethanol-Induced Mice Liver Injury via Regulation of the Nrf2/ARE Pathway. J. Food Biochem. 2021, 45, e13537. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chang, X.; Zhan, H.; Zhang, Q.; Li, C.; Gao, Q.; Yang, M.; Luo, Z.; Li, S.; Sun, Y. Curcumin and Baicalin Ameliorate Ethanol-Induced Liver Oxidative Damage via the Nrf2/HO-1 Pathway. J. Food Biochem. 2020, 44, e13425. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.D.; Zhang, Y.; Dai, Y.D.; Ren, K.; Han, C.; Wang, H.X.; Yi, S.Q. Tamarix Chinensis Lour Inhibits Chronic Ethanol-Induced Liver Injury in Mice. World J. Gastroenterol. 2020, 26, 1286–1297. [Google Scholar] [CrossRef]
- Wang, W.; Zhong, G.Z.; Long, K.B.; Liu, Y.; Liu, Y.Q.; Xu, A.L. Silencing MiR-181b-5p Upregulates PIAS1 to Repress Oxidative Stress and Inflammatory Response in Rats with Alcoholic Fatty Liver Disease through Inhibiting PRMT1. Int. Immunopharmacol. 2021, 101, 108151. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, H.; Xing, R.; Li, P. Hepatoprotective Effect of Oyster Peptide on Alcohol-Induced Liver Disease in Mice. Int. J. Mol. Sci. 2022, 23, 8081. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Liu, Y.; Cong, P.; Xu, J.; Xue, C. Hepatoprotective Effects of Sea Cucumber Ether-Phospholipids against Alcohol-Induced Lipid Metabolic Dysregulation and Oxidative Stress in Mice. Food Funct. 2022, 13, 2791–2804. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Mu, J. Arbutin Attenuates Ethanol-Induced Acute Hepatic Injury by the Modulation of Oxidative Stress and Nrf-2/HO-1 Signaling Pathway. J. Biochem. Mol. Toxicol. 2021, 35, e22872. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Huang, Q.; Huang, R.; Chen, Y.; Lv, S.; Wei, L.; Liang, C.; Liang, S.; Zhuo, L.; Lin, X. Asiatic Acid from Potentilla Chinensis Attenuate Ethanol-Induced Hepatic Injury via Suppression of Oxidative Stress and Kupffer Cell Activation. Biol. Pharm. Bull. 2013, 36, 1980–1989. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, Y.; Jia, R.; Fang, F.; Liu, Y.; Cui, W. Computational and Biological Investigation of the Soybean Lecithin-Gallic Acid Complex for Ameliorating Alcoholic Liver Disease in Mice with Iron Overload. Food Funct. 2019, 10, 5203–5214. [Google Scholar] [CrossRef]
- Wu, C.; Liu, J.; Tang, Y.; Li, Y.; Yan, Q.; Jiang, Z. Hepatoprotective Potential of Partially Hydrolyzed Guar Gum against Acute Alcohol-Induced Liver Injury in Vitro and Vivo. Nutrients 2019, 11, 963. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Zhang, J.; Yao, J.; Zhang, B.; Duan, W.; Xia, M.; Song, J.; Zheng, Y.; Wang, M. Shanxi Aged Vinegar Prevents Alcoholic Liver Injury by Inhibiting CYP2E1 and NADPH Oxidase Activities. J. Funct. Foods 2018, 47, 575–584. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, J.; Xing, F.; Han, T.; Jiao, R.; Liong, E.C.; Fung, M.L.; So, K.F.; Tipoe, G.L. Zeaxanthin Dipalmitate Therapeutically Improves Hepatic Functions in an Alcoholic Fatty Liver Disease Model through Modulating MAPK Pathway. PLoS ONE 2014, 9, e95214. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, R.; Huang, F.; Liu, L.; Deng, Y.; Ma, Y.; Wei, Z.; Tang, X.; Zhang, Y.; Zhang, M. Lychee (Litchi chinensis Sonn.) Pulp Phenolic Extract Confers a Protective Activity against Alcoholic Liver Disease in Mice by Alleviating Mitochondrial Dysfunction. J. Agric. Food Chem. 2017, 65, 5000–5009. [Google Scholar] [CrossRef]
- Xiao, J.; Wu, C.; He, Y.; Guo, M.; Peng, Z.; Liu, Y.; Liu, L.; Dong, L.; Guo, Z.; Zhang, R.; et al. Rice Bran Phenolic Extract Confers Protective Effects against Alcoholic Liver Disease in Mice by Alleviating Mitochondrial Dysfunction via the PGC-1α-TFAM Pathway Mediated by MicroRNA-494-3p. J. Agric. Food Chem. 2020, 68, 12284–12294. [Google Scholar] [CrossRef]
- Xu, J.J.; Li, H.D.; Wu, M.F.; Zhu, L.; Du, X.S.; Li, J.J.; Li, Z.; Meng, X.M.; Huang, C.; Li, J. 3-B-RUT, a Derivative of RUT, Protected against Alcohol-Induced Liver Injury by Attenuating Inflammation and Oxidative Stress. Int. Immunopharmacol. 2021, 95, 107471. [Google Scholar] [CrossRef]
- Yalçinkaya, S.; Ünlüçerçi, Y.; Uysal, M. Methionine-Supplemented Diet Augments Hepatotoxicity and Prooxidant Status in Chronically Ethanol-Treated Rats. Exp. Toxicol. Pathol. 2007, 58, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.L.; Yin, M.C. Protective and Alleviative Effects from 4 Cysteine-Containing Compounds on Ethanol-Induced Acute Liver Injury through Suppression of Oxidation and Inflammation. J. Food Sci. 2007, 72, S511–S515. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Wang, Z.; Zhan, Y.; Wang, T.; Zhou, M.; Xia, L.; Yang, X.; Zhang, J. Endogenous A1 Adenosine Receptor Protects Mice from Acute Ethanol-Induced Hepatotoxicity. Toxicology 2013, 309, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Liao, A.M.; Cui, Y.; Yu, G.; Hou, Y.; Pan, L.; Chen, W.; Zheng, S.; Li, X.; Ma, J.; et al. Wheat Embryo Globulin Protects against Acute Alcohol-Induced Liver Injury in Mice. Food Chem. Toxicol. 2021, 153, 112240. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhou, Z.; Liu, Y.; Xu, X.; Xu, Y.; Zhou, W.; Chen, S.; Mao, J. Non-Alcoholic Components in Huangjiu as Potential Factors Regulating the Intestinal Barrier and Gut Microbiota in Mouse Model of Alcoholic Liver Injury. Foods 2022, 11, 1537. [Google Scholar] [CrossRef]
- Yao, P.; Li, K.; Song, F.; Zhou, S.; Sun, X.; Zhang, X.; Nüssler, A.K.; Liu, L. Heme Oxygenase-1 Upregulated by Ginkgo Biloba Extract: Potential Protection against Ethanol-Induced Oxidative Liver Damage. Food Chem. Toxicol. 2007, 45, 1333–1342. [Google Scholar] [CrossRef] [PubMed]
- Yeh, W.J.; Tsai, C.C.; Ko, J.; Yang, H.Y. Hylocereus Polyrhizus Peel Extract Retards Alcoholic Liver Disease Progression by Modulating Oxidative Stress and Inflammatory Responses in C57Bl/6 Mice. Nutrients 2020, 12, 3884. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.J.; Koh, E.J.; Kim, C.S.; Zee, O.P.; Kwak, J.H.; Jeong, W.J.; Kim, J.H.; Lee, S.M. Agrimonia Eupatoria Protects against Chronic Ethanol-Induced Liver Injury in Rats. Food Chem. Toxicol. 2012, 50, 2335–2341. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Yoo, S.; Yoon, H.G.; Park, J.; Lee, Y.H.; Kim, S.; Oh, K.T.; Lee, J.; Cho, H.Y.; Jun, W. In Vitro and in Vivo Hepatoprotective Effects of the Aqueous Extract from Taraxacum Officinale (Dandelion) Root against Alcohol-Induced Oxidative Stress. Food Chem. Toxicol. 2010, 48, 1632–1637. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Liu, Y.L.; Ai, Z.Y.; Wang, Y.S.; Liu, J.M.; Piao, C.H.; Wang, Y.H. Lactobacillus Fermentum KP-3-Fermented Ginseng Ameliorates Alcohol-Induced Liver Disease in C57BL/6N Mice through the AMPK and MAPK Pathways. Food Funct. 2020, 11, 9801–9809. [Google Scholar] [CrossRef]
- Yu, Y.; Tian, Z.Q.; Liang, L.; Yang, X.; Sheng, D.D.; Zeng, J.X.; Li, X.Y.; Shi, R.Y.; Han, Z.P.; Wei, L.X. Babao Dan Attenuates Acute Ethanol-Induced Liver Injury via Nrf2 Activation and Autophagy. Cell Biosci. 2019, 9, 80. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Yu, R.-J.; Yang, C.; Wang, Q.; Xuan, Y.; Wang, Z.; He, Z.; Xu, Y.; Kou, L.; Zhao, Y.-Z.; et al. Evaluation of the Hepatoprotective Effect of Naringenin Loaded Nanoparticles against Acetaminophen Overdose Toxicity. Drug Deliv. 2022, 29, 3256–3269. [Google Scholar] [CrossRef] [PubMed]
- Yuan, R.; Tao, X.; Liang, S.; Pan, Y.; He, L.; Sun, J.; Wenbo, J.; Li, X.; Chen, J.; Wang, C. Protective Effect of Acidic Polysaccharide from Schisandra Chinensis on Acute Ethanol-Induced Liver Injury through Reducing CYP2E1-Dependent Oxidative Stress. Biomed. Pharmacother. 2018, 99, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Duan, S.; Guan, T.; Yuan, X.; Lin, J.; Hou, S.; Lai, X.; Huang, S.; Du, X.; Chen, S. Vitexin Protects against Ethanol-Induced Liver Injury through Sirt1/P53 Signaling Pathway. Eur. J. Pharmacol. 2020, 873, 173007. [Google Scholar] [CrossRef] [PubMed]
- Zahid, M.; Arif, M.; Rahman, M.A.; Mujahid, M. Hepatoprotective and Antioxidant Activities of Annona Squamosa Seed Extract against Alcohol-Induced Liver Injury in Sprague Dawley Rats. Drug Chem. Toxicol. 2020, 43, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Zeng, T.; Zhang, C.L.; Song, F.Y.; Zhao, X.L.; Yu, L.H.; Zhu, Z.P.; Xie, K.Q. The Activation of HO-1/Nrf-2 Contributes to the Protective Effects of Diallyl Disulfide (DADS) against Ethanol-Induced Oxidative Stress. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 4848–4859. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xue, J.; Wang, H.; Zhang, Y.; Xie, M. Osthole Improves Alcohol-Induced Fatty Liver in Mice by Reduction of Hepatic Oxidative Stress. Phytother. Res. 2011, 25, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Ma, D.; Wang, Y.; Zhang, M.; Qiang, X.; Liao, M.; Liu, X.; Wu, H.; Zhang, Y. Berberine Protects Liver from Ethanol-Induced Oxidative Stress and Steatosis in Mice. Food Chem. Toxicol. 2014, 74, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Qiang, X.; Zhang, M.; Ma, D.; Zhao, Z.; Zhou, C.; Liu, X.; Li, R.; Chen, H.; Zhang, Y. Demethyleneberberine, a Natural Mitochondria-Targeted Antioxidant, Inhibits Mitochondrial Dysfunction, Oxidative Stress, and Steatosis in Alcoholic Liver Disease Mouse Model. J. Pharmacol. Exp. Ther. 2015, 352, 139–147. [Google Scholar] [CrossRef]
- Zhang, L.; Meng, B.; Li, L.; Wang, Y.; Zhang, Y.; Fang, X.; Wang, D. Boletus aereus Protects against Acute Alcohol-Induced Liver Damage in the C57BL/6 Mouse via Regulating the Oxidative Stress-Mediated NF-ΚB Pathway. Pharm. Biol. 2020, 58, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, S.; Fu, Y.; Yan, L.; Feng, Y.; Chen, Y.; Wu, Y.; Deng, Y.; Zhang, G.; Chen, Z.; et al. Computational Repositioning of Dimethyl Fumarate for Treating Alcoholic Liver Disease. Cell Death Dis. 2020, 11, 641. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yang, J.; Liu, J.; Long, X.; Zhang, X.; Li, J.; Hou, C. Red Yeast Rice Prevents Chronic Alcohol-Induced Liver Disease by Attenuating Oxidative Stress and Inflammatory Response in Mice. J. Food Biochem. 2021, 45, e13672. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Chen, H.; Li, Y. Protective Effect of Bicyclol on Acute Alcohol-Induced Liver Injury in Mice. Eur. J. Pharmacol. 2008, 586, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, N.; Yang, D.; Yang, M.; Guo, X.; He, J.; Wu, W.; Ji, B.; Cheng, Q.; Zhou, F. Protective Effects of Five Structurally Diverse Flavonoid Subgroups against Chronic Alcohol-Induced Hepatic Damage in a Mouse Model. Nutrients 2018, 10, 1754. [Google Scholar] [CrossRef]
- Zhao, L.; Mehmood, A.; Soliman, M.M.; Iftikhar, A.; Iftikhar, M.; Aboelenin, S.M.; Wang, C. Protective Effects of Ellagic Acid Against Alcoholic Liver Disease in Mice. Front. Nutr. 2021, 8, 744520. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Liu, S.; Zhao, H.; Liu, Y.; Xue, M.; Zhang, H.; Qiu, X.; Sun, Z.; Liang, H. Protective Effects of Fucoidan against Ethanol-Induced Liver Injury through Maintaining Mitochondrial Function and Mitophagy Balance in Rats. Food Funct. 2021, 12, 3842–3854. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Cui, J.; Chen, A.H.; Zong, Z.M.; Wei, X.Y. Optimization of Ultrasonic-Microwave Assisted Extraction and Hepatoprotective Activities of Polysaccharides from Trametes orientalis. Molecules 2019, 24, 147. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.Y.; Zou, X.; Wang, Y.L.; Zou, M.; Ma, F.; Wang, N.; Li, J.W.; Wang, M.S.; Hung, H.Y.; Wang, Q. Betulinic Acid-Nucleoside Hybrid Prevents Acute Alcohol -Induced Liver Damage by Promoting Anti-Oxidative Stress and Autophagy. Eur. J. Pharmacol. 2022, 914, 174686. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Sun, X.; Kang, Y.J. Metallothionein Protection against Alcoholic Liver Injury through Inhibition of Oxidative Stress. Exp. Biol. Med. 2002, 227, 214–222. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, J.; Wang, C.; Qu, S.; Zhu, Y.; Yang, Z.; Wang, L. Açaí (Euterpe Oleracea Mart.) Attenuates Alcohol-Inducedliver Injury in Rats by Alleviating Oxidative Stressand Inflammatory Response. Exp. Ther. Med. 2018, 15, 166–172. [Google Scholar] [PubMed]
- Zhou, J.; Zhang, N.; Zhao, L.; Wu, W.; Zhang, L.; Zhou, F.; Li, J. Astragalus Polysaccharides and Saponins Alleviate Liver Injury and Regulate Gut Microbiota in Alcohol Liver Disease Mice. Foods 2021, 10, 2688. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, N.; Zhao, L.; Soliman, M.M.; Wu, W.; Li, J.; Zhou, F.; Zhang, L. Protective Effects of Honey-Processed Astragalus on Liver Injury and Gut Microbiota in Mice Induced by Chronic Alcohol Intake. J. Food Qual. 2022, 2022, 5333691. [Google Scholar] [CrossRef]
- Zhu, S.; Ma, L.; Wu, Y.; Ye, X.; Zhang, T.; Zhang, Q.; Rasoul, L.M.; Liu, Y.; Guo, M.; Zhou, B.; et al. FGF21 Treatment Ameliorates Alcoholic Fatty Liver through Activation of AMPK-SIRT1 Pathway. Acta Biochim. Biophys. Sin. 2014, 46, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zhou, W.; Yang, Y.; Wang, K.; Li, F.; Dang, Y. Quantitative Profiling of Oxylipin Reveals the Mechanism of Pien-Tze-Huang on Alcoholic Liver Disease. Evid. Based Complement Alternat. Med. 2021, 2021, 9931542. [Google Scholar] [CrossRef]
- Xie, N.; Zhang, L.; Gao, W.; Huang, C.; Huber, P.E.; Zhou, X.; Li, C.; Shen, G.; Zou, B. NAD+ Metabolism: Pathophysiologic Mechanisms and Therapeutic Potential. Signal Transduct. Target Ther. 2020, 5, 227. [Google Scholar] [CrossRef] [PubMed]
- Bougarne, N.; Weyers, B.; Desmet, S.J.; Deckers, J.; Ray, D.W.; Staels, B.; De Bosscher, K. Molecular Actions of PPARα in Lipid Metabolism and Inflammation. Endocr. Rev. 2018, 39, 760–802. [Google Scholar] [CrossRef]
- Namachivayam, A.; Gopalakrishnan, A.V. A Review on Molecular Mechanism of Alcoholic Liver Disease. Life Sci. 2021, 274, 119328. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Huang, H.; Wang, Y.; Yang, R.; Ke, X. Antioxidant Effects of Se-Glutathione Peroxidase in Alcoholic Liver Disease. J. Trace Elem. Med. Biol. 2022, 74, 127048. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Fu, J.; Li, L.; Chen, C.; Wang, H.; Hou, Y.; Xu, Y.; Pi, J. Nrf2 in Alcoholic Liver Disease. Toxicol. Appl. Pharmacol. 2018, 357, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Bae, T.; Hallis, S.P.; Kwak, M.-K. Hypoxia, Oxidative Stress, and the Interplay of HIFs and NRF2 Signaling in Cancer. Exp. Mol. Med. 2024, 56, 501–514. [Google Scholar] [CrossRef]










| Study Characteristics | Animal Characteristics | Study Design | Total (n) | Outcomes | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Author and Year | Study Location | Funding | Conflict of Interest | Lineage | Gender | Size | Age | Control | AFLD | ||
| Abdelhamid et al., 2020 [10] | Egypt | Any specific grant from funding agencies in the public, commercial, or not-for-profit sectors | No conflicts declared | BALB/c mice | Male | 25 ± 3 g | 10 weeks old | Ethanol-containing liquid diet. Ethanol increased from 1% to 4% (v/v) from day 2 to day 5, and 5% (v/v) on day 6 and for 10 days. After that, mice were gavaged with a single dose of ethanol (5 g/kg) | 12 | 12 | ALT, AST, MDA, SOD, GSH, IL-6, IL-1B, and TNF-a |
| Abdelhamid et al., 2021 [11] | Egypt | No information | No conflicts declared | BALB/c mice | Male | 25 ± 3 g | 10 weeks old | Lieber-DeCarli liquid diet for 10 days. Ethanol increased from 1% to 4% (v/v) from day 2 to day 5, respectively. Then, from day 6 and for 10 days 5% (v/v). After that, mice were gavaged with a single dose of ethanol (5 g/kg)] | 6 | 6 | ALT, AST, SOD, GSH, MDA, TNF-a, IL-6, and IL-1B |
| Al-Rejaie, 2012 [12] | Saudi Arabia | Deanship of Scientific Research at King Saud University and Global Research Network for Medicinal Plantas and King Saud University | No information | Wistar rats | Male | 180–200 g | 8 weeks old | 25% ethanol (5 g/kg/bw) for 5 weeks | 6 | 6 | ALT, AST, TAG, GSH, MDA, SOD, andCAT |
| Atef et al., 2018 [13] | Egypt | No information | No conflicts declared | Albino rats | Male | 120–150 g | 90 days old | 20% ethanol (7.9 g kg/day) once a day orally for 8 weeks | 6 | 6 | ALT, AST, TAG, MDA, GSH, and SOD |
| Bae et al., 2015 [14] | Korea | Korea Institute of Planning and Evaluation for Technology in Food, Agriculture Forestry, and Fisheries | No conflicts declared | Sprague Dawley rats | Male | 220–240 g | Not declared | Ethanol 2.5 g/kg every 12 h for a total of 42 doses | 8 | 8 | ALT, AST, CAT, GST, GPx, GR, GSH, MDA, CYP2E1 and Histopathological score |
| Balasubramaniyan et al., 2003 [15] | India | No information | No information | Swiss mice | Male | 25–30 g | Not declared | 16% ethanol (6.32 g/kg/bw) as an aqueous solution using an intragastric tube daily for 45 days | 6 | 6 | TBARS, CAT, GSH, and GST |
| Baranisrinivasan et al., 2009 [16] | India | No information | No information | Wistar rats | Male | 160–180 g | Not declared | 20% ethanol (7.9 g/kg/bw) for 45 days | 6 | 6 | TBARS, SOD, and CAT |
| Bardag-Gorce et al., 2011 [17] | United States | NIH/NIAAA, USC Research Center for Alcoholic Liver and Pancreatic Disease, Cirrhosis Pilot Project Funding, and Morphologic Core | No information | Wistar rats | Male | 250–300 g | Not declared | Liquid diet containing ethanol (13 g/kg/bw/day) for 4 weeks | 3 | 3 | / |
| Bedi et al., 2017 [18] | India | Mr. Parveen Garg, Chairman, ISF College of Pharmacy | No information | Wistar rats | Either sex | 200–250 g | Not declared | 40% alcohol (2 mL/100 g/day) for 21 days | 6 | 6 | ALT, AST, LPO, TNF-a, IL-1B, and IL-6 |
| Bharrhan et al., 2011 [19] | India | Indian Council of Medical Research | No conflicts declared | Wistar rats | Female | 200–250 g | Not declared | 35% ethanol (10 g/kg/bw) by oral gavage for 2 weeks. Thereafter, the dose was increased to 14 g/kg/bw and was continued for 10 weeks | 6 or 8 | 6 or 8 | ALT, AST, TNF-a, MDA, GSH, SOD, GR, and GPx |
| Bisht et al., 2018 [20] | India | No information | Declare no conflict | Wistar rats | Either sex | 150–200 g | Not declared | Ethanol (3.76 g/kg) for 26 days | 6 | 6 | ALT, AST, TAG, SOD, CAT, and LPO |
| Bispo et al., 2017 [21] | Brazil | Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq); Instituto Nacional de Ciência e Tecnologia de Processos Redox em Biomedicina | No information | Wistar rats | Male | 250 ± 50 g | Not declared | 2.5 mL/kg of ethanol 35% (w/v) twice a day for 4 days | 5 | 5 | ALT, AST, CAT, TBARS, LPO, TAG, GSH, and GSSG/GSH ratio |
| Buko et al., 2019 [22] | Belarus | State Program of Belarus “Fundamental and Applied Sciences for Medicine,” Subprogram 11.1, “Fundamental and Applied Medicine” | No conflicts declared | Wistar rats | Male | 200–230 g | Not declared | 30% Ethanol(4 g/kg/bw) for 8 weeks | 8 | 8 | ALT, AST, TAG, TNFα, IL-1β, TBARS, GSH, and Inflammatory foci |
| Bulle et al., 2015 [23] | India | No information | No information | Wistar rats | Male | 120–140 g | 2 month old | Alcohol 20% (5 g/kg/bw) for 60 days | 8 | 8 | / |
| Cao et al., 2015 [24] | China | Research Committee of the University of Macau and Macao Science and Technology Development Fund | No information | C57BL/6 mice | Female | 25–30 g | 16–17 weeks old | Lieber-DeCarli liquid alcohol diet for 4 weeks | 4 | 4 | ALT, AST, TAG, TNF-a, IL-6, MDA, GSH, SOD, GPx, CYP2E1, and Nrf2 |
| Chandra et al., 2000 [25] | India | No information | No information | Wistar rats | Male | 150–180 g | Not declared | 2 mL of 50% ethanol (v/v) per day for a period of 7 days | 8 | 8 | GSH and GST |
| Chang et al., 2017 [26] | Taiwan | Ministry of Science and Technology | No conflicts declared | C57BL/6J mice | Male | 20–22 g | 8 weeks old | Lieber-DeCarli ethanol liquid diet for 4 weeks | 8 | 8 | ALT, AST, TAG, TBARS, GSH, SOD, CAT, GPx, TNF-a, IL-1B, CYP2E1, and inflammation score |
| Chang et al., 2021 [27] | Korea | Technology Development Program funded by the Ministry of SMEs and Startups | No conflicts declared | Sprague Dawley rats | Male | 160–170 g | 6–7 weeks old | Alcohol was administered intragastrically at a dose of 5 g/kg every 12 h for a total of 3 doses | 10 | 10 | ALT, AST, TAG, MDA, and GSH |
| Chaturvedi et al., 2007 [28] | Africa | University of Botswana | No information | Wistar rats | Male | 200–250 g | Not declared | Ethanol (5 g/kg/bw) for 30 days | 5 | 5 | ALT and AST |
| Chavan et al., 2017 [29] | India | Bharati Vidyapeeth Deemed University | No conflicts declared | Wistar rats | Male | 150–200 g | Not declared | 1 mL of 30% alcohol per 100 g/bw/day for 15 days | 6 | 6 | ALT, AST, TAG, SOD, and CAT |
| Chen et al., 2013 [30] | Taiwan | Gold Nanotech, Inc., Taiwan, Republic of China | No conflicts declared | Wistar rats | Male | Not declared | 6 weeks old | Liber-DeCarli liquid diet for 10 weeks. Ethanol contributed 35% of the total calories, 8/125, (v/v) | 6 | 6 | ALT, AST, TAG, GPx, GR, SOD, CAT, GSH/GSSG ratio, TBARS, CYP2E1, and TNF-a |
| Chen et al., 2016 [31] | United States | NIH and NIAAA | No conflicts declared | C57BL/6J mice | Male | Not declared | 10–12 weeks old | Modified Lieber-DeCarli for 6 weeks. Ethanol was increased 1% weekly until it reached 5% (v/v)] | 4, 5 or 6 | 4, 5 or 6 | ALT, AST, TAG, CYP2E1, GSH, andNrf2 |
| Cheng and Khong, 2011 [32] | China | Department of Education, Liaoning Province | No information | Rats (lineage not specified) | Male | 200–220 g | 12 weeks old | 56% (v/v) ethanol administered by gastric infusion (7 g/kg/bw) three times a day for 30 consecutive days | 12 | 12 | ALT, AST, TAG, MDA, SOD, CAT, GPx, and GSH |
| Chiu et al., 2011 [33] | China | Lee Kum Kee Health Products Group Ltd. | No conflicts declared | Sprague Dawley rats | Female | 250–300 g | Not declared | Ethanol intragastrically at 7.9 g/kg/day (20% v/v) for 45 days | 6 | 6 | ALT, MDA, SOD< GSH, GR, GPx, and GST |
| Chu et al., 2021 [34] | China | Chinese National Natural Science Foundation and the Natural Science Foundation from the Department of Science and Technology of Liaoning Province | No information | C57BL/6 mice | Male | Not declared | 8 weeks old | Lieber-DeCarli liquid diet containing 5% ethanol (v/v) (EtOH) for 8 weeks | 3, 6 or 8 | 3, 6 or 8 | ALT, AST, TAG, Nrf2, GSH, and MDA |
| Colontoni et al., 2000 [35] | United States | National Institute on Alcohol Abuse and Alcoholism | No information | Sprague Dawley rats | Either sex | 218 ± 3.3 g (female) 126 ± 1.7 g (male) | 30–35 days old | Lieber-DeCarli liquid diet for 8 weeks | 12 or 16 | 12 or 16 | MDA, GSH, and GSH/GSSG ratio |
| Cui et al., 2014 [36] | China | No information | No information | Kunming mice | Male | 18–22 g | Not declared | Alcohol (50 %, v/v) administered intragastrically by gavage twice daily as in previous studies. The amount of the 50% alcohol was initially 10 mL/kg/bw/day (4.0 g/kg/bw/day) and gradually increased as tolerance developed during the first 3 weeks to a maintenance dose of 16 mL/kg/bw/day (6.3 g/kg/bw/day) that was continued for 8 more weeks. | 12 | 12 | ALT, AST, TAG, MDA, GSH, SOD, TNF-a, IL-1B, and IL-10 |
| Cui et al., 2014 [37] | China | National Science and Technology Support Program, the Priority Academic Program Development of Jiangsu Higher Education Institution, and the Fundamental Research Funds for the Central Universities of China | No information | Kunming mice | Male | 18–22 g | Not declared | Alcohol (50% v/v) twice a day for 11 weeks. The 50% alcohol administered was gradually increased every week from 10 to 16 mL/kg/day according to animal tolerance | 10 | 10 | ALT, AST, TAG, MDA, GSH, SOD, TNF-α, IL-1β, and IL-10 |
| Das et al., 2006 [38] | India | No information | No information | BALB/c mice | Male | 20–30 g | 8–10 weeks old | 1.6 g ethanol/kg/bw/day for 12 weeks | 6 | 6 | ALT, AST, and IL-10 |
| Das et al., 2012 [39] | India | Kerala State Council for Science, Technology, and Environment, Government of Kerala, India, and the Van Slyke Foundation of the American Association for Clinical Chemistry | No conflicts declared | Wistar rats | Male | 200–220 g | 16–18 weeks old | 1.6 g ethanol/kg/bw/day administered intragastrically for 4, 12, or 36 weeks | 3 or 6 | 3 or 6 | TBARS, GSH, GSH/GSSG ratio, GPx, GR, GST, CAT, SOD, IL-10, IL-1B, and TNF-a |
| De Souza et al., 2015 [40] | Brazil | Fundação Araucária and CAPES | No information | Wistar rats | Male | 200 ± 20 g | Not declared | 10% ethanol for 4 weeks | 10 | 10 | ALT, AST, TAG, SOD, GST, GSH, and LPO |
| Develi et al., 2014 [41] | Turkey | Research Fund of Istanbul University | No conflicts declared | Sprague Dawley rats | Female | 250–300 g | 16 weeks old | Ethanol 40% (5 g/kg) every 12 h for three doses in total | 8 | 8 | ALT, AST, MDA, GSH, SOD, GPx, and GST |
| Dou et al., 2013 [42] | China | National Institutes of Health NIAAA | No conflicts declared | C57BL/6 mice | Male | 25 ± 0.5 g | Not declared | Animals were fed ad libitum with ethanol for 4 weeks Ethanol-derived calories were increased from 30% to 36% during the first 4 weeks, with a 2% increase each week | 6 | 6 | ALT, TBARS, GSH, and GSH/GSSG ratio |
| Du et al., 2015 [43] | China | China–Japan Friendship Hospital Youth Science and Technology Excellence Project and the Research Fund of the China–Japan Friendship Hospital | No conflicts declared | Wistar rats | Not declared | 150–200 g | Not declared | Ethanol [5 g/kg/bw] by gavage every 12 h for a total of 3 doses | 6 | 6 | ALT, AST, Protein carbonyl, Lipid peroxidation, SOD, CAT, GPx, GST, Nrf-2, TNF-α, IL-6, and IL-1β |
| Duryee et al., 2018 [44] | United States | U.S. Department of Veterans Affairs Rehabilitation Research and Development Service VA Merritt Application | No information | Wistar rats | Male | Not declared | 270 days old | Ethanol liquid diet daily for 7 weeks | 4 or 6 | 4 or 6 | ALT, AST, TNF-a, and IL-6 |
| Feng et al., 2019 [45] | China | Research Committee of the University of Macau and Health Nutrition Research | No conflicts declared | C57BL/6 mice | Male | Not declared | 8–10 weeks old | Lieber-DeCarli liquid diet for 10 days. On day 11, the mice orally received a single dose of 31.5% (v/v) ethanol (5 g/kg/bw) | 8 | 8 | ALT, AST, TBARS, GSH, GSH/GSSG ratio, SOD, CAT, GR, GPx, TAG, TNF-a, IL-6, and Il-1B |
| Galligan et al., 2012 [46] | United States | National Institutes of Health/National Institutes of Alcoholism and Alcohol Abuse | No information | C57/BL6J mice | Male | Not declared | Not declared | Modified Lieber-DeCarli liquid diet for 6 weeks [2% (v/v) ethanol in the first week, increased on a weekly basis; week 6 consisted of 6% ethanol (v/v)] | 6 or 12 | 6 or 12 | ALT, TAG, TBARS, GSH, GSH/GSSG ratio, GR, and GST |
| Gao et al., 2021 [47] | China | Natural Science Foundation of Jiangsu Province | No conflicts declared | ICR mice | Male | Not declared | 4 weeks old | Ethanol (30%, v/v) by gavage (10 mL/kg/bw/day) for 8 weeks | 9 | 9 | ALT, AST, TAG, GPx, CAT, GSH, and MDA |
| George and Chaturvedi, 2009 [48] | Africa | Office of Research and Development, University of Botswana | No conflicts declared | Wistar rats | Male | 200–250 g | Not declared | Alcohol (5 g/kg/bw) for 30 days | 6 | 6 | ALT and AST |
| Gustot et al., 2006 [49] | Belgium | No information | No information | C57Bl6/J mice | Female | Not declared | 8 weeks old | Lieber-DeCarli ethanol liquid diet for 10 days | 13 | 13 | / |
| Han et al., 2021 [50] | China | National Natural Science Foundation of China | No conflicts declared | C57BL/6J mice | Male | 18 ± 0.5 g | 4 weeks old | Mice were oral gavaged with 30% ethanol for 15 days. On the 16th day, 50% ethanol (10 mL/kg) was administrated | 10 | 10 | ALT, AST, SOD, MDA, CAT, GSH, TNF-a, IL-1B, IL-6, and number of inflammatory cells |
| Hao et al., 2018 [51] | United States | National Institutes of Health | No conflicts declared | C57BL/6J mice | Male | Not declared | 10 weeks old | Lieber-DeCarli liquid alcohol diet for 8 weeks [the ethanol content (%, w/v) in the diet was 3.6 for the first 2 weeks and increased by 0.3% every 2 weeks, reaching 4.5% for the last 2 weeks] | 8 | 8 | ALT, AST, GSH, CYP2E1, PPAR-a, TAG, and Caspase 3 |
| Hao et al., 2021 [52] | United States | National Institutes of Health | No information | C57BL/6 mice | Male | Not declared | 12 weeks old | Lieber-DeCarli liquid diet for 8 weeks and 4 h before tissue collection, the mice were gavaged with one dose of ethanol (4 g/kg) | 5 | 5 | ALT, AST, TAG, caspase 3, and CYP2E1 |
| Hasanein et al., 2018 [53] | Iran | Not declared | No conflicts declared | Wistar rats | Male | 220–250 g | 8 weeks old | Ethanol (4 g/bw) via gavage for 30 days | 7 | 7 | ALT, AST, TNF-a, IL-6, MDA, GSH, SOD, and CAT |
| He et al., 2021 [54] | China | National Natural Science Foundation of China and Jilin Province Administration of Traditional Chinese Medicine Projects | No information | Sprague Dawley rats | Male | 180–220 g | Not declared | 10 mL/kg of 60% ethanol solution orally every day for 30 days | 12 | 12 | ALT, AST, MDA, GSH, TAG, Bax/Bcl-2 ratio, Caspase-3, CYP2E1, and Nrf2 |
| Hsu et al., 2018 [55] | Taiwan | No external funding | No conflicts declared | C57BL/6J mice | Male | Not declared | 5 weeks old | Lieber–DeCarli alcohol-containing liquid diet for 5 weeks (alcohol was gradually increased to 10% of total energy on days 1 and 2, 20% on days 3 and 4, 30% on days 5 and 6, and 36% on day 7 and thereafter) | 10 | 10 | ALT, AST, TAG, PPAR-a, SREBP-1, CYP2E1, SOD, CAT, GPx, GSH, and MDA |
| Hu et al., 2021 [56] | China | National Key Research and Development Program of China | No conflicts declared | Kunming mice | Male | 20 ± 2 g | Not declared | 56% (v/v) alcohol for 21 consecutive days | 6 | 6 | ALT, AST, TAG, SOD, MDA, GSH, GPx, IL-6, TNF-a, IL-1B, and Nrf-2 |
| Huang et al., 2017 [57] | China | Hong Kong, Macao, and Taiwan Science and Technology Cooperation Program of China, Science and Technology Major Project of Guangdong Province, Science and Technology Planning Project of Guangdong Province, China, Guangdong International Cooperation Project, Guangdong Provincial Department of Education Feature Innovation Project, and Key Disciplines Construction Projects of High-level University of Guangdong Province | No conflicts declared | Wistar rats | Male | 210 ± 10 g | Not declared | Ethanol (7 mL/kg) intragastrically every 12 h at 5 different time points for 9 days | 12 | 12 | ALT, AST, TAG, CAT, SOD, GPx, MDA, CYP2E1, Nrf2, TNF-α, IL-1β, and IL-6 |
| Ilaiyaraja and Khanum, 2011 [58] | India | No information | No conflicts declared | Wistar rats | Male | 250–280 g | Not declared | Rats received 20% ethanol (7.9 g/kg/bw) orally for 6 weeks | 6 | 6 | ALT, AST, MDA, GR, GSH, and Protein carbonyl |
| Jayaraman et al., 2009 [59] | India | No significant financial support for this work | No conflicts declared | Wistar rats | Male | 150–170 g | Not declared | 20% ethanol (6 g/kg/bw) as an aqueous solution by intragastric intubation for 60 days | 8 | 8 | ALT, AST, TBARS, LOOH, Protein carbonyl, CAT, GR, GST, and GSH |
| Jiang et al., 2016 [60] | China | High-end Foreign Experts Recruitment Program of State Administration of Foreign Expert Affairs, the Ministry of Education and State Administration, the Key Construction Program of International Cooperation Base in S&T, Shanxi Provincial Science and Technology Coordinating Innovative Engineering Project | No conflicts declared | Kunming mice | Either sex | 16–18 g | 3 weeks old | Increasing dose of alcohol 25% v/v per week (5, 8, 10, 12, and 15 mL/kg of body weight) for a total of 5 weeks | 8 | 8 | ALT, AST, GSH, SOD, MDA, TAG, and CYP2E1 |
| Jiang et al., 2019 [61] | China | National Natural Science Foundation of China, Science and Technology Innovation as a Whole Plan Project of Yulin City, International Scientific and Technological Cooperation and Exchange Program, the Postdoctoral Program of China, the Excellent Doctoral Dissertation Funded Projects of Shaanxi Normal University, and the Development Program for Innovative Research Team of Shaanxi Normal University | No conflicts declared | Kunming mice | Male | 18–22 g | Not declared | 28% (v/v) ethanol (10 mL/kg/bw) by intragastricgavage for 10 weeks | 10 | 10 | ALT, AST, TAG, MDA, SOD, GPx, TNF-a, and IL-6 |
| Jin et al., 2010 [62] | Korea | No information | No information | Sprague Dawley rats | Male | 250 ± 20 g | Not declared | Acute experiment: single dose of 4 mL of 30% ethanolChronic experiment: 4 mL of 30% ethanol 10 times every 2 days for 20 days | 5 | 5 | ALT, AST, TNF-a, GSH, SOD, CAT, GPx, and TBARS |
| Jose et al., 2018 [63] | India | M/s Akay Flavours and Aromatics Pvt Ltd, Cochin, India | One conflict declared | Wistar rats | Male | 150 ± 10 g | Not declared | Ethanol 90% (12.5 g/kg/bw) by oral gavage for 30 days | 8 | 8 | ALT, AST, TBARS, TNF-a, IL-6, SOD, CAT, and GPx |
| Kanbak et al., 2001 [64] | Turkey | Osmangazi University | No information | Wistar rats | Male | 150–250 g | Not declared | Animals consumed an approximately 60 mL diet (containing 2.5–4% ethanol) per day over 60 days (corresponding to 8 g/kg/day) | 8 | 8 | ALT, GSH, and MDA |
| Kanchana and Jayapriya, 2013 [65] | India | No information | No information | Wistar rats | Female | 140–150 g | Not declared | Ethanol (3 g/kg/bw) for 35 days | 6 | 6 | TBARS, GSH, SOD, CAT, and GPx |
| Kang et al., 2009 [66] | United States | National Institutes of Health, Office of Dietary Supplements grants, and the Veterans Administration | No information | 129S mice | Male | Not declared | Not declared | Lieber-DeCarli liquid alcohol diet for 4 weeks | 4 | 4 | ALT, TAG, SOD, GPx, CAT, and MDA |
| Kang et al., 2022 [67] | United States | USDA Multi-State Hatch | No conflicts declared | C57BL/6J mice | Male | 25–30 g | 12 weeks old | 5% ethanol (v/v) Lieber-DeCarli diet for 10 days | 4 | 4 | ALT, TAG, CYP2E1, and Nrf-2 |
| Kaviarasan et al., 2008 [68] | India | Indian Council of Medical Research | No conflicts declared | Wistar rats | Male | 150–170 g | Not declared | Ethanol (6 g/kg) as an aqueous solution for 60 days | 6 | 6 | TBARS, Protein carbonyl, SOD, CAT, GR, and GSH |
| Khanal et al., 2009 [69] | Republic of Korea | Jangsaeng Doraji Co. Ltd., Jinju, South Korea, provided the Changkil | No conflicts declared | C57BL/6 mice | Male | 23–25 g | Not declared | Ethanol (50%) was administered orally to mice at a dose of 5 g/kg every 12 h for a total of 3 doses | 4 | 4 | ALT, TNF-a, Steatosis score, Inflammation score, TBARS, GSH, TAG, and CYP2E1 |
| Kim et al., 2012 [70] | Korea | Basic Science Research Program through the National Research Foundation of Korea | No conflicts declared | Sprague Dawley rats | Male | 150–170 g | Not declared | Lieber-DeCarli ethanol liquid diet. Ethanol was introduced progressively, with 30 g/L for 2 days, 40 g/L for the subsequent 2 days, followed by the final formula containing 50 g/L | 8 | 8 | MDA, GSH, and Nrf2 |
| Kim et al., 2016 [71] | United States | No information | No conflicts declared | Wister rats | Male | 80 ± 5 g | 4 weeks old | Ethanol 20% (3.95 g/kg/bw) daily for 42 days | 5 | 5 | ALT, AST, TAG, TBARS, SOD, and CAT |
| Kumar et al., 2019 [72] | India | No significant financial support | No conflicts declared | Wistar rats | Male | 175 ± 25 g | Not declared | Ethanol 3% to 15% (in water) gradually increased weekly for 12 weeks | 3 or 6 | 3 or 6 | ALT, AST, TAG, GSH, IL-1B, and Nrf2 |
| Lai et al., 2019 [73] | Taiwan | No information | No conflicts declared | C57BL/6J mice | Male | Not declared | 6 weeks old | Lieber-DeCarli ethanol liquid diet for 6 weeks | 8 | 8 | ALT, AST, TAG, CAT, SOD, GPx, GR, Nrf2, PPAR-a, and SREBP |
| Lee et al., 2015 [74] | Korea | Ministry of Agriculture, Food, and Rural Affairs of Korea | No information | C57BL/6 mice | Male | 22 ± 1 g | 8 weeks old | Ethanol (5 g/kg/bw) for 3 days | 8 | 8 | ALT, AST, SOD, CAT, GR, GSH, MDA, and CYP2E1 |
| Lee et al., 2016 [75] | Korea | No information | No information | C57BL/6J mice | Male | Not declared | 7 weeks old | Lieber-DeCarli ethanol liquid diet for 6 weeks | 10 | 10 | ALT, AST, MDA, CAT, GST, GPx, GR, and CYP2E1 |
| Lee et al., 2016 [76] | Taiwan | Ministry of Science and Technology | No conflicts declared | C57BL/6 mice | Male | 12–16 g | 4–5 weeks old | Lieber-DeCarli formulation 5% (v/v) ethanol for 6 weeks | 10 | 10 | ALT, AST, TAG, TBARS, and SOD |
| Lee et al., 2020 [77] | Korea | Basic Science Research Program through the National Research Foundation of Korea and the Chung-Ang University Graduate Research Scholarship | No conflicts declared | Sprague Dawley rats | Male | Not declared | 7 weeks old | Ethanol 70% was administered orally (7 g/kg) for 42 days | 7 | 7 | ALT, AST, TAG, TNF-α, and IL-1β |
| Lee et al., 2020 [78] | Republic of Korea | National Research Foundationof Korea | No conflicts declared | C57BL/6 mice | Male | Not declared | 9 weeks old | Lieber-DeCarli liquid ethanol diet for 10 days | 20 | 20 | ALT, AST, TAG, GSH/GSSG ratio, and MDA |
| Lee et al., 2021 [79] | Taiwan | Ministry of Science and Technology and the Chung Shan Medical University Hospital | No conflicts declared | C57BL/6J mice | Male | 22 ± 2 g | Not declared | Lieber-DeCarli liquid ethanol diet for 8 weeks | 8 | 8 | ALT, AST, TAG, CAT, GPx, SOD, TBARS, Leukocyte infiltration, Accumulation of hepatic lipids, TAG, and SREBP1 |
| Li et al., 2013 [80] | China | National Natural Science Foundation of China, the Program for New Century Excellent Talents in the University of China, and the Wuhan Planning Project of Science and Technology | No conflicts declared | Balb/c mice | Male | 18–22 g | Not declared | Ethanol 50% (v/v) (5 g/kg/bw) three times with 12 h of interval | 12 | 12 | ALT, AST, GSH, SOD, MDA, TNF-a, and IL-6 |
| Li et al., 2015 [81] | China | No information | No information | Sprague Dawley rats | Male | Not declared | 8 weeks old | Different alcohol doses [10%, v/v, 0.8 g/kg/bw; or 20%, 1.6 g/kg/bw; or 30%, 2.4 g/kg/bw] for 90 days | 10 | 10 | ALT, AST, GSH, MDA, SOD, and CYP2E1 |
| Li et al., 2016 [82] | China | National Natural Science Foundation of China | No conflicts declared | C57BL/6J mice | Male | 18–20 g | Not declared | Lieber-DeCarli ethanol liquid diet for 15 weeks | 12 | 12 | MDA and GSH/GSSG ratio |
| Li et al., 2017 [83] | China | National Natural Science Foundation of China | No conflicts declared | ICR mice | Male | 18–22 g | 6–7 weeks old | Ethanol (50%, v/v, 12 mL/kg) for 1 or 7 days | 8 | 8 | ALT, AST, TAG, TBARS, GSH, SOD, CAT, GR, and GPx |
| Li et al., 2018 [84] | China | China Agriculture Research System | Declare no conflict | C57 mice | Not declared | 20–32 g | Not declared | 10 mL/kg alcohol (55%, v/v) by gavage for 4 weeks | 8 | 8 | ALT, AST, TAG, MDA, GPx, SOD, TNF-a, IL-1B, and IL-6 |
| Li et al., 2021 [85] | China | National Key R&D Program of China and the Key Project of Guangdong Provincial Science and Technology Program | No conflicts declared | C57BL/6J mice | Male | Not declared | 8 weeks old | Lieber-DeCarli diet for 11 days and then Lieber-DeCarli ethanol liquid diet containing 4% (w/v) ethanol for 4 weeks | 9 | 9 | ALT, AST, TAG, CYP2E1, MDA, SOD, CAT, GPx, GSH, IL-6, and TNF-a |
| Li et al., 2021 [86] | China | National Key R&D Program of China and the Key Project of Guangdong Provincial Science and Technology Program | No conflicts declared | C57BL/6 J mice | Male | Not declared | 8 weeks old | Lieber–DeCarli liquid diet (4% ethanol w/v) for 11 days, and distilled water (10 mL/kg) for 4 weeks | 9 | 9 | ALT, AST, TAG, MDA, GSH, GPx, SOD, CAT, TNF-a, IL-6, and CYP2E1 |
| Li et al., 2021 [87] | China | National Key Research and Development Program of China, Natural Science Foundation of Heilongjiang Province, National Natural Science Foundation of China, and Academic Backbone Plan of Northeast Agricultural University | No conflicts declared | C57BL/6J mice | Male | Not declared | 6 weeks old | Lieber-DeCarli liquid diet. Alcohol was gradually increased to 4% (w/v) by the end of the week and was maintained at 4% for 6 weeks | 12 | 12 | ALT, AST, and TAG |
| Li et al., 2021 [88] | China | National Key R&D Program of China, China Central Public-Interest Scientific Institution Basal Research Fund, Chinese Academy of Agricultural Sciences, and the Key Project of Guangdong Provincial Science and Technology Program | No conflicts declared | C57BL/6J mice | Male | 20 g | Not declared | Lieber-DeCarli ethanol liquid for 6 days and 4% ethanol liquid diet plus distilled water (10 mL/kg) for 4 weeks | 9 | 9 | ALT, AST, TAG, CYP2E1, SOD, CAT, GPx, GSH, and MDA |
| Lian et al., 2010 [89] | China | No information | No information | C57BL/6mice | Male | Not declared | Not declared | Ethanol (5 g/kg/bw) every 12 h for a total of three doses | 10 | 10 | ALT, AST, TAG, MDA, GSH, GPx, SOD, CAT, CYP2E1, and SREBP-1 |
| Lin et al., 2017 [90] | Taiwan | Chung Shan Medical University | Declare no conflict | C57BL/6 mice | Female | Not declared | 5 weeks old | Ethanol content in the diet was graded from 7.2% to 36% of energy composition for 10 weeks. After that, mice were gavaged with a single dose of ethanol (5 g/kg) | 8 | 8 | ALT, AST, TAG, TNF-a, IL-6, IL-10, SOD, GPx, GSH, and MDA |
| Lin et al., 2021 [91] | Taiwan | No significant financial support for this work | No conflicts declared | C57BL/6J mice | Male | Not declared | 7 weeks old | Lieber-DeCarli liquid ethanol diet for 6 weeks | 8 | 8 | ALT, AST, TAG, MDA, CAT, SOD, GPx, GSH, and PPPAR-a |
| Liu et al., 2014 [92] | China | Program for Changjiang Scholars, Innovative Research Team in University and National Nature Scientific Foundation | No conflicts declared | Sprague Dawley rats | Not declared | 180–200 g | Not declared | Ethanol 51.3% (4 g/kg/day) via an intragastric administration tube for 30 days | 7 | 7 | AL, AST, TAG, MDA, GSH, GSH/GSSG ratio, SOD, CAT, GR, Nrf-2, and CYP2E1 |
| Liu et al., 2015 [93] | China | Program for Changjiang Scholars, National Nature Scientific Foundation and Natural Science Foundation of Shanxi Province | No conflicts declared | C57BL/6 mice | Male | 33–34 g | 12–14 weeks old | Ethanol (5 g/kg) intragastrically for 7 days | 3 or 8 | 3 or 8 | ALT, AST, Caspase-3, Bax/Blc-2 ratio, MDA, GSH, GSH/GSSG, Nrf-2, CAT, SOD, GR, CYP2E1, TAG, and SREBP-1c |
| Liu et al., 2020 [94] | China | National Natural Science Foundation of China | No conflicts declared | C57BL/6J mice | Male | 18–20 g | Not declared | Ethanol-containing Lieber-DeCarli liquid diet (30% of total calories from ethanol) for 15 weeks | 9 | 9 | GSH |
| Liu et al., 2022 [95] | China | National Natural Science Foundation of China | No conflicts declared | C57BL/6 mice | Male | Not declared | Not declared | Ethanol liquid diet for 17 days. Ethanol was gradually increased from 0 to 5% (v/v) during one week; after that, mice were fed with ethanol (5%) for 10 consecutive days. On the 11th day, mice were gavaged with 5 g/kg ethanol | 9 | 9 | AL, AST, TAG, MDA, GSH, GSH/GSSG ratio, Nrf2, TNF-a, PPAR-a, and SREBP-1c |
| Liu et al., 2022 [96] | China | Program for Changjiang Scholars, Innovative Research Team in University and National Nature Scientific Foundation | No conflicts declared | C57BL/6 mice | Male | 20–21 g | 6–8 weeks old | Ethanol 51.3% (5 g/kg, intragastrically) twice a day for 7 days | 3 or 8 | 3 or 8 | ALT, AST, TAG, caspase 3, Bax/Bcl2 ratio, MDA, GSH, GSH/GSSG ratio, SOD, GR, Nrf-2, and CYP2E1 |
| Lu et al., 2014 [97] | Taiwan | National Science Council and National Taiwan University | No information | C57BL/6 mice | Male | 23–25 g | 6 weeks old | Lieber-DeCarli ethanol liquid diet (alcohol-containing liquid in the mixture increased gradually from 20% to 100%) for 4 weeks | 3 or 12 | 3 or 12 | ALT, AST, TAG, GSH, TBARS, TNF-a, IL-1B, IL-6, GPx, GR, CAT, SOD, CYP2E1, and SREBP-1c |
| Lu et al., 2015 [98] | China | National Natural Science Foundation of China, Priority Academic Program Development of Jiangsu Higher Education Institutions, Youth Natural Science Foundation of Jiangsu Province, 2013 Program for Excellent Scientific, Technological Innovation Team of Jiangsu Higher Education, Youth Natural Science Foundation of Nanjing University of Chinese Medicine, and the Natural Science Research General Program of Jiangsu Higher Education Institutions | No conflicts declared | Sprague Dawley rats | Male | 200 ± 20 g | Not declared | Alcohol (56%, v/v, 10 mL/kg) by gavage every day for 9 weeks | 10 | 10 | ALT, AST, TAG, SREBP-1c, PPAR-a, MDA, GSH, GR, SOD, CAT, Nrf2, Bax/Bcl-2 ratio, and Caspase-3 |
| Lu et al., 2020 [99] | Taiwan | Ministry of Science and Technology | No conflicts declared | Wistar rats | Male | Not declared | 8 weeks old | Lieber-DeCarli ethanol liquid diet for 8 weeks | 5 | 5 | ALT, AST, Steatosis score, Inflammation score, TAG, GSH/GSSG ratio, TNF-a, IL-1β, IL-6, IL-10, and CYP2E1 |
| Ma et al., 2007 [100] | Korea | Ministry of Commerce, Industry, and Energy and Korea Institute of Industrial Technology Evaluation and Planning through the Biohealth Products Research Center of Inje University | No information | C57BL/6 mice | Male | 20–25 g | 9 weeks old | Single dose of 50% ethanol (5 g/kg/bw) | 6 | 6 | ALT, AST, TAG, MDA, CAT, GPx, and GR |
| Madushani Herath et al., 2018 [101] | Republic of Korea | Ministry of Trade, Industry, and Energy and Korea Institute for Advancement of Technology through the Promoting Regional Specialized Industry | Declare no conflict | C57BL/6 mice | Not declared | 20–25 g | 8–9 weeks old | 30% ethanol (5 g/kg/bw) by gavage every 12 h for a total of 3 doses | 3 | 3 | CYP2E1 |
| Mai et al., 2022 [102] | China | Guangdong Province Rural Science and Technology Commissioner Project, Guangdong Modern Agricultural Industrial Technology System Innovation Team Construction Project with Agricultural Products as the Unit, Guangdong Province Lingnan Chinese Herbal Medicine Protection Fund Talents Training Special Project, and Project of Traditional Chinese Medicine Bureau of Guangdong Province | Declared some conflicts | BALB/c mice | Male | 18–20 g | Not declared | Ethanol (6 mL/kg) for the first week and then increased by 1 mL every week (up to 10 mL/kg) for 7 weeks | 6 | 6 | ALT, AST, MDA, GSH, SOD, TNF-a, IL-6, IL-1B, and CYP2E1 |
| Maimaitimin et al., 2018 [103] | China | National Natural Science Foundation, SCO Regional Collaborative Innovation Project, Xinjiang, Urumqi Science Project; the High-End Foreign Experts Recruitment Program of State Administration of Foreign Expert Affairs | No information | Kunming mice | Female | 28 ± 2 g | 6 weeks old | Single dose of 50% alcohol (10 mL/kg) | 7 | 7 | ALT, AST, MDA, SOD, GSH, and CYP2E1 |
| Mallikarjuna et al., 2008 [104] | India | No information | No information | Wistar rats | Male | 170 ± 10 g | Not declared | Absolute ethanol (2.0 g/kg/bw) via orogastric tube for 4 weeks | 6 | 6 | SOD, CAT, GPx, GR, GSH, and MDA |
| Mandal et al., 2013 [105] | India | Council of Scientific and Industrial Research and CSIR fellowships | No conflicts declared | Sprague Dawley rats | Female | 110–130 g | Not declared | Lieber-DeCarli diet for 8 weeks | 5 | 5 | ALT, AST, Protein carbonyl, TBARS, and GSH |
| Mehanna et al., 2021 [106] | Egypt | No external funding | No conflicts declared | Albino rats | Male | 180–200 g | Not declared | Ethanol (70% w/v) daily at a dose of 3 or 5 g/kg through intra-gastric gavage for 28 days | 8 | 8 | ALT, AST, MDA, GSH, CAT, SOD, TNF-a, IL-6, and Inflammation score |
| Meng et al., 2020 [107] | China | No information | No conflicts declared | Kunming mice | Male | 18–22 g | Not declared | Single dose of alcohol (10 mL/kg, 52%, v/v) intragastrically | 8 | 8 | ALT, AST, TAG, SOD, CAT, GSH, and MDA |
| Miñana et al., 2002 [108] | Spain | No information | No information | Wistar rats | Male | Not declared | 4–6 months | Liquid ethanol diet (12 g/k/ bw) for 8 or 18 weeks | 8 | 8 | MDA |
| Ming et al., 2021 [109] | China | National Key Research and Development Project, and the High-level Talents Introduction to Scientific Research Start-up Project | No conflicts declared | C57BL/6NCr mice | Male | 20 ± 2 g | 8–10 weeks old | Lieber-DeCarli ethanol liquid diet for 8 weeks, then animals received 31.5% (v/v) ethanol by oral gavage at a dose of 7.3 g/kg | 8 | 8 | ALT, AST, TNF-a, IL-6, IL-1B, IL-10, TAG, MDA, SOD, and GSH |
| Mohan et al., 2019 [110] | India | M/s Akay Flavours and Aromatics Pvt Ltd., Cochin | One conflict declared | Wistar rats | Male | 250 ± 10 g | Not declared | 38% ethanol (12.5 g/kg/bw) for 30 days | 8 | 8 | ALT, AST, SOD, CAT, GPx, GSH, and TBARS |
| Nagappan et al., 2018 [111] | Korea | National Research Foundation of Korea | No conflicts declared | C57BL/6N mice | Male | 20–22 g | 8-week-old | Ethanol Lieber-DeCarli diet (gradually increasing ethanol concentrations of 0–5%) for the first 5 days. Then, mice were allowed free access to the ethanol Lieber-DeCarli diet containing 5% (v/v) ethanol for 10 days | 6 | 6 | ALT, AST, TAG, SREBP-1c, PPAR-a, TBARS, CYP2E1, and GSH |
| Nie et al., 2021 [112] | China | R&D and Demonstration of Key Technologies and Equipment for Green Manufacturing of Chinese Traditional Meat Products, and Anhui Qiangwang Flavouring Food CO | No conflicts declared | C57BL/6 mice | Male | 20 ± 2 g | 8 weeks old | Lieber-DeCarli 3–5 % (v/v) liquid alcohol diet by daily oral gavage for 21 days. Then, a 31.5 % (v/v) alcohol solution (5 g/kg/bw) was given twice by oral gavage for 1 week | 8 | 8 | ALT, AST, TNF-a, IL-1B, IL-6, GPx, SOD, Nrf-2, TAG, SREBP-1, and CYP2E1 |
| Nie et al., 2022 [113] | China | Anhui province, and Anhui Qiangwang Flavouring Food Co., Ltd. | No conflicts declared | C57BL/6 mice | Male | 20 ± 2 g | 8 weeks old | Daily oral gavage of 3.0 g/kg/bw alcohol for 15 days and 5.0 g/kg/bw alcohol for 20 days | 8 | 8 | ALT, AST, TNF-a, IL-6, IL-1B, SOD, GPx, and TAG |
| Oh et al., 2002 [114] | Korea | Korea Research Foundation for Health Science and Seoul National University Hospital | No information | Sprague Dawley rats | Male | 120–180 g | Not declared | Liber-DeCarli liquid diet for 41 days. Ethanol was increased from 0 to 5% over a 1-week period) | 6 | 6 | ALT and AST |
| Osaki et al., 2016 [115] | Korea | Bigenhwaseong Co., Ltd | No information | Wistar rats | Male | Not declared | 4 weeks old | 40% ethanol 5 g/kg/bw for 6 weeks | 12 | 12 | ALT, AST, Numbers of fatty changed hepatocytes, SOD, GPx, GSH, MDA, andCYP2E1 |
| Panda et al., 2012 [116] | India | No information | No information | Wistar rats | Either sex | 150–200 g | Not declared | Ethanol (5 g/kg, 20% w/v) once daily for 21 days | 6 | 6 | ALT, AST, TAG, TBARS, GSH, SOD, CAT, GPx, and GR |
| Panda et al., 2015 [117] | India | No information | No information | Wistar rats | Either sex | 150–200 g | Not declared | 20% Ethanol (4 g/kg) once daily for 21 days | 6 | 6 | ALT, AST, TBARS, GSH, SOD, CAT, GPx, and GR |
| Pari and Suresh, 2008 [118] | India | No information | No conflicts declared | Wistar rats | Male | 150–170 g | Not declared | 20% ethanol (3.95 g/kg/bw) twice daily for 45 days | 6 | 6 | ALT, AST, TBARS, GSH, SOD, CAT, GPx, and GST |
| Park et al., 2013 [119] | Korea | No information | No conflicts declared | ICR mice | Male | 27–28 g | 8 weeks old | 40% ethanol (6.5 g/kg/bw) for 8 weeks | 10 | 10 | ALT, AST, TAG, GSH, MDA, TNF-a, and IL-1B |
| Park et al., 2017 [120] | Republic of Korea | National Institute of Fisheries Science | No conflicts declared | Balb/c mice | Either sex | 19–21 g | 6 weeks old | Ethanol 4 g/kg for 20 days | 6 | 6 | ALT, AST, TAG, SOD, CAT, GPx, and TBARS |
| Park et al., 2019 [121] | Korea | Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology | No conflicts declared | Balb/c mice | Male | 23–26 g | 6 weeks old | Ethanol 3 g/kg/day for 10 days | 6 | 6 | ALT, AST, MDA, Bax/Bcl-2 ratio, Caspase-3, SOD, CAT, GPx, and Nrf-2 |
| Patere et al., 2011 [122] | India | University of Mumbai | No information | Wistar rats | Male | 120–150 g | Not declared | Animals received 10%, 15%, and 20% (v/v) ethanol for 30 days (8–10 g/kg/day during the first week, gradually increasing to 14–16 g/kg/day). The group that maintained the alcohol level between 150–350 mg/dL was selected for the next steps [received increasing concentrations of alcohol through drinking water (10–30%)] | 6 | 6 | ALT, MDA, SOD, and CAT |
| Peng et al., 2011 [123] | Taiwan | National Science Council of Taiwan | No information | Wistar rats | Male | 160 g | Not declared | Lieber-DeCarli ethanol liquid diet for 7 weeks | 10 | 10 | ALT, AST, Fatty change, Inflammation, TAG, GSH/GSSG ratio, TNF-α, IL-1β, IL-6, TNF-α, IL-1β, IL-6, and CYP2E1 |
| Peng et al., 2013 [124] | Taiwan | Cathay General Hospital | No conflicts declared | Wistar rats | Male | Not declared | 6 weeks old | Modified Lieber-DeCarli liquid diet for 12 weeks | 10 | 10 | ALT, AST, Fatty change, Inflammation, TNF-α, TBARS, GSH/GSSG ratio, CYP2E1, and Caspase-3 |
| Pi et al., 2021 [125] | China | Natural Science Foundation of China, Zhejiang Natural Science Foundation for Distinguished Young Scholars, Special Support Program for High Level Talents in Zhejiang Province, and Research Project of Zhejiang Chinese Medical University | No conflicts declared | C57BL/6J mice | Male | 18.51 ± 1.21 g | Not declared | Lieber-DeCarli ethanol diet for 4 weeks | 8 | 8 | ALT, TAG, MDA, SOD, GPx, Caspase-3, Bax/Bcl2 ratio, PPAR-a, and SREBP-1c |
| Prathibha et al., 2013 [126] | India | District Development Office for Scheduled Castes, Trivandrum Kerala | No information | Sprague Dawley rats | Male | 100–140 g | Not declared | Ethanol diluted with distilled water (1:1) (4 g/kg/bw/day) was given orally by gastric intubation for 90 days | 6 | 6 | MDA, Protein carbonyls, CAT, SOD, GPx, GR, GSH, and CYP2E1 |
| Qi et al., 2017 [127] | China | National Natural Science Foundation of China, Provincial Natural Science Research Project of Anhui, and National Undergraduate Training Programs for Innovation and Entrepreneurship of China | No conflicts declared | Kunming mice | Male | 18–22 g | Not declared | 10 mL (5.14 mol/L alcohol)/kg body weight in the first 4 weeks, 11 mL (6.85 mol/L alcohol)/kg body weight in the second 4 weeks, and 12 mL (8.56 mol/L alcohol)/kg body weight in the final 4 weeks | 10 | 10 | ALT, AST, TAG, MDA, SOD, GPx, and Caspase-3 |
| Qu et al., 2019 [128] | China | National Natural Science Foundation of China | No information | ICR mice | Male | 18–22 g | 6 weeks old | Ethanol 50% (10 mL/kg/bw) for 6 weeks | 8 | 8 | ALT, AST, TNF-a, IL-6, IL-1B, SOD, MDA, GSH, CYP2E1, Bax/Bcl2 ratio, and Caspase-3 |
| Rabelo et al., 2018 [129] | Brazil | Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Universidade Federal de Ouro Preto (UFOP | No conflicts declared | Fisher rats | Male | 220–250 g | Not declared | Acute experiment: 5 mL/kg of absolute ethanol by gavage for 2 days Chronic experiment: 5 mL/kg of diluted ethanol (in the first week they received 20% ethanol (v/v), in the second 40% and third and fourth 60%) for 28 days | 7 | 5 | ALT, AST, TAG, SOD, CAT, GSH/GSSG ratio, GPx, GR, TBARS, Carbonylated protein |
| Rejitha et al., 2012 [130] | India | Council of Scientific and Industrial Research | No conflicts declared | Sprague Dawley rats | Male | 100–140 g | Not declared | Alcohol (4 g/kg/bw) for 90 days | 6 | 6 | SOD, CAT, GPx, GR, MDA, andProtein carbonyls |
| Roede et al., 2008 [131] | United States | NIH/NIAAA RO1AA09300, NIH/NIDDK 074407, and NIH/NIAAA F31AA016710 | No information | C57/Bl6 mice | Male | Not declared | Not declared | Modified Lieber-DeCarli diet for 9 weeks. Animals began the study on a diet containing 2% ethanol (v/v), and the amount of ethanol was increased each week until the diet contained 5% ethanol (v/v) | 3 or 8 | 3 or 8 | ALT, TAG, CYP2E1, and GSH |
| Roede et al., 2009 [132] | United States | National Institutes of Health National Institute of Alcohol Abuse and Alcoholism, and National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases | No information | C57/BL6 mice | Male | Not declared | Not declared | Lieber-DeCarli diet for 9 weeks. Animals began the study on a diet containing 2% ethanol (v/v), and the amount of ethanol was increased each week until the diet contained 5% ethanol (v/v) | 4 or 8 | 4 or 8 | ALT, CYP2E1, GSH, SOD, GPx, and GST |
| Rong et al., 2012 [133] | China | Graduates’ Innovation Fund of HUST, National Natural Science Foundation of China, and Program for New Century Excellent Talents in the University of China | No information | Balb/c mice | Male | 18–22 g | 6 weeks old | 2.4 g/kg/day ethanol for the initial 4 weeks and 4 g/kg/day for another 2 weeks | 12 | 12 | ALT, AST, TAG, GSH, GPx, and GST |
| Ronis et al., 2005 [134] | United States | Supported in part by R01 AA088645 | No information | Sprague Dawley rats | Male | 250–300 g | Not declared | Ethanol beginning at 10 g/kg and increased by 0.5 g/kg a week to attain a final concentration of 12.5 g/kg (39% of total energy) for 70 days | 8 | 8 | Steatosis score, Inflammation score, CYP2E1, GSH, GSH/GSSG ratio, TBARS, and TAG |
| Ronis et al., 2010 [135] | United States | Supported in part by the National Institute on Alcohol Abuse and Alcoholism | No information | Sprague Dawley rats | Male | 300–350 g | Not declared | Ethanol (10–12 g/kg/day) by total enteral nutrition for 45 days | 15 | 18 | ALT, TAG, CYP2E1, Steatosis score, Inflammation score, TBARS, and GSH |
| Samuhasaneeto et al., 2009 [136] | Thailand | 90th Anniversary of Chulalongkorn University Fund (Ratchada phiseksomphot Endowment Fund) and Grant of Ratchada Phiseksomphot, Faculty of Medicine, Chulalongkorn University | No information | Sprague Dawley rats | Female | 180–220 g | Not declared | 50% ethanol (7.5 g/kg/bw a day) orally via an intragastric tube twice a day for 4 weeks | 8 | 8 | MDA and SOD |
| Saravanan, 2007 [137] | India | No information | No information | Wistar rats | Male | 130–180 g | 90 days | 20% ethanol (5.0 g/kg daily) using an intragastric tube daily for 60 days | 10 | 10 | ALT, AST, TBARS, LOOH, SOD, CAT, GPx, and GSH |
| Saravanan and Nalini, 2007 [138] | India | No information | No information | Wistar rats | Male | 130–180 g | Not declared | Ethanol 20% (5.0 g/kg/bw/day) for 60 days | 10 | 10 | AL, AST, TBARS, LOOH, SOD, CAT, GPx, and GSH |
| Sathiavelu et al., 2009 [139] | India | No specific grant from any funding agency in the public, commercial, or not-for-profit sectors | No conflicts declared | Wistar rats | Female | 160–180 g | Not declared | 20% ethanol (5 g/kg/bw) by intragastric intubation for 60 days | 8 | 8 | TBARS, Lipid hydroperoxides, CAT, GSH, GR, and GST |
| Senthilkumar et al., 2004 [140] | India | No information | No information | Wistar rats | Male | 150–170 g | 90 days | 20% ethanol, 5 mL each (7.9 g/kg/bw) for 60 days | 10 | 10 | TBARS, GSH, GR, SOD, and CAT |
| Shankari et al., 2010 [141] | India | No information | No information | Wistar rats | Male | 180–220 g | Not declared | 20% ethanol (2.5 mL twice daily), equivalent to 7.9 g/kg/bw for 60 days | 6 | 6 | ALT and AST |
| Shearn et al., 2014 [142] | United States | University of Colorado Anschutz Medical Campus, University of Colorado Denver Cancer Center Research Histology Core, and Colorado Clinical Translational Science Institute | No information | C57BL/6J mice | Male | Not declared | 6–8 weeks old | Modified Lieber-DeCarli diet. The ethanol-derived caloric content was ramped from week 1 of 10.8%, with incremental increases weekly to 16.2, 21.5, 26.9, 29.2, 31.8, and 34.7% for the last 1.5 weeks of feeding | 6 | 6 | ALT, TAG, GSH, and GSH/GSSG ratio |
| Shenbagam and Nalini, 2010 [143] | India | No information | No information | Wistar rats | Male | 150–180 g | Not declared | 20% ethanol twice a day (7.9 g/kg/bw) for 60 days | 6 | 6 | ALT, AST, TBARS, LOOH, CAT, GPx, GST, and GSH |
| Shi et al., 2018 [144] | China | Chinese National Natural Science Foundation and the Natural Science Foundation of Liaoning Province | No conflicts declared | Sprague Dawley rats | Male | 180–220 g | Not declared | Lieber-DeCarli diet for 8 weeks | 3 or 8 | 3 or 8 | ALT, AST, TAG, and CYP2E1 |
| Smathers et al., 2013 [145] | United States | National Institutes of Health | No information | C57BL/6 mice | Male | Not declared | 10 weeks old | Single dose of modified 45% fat-containing Lieber-DeCarli liquid diet | 6 | 6 | ALT, TAG, CYP2E1, TBARS, GSH, GSH/GSSG ratio, GPx, and PPAR-a |
| Sönmez et al., 2012 [146] | Turkey | No information | No conflicts declared | Wistar rats | Male | 200–250 g | Not declared | Alcohol-containing liquid diet for 28 days (2.4% ethanol was administered for 3 days, then the ethanol was increased to 4.8% and 7.2% for the following 4 and 21 days on a liquid diet, respectively) | 6 | 6 | / |
| Song et al., 2006 [147] | United States | No information | No information | C57BL/6 mice | Male | Not declared | 9 weeks old | Ethanol (5 g/kg/bw) by gavage every 12 h for a total of 3 doses | 6 | 6 | ALT, TAG, TBARS, GSH, TNF-a, and CYP2E1 |
| Song et al., 2018 [148] | China | The Central Hospital of Taian and Mushroom Technology System ofShandong Province | No conflicts declared | Kunming mice | Male | 20 ± 2 g | 8 weeks old | Alcohol intragastric (50%, v/v, 12 mL/kg/bw) three times at 8-h intervals | 10 | 10 | ALT, AST, TAG, TNF-a, IL-6, IL-1B, SOD, GPx, CAT, MDA, LPO |
| Song et al., 2020 [149] | China | National Natural Science Foundation of China and Jilin Province Health Science and Technology Capacity Improvement Project. | No conflicts declared | C57BL/6J mice | Male | 16–20 g | 8 weeks old | Alcohol solution 52% [v/v], 7.5 mL/kg/bw, oral gavage | 10 | 10 | ALT, AST, MDA, and SOD |
| Song et al., 2021 [150] | China | Mushroom Technology System of Shandong Province and Shandong Key Research and Development Program | No conflicts declared | Kunming mice | Male | 18–22 g | 8–10 weeks old | Intragastrically injected daily with ethanol (50%, v/v, 10 mL/kg) for 6 weeks | 10 | 10 | ALT, AST, SOD, GPx, CAT, MDA, Nrf2, TNF-a, IL-1B, and IL-6 |
| Sudha et al., 2012 [151] | India | No information | No information | Wistar rats | Male | 150–180 g | Not declared | 20% ethanol (5 g/kg/bw) for 3 weeks | 6 | 6 | ALT, AST, MDA, GSH, GR, GSH, and SOD |
| Sun et al., 2016 [152] | United States | National Institutes of Health | No conflicts declared | Wistar rats | Male | Not declared | 8 weeks old | Lieber-DeCarli liquid alcohol diet for 5 months. the ethanol content (%, w/v) in the diet started at 1.6 and increased by 1 every 2 days to reach 3.6 at the end of prefeeding. On the day of feeding, the ethanol content in the diet was 5.0 (36% of total calories) and gradually increased to 6.3 (44% of total calories) | 6 | 6 | TAG |
| Tahir et al., 2013 [153] | India | No information | No information | Wistar rats | Female | 150–200 g | 6–8 weeks old | Increased dose of ethanol 25% v/v (5, 8, 10, and 12 g/kg/bw per week) for 28 days | 6 | 6 | ALT, AST, CYP2E1, LPO, GSH, GPx, GR, CAT, and TNF-a |
| Tan et al., 2017 [154] | China | National Natural Science Foundation of China, the Scientific Research Foundation for the Returned Overseas Chinese Scholars of Heilongjiang Province, and the Graduate Innovation Foundation of Harbin Medical University. Dr. Ying Liu was supported by the Scientific Research Foundation of Heilongjiang Province | One conflict declared | C57BL/6 mice | Male | Not declared | 8–10 weeks old | 5% alcohol solution during the first week. Then, alcohol was increased every two weeks by 5% until the alcohol concentration reached 15% (v/v). The final concentration was continued for up to 9 or 12 months | 5 or 6 | 5 or 6 | TAG and SOD |
| Tang et al., 2012 [155] | China | National Natural Science Foundation of China and Program for New Century Excellent Talents in University of China | No conflicts declared | Sprague Dawley rats | Male | 140–160 g | Not declared | Ethanol 4.0 g/kg (50%, 10 mL/kg/bw) intragastrically for 90 days | 8 | 8 | ALT, AST, TAG, GSH, GPx, SOD, and GST |
| Tang et al., 2014 [156] | Taiwan | National Science Council | No information | C57BL/6J mice | Male | 20 g | 8 weeks old | Lieber-DeCarli ethanol diet (36% ethanol-derived calories) for 6 weeks | 3 or 8 | 3 or 8 | ALT, AST, TAG, TBARS, GSH, GPx, CAT, SREBP1, and PPAR-a |
| Tang et al., 2014 [157] | China | National Natural Science Foundation of China, Program for New Century Excellent Talents in the University of China, and Wuhan Planning Project of Science and Technology | No conflicts declared | C57BL/6J mice | Male | 18–20 g | Not declared | Lieber-Decarli liquid diet (the ethanol content was gradually increased over a 12-day period, reaching 30% of total calories as ethanol) | 12 or 15 | 12 or 15 | ALT, AST, MDA, and GSH |
| Tang et al., 2017 [158] | China | Xiamen Science and Technology Program, Education Department of Hunan Province, Joint Funds of Hunan Provincial Natural Science Foundation of China, and the Fujian Province Young and Middle-aged Teacher Education Research Project | No conflicts declared | ICR mice | Male | 25 ± 2 g | 7 weeks old | Gavage with 12 mL/kg/bw alcohol for 10 consecutive days | 7 or 10 | 7 or 10 | ALT, AST, TAG, Steatosis score, Inflammation score, and SOD |
| Tao et al., 2021 [159] | China | National Key Research and Development Program of China, Jiangsu ‘‘333” Project of Cultivation of High-level Talents, and 11th Six Talents Peak Project of Jiangsu Province | No conflicts declared | C57BL/6 mice | Male | 20–25 g | Not declared | Lieber-DeCarli ethanol liquid diet for 10 days. On the 11th day, the animals were administered 31.6% ethanol | 3 or 8 | 3 or 8 | ALT, AST, CYP2E1, MDA, PPARa, and SREBP-1c |
| Valansa et al., 2020 [160] | Cameroon | No significant financial support for this work | No conflicts declared | Albino mice | Both sexes | 20–25 g | Not declared | Acute experiment: ethanol 40% for 3 daysChronic experiment: 40% ethanol (10 mL/kg) for 28 days | 4 or 5 | 4 or 5 | MDA and TNF-a |
| Varghese et al., 2016 [161] | India | Department of Biotechnology, Government of India and a Fluid Research Grant | No conflicts declared | Swiss albino mice | Male | 28–30 g | Not declared | Lieber-DeCarli liquid alcohol diet for 2, 4, 8, or 12 weeks | 3 | 3 | CYP2E1 and GSH/GSSG ratio |
| Velvizhi et al., 2002 [162] | India | No information | No information | Wistar rats | Male | 180–220 g | Not declared | 20% ethanol (5 mL/day) with an intragastric tube for 60 days | 6 | 6 | ALT and AST |
| Wang et al., 2014 [163] | China | Beijing Natural Science Foundation | No conflicts declared | Wistar rats | Male | 250 ± 20 g | 8 weeks old | Lieber-Decarli liquid alcohol diet (4.8, wt/v) for 1 week, and ethanol content increased up to 5.4 in the next 7 weeks | 8 | 8 | ALT, AST, IL-1β, IL-6, and TNF-α |
| Wang et al., 2015 [164] | China | No information | No conflicts declared | C57BL/6 mice | Female | 20–24 g | 6 weeks old | Lieber-DeCarli diet, where in the first 3 days, the concentration of alcohol was 1% (v/v), followed by 5% (v/v) for the remaining 9 days | 9 | 9 | ALT, AST, TAG, MDA, and SOD |
| Wang et al., 2016 [165] | China | China–Japan Friendship Hospital Youth Science, Technology Excellence Project, and the Research Fund of the China–Japan Friendship Hospital | No conflicts declared | Wistar rats | Male | 180–220 g | Not declared | Alcohol 65 % for 4 or 8 weeks [(5 mL/kg/day) in the first 3 days, and then 10 mL/kg/day in the following days] | 6 | 6 | ALT, AST, TAG, MDA, SOD, GPx, TNF-a, IL-1B, IL-6, Caspase-3, and Bax/Bcl-2 ratio |
| Wang et al., 2018 [166] | China | Mushroom Technology System of Shandong Province | No conflicts declared | Kunming mice | Male | 20 ± 2 g | Not declared | 50% alcohol solution (8 mL/kg) four times at 6-h intervals | 10 | 10 | ALT, AST, SOD, GPx, CAT, LPO, MDA, TAG, and CYP2E1 |
| Wang et al., 2021 [167] | China | Natural Science Research Project of Colleges and Universities of the Department of Education, Anhui Province | No conflicts declared | ICR mice | Female | Not declared | 8 weeks old | Free access to a liquid diet containing 5% (v/v) ethanol for 10 days. Then, mice were gavaged with a megadose of ethanol (5 g/kg) | 6, 7 or 8 | 6, 7 or 8 | ALT, AST, TAG, MDA, GSH, CAT, SOD, GPx, and Nrf2 |
| Wang et al., 2020 [168] | China | Talent Innovation and Entrepreneurship Project and the Science and Technology Bureau | No conflicts declared | Wistar rats | Male | 190–230 g | Not declared | Ethanol was administered by gavage twice daily with an initial dose of 2 g/kg/d for 3 days, and the dose was gradually increased to 4 g/kg/d for 5 days, 6 g/kg/d for 6 days, and 8 g/kg/d for 28 days (1 mL per 100 g bw) | 5 or 10 | 5 or 10 | SOD, GPx, LPO, and Nrf2 |
| Wang et al., 2020 [169] | Japan | No significantfinancial support for this work | No conflicts declared | C57BL/6J mice | Male | Not declared | 8–10 weeks old | Modified Lieber-DeCarli liquid alcohol diet for 4 weeks | 7 or 16 | 7 or 16 | ALT, AST, TAG, MDA, and SOD |
| Wang et al., 2021 [170] | China | No information | No conflicts declared | Wistar rats | Male | 200–240 g | Not declared | Lieber-DeCarli liquid ethanol diet for 5 weeks | 12 | 12 | ALT, AST, IL-1β, IL-6, and TNF-α |
| Wang et al., 2022 [171] | China | Key Projects for Major Projects on the Transformation of Old and Novel Kinetic Energy of Shandong Province, STS Project of the Chinese Academy of Sciences | No conflicts declared | C57BL/6 mice | Not declared | 18–22 g | 4 weeks old | Orally administered daily dose of 50% (v/v) ethanol (10 mL/kg/bw) for 6 weeks | 10 | 10 | ALT, AST, TAG, SOD, GSH, MDA, IL-1β, IL-6, and TNF-α |
| Wang et al., 2022 [172] | China | National Key R&D Program of China | No conflicts declared | C57BL/6N mice | Male | Not declared | 6 weeks old | Ethanol liquid diet (for the first week, 2 g ethanol/kg/bw/day; for the second week, 4 g ethanol/kg/bw/day; and for 3–5 weeks, 6 g ethanol/kg/bw/day) | 10 | 10 | ALT, AST, TAG, SOD, and MDA |
| Wang and Mu, 2021 [173] | China | No information | No conflicts declared | Wistar rats | Not declared | 180–210 g | Not declared | 3 g/kg/day (40% v/v) ethanol challenge for 4 weeks | 6 | 6 | ALT, SOD, GPx, LPO, TNF-a, and IL-6 |
| Wei et al., 2013 [174] | China | National Natural Science Foundation of China, the Guangxi Natural Science Foundation, and the Foundation for the Guangxi Key Laboratory for Prevention and Treatment of Regional High-Incidence Diseases | No conflicts declared | Wistar rats | Male | 180–200 g | Not declared | Ethanol 5.0 g/kg/day from 1 to 4 weeks, 7.0 g/kg/day from 5 to 8 weeks, and 9.0 g/kg/day from 9 to 12 weeks, for a total of 24 weeks | 15 | 15 | ALT, AST, TNF-a, IL-1B, SOD, GPx, GR, CAT, MDA, and CYP2E1 |
| Wu et al., 2019 [175] | China | National Natural Science Foundation of China | No conflicts declared | C57BL/6J mice | Male | 18.0 ± 2.0 g | 8 weeks old | Alcohol solution (52%, 7.5 mL/kg/bw) for 12 weeks | 10 | 10 | ALT, AST, MDA, and SOD |
| Wu et al., 2019 [176] | China | The National Key Research and Development Program of China and National Natural Science Foundation of China | No conflicts declared | Kunming mice | Female | 21–25 g | 4 weeks old | 50% ethanol (14 mL/kg) for 4 weeks | 3 or 10 | 3 or 10 | ALT, AST, SOD, CAT, GPx, MDA, TAG, TNF-a, IL-6, IL-1B, CYP2E1, and Bax/Blc-2 ratio |
| Xia et al., 2018 [177] | China | National Natural Science Foundation of China, Tianjin Municipal Science and Technology Commission, Rural Affairs Committee of Tianjin, and the Innovative Research Team of Tianjin Municipral Education Commission | No conflicts declared | ICR mice | Male | Not declared | 8 weeks old | Single dose of ethanol 50% (w/v), 4.8 g/kg bw | 6 | 6 | ALT, AST, MDA, SOD, CYP2E1, Caspase 3, TNF-a, and IL-6 |
| Xiao et al., 2014 [178] | China | Zhejiang Provincial Natural Science Foundation of China; Small Project Funding, University Research Committee, HKU; General Research Fund, University Grant Council, Hong Kong SAR; and the Azalea Endowment Fund to KFS | No conflicts declared | Sprague Dawley rats | Female | 180–200 g | Not declared | Ethanol 4.0 g/kg for 10 weeks | 6 | 6 | ALT, AST, TAG, and CYP2E1 |
| Xiao et al., 2017 [179] | China | Joint Fund from the NSFC and Guangdong Provincial Government, the National Nature Science Foundation of China, the PhD Start-up Fund of the Natural Science Foundation of Guangdong, the China Postdoctoral Science Foundation, and the Guangdong | No conflicts declared | C57BL/6 mice | Male | 26 ± 2 g | 10 weeks old | Lieber-DeCarli 4% (w/v) ethanol-containing liquid diet for 8 weeks | 4 or 10 | 4 or 10 | ALT, AST, TAG, TBARS, SOD, GPx, CAT, GSH, GSH/GSSH ratio, Caspase-3, and Bax/bcl2 ratio |
| Xiao et al., 2020 [180] | China | Key Research and Development Program of Guangdong Province, the National Natural Science Foundation of China, the Scientific Research Startup Fund of Hainan University, the Guangdong Special Support Program, the Group Program of Natural Science Foundation of Guangdong Province, the Special Fund for Scientific Innovation Strategy-Construction of the High-level Academy of Agriculture Science, the Discipline Team Building Projects of Guangdong Academy of Agricultural Sciences in the 13th Five-Year Period, and the Open Fund of the Key Laboratory of Food Nutrition and Functional Food of Hainan Province | No conflicts declared | C57BL/6 mice | Male | 18 ± 2 g | 6 weeks old | Lieber-DeCarli ethanol liquid diet (4%, w/v) for 8 weeks | 4 or 8 | 4 or 8 | ALT, AST, TAG, TBARS, SOD, GPx, CAT, Caspase 3, Bax/Bcl2 ratio, GSH, and GSH/GSSG ratio |
| Xu et al., 2021 [181] | China | National Natural Science Foundation of China, Anhui Medical University of Science and Technology, and the University Synergy Innovation Program of Anhui Province | No conflicts declared | C57BL/6J mice | Male | 18–22 g | 6–8 weeks old | 5% ethanol liquid diet for 16 days, and a single alcohol plus binge (5 g/kg, 33% ethanol) on the last day | 6 | 6 | Steatosis score, ALT, AST, IL-1β, IL-6, and TNF-α |
| Yalçinkaya et al., 2007 [182] | Turkey | Research Fund of the University of İstanbul | No information | Wistar rats | Male | 180–200 g | Not declared | Ethanol was added to drinking water 20% (v/v) for 75 days (approximately 8.5 g/kg/bw/day) | 6 | 8 | ALT, AST, MDA, Protein carbonyl, GSH, SOD, GPx, and GST |
| Yan and Yin, 2007 [183] | Taiwan | No information | No information | Balb/cA mice | Male | Not declared | 5–6 weeks old | Three doses of 25% (w/v) ethanol were administered at 5 g/kg/bw by gavage every 12 h | 15 | 15 | ALT, AST, GSH, GSH/GSSG ratio, GPx, and CAT |
| Yang et al., 2013 [184] | China | 973 Program | No conflicts declared | C57BL/6 mice | Male | Not declared | 8–10 weeks old | A single dose of ethanol (5 g/kg) | 5 | 5 | ALT, AST, TAG, MDA, GSH, and SOD |
| Yang et al., 2021 [185] | China | Zhongyuan Scholars, Strategic Consulting Research Project of Henan Institute of Chinese Engineering Development Strategies, Major Science and Technology Projects for Public Welfare of Henan Province, Youth Talent Support Program, Key Project Foundation of Natural Science Research, Key Scientific and Technological Research Projects of Henan Province, Fundamental Research Funds for the Henan Provincial Colleges and Universities in Henan University of Technology, High-Level Talents Research Fund of HAUT, and Open Research Subject of the National Engineering Laboratory for Wheat and Corn Further Processing | No conflicts declared | Kunming mice | Male | 20 ± 2 g | 6 weeks old | 52% ethanol (5 mL/kg/bw) thrice every 12 h | 10 | 10 | TAG, GSH, MDA, and SOD |
| Yang et al., 2022 [186] | China | National Natural Science Foundation of China | No conflicts declared | C57BL/6J mice | Male | 22 ± 2 g | 8 weeks old | Lieber-DeCarli liquid alcohol diet for 5 weeks [alcohol was gradually increased from 1% to 4% (w/v)] | 10 | 10 or 12 | ALT, AST, TAG, GSH, GPx, SOD, MDA, TNF-a, IL-6, IL-1B, and CAT |
| Yao et al., 2007 [187] | China | National Natural Science Foundation of China, and Program for New Century Excellent Talents in the University of China | No information | Sprague Dawley rats | Male | 140–160 g | Not declared | Ethanol 2.4 g/kg (30% v/v, 10 mL/kg) for 90 days | 3 or 8 | 3 or 8 | ALT, AST, GPx, CAT, GSH, and MDA |
| Yeh et al., 2020 [188] | Taiwan | Ministry of Science and Technology and National Taiwan Normal University | No conflicts declared | C57BL/6 mice | Male | Not declared | 7 weeks old | Modified Lieber-DeCarli ethanol liquid diet (500 mg/kg/bw) for 11 weeks | 10 | 10 | ALT, AST, TAG, TNF-α, IL-1β, Histology (liver steatosis score and liver inflammation score), PPAR-a, SREBP-1, MDA, GSH, CYP2E1, and Nrf2 |
| Yoon et al., 2012 [189] | South Korea | Technology Development Program for Food, Ministry for Food, Agriculture, Forestry, and Fisheries | No conflicts declared | Sprague Dawley rats | Male | 150–170 g | Not declared | Lieber-DeCarli ethanol liquid diet for 8 weeks, Ethanol was introduced progressively at 3% (w/v) of the liquid diet for 2 days, 4% for the subsequent 2 days, and 5% thereafter | 8 | 8 | ALT, AST, Steatosis score, Inflammation score, TNF-a, IL-6, MDA, and GSH |
| You et al., 2010 [190] | Republic of Korea | Jeollanam-Do | No conflicts declared | ICR mice | Male | 30 ± 2 g | 8 weeks old | 5 g/kg/bw/day of ethanol by gastric intubation for 8 days | 8 | 8 | ALT, AST, CAT, GST, GPx, GR, GSH, and MDA |
| You et al., 2020 [191] | China | National Key R&D Program of China | No conflicts declared | C57BL/6 mice | Male | Not declared | Not declared | Lieber-DeCarli liquid diet for 8 weeks | 3 or 10 | 3 or 10 | ALT, AST, TAG, IL-6, TNF-a, MDA, Protein Carbonyl, and GPx |
| Yu et al., 2019 [192] | China | National Natural Science Foundation of China, Special Funds for National Key Sci-Tech Special Project of China, Shanghai Science and Technology Committee, Science Fund for Creative Research Groups | No conflicts declared | C57BL/6J mice | Male | 20–22 g | 6–8 weeks old | Single dose of ethanol 50% (v/v) (5 g/bw) by gavage | 10 | 10 | ALT, AST, Histology, TAG, and MDA |
| Yu et al., 2022 [193] | China | No information | No information | Sprague Dawley rats | Male | 185–200 g | 6–7 weeks old | 8 mL/kg/day ethanol twice daily, changing weekly, for 4 weeks (10%, 15%, 30%, and 56% alcohol v/v) | 5 or 10 | 5 or 10 | ALT, AST, SOD, GPx, MDA, and Nrf-2 |
| Yuan et al., 2018 [194] | China | Jilin Pharmaceutical Industry Promotion Plan | No conflicts declared | ICR mice | Male | 19–21 g | Not declared | Single dose of 50% ethanol solution (12 mL/bw) intragastrically | 10 | 10 | ALT, AST, TAG, MDA, SOD, and CYP2E1 |
| Yuan et al., 2020 [195] | China | Guangdong Province Key Laboratory for New Drugs Research and Development of Chinese Medicine, China, Project of Guangzhou University of Chinese Medicine, Science and Technology Project Scheme of Guangdong Province, China, and Natural Science Foundation of Guangdong Province, China | No conflicts declared | Kunming mice | Male | 18–22 g | Not declared | 30% ethanol (10 mL/kg) intragastrically for one week, after that, the ethanol concentration iwas ncreased gradually (the next 3 weeks were 40%, 50%, and 55%) | 3, 5 or 6 | 3, 5, or 6 | ALT, AST, TAG, Bax/Bcl-2 ratio, Caspase3, and TNF-a |
| Zahid et al., 2018 [196] | India | No significantfinancial support for this work | No conflicts declared | Sprague Dawley rats | Not declared | 150–210 g | Not declared | 50% ethanol (12 mL/kg/bw) administered once a day for 8 days | 5 | 5 | ALT, AST, SOD, CAT, GSH, and TBARS |
| Zeng et al., 2013 [197] | China | Shandong Province Science Foundation and Postdoctoral Science Foundation Funded Project of Shandong Province | No conflicts declared | Kunming mice | Male | 18–22 g | Not declared | Ethanol (5 g/kg/bw) at 12-h intervals for a total of three doses | 10 | 10 | ALT, AST, MDA, and GSH |
| Zhang et al., 2011 [198] | China | Science and Technology Funds of Suzhou City and Jiangsu Province | No conflicts declared | Kunming mice | Male | 22 ± 2 g | Not declared | 52% alcohol for 4 weeks (the amount of alcohol was gradually increased from 0.2 mL (10 g/day) to 0.4 mL (10 g/day) over 1 week) | 6 or 10 | 6 or 10 | TAG, SOD, MDA, and GPx |
| Zhang et al., 2014 [199] | China | National Natural Science Foundation of China, Priority Academic Program Development of Jiangsu Higher Education Institutions and College Students Innovation Project for the R&D of Novel Drugs | No conflicts declared | ICR mice | Male | 24–16 g | 8 weeks old | Acute experiment: Three doses of ethanol (6 g/kg) at 12-h intervalsChronic experiment: Lieber–DeCarli liquid diets containing 36% ethanol for 5 weeks | 7 or 8 | 7 or 8 | ALT, TBARS, GSH, Steatosis score, TAG, TBARS, and CYP2E1 |
| Zhang et al., 2015 [200] | China | No information | No information | ICR mice | Male | 24–26 g | 8 weeks old | Acute experiment: ethanol (6 g/kg orally gavage) three times at 12-h intervalsChronic experiment: Lieber-DeCarli liquid diet containing ethanol at 36% of the caloric content for 5 weeks | 3,4 or 6 | 3,4 or 6 | ALT, AST, GSH, GPx, TBARS, TAG, and CYP2E1 |
| Zhang et al., 2020 [201] | China | Science and Technology Develop Project in Jilin Province of China, the Special Projects of Cooperation between Jilin University and Jilin Province in China, Innovation Training Program of Zhuhai College of Jilin University, and “Three levels” Talent Construction Projects in Zhuhai College of Jilin University | No conflicts declared | C57BL/6 mice | Male | 18–22 g | 8–10 weeks old | Mice were intragastrically administrated with 13 g/kg of 56% ethanol for 14 days | 6 or 10 | 6 or 10 | ALT, AST, MDA, SOD, GPx, and CAT |
| Zhang et al., 2020 [202] | China | National Natural Science Foundation of China and Changsha Science and Technology Bureau | No conflicts declared | C57BL/6 mice | Male | Over 20 g | 8 weeks old | Lieber-DeCarli ethanol liquid diet for 10 days | 6 | 6 | / |
| Zhang et al., 2021 [203] | China | National Natural Science Foundation of China and the Science and Technology Research of Shanxi Province | No conflicts declared | C57/B6 mice | Male | Not declared | 6 weeks old | Daily oral gavage of 50% (v/v) ethanol (4 g/kg) for 8 weeks | 7 | 7 | ALT, AST, TAG, TNF-α, IL−1β, IL−10, SOD, and GPx |
| Zhao et al., 2008 [204] | China | National Grand Fundamental Research 973 Program of China | No information | ICR mice | Male | 22–24 g | Not declared | Single dose of alcohol 6 g/kg | 8 | 8 | ALT, TAG, TBARS, GSH, SOD, CAT, GR, GPx, TNF-a, and IL-1B |
| Zhao et al., 2018 [205] | China | National Natural Science Foundation of China and the Study and Demonstration of Introduction and Deep Processing Technology of Quinoa in Mountainous Regions | No conflicts declared | ICR mice | Male | 20–22 g | Not declared | 50% alcohol (10 mL/kg/bw) by oral gavage for 5 weeks | 10 | 10 | ALT, AST, TAG, MDA, SOD, CAT, GSH, GPx, TNF-a, and IL-6 |
| Zhao et al., 2021 [206] | China | China Postdoctoral Science Foundation, Beijing Postdoctoral Research Foundation, Technological Innovation Service Capacity Building-Basic Scientific Research Expenses, and Taif University Researchers Supporting Project | No conflicts declared | ICR mice | Male | 22–24 g | 7–9 weeks old | 50% (v/v) alcohol (10 mL/kg/bw daily) by oral route for 4 weeks | 6 | 6 | ALT, AST, TAG, MDA, SOD, GPx, GSH, CAT, IL-6, IL-1β, and TNF-α |
| Zhao et al., 2021 [207] | China | National Natural science Foundation of China, the Major Science, Technology Innovation Project of Shandong Province, and the Shandong Provincial Natural Science Foundation | No conflicts declared | Rats (lineage not specified) | Male | 200 ± 20 g | 8 weeks old | Oral gavage of 7 mL per kg/bw ethanol 56% (v/v) for the first 4 weeks, and then gavage of 9 mL per kg/bw alcohol for the remaining 16 weeks | 5 or 10 | 5 or 10 | ALT, AST, TAG, SOD, GPx, CAT, MDA, Bax/Bcl2 ratio, and Caspase-3 |
| Zheng et al., 2019 [208] | China | National Natural Science Foundation of China; the Priority Academic Program Development of Jiangsu Higher Education Institutions, the Universities Natural Science Research Project of Jiangsu Province; the Primary Research and Development Plan of Jiangsu Province; the Northern Jiangsu Project of Science and Technology Development | No conflicts declared | Kunming mice | Male | 20 ± 2 g | Not declared | 12 mL/kg of 50% alcohol every 12 h for a total of three times | 10 | 10 | ALT, AST, TNF-a, IL-1B, SOD, CAT, GPx, and MDA |
| Zheng et al., 2022 [209] | China | Basal Research Fund of the National Health Commission Key Laboratory of Birth Defect Prevention, the Medical Science and Technology Research Project of Henan Province, the Project of Basal Research Fund of Henan Institute of Medical and Pharmacological Sciences, the Basal Research Fund of Henan Academy of Sciences | No conflicts declared | Kunming mice | Male | 20–22 g | 8 weeks old | Single dose of 70% ethanol (12 mL/kg/bw) | 8 | 8 | ALT, AST, TAG, CAT, GPx, Nrf2, and Caspase 3 |
| Zhou et al., 2002 [210] | United States | No information | No information | C57BL/6 mice | Male | Not declared | 9 weeks old | Three doses of 25% (w/v) ethanol were administered at 5 g/kg body weight by gavage every 12 h | 5 | 5 | ALT, GSH, GSH/GSSG ratio, TBARS, Protein carbonyl, and CYP2E1 |
| Zhou et al., 2018 [211] | China | No information | No information | Wistar rats | Not declared | 220–240 g | 7 weeks old | In the first week, rats were treated with alcohol (56%; 0.8 mL/100 g) daily by oral gavage. The amount of alcohol was increased by 0.1 mL every other week until the 8th week (1.5 mL/100 g in the 8th week) | 6 or 10 | 6 or 10 | ALT, AST, TAG, GSH, SOD, MDA, amd TNF-a |
| Zhou et al., 2021 [212] | China | Deep Process and Functional Food Development of Daylily and Astragalus | No conflicts declared | ICR mice | Male | 18–21 g | Not declared | 10 mL/kg of 50% alcohol, by oral gavage for 4 weeks | 12 | 12 | ALT, AST, TAG, SOD, CAT, GSH, GPx, MDA, TNF-a, IL-6, and IL-1B |
| Zhou et al., 2022 [213] | China | Deep Process and Functional Food Development of Daylily and Astragalus, Taif University Researchers Supporting Project, and the National Dairy Industry and Technology System of China | No conflicts declared | ICR mice | Male | 20 ± 1 g | 5 weeks old | 50% alcohol (10 mL/kg/bw) for 4 weeks | 12 | 12 | ALT, AST, TAG, CAT, SOD, GSH, GPx, MDA, IL-1B, IL-6, and TNF-a |
| Zhu et al., 2014 [214] | China | Heilongjiang Development and Reform Commission, Heilongjiang Education Department, the Heilongjiang Education Department of Science and Technology Research Project; the Harbin Special Funds for Technological Innovation Research Projects, the Heilongjiang Postdoctoral Scientific Research Foundation, and the National Natural Science Foundation of China | No information | Kunming mice | Male | Not declared | 8–10 weeks old | 40% ethanol (5 g/kg/bw) for 6 weeks | 8 | 8 | ALT, AST, TAG, SOD, and GPx |
| Zhu et al., 2021 [215] | China | National Natural Science Foundation of China | No conflicts declared | C57BL/6 mice | Male | 22–25 g | Not declared | Lieber-DeCarli ethanol diet for 10 days. On day 11, mice were gavaged with a single dose of 31.5% (v/v) ethanol (20 μL/gbw) | 8 | 8 | ALT, AST, TAG, MDA, SREBP-1, and PPAR-a |
| Author and Year | Selection Bias | Performance Bias | Detection Bias | Attrition Bias | Reporting Bias | Other | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| Abdelhamid et al., 2020 [10] | Y | U | U | Y | U | U | U | Y | Y | Y |
| Abdelhamid et al., 2021 [11] | Y | U | U | Y | U | U | U | Y | Y | Y |
| Al-Rejaie, 2012 [12] | Y | U | U | U | U | U | U | Y | Y | U |
| Atef et al., 2018 [13] | Y | U | U | Y | U | U | U | Y | Y | Y |
| Bae et al., 2015 [14] | Y | U | U | Y | U | U | U | Y | Y | Y |
| Balasubramaniyan et al., 2003 [15] | U | U | U | Y | U | U | U | N | Y | U |
| Baranisrinivasan et al., 2009 [16] | Y | U | U | U | U | U | U | Y | Y | U |
| Bardag-Gorce et al., 2011 [17] | U | U | U | U | U | U | U | Y | Y | U |
| Bhakuni et al., 2017 [18] | Y | U | U | U | U | U | U | Y | Y | U |
| Bharrhan et al., 2011 [19] | Y | U | U | U | U | U | U | N | Y | Y |
| Bisht et al., 2018 [20] | U | U | U | U | U | U | U | Y | Y | Y |
| Bispo et al., 2017 [21] | U | U | U | U | U | U | U | Y | Y | U |
| Buko et al., 2019 [22] | U | U | U | U | U | U | U | Y | Y | Y |
| Bulle et al., 2015 [23] | U | U | U | U | U | U | U | Y | Y | U |
| Cao et al., 2015 [24] | Y | U | U | Y | U | U | U | N | Y | U |
| Chandra et al., 2000 [25] | U | U | U | U | U | U | U | Y | Y | U |
| Chang et al., 2017 [26] | Y | U | U | Y | U | Y | U | N | Y | Y |
| Chang et al., 2021 [27] | U | U | U | U | U | U | U | N | Y | Y |
| Chaturvedi et al., 2007 [28] | U | U | U | U | U | U | U | Y | Y | U |
| Chavan et al., 2017 [29] | Y | U | U | U | U | U | U | Y | Y | Y |
| Chen et al., 2013 [30] | N | Y | U | Y | U | U | U | N | Y | Y |
| Chen et al., 2016 [31] | U | U | U | Y | U | U | U | N | Y | Y |
| Cheng and Khong, 2011 [32] | U | U | U | U | U | U | U | Y | Y | U |
| Chiu et al., 2011 [33] | Y | U | U | U | U | U | U | Y | Y | Y |
| Chu et al., 2021 [34] | U | U | U | U | U | U | U | N | Y | U |
| Colontoni et al., 2000 [35] | N | Y | U | U | U | U | U | Y | Y | U |
| Cui et al., 2014 [36] | Y | U | U | U | U | U | U | N | Y | U |
| Cui et al., 2014 [37] | Y | U | U | U | U | U | U | N | Y | U |
| Das et al., 2007 [38] | U | U | U | U | U | U | U | Y | Y | U |
| Das et al., 2012 [39] | Y | U | U | U | U | U | U | N | Y | Y |
| De Souza et al., 2015 [40] | U | U | U | Y | U | U | U | N | Y | U |
| Develi et al., 2014 [41] | U | U | U | U | U | U | U | Y | Y | Y |
| Dou et al., 2013 [42] | U | U | U | U | U | U | U | Y | Y | Y |
| Du et al., 2015 [43] | U | U | U | U | U | U | U | N | Y | Y |
| Duryee et al., 2018 [44] | U | U | U | U | U | U | U | N | Y | U |
| Feng et al., 2019 [45] | Y | U | U | Y | U | U | U | Y | Y | Y |
| Galligan et al., 2012 [46] | U | U | U | U | U | U | U | N | Y | U |
| Gao et al., 2021 [47] | Y | U | U | Y | U | U | U | Y | Y | Y |
| George and Chaturvedi, 2009 [48] | Y | U | U | U | U | U | U | Y | Y | Y |
| Gustot et al., 2006 [49] | U | U | U | U | U | U | U | N | Y | U |
| Han et al., 2021 [50] | Y | U | U | U | U | U | U | Y | Y | Y |
| Hao et al., 2018 [51] | U | U | U | U | U | U | U | Y | Y | Y |
| Hao et al., 2021 [52] | U | U | U | U | U | U | U | Y | Y | U |
| Hasanein and Seifi, 2018 [53] | Y | U | U | U | U | U | U | Y | Y | Y |
| He et al., 2021 [54] | U | U | U | U | U | U | U | Y | Y | U |
| Hsu et al., 2018 [55] | Y | U | U | U | U | U | U | N | Y | Y |
| Hu et al., 2021 [56] | Y | U | U | U | U | U | U | N | Y | Y |
| Huang et al., 2017 [57] | Y | U | U | U | U | U | U | N | Y | Y |
| Ilaiyaraja and Khanum, 2011 [58] | Y | U | U | U | U | U | U | Y | Y | Y |
| Jayaraman et al., 2009 [59] | U | U | U | U | U | U | U | Y | Y | Y |
| Jiang et al., 2016 [60] | Y | U | U | U | U | U | U | Y | Y | Y |
| Jiang et al., 2019 [61] | Y | U | U | Y | U | U | U | Y | Y | Y |
| Jin et al., 2010 [62] | U | U | U | U | U | U | U | Y | Y | U |
| Jose et al., 2018 [63] | Y | U | U | U | U | U | U | N | Y | N |
| Kanbak et al., 2001 [64] | U | U | U | Y | U | U | U | Y | Y | U |
| Kanchana and Jayapriya, 2013 [65] | U | U | U | U | U | U | U | Y | Y | U |
| Kang et al., 2010 [66] | U | U | U | U | U | U | U | N | Y | U |
| Kang et al., 2021 [67] | U | U | U | U | U | U | U | Y | Y | Y |
| Kaviarasan et al., 2008 [68] | Y | U | U | U | U | U | U | Y | Y | Y |
| Khanal et al., 2009 [69] | U | U | U | U | U | U | U | Y | Y | Y |
| Kim et al., 2012 [70] | U | U | U | U | U | U | U | N | Y | Y |
| Kim et al., 2016 [71] | Y | U | U | U | U | U | U | Y | Y | Y |
| Kumar et al., 2019 [72] | Y | U | U | U | U | U | U | Y | Y | Y |
| Lai et al., 2019 [73] | Y | U | U | U | U | U | U | Y | Y | Y |
| Lee et al., 2015 [74] | U | U | U | U | U | U | U | Y | Y | U |
| Lee et al., 2016 [75] | U | U | U | U | U | U | U | Y | Y | U |
| Lee et al., 2016 [76] | Y | U | U | U | U | U | U | Y | Y | Y |
| Lee et al., 2020 [77] | Y | U | U | U | U | U | U | N | Y | Y |
| Lee et al., 2020 [78] | Y | U | U | U | U | U | U | N | Y | Y |
| Lee et al., 2021 [79] | N | Y | U | Y | U | U | U | Y | Y | Y |
| Li et al., 2013 [80] | Y | U | U | U | U | U | U | Y | Y | Y |
| Li et al., 2015 [81] | Y | U | U | U | U | U | U | Y | Y | U |
| Li et al., 2016 [82] | Y | U | U | U | U | U | U | N | Y | Y |
| Li et al., 2017 [83] | Y | U | U | U | U | U | U | N | Y | Y |
| Li et al., 2018 [84] | N | Y | U | U | U | U | U | Y | Y | Y |
| Li et al., 2021 [85] | N | Y | U | U | U | U | U | Y | Y | Y |
| Li et al., 2021 [86] | N | Y | U | U | U | U | U | Y | Y | Y |
| Li et al., 2021 [87] | U | U | U | U | U | U | U | Y | Y | Y |
| Li et al., 2021 [88] | Y | U | U | Y | U | U | U | Y | Y | Y |
| Lian et al., 2010 [89] | Y | U | U | U | U | U | U | Y | Y | U |
| Lin et al., 2017 [90] | U | U | U | U | U | U | U | Y | Y | Y |
| Lin et al., 2021 [91] | Y | U | U | Y | U | U | U | Y | Y | Y |
| Liu et al., 2014 [92] | Y | U | U | Y | U | U | U | N | Y | Y |
| Liu et al., 2015 [93] | Y | U | U | U | U | U | U | N | Y | Y |
| Liu et al., 2020 [94] | Y | U | U | U | U | U | U | N | Y | Y |
| Liu et al., 2022 [95] | Y | U | U | U | U | U | U | Y | Y | Y |
| Liu et al., 2022 [96] | Y | U | U | U | U | U | U | N | Y | Y |
| Lu et al., 2014 [97] | Y | U | U | Y | U | U | U | N | Y | U |
| Lu et al., 2015 [98] | Y | U | U | U | U | U | U | Y | Y | Y |
| Lu et al., 2020 [99] | N | Y | U | Y | U | U | U | Y | Y | Y |
| Ma et al., 2007 [100] | U | U | U | U | U | U | U | Y | Y | U |
| Madushani Herath et al., 2018 [101] | U | U | U | U | U | U | U | Y | Y | Y |
| Mai et al., 2022 [102] | Y | U | U | U | U | U | U | N | Y | N |
| Maimaitimin et al., 2018 [103] | Y | U | U | U | U | U | U | Y | Y | U |
| Mallikarjuna et al., 2008 [104] | U | U | U | Y | U | U | U | Y | Y | U |
| Mandal et al., 2013 [105] | U | U | U | U | U | U | U | Y | Y | Y |
| Mehanna et al., 2021 [106] | Y | U | U | U | U | U | U | N | Y | Y |
| Meng et al., 2020 [107] | Y | U | U | U | U | U | U | Y | Y | Y |
| Miñana et al., 2002 [108] | U | U | U | U | U | U | U | N | Y | U |
| Ming et al., 2021 [109] | Y | U | U | U | U | U | U | Y | Y | Y |
| Mohan et al., 2019 [110] | Y | U | U | U | U | U | U | N | Y | N |
| Nagappan et al., 2018 [111] | Y | U | U | U | U | U | U | N | Y | Y |
| Nie et al., 2021 [112] | Y | U | U | U | U | U | U | Y | Y | Y |
| Nie et al., 2022 [113] | Y | U | U | U | U | U | U | Y | Y | Y |
| Oh et al., 2002 [114] | N | Y | U | Y | U | U | U | Y | Y | U |
| Osaki et al., 2016 [115] | U | U | U | U | U | U | U | Y | Y | U |
| Panda et al., 2012 [116] | Y | U | U | U | U | U | U | Y | Y | U |
| Panda et al., 2015 [117] | Y | U | U | U | U | U | U | Y | Y | U |
| Pari and Suresh, 2008 [118] | Y | U | U | U | U | U | U | Y | Y | Y |
| Park et al., 2013 [119] | Y | U | U | Y | U | U | U | Y | Y | Y |
| Park et al., 2017 [120] | Y | U | U | U | U | U | U | Y | Y | Y |
| Park et al., 2019 [121] | Y | U | U | U | U | U | U | Y | Y | Y |
| Patere et al., 2011 [122] | Y | U | U | U | U | U | U | Y | Y | U |
| Peng et al., 2011 [123] | N | Y | U | Y | U | U | U | Y | Y | U |
| Peng et al., 2013 [124] | N | Y | U | U | U | U | U | Y | Y | Y |
| Pi et al., 2021 [125] | Y | U | U | U | U | U | U | N | Y | Y |
| Prathibha et al., 2013 [126] | N | Y | U | U | U | U | U | Y | Y | U |
| Qi et al., 2017 [127] | U | U | U | U | U | U | U | Y | Y | Y |
| Qu et al., 2019 [128] | Y | U | U | U | U | U | U | N | Y | U |
| Rabelo et al., 2018 [129] | U | U | U | U | U | U | U | U | Y | Y |
| Rejitha et al., 2012 [130] | N | Y | U | U | U | U | U | Y | Y | Y |
| Roede et al., 2008 [131] | U | U | U | U | U | U | U | N | Y | U |
| Roede et al., 2009 [132] | U | U | U | U | U | U | U | N | Y | U |
| Rong et al., 2012 [133] | Y | U | U | Y | U | U | U | Y | Y | U |
| Ronis et al., 2004 [134] | U | U | U | U | U | U | U | N | Y | U |
| Ronis et al., 2010 [135] | U | U | U | U | U | U | U | N | Y | U |
| Samuhasaneeto et al., 2009 [136] | Y | U | U | U | U | U | U | Y | Y | U |
| Saravanan, 2007 [137] | U | U | U | U | U | U | U | Y | Y | U |
| Saravanan and Nalini, 2007 [138] | U | U | U | U | U | U | U | Y | Y | U |
| Sathiavelu et al., 2009 [139] | U | U | U | U | U | U | U | Y | Y | Y |
| Senthilkumar et al., 2004 [140] | U | U | U | U | U | U | U | Y | Y | U |
| Shankari et al., 2010 [141] | Y | U | U | U | U | U | U | Y | Y | U |
| Shearn et al., 2014 [142] | U | U | U | U | U | U | U | Y | Y | U |
| Shenbagam and Nalini, 2010 [143] | U | U | U | U | U | U | U | Y | Y | U |
| Shi et al., 2018 [144] | Y | U | U | U | U | U | U | N | Y | Y |
| Smathers et al., 2013 [145] | U | U | U | U | U | U | U | Y | Y | U |
| Sönmez et al., 2012 [146] | Y | U | U | U | U | U | U | Y | Y | Y |
| Song et al., 2006 [147] | U | U | U | U | U | U | U | Y | Y | U |
| Song et al., 2018 [148] | Y | U | U | U | U | U | U | Y | Y | Y |
| Song et al., 2020 [149] | Y | U | U | U | U | U | U | Y | Y | Y |
| Song et al., 2021 [150] | Y | U | U | U | U | U | U | Y | Y | Y |
| Sudha et al., 2012 [151] | Y | U | U | U | U | U | U | Y | Y | U |
| Sun et al., 2016 [152] | U | U | U | U | U | U | U | N | Y | Y |
| Tahir et al., 2013 [153] | U | U | U | U | U | U | U | Y | Y | U |
| Tan et al., 2016 [154] | Y | U | U | U | U | U | U | N | Y | N |
| Tang et al., 2012 [155] | Y | U | U | U | U | U | U | Y | Y | Y |
| Tang et al., 2014 [156] | U | U | U | U | U | U | U | N | Y | U |
| Tang et al., 2014 [157] | Y | U | U | U | U | U | U | N | Y | Y |
| Tang et al., 2017 [158] | Y | U | U | U | U | U | U | Y | Y | Y |
| Tao et al., 2021 [159] | Y | U | U | U | U | U | U | Y | Y | Y |
| Valansa et al., 2020 [160] | Y | U | U | U | U | U | U | Y | Y | Y |
| Varghese et al., 2016 [161] | U | U | U | Y | U | U | U | N | Y | Y |
| Velvizhi et al., 2002 [162] | Y | U | U | U | U | U | U | Y | Y | U |
| Wang et al., 2014 [163] | Y | U | U | U | U | U | U | Y | Y | Y |
| Wang et al., 2015 [164] | Y | U | U | U | U | U | U | Y | Y | Y |
| Wang et al., 2016 [165] | Y | U | U | U | U | U | U | Y | Y | Y |
| Wang et al., 2018 [166] | Y | U | U | U | U | U | U | Y | Y | Y |
| Wang et al., 2020 [167] | Y | U | U | Y | U | U | U | N | Y | Y |
| Wang et al., 2020 [168] | N | Y | U | U | U | U | U | N | Y | Y |
| Wang et al., 2020 [169] | Y | U | U | Y | U | U | U | N | Y | Y |
| Wang et al., 2021 [170] | U | U | U | U | U | U | U | Y | Y | Y |
| Wang et al., 2022 [171] | Y | U | U | U | U | U | U | Y | Y | Y |
| Wang et al., 2022 [172] | U | U | U | U | U | U | U | N | Y | Y |
| Wang and Mu, 2021 [173] | U | U | U | U | U | U | U | N | Y | Y |
| Wei et al., 2013 [174] | U | U | U | U | U | U | U | Y | Y | Y |
| Wu et al., 2019 [175] | Y | U | U | U | U | U | U | N | Y | Y |
| Wu et al., 2019 [176] | Y | U | U | U | U | U | U | N | Y | Y |
| Xia et al., 2018 [177] | Y | U | U | U | U | U | U | N | Y | Y |
| Xiao et al., 2014 [178] | Y | U | U | U | U | U | U | Y | Y | Y |
| Xiao et al., 2017 [179] | Y | U | U | Y | U | U | U | N | Y | Y |
| Xiao et al., 2020 [180] | Y | U | U | Y | U | U | U | N | Y | Y |
| Xu et al., 2021 [181] | Y | U | U | U | U | U | U | N | Y | Y |
| Yalçinkaya et al., 2007 [182] | U | U | U | U | U | U | U | Y | Y | U |
| Yan and Yin, 2007 [183] | U | U | U | U | U | U | U | Y | Y | U |
| Yang et al., 2013 [184] | U | U | U | U | U | U | U | Y | Y | Y |
| Yang et al., 2021 [185] | Y | U | U | U | U | U | U | Y | Y | Y |
| Yang et al., 2022 [186] | Y | U | U | Y | U | U | U | N | Y | Y |
| Yao et al., 2007 [187] | Y | U | U | U | U | U | U | N | Y | U |
| Yeh et al., 2020 [188] | Y | U | U | U | U | U | U | Y | Y | Y |
| Yoon et al., 2012 [189] | Y | U | U | U | U | U | U | N | Y | Y |
| You et al., 2010 [190] | U | U | U | U | U | U | U | Y | Y | Y |
| You et al., 2020 [191] | Y | U | U | U | U | U | U | N | Y | Y |
| Yu et al., 2019 [192] | U | U | U | U | U | U | U | N | Y | Y |
| Yu et al., 2021 [193] | Y | U | U | U | U | U | U | N | Y | U |
| Yuan et al., 2018 [194] | Y | U | U | U | U | U | U | Y | Y | Y |
| Yuan et al., 2020 [195] | U | U | U | U | U | U | U | N | Y | Y |
| Zahid et al., 2018 [196] | Y | U | U | U | U | U | U | Y | Y | Y |
| Zeng et al., 2013 [197] | Y | U | U | U | U | U | U | Y | Y | Y |
| Zhang et al., 2010 [198] | Y | U | U | U | U | U | U | N | Y | Y |
| Zhang et al., 2014 [199] | Y | U | U | U | U | U | U | N | Y | Y |
| Zhang et al., 2015 [200] | U | U | U | U | U | U | U | N | Y | U |
| Zhang et al., 2020 [201] | Y | U | U | U | U | U | U | N | Y | Y |
| Zhang et al., 2020 [202] | U | U | U | U | U | U | U | Y | Y | Y |
| Zhang et al., 2021 [203] | Y | U | U | U | U | U | U | N | Y | Y |
| Zhao et al., 2008 [204] | U | U | U | U | U | U | U | N | Y | U |
| Zhao et al., 2018 [205] | Y | U | U | U | U | U | U | Y | Y | Y |
| Zhao et al., 2021 [206] | U | U | U | U | U | U | U | N | Y | Y |
| Zhao et al., 2021 [207] | Y | U | U | U | U | U | U | N | Y | Y |
| Zheng et al., 2019 [208] | Y | U | U | U | U | U | U | Y | Y | Y |
| Zheng et al., 2022 [209] | Y | U | U | U | U | U | U | Y | Y | Y |
| Zhou et al., 2002 [210] | U | U | U | U | U | U | U | N | Y | U |
| Zhou et al., 2018 [211] | Y | U | U | U | U | U | U | N | Y | U |
| Zhou et al., 2021 [212] | Y | U | U | U | U | U | U | Y | Y | Y |
| Zhou et al., 2022 [213] | Y | U | U | U | U | U | U | Y | Y | Y |
| Zhu et al., 2014 [214] | U | U | U | U | U | U | U | Y | Y | U |
| Zhu et al., 2021 [215] | Y | U | U | U | U | U | U | Y | Y | Y |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rabelo, A.C.S.; Andrade, A.K.d.L.; Costa, D.C. The Role of Oxidative Stress in Alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis of Preclinical Studies. Nutrients 2024, 16, 1174. https://doi.org/10.3390/nu16081174
Rabelo ACS, Andrade AKdL, Costa DC. The Role of Oxidative Stress in Alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis of Preclinical Studies. Nutrients. 2024; 16(8):1174. https://doi.org/10.3390/nu16081174
Chicago/Turabian StyleRabelo, Ana Carolina Silveira, Amanda Kelly de Lima Andrade, and Daniela Caldeira Costa. 2024. "The Role of Oxidative Stress in Alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis of Preclinical Studies" Nutrients 16, no. 8: 1174. https://doi.org/10.3390/nu16081174